SlTDC1 Overexpression Promoted Photosynthesis in Tomato under Chilling Stress by Improving CO2 Assimilation and Alleviating Photoinhibition
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrolyte Leakage Rate and H2O2 Content
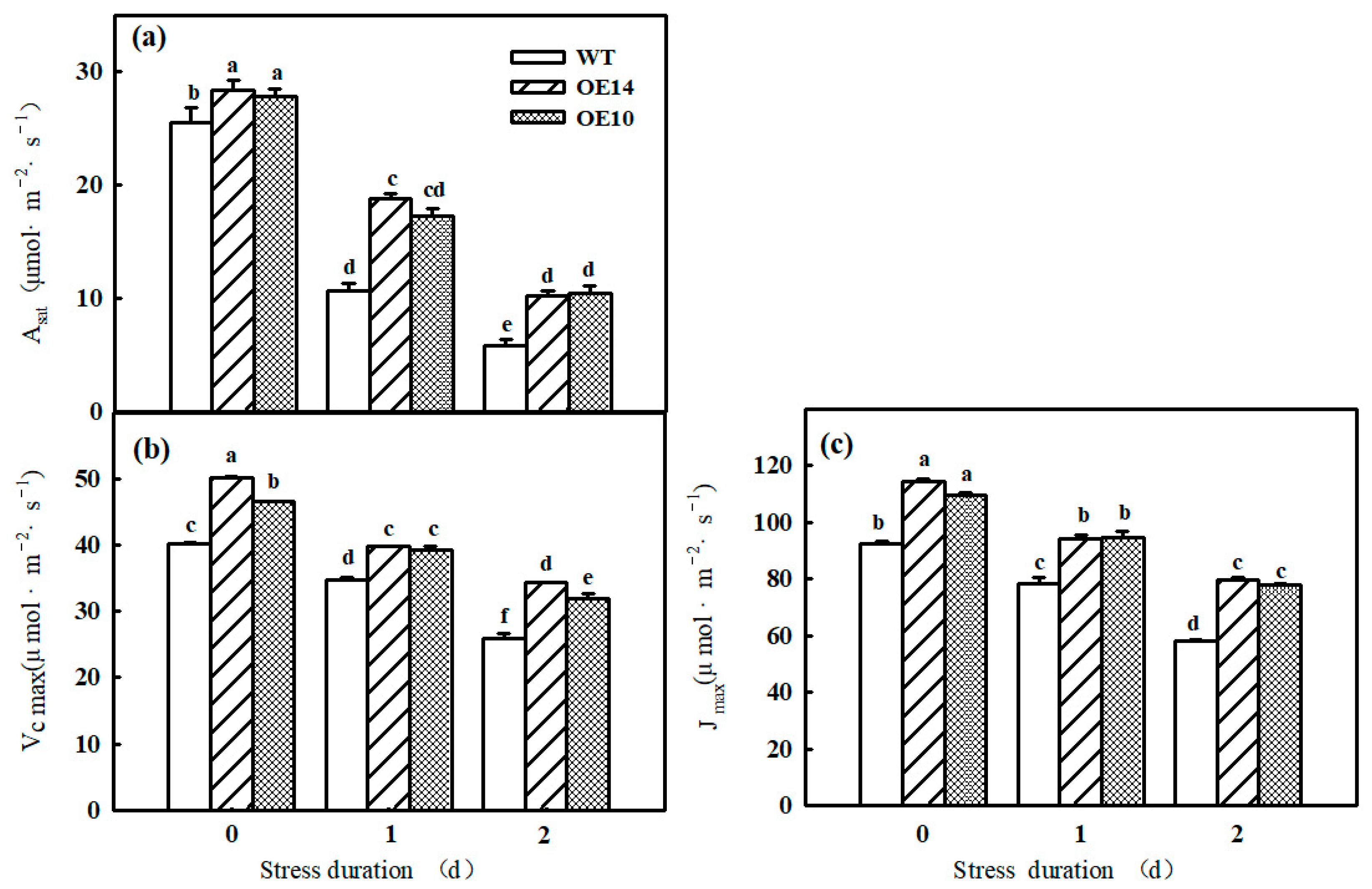
2.2. Photosynthetic Parameter
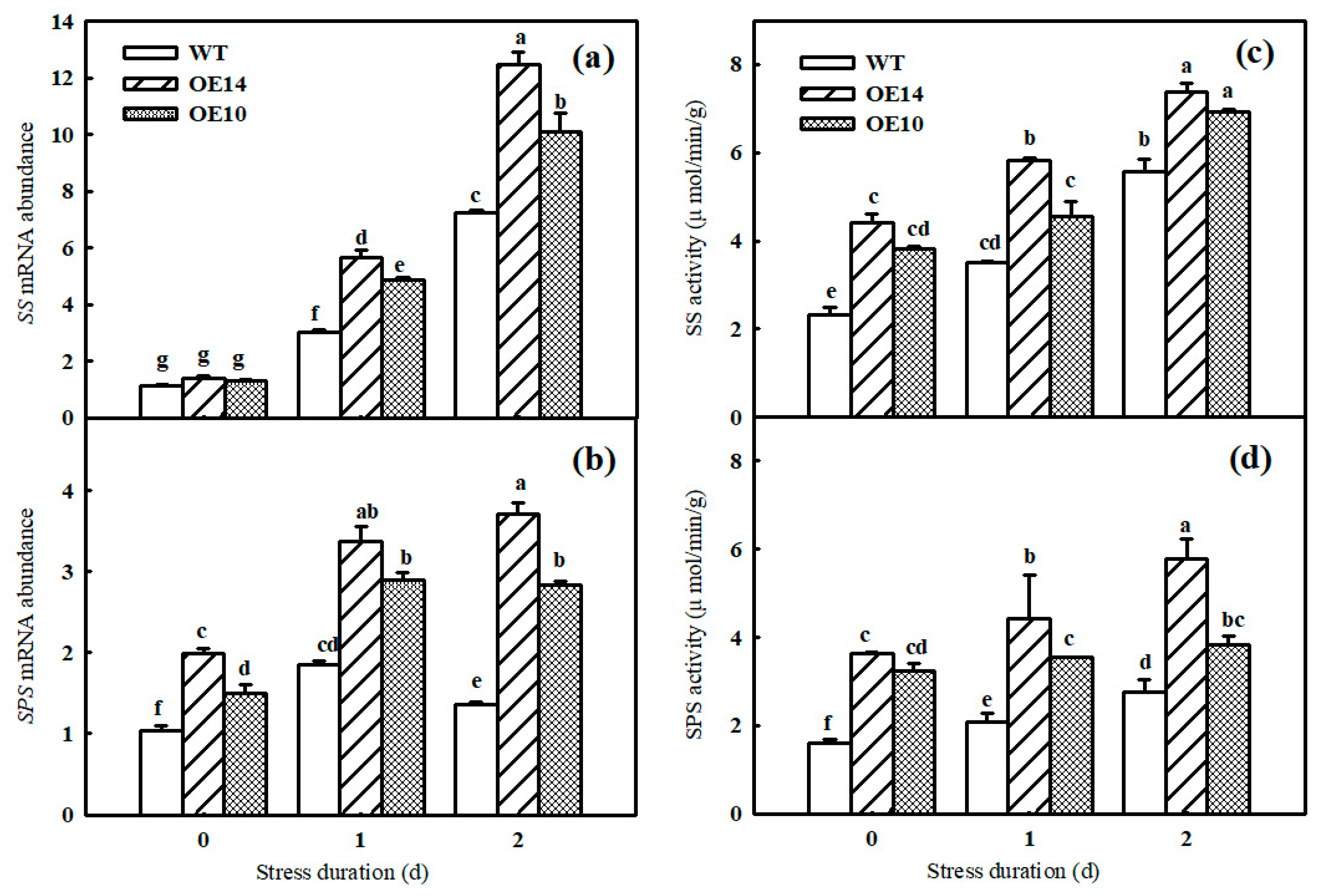
2.3. Gene Expression and Photosynthetic Enzyme Activity
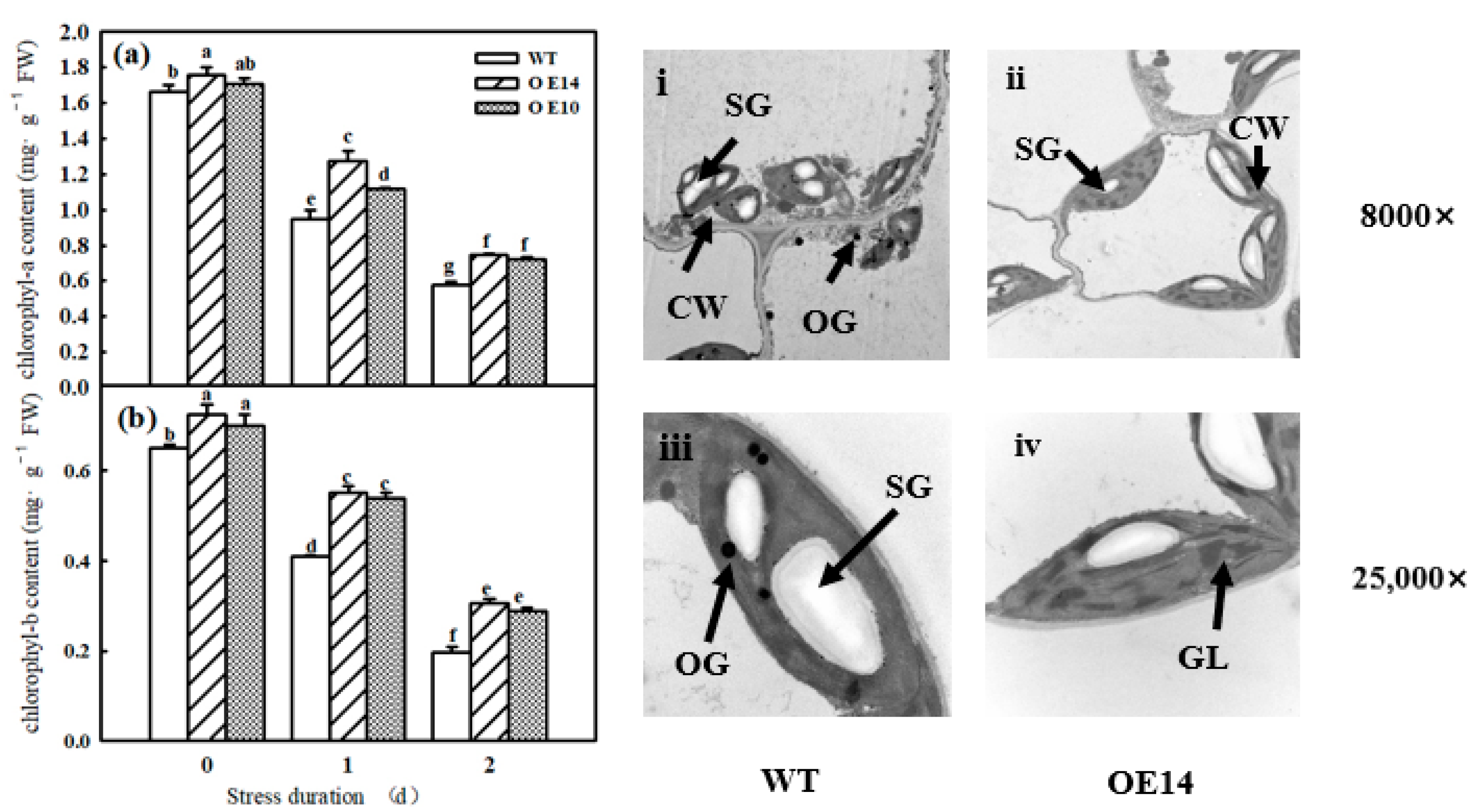
2.4. Chlorophyll Content and Chloroplast Ultrastructure
2.5. Carbon Metabolism
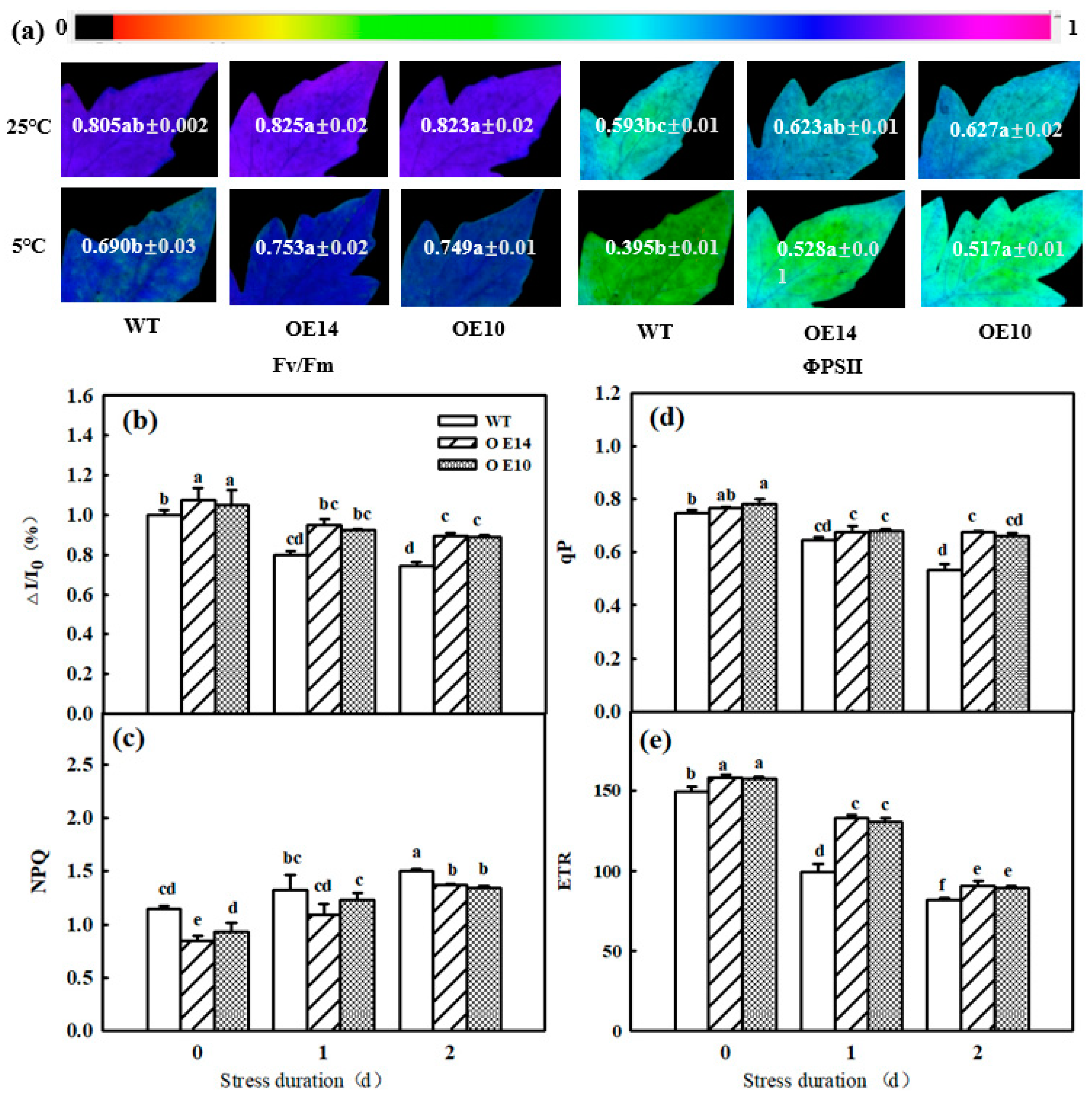
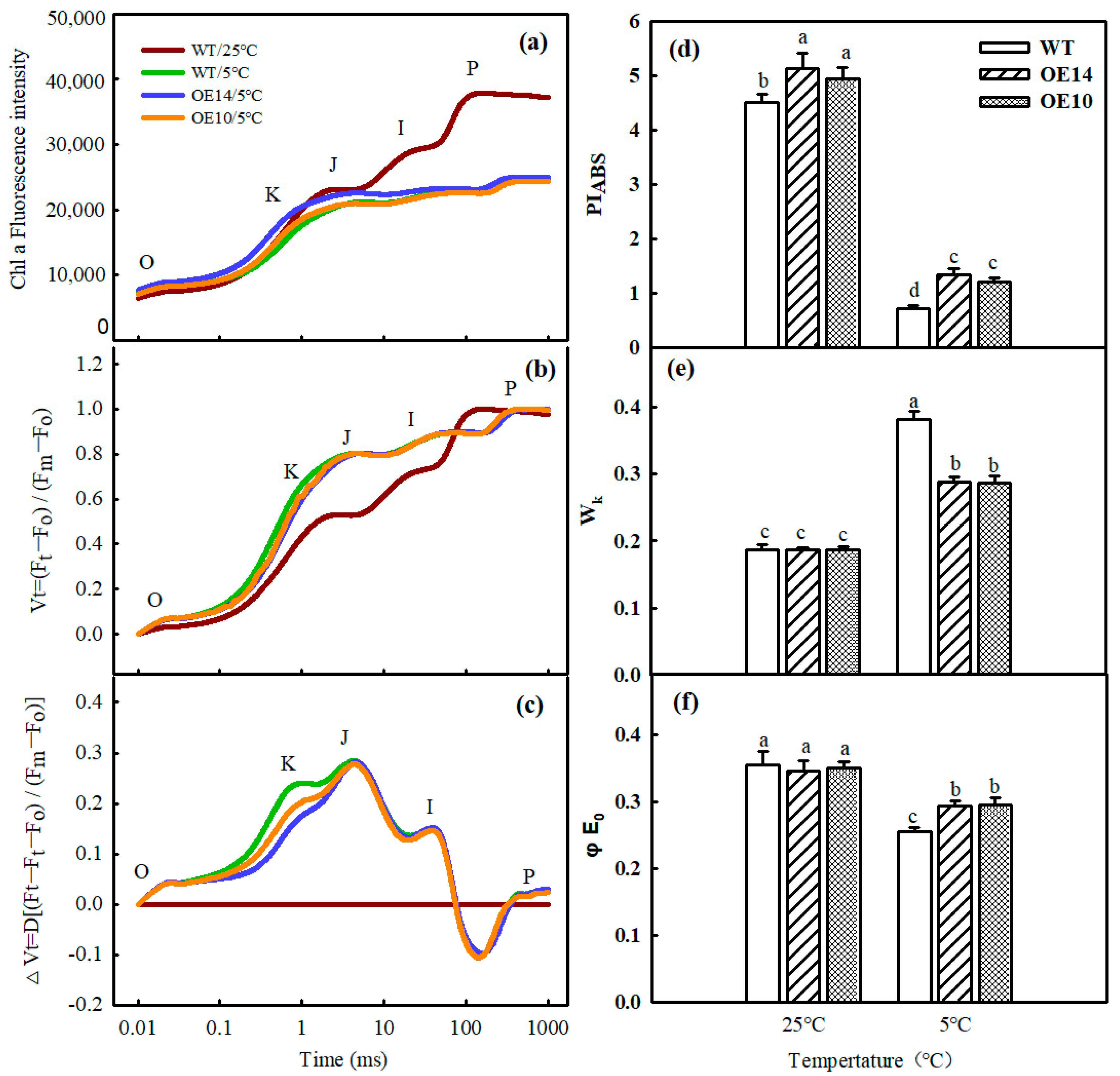
2.6. Photoinhibition
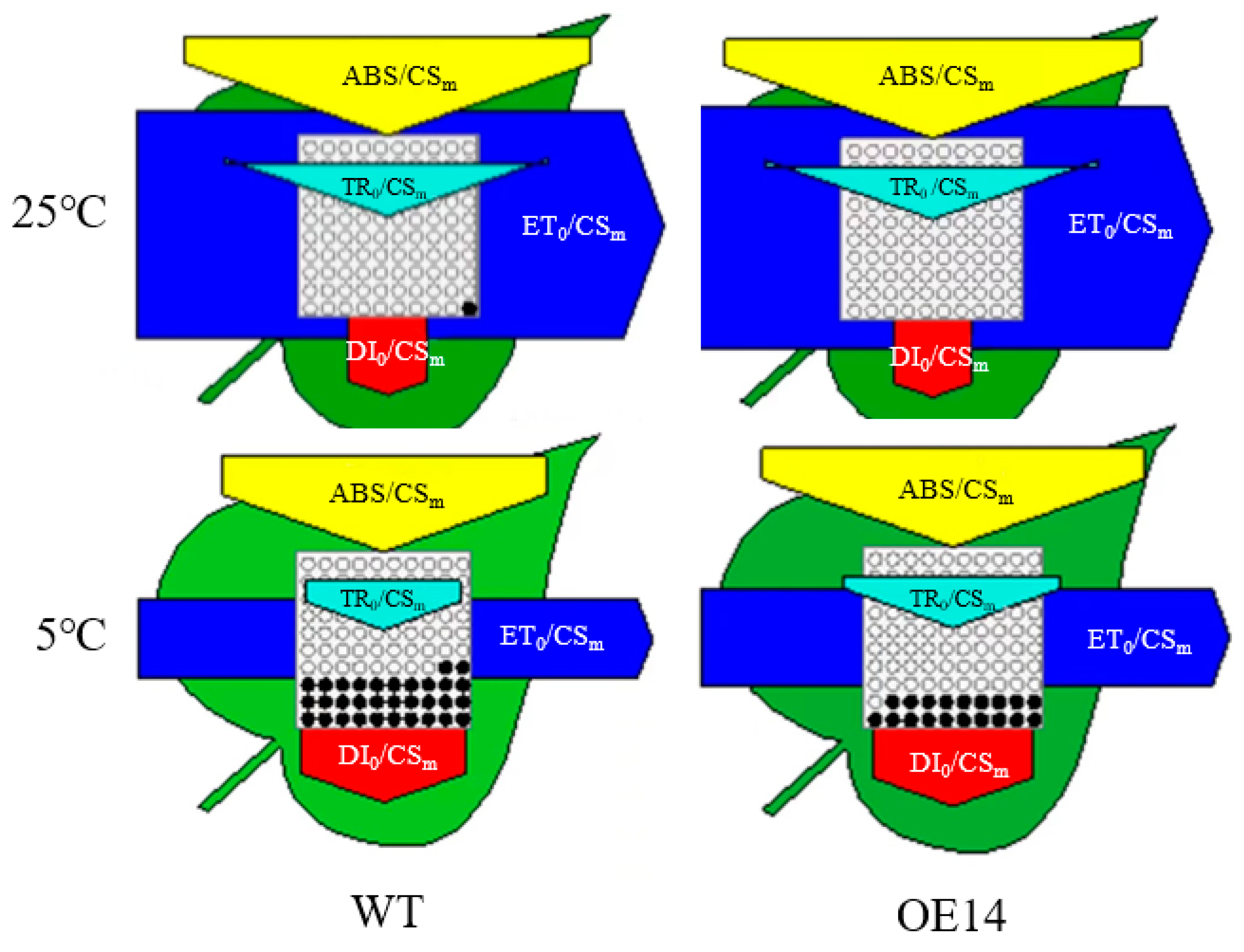
2.7. Electron Transport and Energy Distribution
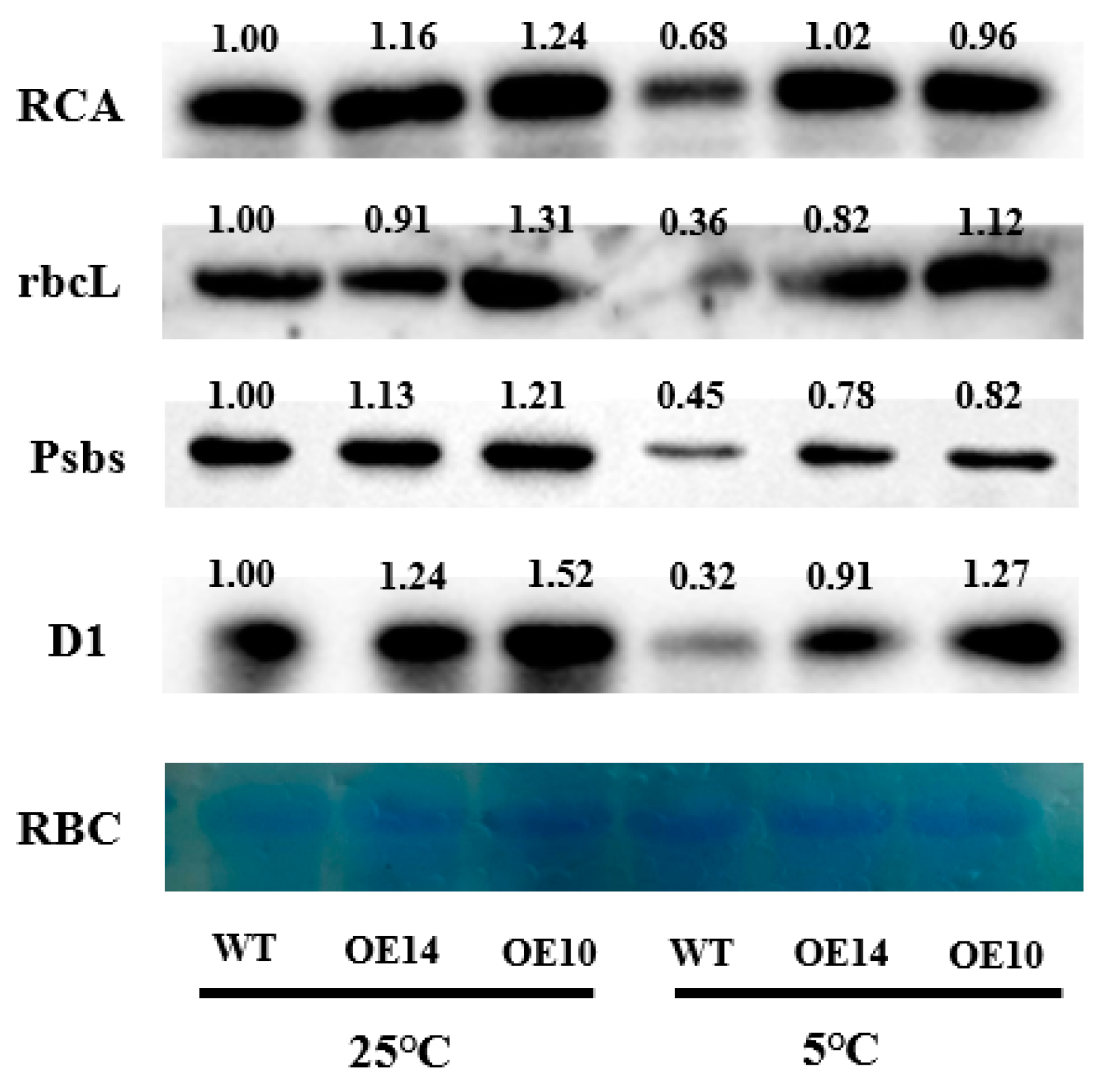
2.8. Photosynthesis-Related Protein Level
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Plant Transformation
3.3. Determination of MT Content
3.4. Determination of EL, H2O2, and Chlorophyll Content
3.5. Determination of Chloroplast Ultrastructure
3.6. Determination of Gas-Exchange Parameters and Photosynthetic Enzyme Activity
3.7. Determination of Chlorophyll Fluorescence Parameters and OJIP Curve
3.8. Determination of Sugar Content and Related Enzyme Activities
3.9. Determination of Gene Expression and Protein Level
3.10. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amin, B.; Atif, M.J.; Meng, H.; Ali, M.; Li, S.; Alharby, H.F.; Majrashi, A.; Hakeem, K.R.; Cheng, Z. Melatonin Rescues Photosynthesis and Triggers Antioxidant Defense Response in Cucumis sativus Plants Challenged by Low Temperature and High Humidity. Front Plant Sci. 2022, 13, 855900. [Google Scholar] [CrossRef]
- Dreyer, A.; Dietz, K.J. Reactive Oxygen Species and the Redox-Regulatory Network in Cold Stress Acclimation. Antioxidants 2017, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Lukatkin, A.S.; Brazaityte, A.; Bobinas, C.; Duchovskis, P. Chilling injury in chilling-sensitive plants: A review. Zemdirbyste 2012, 99, 111–124. [Google Scholar]
- Jahan, M.S.; Guo, S.R.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Bioch. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Bba-Bioenerg. 2012, 1817, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Che, Y.F.; Nakano, T.; Miyake, C.; Ifuku, K. The ability of P700 oxidation in photosystem I reflects chilling stress tolerance in cucumber. J. Plant Res. 2022, 135, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Shahzadi, A.K.; Fatima, S. Uncovering the role of melatonin in plant stress tolerance. Theor. Exp. Plant Physiol. 2022, 34, 335–346. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2019, 10, 54. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.Y.; Hao, Y.Y.; Mumtaz, M.A.; Lu, X.; Wang, Z.W. Melatonin Affects the Photosynthetic Performance of Pepper (Capsicum annuum L.) Seedlings under Cold Stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.R.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotox. Environ. Safe 2020, 197, 110593. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin Application to Pisum sativum, L. Seeds Positively Influences the Function of the Photosynthetic Apparatus in Growing Seedlings during Paraquat-Induced Oxidative Stress. Front. Plant Sci. 2016, 7, 1663. [Google Scholar] [CrossRef]
- Lei, X.Y.; Zhu, R.Y.; Zhang, G.Y.; Dai, Y.R. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: The possible involvement of polyamines. J. Pineal Res. 2004, 36, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Deger, O.; Szafranska, K.; Koklu, S.; Karaca, A.; Yakupoglu, G.; Kocacinar, F. Melatonin effects in enhancing chilling stress tolerance of pepper. Sci. Hortic. 2021, 289, 110434. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Balabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Qari, S.H.; Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Naqve, M.; Nawaz, M.; Barbanti, L.; Alahdal, M.A.; Aljabri, M. Melatonin Induced Cold Tolerance in Plants: Physiological and Molecular Responses. Front. Plant Sci. 2022, 13, 843071. [Google Scholar] [CrossRef]
- Ding, F.; Wang, M.; Liu, B.; Zhang, S. Exogenous Melatonin Mitigates Photoinhibition by Accelerating Non-photochemical Quenching in Tomato Seedlings Exposed to Moderate Light during Chilling. Front Plant Sci. 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Lazar, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal Behav. 2013, 8, e23279. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Xu, W.; Liu, A.R.; Chen, S.C. COMT1 Silencing Aggravates Heat Stress-Induced Reduction in Photosynthesis by Decreasing Chlorophyll Content, Photosystem II Activity, and Electron Transport Efficiency in Tomato. Front. Plant Sci. 2018, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Park, S.; Lee, H.Y.; Kim, Y.S.; Back, K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 2014, 56, 275–282. [Google Scholar] [CrossRef]
- Xu, W.; Cai, S.Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Reiter, R.J.; et al. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 2016, 61, 457–469. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef]
- Kang, K.; Kang, S.; Lee, K.; Park, M.; Back, K. Enzymatic features of serotonin biosynthetic enzymes and serotonin biosynthesis in plants. Plant Signal Behav. 2008, 3, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Kim, Y.S.; Park, S.; Back, K. Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant Physiol. 2009, 150, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, Y.; Hano, S.; Imoto, N.; Shibuya, T.; Ikeda, H.; Amagaya, K.; Kato, K.; Shirakawa, H.; Aso, H.; Kanayama, Y. Physiological roles of tryptophan decarboxylase revealed by overexpression of SlTDC1 in tomato. Sci. Hortic. 2021, 275, 109672. [Google Scholar] [CrossRef]
- Cai, B.B.; Li, Q.; Liu, F.J.; Bi, H.G.; Ai, X.Z. Decreasing fructose-1,6-bisphosphate aldolase activity reduces plant growth and tolerance to chilling stress in tomato seedlings. Physiol. Plant. 2018, 163, 247–258. [Google Scholar] [CrossRef]
- Liu, K.; Jing, T.T.; Wang, Y.A.; Ai, X.Z.; Bi, H.G. Melatonin delays leaf senescence improves cucumber yield by modulating chlorophyll degradation photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915. [Google Scholar] [CrossRef]
- Liu, F.J.; Zhang, X.W.; Cai, B.B.; Pan, D.Y.; Fu, X.; Bi, H.G.; Ai, X.Z. Physiological response and transcription profiling analysis reveal the role of glutathione in H2S-induced chilling stress tolerance of cucumber seedlings. Plant Sci. 2020, 291, 110363. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, J.A.R.; Long, S.P. Improved temperature response functions for models of rubisco-limited photosynthesis. Plant Cell Environ. 2001, 24, 253–260. [Google Scholar] [CrossRef]
- McMurtrie, R.E.; Wang, Y.P. Mathmatical models of the photosynthetic response of tree stands to rising CO2 concentrations and temperature. Plant Cell Environ. 1993, 16, 1–13. [Google Scholar] [CrossRef]
- Tian, Y.; Ungerer, P.; Zhang, H.; Ruban, A.V. Direct impact of the sustained decline in the photosystem II efficiency upon plant productivity at different developmental stages. J. Plant Physiol. 2017, 212, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, E.; Fusaro, L.; Gottardini, E.; Pollastrini, M.; Goltsev, V.; Strasser, R.J.; Bussotti, F. Plant stress analysis: Application of prompt, delayed chlorophyll fluorescence and 820 nm modulated reflectance. Plant Physiol. Biochem. 2014, 85, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Strasser, R.J.; Govindjee. Differential effects of dimethylbenzoquinone dichlorobenzoquinone on chlorophyll fluorescence transient in spinach thylakoids. J. Photochem. Photobiol. B 1995, 31, 163–169. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis; CRC Press: Boca Raton, FL, USA, 2000; pp. 445–483. [Google Scholar]
- El-Dakak, R.A.; Badr, R.H.; Zeineldein, M.H.; Swedan, E.A.; El Batrawy, O.; Hassaballah, A.F. Effect of chilling and salinity stress on photosynthetic performance and ultrastructure of chloroplast in faba beans (Vicia faba L.) leaves. Sci. Fis. Nat. 2023, 34, 447–456. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin Increases the Chilling Tolerance of Chloroplast in Cucumber Seedlings by Regulating Photosynthetic Electron Flux and the Ascorbate-Glutathione Cycle. Front Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [PubMed]
- Zoufan, P.; Zare Bavani, M.R.; Tousi, S.; Rahnama, A. Effect of exogenous melatonin on improvement of chlorophyll content and photochemical efficiency of PSII in mallow plants (Malva parviflora L.) treated with cadmium. Physiol. Mol. Biol. Pla. 2023, 29, 145–157. [Google Scholar] [CrossRef]
- Ozaki, K.; Uchida, A.; Takabe, T.; Shinagawa, F.; Tanaka, Y.; Takabe, T.; Hayashi, T.; Hattori, T.; Rai, A.K.; Takabe, T. Enrichment of sugar content in melon fruits by hydrogen peroxide treatment. J. Plant Physiol. 2009, 166, 569–578. [Google Scholar] [CrossRef]
- Stitt, M. Limitation of photosynthesis by carbon metabolism: Ⅰ. evidence for excess electron transport capacity in leaves carrying out photosynthesis in saturating light and CO2. Plant Physiol. 1986, 81, 1115–1122. [Google Scholar] [CrossRef]
- Ye, Z.P.; Robakowski, P.; Suggett, D.J. A mechanistic model for the light response of photosynthetic electron transport rate based on light harvesting properties of photosynthetic pigment molecules. Planta 2013, 237, 837–847. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Li, P.M.; Gao, H.Y.; Strasser, R.J. Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2005, 31, 559–566. [Google Scholar] [PubMed]
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S. Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 2020, 6, e04364. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R.; Vogelmann, T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010, 37, 403–412. [Google Scholar] [CrossRef]




| Stress Days (d) | Strains | Asat (μmol·m−2·s−1) | Total Sugar Content (mg·g−1DW) | Sucrose Content (mg·g−1DW) | Glucose Content (mg·g−1DW) | Fructose Content (mg·g−1DW) | Starch Content (mg·g−1DW) |
|---|---|---|---|---|---|---|---|
| 0 d | WT | 25.53 ± 1.28 b | 234.30 ± 3.50 b | 74.26 ± 1.35 b | 6.61 ± 0.16 b | 5.49 ± 0.05 c | 128.54 ± 0.68 b |
| OE14 | 28.3 ± 0.98 a | 243.00 ± 2.14 a | 84.21 ± 2.31 a | 8.69 ± 0.40 a | 7.10 ± 0.20 a | 116.93 ± 2.05 c | |
| OE10 | 27.83 ± 0.6 a | 244.56 ± 4.12 a | 82.78 ± 3.96 a | 8.92 ± 0.21 a | 6.20 ± 0.15 b | 117.58 ± 2.02 c | |
| 1 d | WT | 10.73 ± 0.55 c | 269.56 ± 1.56 b | 115.11 ± 2.47 c | 16.58 ± 0.49 b | 8.49 ± 0.13 c | 164.51 ± 2.50 d |
| OE14 | 18.8 ± 0.45 a | 353.27 ± 1.23 a | 135.40 ± 1.26 a | 19.25 ± 1.01 a | 10.74 ± 0.03 a | 142.98 ± 1.93 f | |
| OE10 | 17.26 ± 0.68 b | 348.97 ± 6.95 a | 129.55 ± 1.24 b | 19.49 ± 0.17 a | 9.87 ± 0.16 b | 149.27 ± 0.79 e | |
| 2 d | WT | 5.83 ± 0.6 b | 324.40 ± 4.61 b | 133.87 ± 4.67 b | 31.04 ± 0.32 c | 11.53 ± 0.07 c | 215.72 ± 0.11 c |
| OE14 | 10.26 ± 0.45 a | 393.76 ± 5.09 a | 150.97 ± 1.28 a | 36.47 ± 0.47 a | 17.54 ± 0.05 a | 198.22 ± 2.73 d | |
| OE10 | 10.43 ± 0.68 a | 393.60 ± 4.25 a | 149.35 ± 3.56 a | 33.91 ± 0.66 b | 15.56 ± 0.13 b | 202.66 ± 0.34 d |
| Genes | Accession Numbers | Primer Pairs (5′-3′) |
|---|---|---|
| Actin | XM_001321306 | TTTGCTGGTGATGATGCC |
| CCTTAGGGTTGAGAGGTGCTT | ||
| TDC | XM_004243253 | GCCTAAGCCCTCATAAGTGG |
| TGCCAGTCCTTGTAATCCA | ||
| SS | XM_019211274 | CGCCAAGAATCCACGACTAA |
| TCTGCCTGCTCTTCCAAATC | ||
| SPS | XM_0012469991 | CAGTCAGCAGAGAGGAAAGAAG |
| CAGTATCAGAATCCCGTCCAAG | ||
| RCA | XM_010327541 | GAAGAAACAGACTGATGGGGAC |
| CGTTGGAGCCTGGAAAAGC | ||
| RbcL | XM_012015910 | TTTCCAAGGTCCGCCTCA |
| CCACCGCGAAGACATTCATA | ||
| RbcS | XM_026029055 | CCATTGCTAGCAACGGTGGAAGA |
| TGCTCGTCGGACAAATCAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, Y.; Feng, Y.; Zhang, X.; Bi, H.; Ai, X. SlTDC1 Overexpression Promoted Photosynthesis in Tomato under Chilling Stress by Improving CO2 Assimilation and Alleviating Photoinhibition. Int. J. Mol. Sci. 2023, 24, 11042. https://doi.org/10.3390/ijms241311042
Liu X, Wang Y, Feng Y, Zhang X, Bi H, Ai X. SlTDC1 Overexpression Promoted Photosynthesis in Tomato under Chilling Stress by Improving CO2 Assimilation and Alleviating Photoinhibition. International Journal of Molecular Sciences. 2023; 24(13):11042. https://doi.org/10.3390/ijms241311042
Chicago/Turabian StyleLiu, Xutao, Yanan Wang, Yiqing Feng, Xiaowei Zhang, Huangai Bi, and Xizhen Ai. 2023. "SlTDC1 Overexpression Promoted Photosynthesis in Tomato under Chilling Stress by Improving CO2 Assimilation and Alleviating Photoinhibition" International Journal of Molecular Sciences 24, no. 13: 11042. https://doi.org/10.3390/ijms241311042
APA StyleLiu, X., Wang, Y., Feng, Y., Zhang, X., Bi, H., & Ai, X. (2023). SlTDC1 Overexpression Promoted Photosynthesis in Tomato under Chilling Stress by Improving CO2 Assimilation and Alleviating Photoinhibition. International Journal of Molecular Sciences, 24(13), 11042. https://doi.org/10.3390/ijms241311042




