Computational Exploration of the Effects of Mutations on GABA Aminotransferase in GABA Aminotransferase Deficiency
Abstract
1. Introduction
2. Results and Discussion
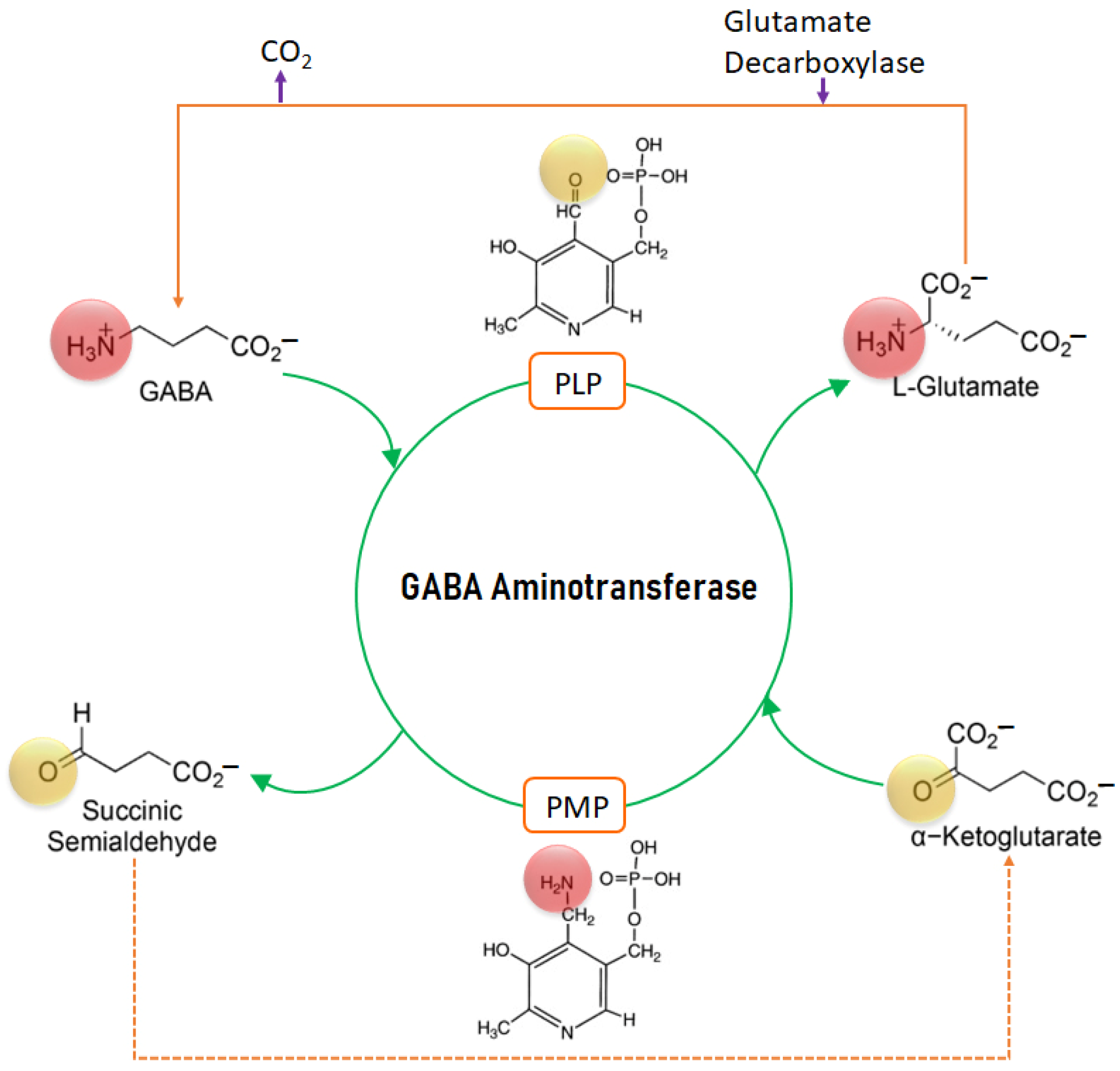
2.1. Mechanism of Action of GABA-AT
2.2. Prediction and Evaluation of Mutated Tyrosinase Structures
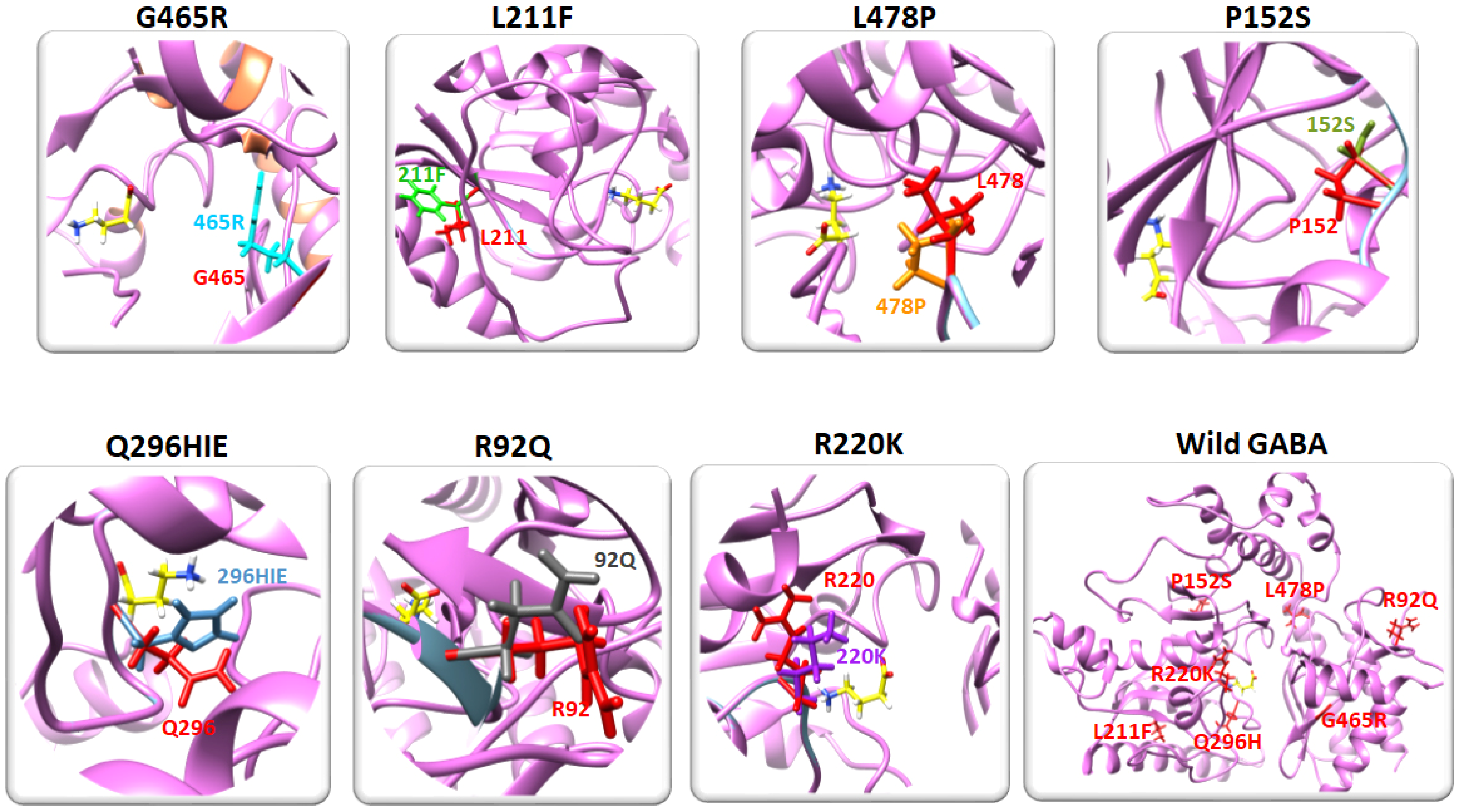
2.3. Residual Mutation Analysis
2.4. Prediction of the Physiochemical Properties of the Predicted Mutated Models
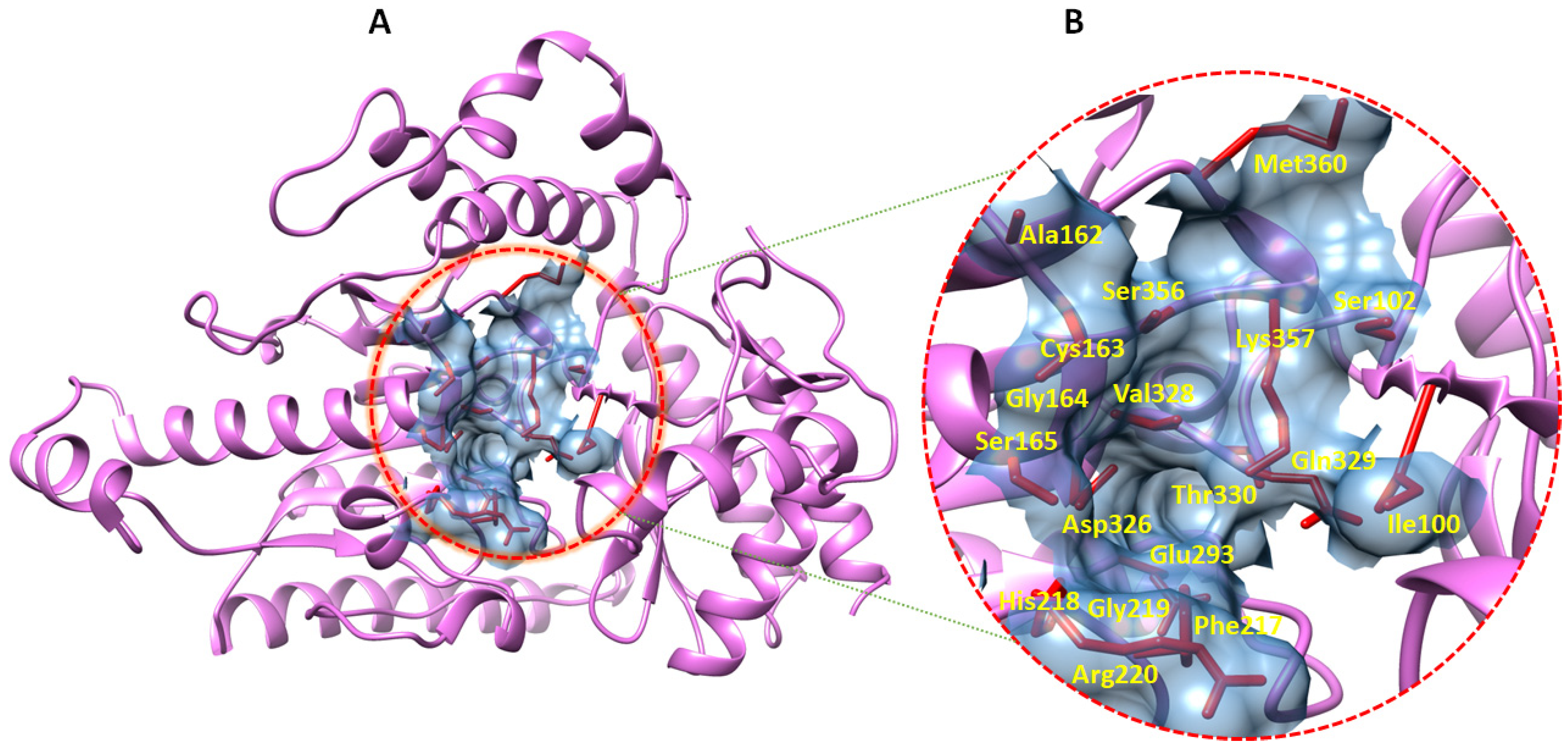
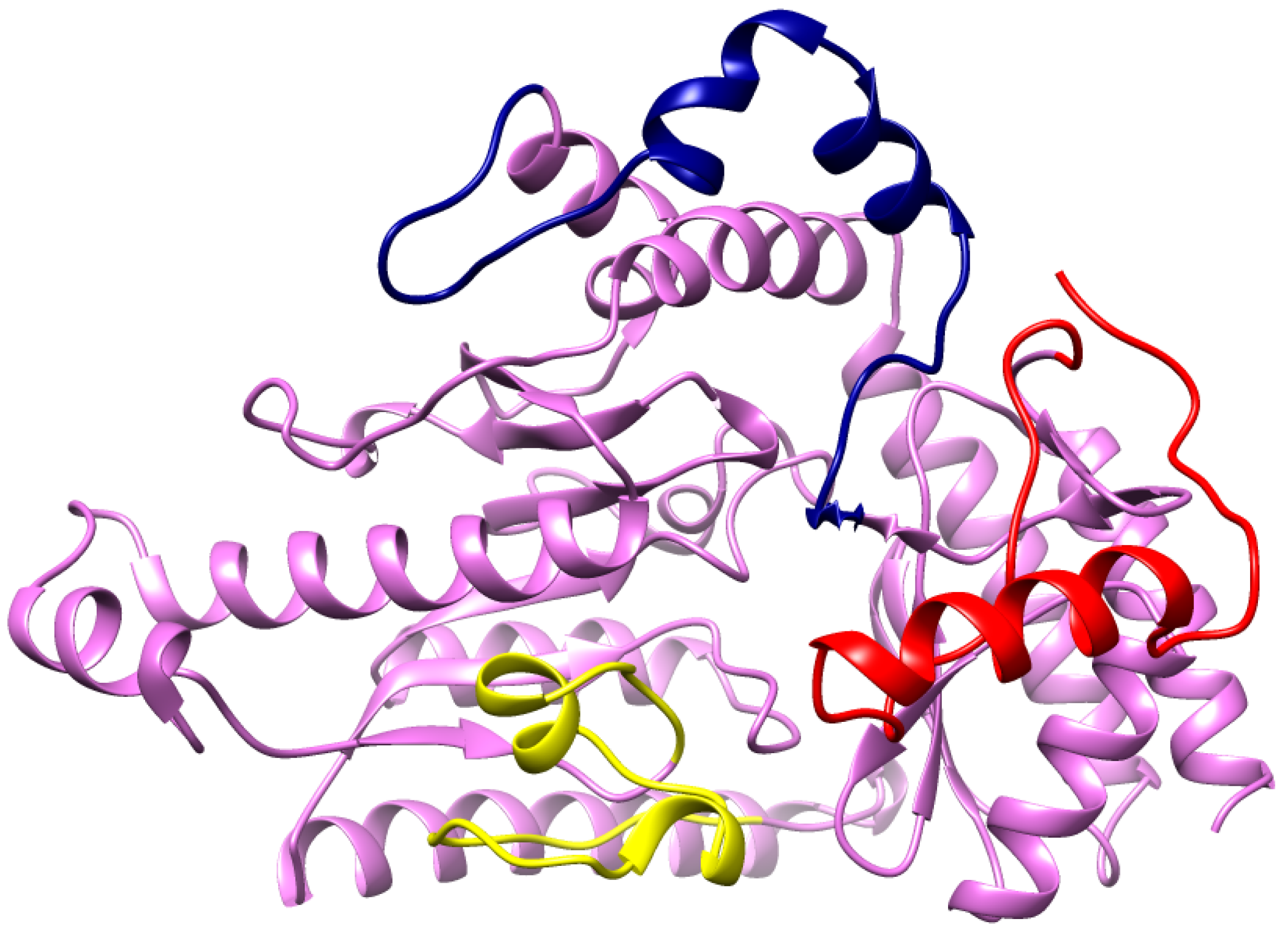
2.5. Prediction of GABA-AT Binding Site
2.6. Molecular Dynamics Simulations
2.6.1. Root Mean Square Deviation Analysis
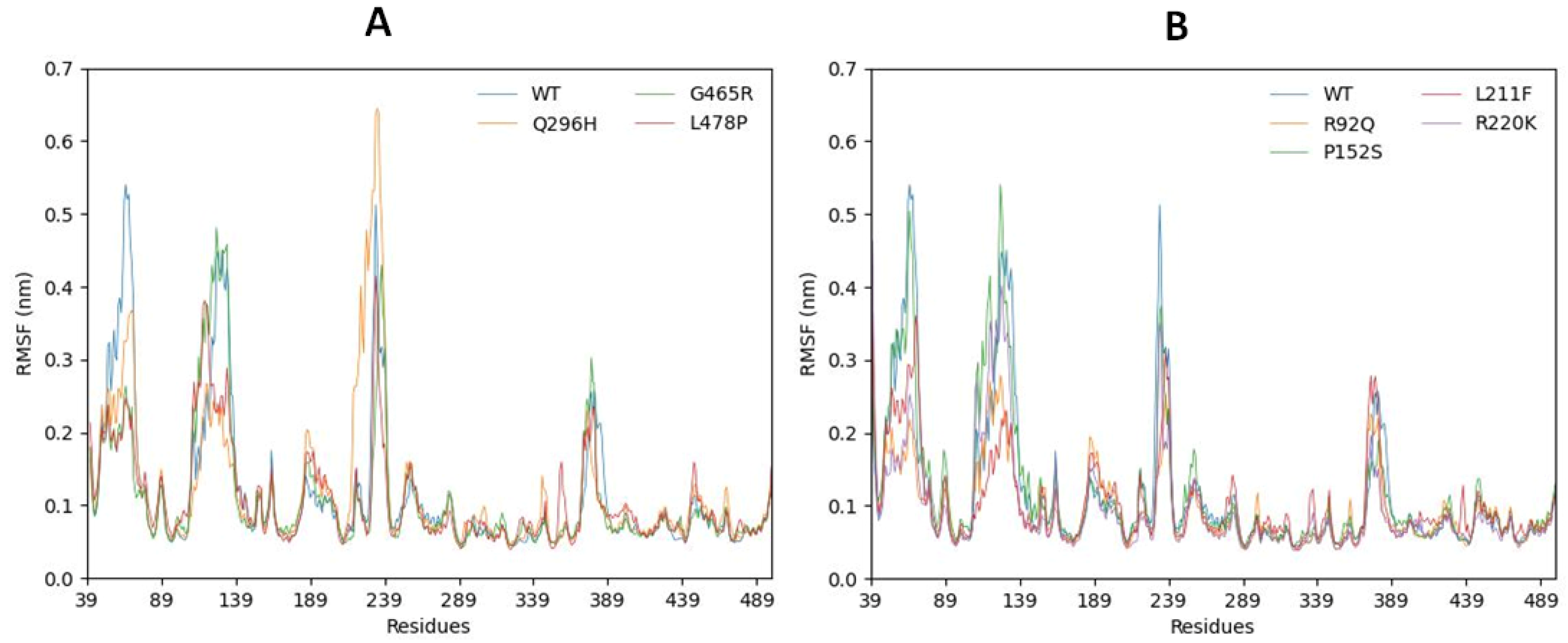
2.6.2. Root Mean Square Fluctuations
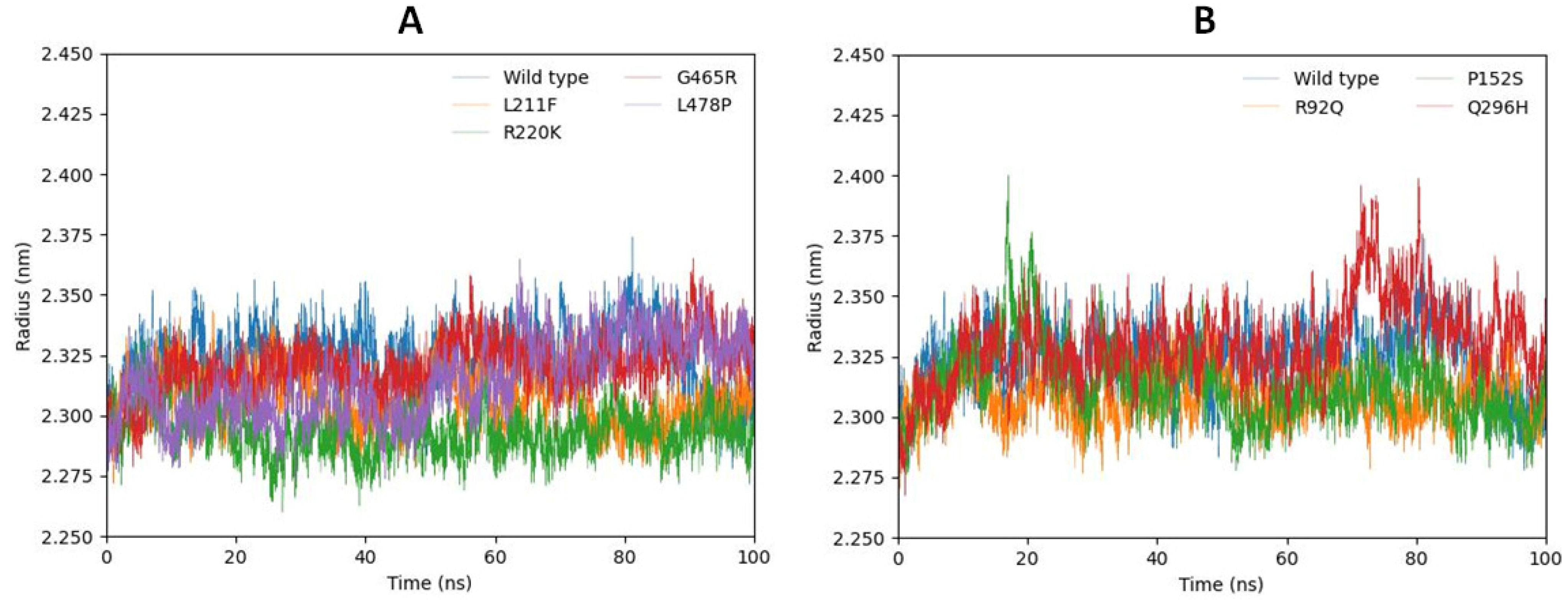
2.6.3. Radius of Gyration (Rg)
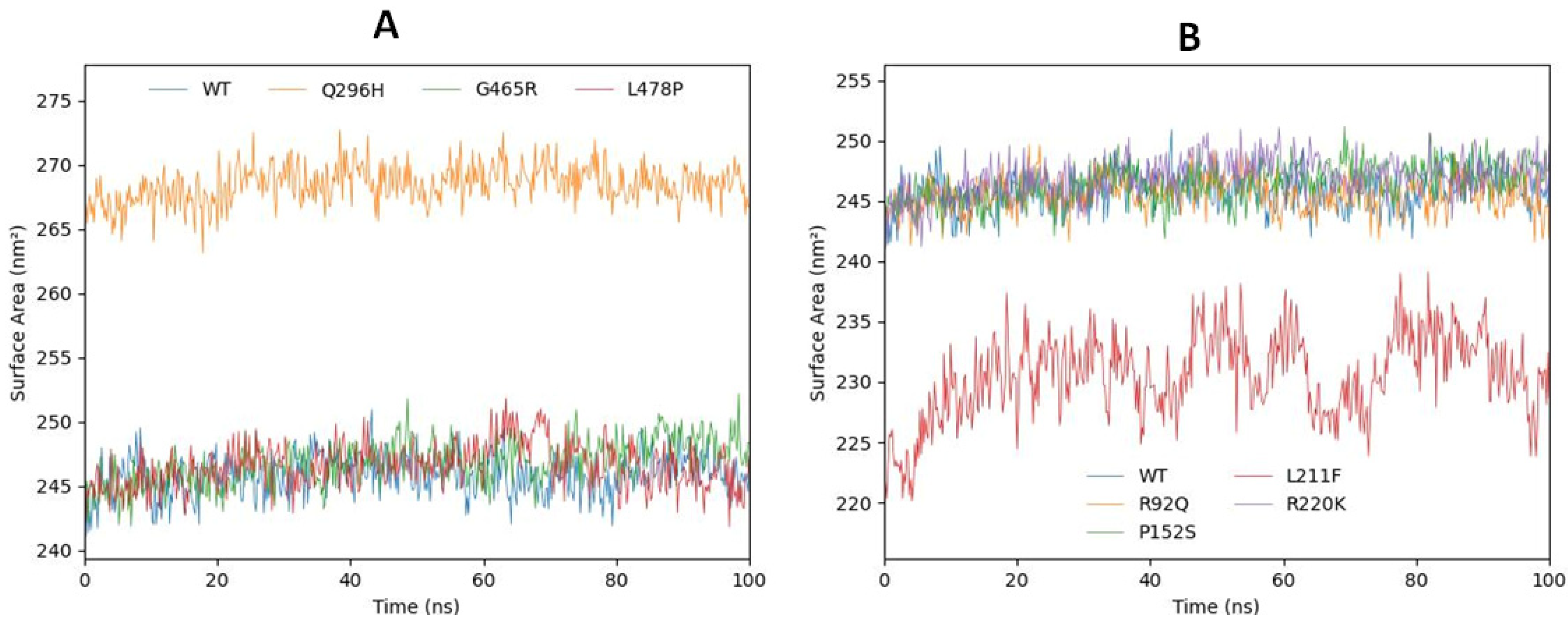
2.6.4. Solvent-Accessible Surface Area
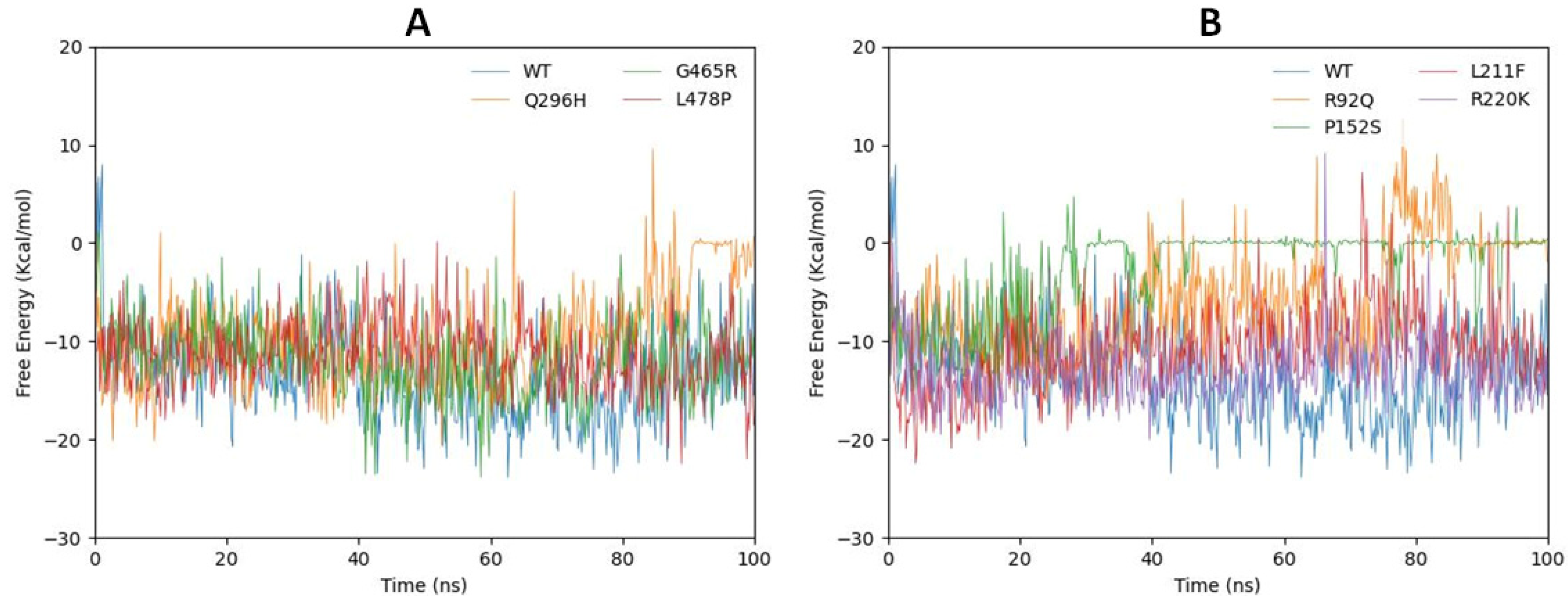
2.7. gmxMMPBSA Free Energy Calculation
2.8. Comparative Analysis
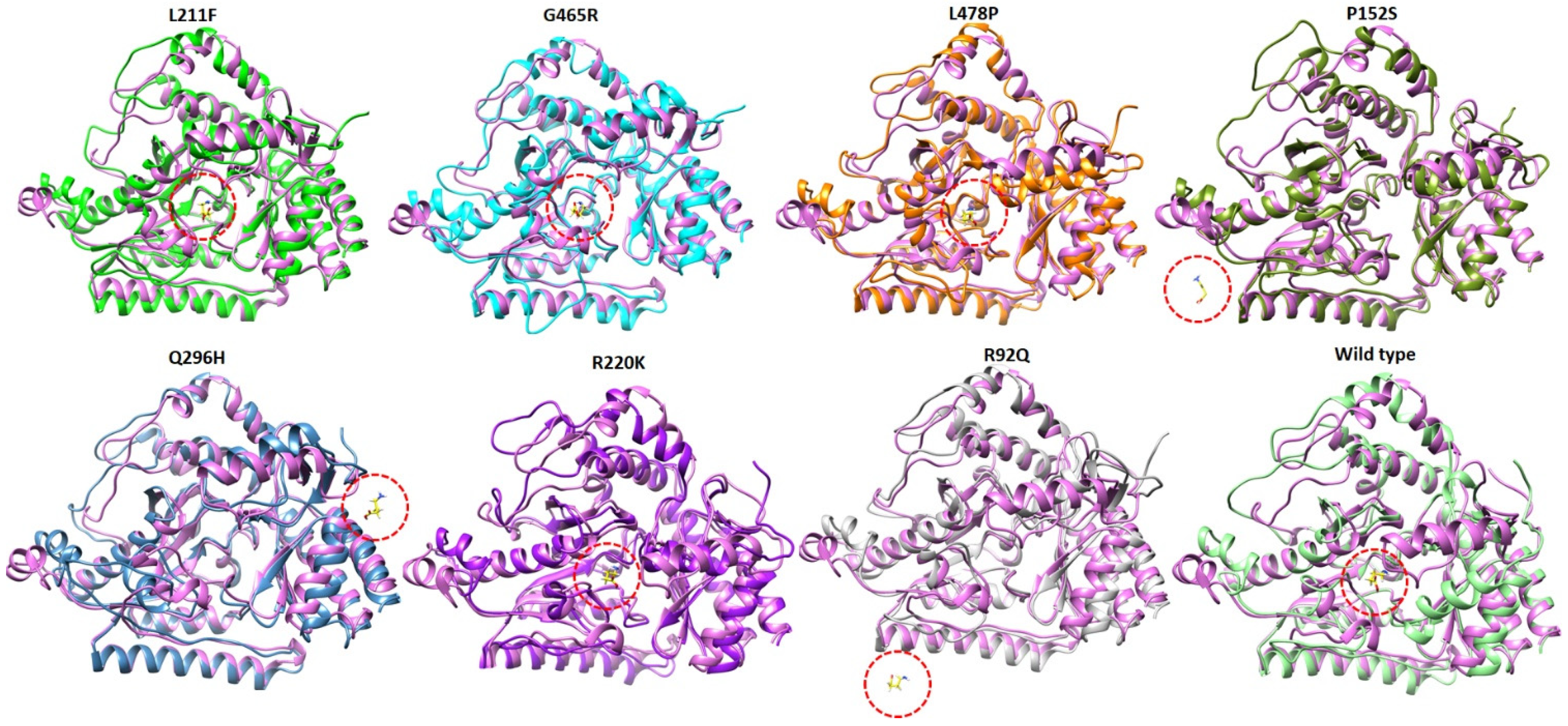
2.9. Structural Analysis at 100 ns of MD Simulation
3. Methods and Materials
3.1. Prediction of Human Tyrosinase Structure
3.2. Residual Mutation Analysis
3.3. Binding Pocket Prediction Analysis
3.4. Molecular Dynamic Simulation
3.5. Free Energy Calculation Using gmxMMPBSA
3.6. Comparative Analysis of Mutated Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegde, A.U.; Karnavat, P.K.; Vyas, R.; DiBacco, M.L.; Grant, P.E.; Pearl, P.L. GABA Transaminase Deficiency With Survival Into Adulthood. J. Child Neurol. 2019, 34, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Oshi, A.; Alfaifi, A.; Seidahmed, M.Z.; Al Hussein, K.; Miqdad, A.; Samadi, A.; Abdelbasit, O.J. GABA transaminase deficiency. Case report and literature review. Clin. Case Rep. 2021, 9, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Hampe, C.S.; Mitoma, H.; Manto, M.J.G. GABA and Glutamate: Their Transmitter Role in the CNS and Pancreatic Islets; 2018. Available online: https://www.intechopen.com/chapters/57103 (accessed on 30 May 2023).
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E. GABA(A) receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Herbison, A.E.; Moenter, S.M. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: Towards an emerging consensus. J. Neuroendocrinol. 2011, 23, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Gokulgandhi, M.; Khurana, V.; Mitra, A.K. 5-Transporters and receptors in the posterior segment of the eye. In Ocular Transporters and Receptors; Mitra, A.K., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 169–205. [Google Scholar]
- Magnaghi, V. GABA and neuroactive steroid interactions in glia: New roles for old players? Curr. Neuropharmacol. 2007, 5, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Liu, L.; Wang, T.; Cannon, M.; Lin, P.; Fan, T.W.M.; Scott, D.A.; Wu, H.-J.J.; Lane, A.N.; Wang, R. GAB functions as a bioenergetic and signalling gatekeeper to control T cell inflammation. Nat. Metab. 2022, 4, 1322–1335. [Google Scholar] [CrossRef]
- Silverman, R.B. Design and Mechanism of GABA Aminotransferase Inactivators. Treatments for Epilepsies and Addictions. Chem. Rev. 2018, 118, 4037–4070. [Google Scholar] [CrossRef]
- Storici, P.; De Biase, D.; Bossa, F.; Bruno, S.; Mozzarelli, A.; Peneff, C.; Silverman, R.B.; Schirmer, T. Structures of gamma-aminobutyric acid (GABA) aminotransferase, a pyridoxal 5’-phosphate, and [2Fe-2S] cluster-containing enzyme, complexed with gamma-ethynyl-GABA and with the antiepilepsy drug vigabatrin. J. Biol. Chem. 2004, 279, 363–373. [Google Scholar] [CrossRef]
- Aldana, B.I.; Zhang, Y.; Jensen, P.; Chandrasekaran, A.; Christensen, S.K.; Nielsen, T.T.; Nielsen, J.E.; Hyttel, P.; Larsen, M.R.; Waagepetersen, H.S.; et al. Glutamate-glutamine homeostasis is perturbed in neurons and astrocytes derived from patient iPSC models of frontotemporal dementia. Mol. Brain 2020, 13, 125. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate Decarboxylase from Lactic Acid Bacteria-A Key Enzyme in GABA Synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.B. The 2011 EB Hershberg Award for important discoveries in medicinally active substances:(1 S, 3 S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA aminotransferase inactivator and new treatment for drug addiction and infantile spasms. J. Med. Chem. 2012, 55, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Alexander, P.B.; Li, Q.-J.; Wang, X.-F. GABAergic signaling beyond synapses: An emerging target for cancer therapy. Trends Cell Biol. 2023, 33, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, Z.-H.; Liu, C.; Li, G.-Y.; Qiao, X.-Z.; Gan, Y.-J.; Zhang, C.-C.; Deng, Y.-C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef]
- Lee, H.H.C.; McGinty, G.E.; Pearl, P.L.; Rotenberg, A. Understanding the Molecular Mechanisms of Succinic Semialdehyde Dehydrogenase Deficiency (SSADHD): Towards the Development of SSADH-Targeted Medicine. Int. J. Mol. Sci. 2022, 23, 2606. [Google Scholar] [CrossRef]
- Koenig, M.K.; Hodgeman, R.; Riviello, J.J.; Chung, W.; Bain, J.; Chiriboga, C.A.; Ichikawa, K.; Osaka, H.; Tsuji, M.; Gibson, K.M.; et al. Phenotype of GABA-transaminase deficiency. Neurology 2017, 88, 1919–1924. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Kwon, Y.-S.; Lee, H.-J.; Hassan, M.; Kloczkowski, A.; Chun, W. Investigation of Flavonoid Scaffolds as DAX1 Inhibitors against Ewing Sarcoma through Pharmacoinformatic and Dynamic Simulation Studies. Int. J. Mol. Sci. 2023, 24, 9332. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Kwon, Y.-S.; Lee, H.-J.; Chun, W. Computational Exploration of Licorice for Lead Compounds against Plasmodium vivax Duffy Binding Protein Utilizing Molecular Docking and Molecular Dynamic Simulation. Molecules 2023, 28, 3358. [Google Scholar] [CrossRef]
- Willard, L.; Ranjan, A.; Zhang, H.; Monzavi, H.; Boyko, R.F.; Sykes, B.D.; Wishart, D.S. VADAR: A web server for quantitative evaluation of protein structure quality. Nucleic Acids Res. 2003, 31, 3316–3319. [Google Scholar] [CrossRef]
- Studio, D.J.A. Discovery Studio. 2008. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Studio%2C+Discovery.+%22Discovery+studio.%22+Accelrys+%5B2.1%5D+%282008%29.&btnG= (accessed on 30 May 2023).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Yasir, M.; Park, J.; Han, E.T.; Park, W.S.; Han, J.H.; Kwon, Y.S.; Lee, H.J.; Hassan, M.; Kloczkowski, A.; Chun, W. Exploration of Flavonoids as Lead Compounds against Ewing Sarcoma through Molecular Docking, Pharmacogenomics Analysis, and Molecular Dynamics Simulations. Molecules 2023, 28, 414. [Google Scholar] [CrossRef] [PubMed]
- Jendele, L.; Krivak, R.; Skoda, P.; Novotny, M.; Hoksza, D.J. PrankWeb: A web server for ligand binding site prediction and visualization. Nucleic Acids Res. 2019, 47, W345–W349. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Velankar, S.; Best, C.; Beuth, B.; Boutselakis, C.H.; Cobley, N.; Sousa Da Silva, A.W.; Dimitropoulos, D.; Golovin, A.; Hirshberg, M.; John, M.; et al. PDBe: Protein Data Bank in Europe. Nucleic Acids Res. 2010, 38, D308–D317. [Google Scholar] [CrossRef] [PubMed]
- Witvliet, D.K.; Strokach, A.; Giraldo-Forero, A.F.; Teyra, J.; Colak, R.; Kim, P.M. ELASPIC web-server: Proteome-wide structure-based prediction of mutation effects on protein stability and binding affinity. Bioinformatics 2016, 32, 1589–1591. [Google Scholar] [CrossRef] [PubMed]
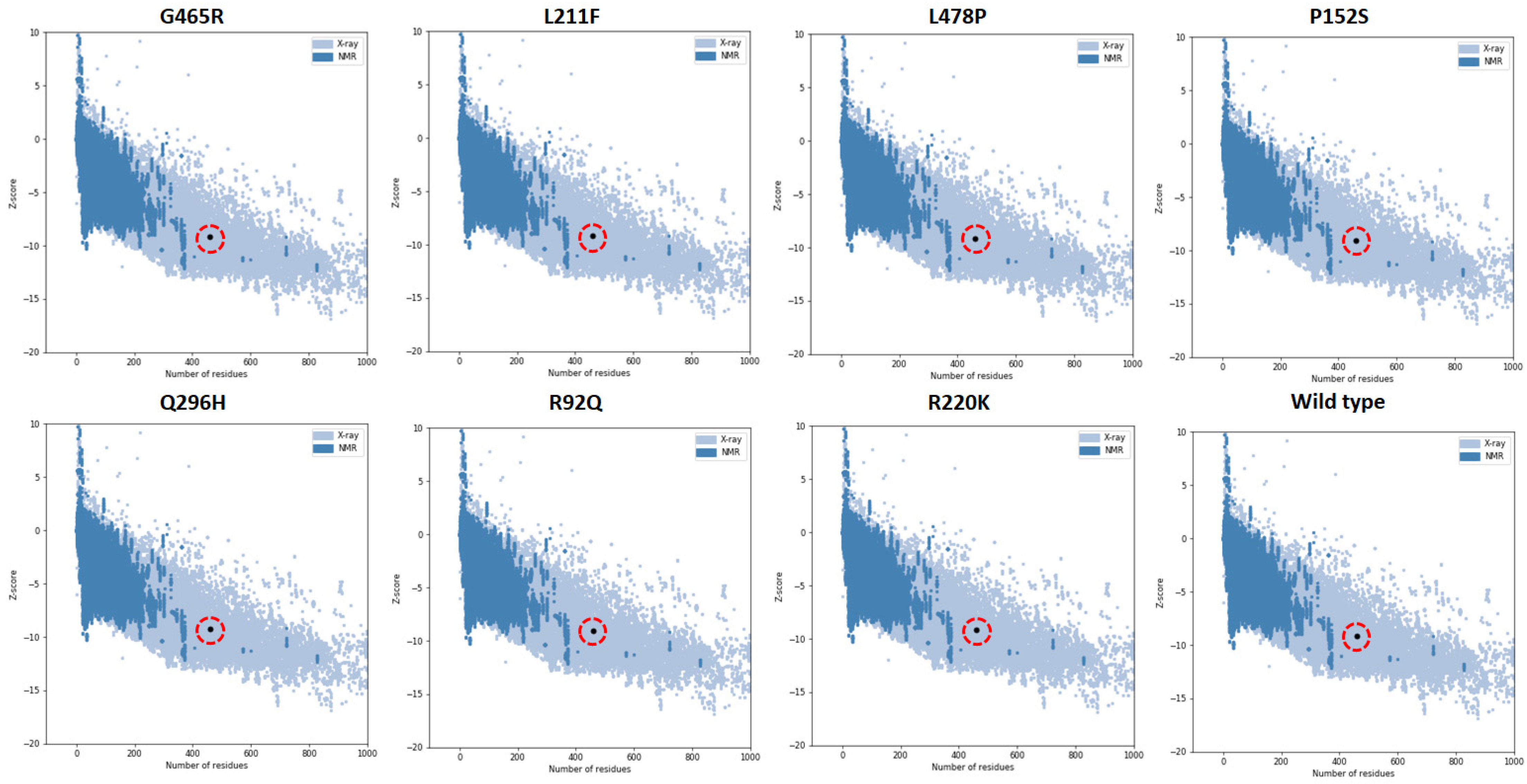

| Sr No | Mutated Structures | MolProbity Score | ProSA Z-Score | ERRAT Score | Ramachandran Favored |
|---|---|---|---|---|---|
| 1 | G465R | 2.13 | −9.14 | 89.95 | 90.41% |
| 2 | L211F | 1.80 | −9.17 | 87.26 | 90.63% |
| 3 | L478P | 1.75 | −9.16 | 91.27 | 90.63% |
| 4 | P152S | 1.70 | −9.05 | 91.53 | 90.63% |
| 5 | Q296H | 1.72 | −9.28 | 90.07 | 90.79% |
| 6 | R92Q | 1.70 | −9.12 | 91.02 | 90.63% |
| 7 | R220K | 1.70 | −9.21 | 91.27 | 90.63% |
| 8 | Wild Type | 1.70 | −9.17 | 91.27 | 90.63% |
| Sr No | Mutated Structures | Theoretical pI | GRAVY | AI |
|---|---|---|---|---|
| 1 | G465R | 7.68 | −0.304 | 82.32 |
| 2 | L211F | 7.31 | −0.297 | 81.43 |
| 3 | L478P | 7.31 | −0.307 | 81.48 |
| 4 | P152S | 7.31 | −0.293 | 82.32 |
| 5 | Q296H | 7.31 | −0.288 | 82.32 |
| 6 | R92Q | 6.98 | −0.293 | 82.32 |
| 7 | R220K | 7.30 | −0.294 | 82.32 |
| 8 | Wild Type | 7.31 | −0.295 | 82.32 |
| Rank | Name | Score | Probability | Amino Acid Residues |
|---|---|---|---|---|
| 1 | Pocket1 | 9.23 | 0.54 | Ile_100, Ser_102, Ala_162, Cys_163, Gly_164, Ser_165, Phe_217, His_218, Gly_219, Arg_220, Glu_293, Asp_326, Val_328, Gln_329, Gln_330, Ser_356, Lys_357, Met_360 |
| 2 | Pocket2 | 3.64 | 0.143 | Glu_169, Leu_172, Lys_173, Phe_176, Met_177, Cys_205, Pro_206, Asp_207, Tyr_208, Gly_223, Phe_241, Trp_243 |
| 3 | Pocket3 | 3.37 | 0.124 | Val_463, His_72, Phe_73, Cys_75, Leu_85, Asp_95, Tyr_97, Gln_99 |
| 4 | Pocket4 | 3.01 | 0.1 | Met_177, Arg_180, Ser_181, Arg_184, Phe_189, Leu_194, Cys_197, Gly_204, Pro_206, Asn_373, Arg_377 |
| 5 | Pocket5 | 2.96 | 0.097 | Ser_102, Val_103, Pro_104, Gly_106, Ser_108, His_109, Leu_112, Ile_116, Met_360, Lys_388, Leu_391 |
| Sr No | Mutated Models | Consensus RMSD | Gibbs Free Energy Change (ΔG) |
|---|---|---|---|
| 1 | Wild type | 0.0010 | – |
| 2 | R92Q | 0.0026 | 1.3152 |
| 3 | P152S | 0.0016 | 1.6217 |
| 4 | L211F | 0.0024 | 1.3274 |
| 5 | R220K | 0.0013 | 0.2570 |
| 6 | Q296H | 0.0041 | 0.1922 |
| 7 | G465R | 0.0015 | 0.6539 |
| 8 | L478P | 0.0037 | 2.9431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasir, M.; Park, J.; Han, E.-T.; Park, W.S.; Han, J.-H.; Kwon, Y.-S.; Lee, H.-J.; Chun, W. Computational Exploration of the Effects of Mutations on GABA Aminotransferase in GABA Aminotransferase Deficiency. Int. J. Mol. Sci. 2023, 24, 10933. https://doi.org/10.3390/ijms241310933
Yasir M, Park J, Han E-T, Park WS, Han J-H, Kwon Y-S, Lee H-J, Chun W. Computational Exploration of the Effects of Mutations on GABA Aminotransferase in GABA Aminotransferase Deficiency. International Journal of Molecular Sciences. 2023; 24(13):10933. https://doi.org/10.3390/ijms241310933
Chicago/Turabian StyleYasir, Muhammad, Jinyoung Park, Eun-Taek Han, Won Sun Park, Jin-Hee Han, Yong-Soo Kwon, Hee-Jae Lee, and Wanjoo Chun. 2023. "Computational Exploration of the Effects of Mutations on GABA Aminotransferase in GABA Aminotransferase Deficiency" International Journal of Molecular Sciences 24, no. 13: 10933. https://doi.org/10.3390/ijms241310933
APA StyleYasir, M., Park, J., Han, E.-T., Park, W. S., Han, J.-H., Kwon, Y.-S., Lee, H.-J., & Chun, W. (2023). Computational Exploration of the Effects of Mutations on GABA Aminotransferase in GABA Aminotransferase Deficiency. International Journal of Molecular Sciences, 24(13), 10933. https://doi.org/10.3390/ijms241310933








