Abstract
Copper (Cu)-based antimicrobial compounds (CBACs) have been widely used to control phytopathogens for nearly fourteen decades. Since the first commercialized Bordeaux mixture was introduced, CBACs have been gradually developed from highly to slightly soluble reagents and from inorganic to synthetic organic, with nanomaterials being a recent development. Traditionally, slightly soluble CBACs form a physical film on the surface of plant tissues, separating the micro-organisms from the host, then release divalent or monovalent copper ions (Cu2+ or Cu+) to construct a secondary layer of protection which inhibits the growth of pathogens. Recent progress has demonstrated that the release of a low concentration of Cu2+ may elicit immune responses in plants. This supports a triple-tiered protection role of CBACs: break contact, inhibit microorganisms, and stimulate host immunity. This spatial defense system, which is integrated both inside and outside the plant cell, provides long-lasting and broad-spectrum protection, even against emergent copper-resistant strains. Here, we review recent findings and highlight the perspectives underlying mitigation strategies for the sustainable utilization of CBACs.
1. Introduction
Agriculture is the most important basic industry globally, as it plays an important role in supporting the demand for food and raw materials in other industries. However, with the global population projected to reach 9.7 billion by 2050, the demand for food by humans has also been increasing [1,2]. However, annual increases in production yield for the vast majority of crops have been steadily declining [3,4,5]. In order to meet the growing needs of humanity and industry, certain measures must be urgently taken to increase food production. Although breeding high-yield varieties is an effective strategy, the yield losses caused by crop diseases have been estimated as 11–30% [6], and the losses of fruits, vegetables, and grains caused by pests and diseases may reach as high as 78%, 54%, and 32% without fungicides [7]. Therefore, finding effective ways to prevent and control plant disease is expected to be useful in improving food production.
Copper ions are used as broad-spectrum protectant fungicides in agricultural systems to control a series of plant diseases. They appear in some forms of copper-based antimicrobial compounds (CBACs), which have been commercially used for nearly 14 decades [8]. With broad-spectrum antimicrobial activity, CBACs can control a wide range of plant diseases, such as grape downy mildew [9], citrus black spot [10], fire blight of pome fruits [11], walnut blight [12,13], potato late blight [14], stone fruit canker [15], coffee berry disease [16], olive leaf spot [17], and powdery mildew of many other crops [18,19,20]. At present, not considering metal contaminants, CBACs are still at the forefront as the main pesticides sold in Europe [8,21].
In this review, we focus on recent advances regarding the function of copper ions in CBACs, emphasizing the historical development and activation of plant immunity, which helps in understanding the physics and molecular mechanisms of copper-mediated protection to promote the sustainable future use of CBACs.
2. Development of Copper-Based Antimicrobial Compounds
The control of plant diseases through the use of copper agents has a long history. In fact, the earliest known record of bluestone (copper sulfate) being used to kill smut spores on wheat grains was in France [22] when, in 1807, Prevost began to use bluestone to disinfect grain seeds [22]. In 1838, the Boucherie of France found that adding 1 part copper sulfate (CuSO4) to 100 parts water could effectively protect wood [23]. After that, CBACs consisting of CuSO4 were widely used to control grapevine downy mildew (Table 1), establishing the rudiments of CBACs.
In 1873, Dreisch added a lime water bath after the application of bluestone, thus improving Prevost’s method of treating wheat grain seeds [22]. In 1883, the beneficial effect of mixing lime with CuSO4 was proven [24], and in 1885, the French botanist Pierre-Marie-Alexis Millardet published his famous discovery that CuSO4 and lime mixed could protect grapes from downy mildew [24]. This mixture became known as the Bordeaux mixture and was the first commercial fungicide made of CBACs [24]. From 1887 to 1890, extensive tests using the Bordeaux mixture were conducted at several experimental agricultural stations, and it was shown to be effective in controlling various diseases, including potato late blight and many other leaf spots and blights [24,25]. As an excellent fungicide and bactericide, the Bordeaux mixture has been widely used for the past thirteen decades all over the world (see Table 1), representing the first generation of inorganic copper fungicides.
As excess free Cu2+ is toxic to plants, lime can help to reduce the concentration of free Cu2+ and cover the surface of plant tissues more effectively and stably. The ratio between CuSO4 and lime has been continuously improved in the development of CBACs. In the beginning, Millardet’s 8:8:100 formula involved mixing 8 pounds of CuSO4, 8 pounds of hydrated lime, and 100 gallons of water; however, the concentration of free copper ions was still too high to be used on young and copper-sensitive plants. To use the Bordeaux mixture on copper-sensitive plants, the relative amount of hydrated lime was increased in the formula (to a ratio of 4:4:100) to fix the Cu2+. To reduce the amount of CuSO4 and hydrated lime, a ratio of 2:6:100 was also used for spraying copper-sensitive seedlings [26].
Since the development of the Bordeaux mixture, second- and third-generation inorganic copper fungicides have gradually taken over in the management of plant diseases, such as copper oxychloride, copper oxide, and copper hydroxide [8,27]. These inorganic copper fungicides follow the principles of progressing from high to low concentrations and from soluble to insoluble from generation to generation. To cope with the disadvantages of inorganic copper fungicides, including complex preparation processes, instability, and difficulty combining them with other fungicides, two other types—synthetic organic copper and natural organic copper—have been developed [28]. Compared with inorganic CBACs, organic copper fungicides such as Cueva copper abietate and thiodiazole–copper have low copper content and greater stability, resulting in less environmental pollution and phytotoxicity (Table 1). With technological advancement, advanced nanotechnology has been introduced into the production of CBACs. Some papers have reported that, although the concentration of copper is low in nanoparticles (NPs), they are still effective in controlling diseases in tomatoes, pepper, rice, and many other plants and have reduced impacts on the environment due to their easy uptake into plant cells [29,30,31,32,33]. Copper nanoparticles are more efficient than conventional CBACs in preventing fungal-induced diseases [34]. For example, relevant studies have shown that Cu–chitosan NPs exhibit higher antifungal activity, due to both the chitosan and copper ions. On one hand, the chitosan component of the NPs can induce plant-defense-related enzymes, leading to an increase in plant antifungal activity. On the other hand, fungi have a tendency to produce different levels of acids during their infection of plants. The resulting acidic pH induces the protonation of chitosan amino groups, resulting in the release of free copper ions from the chitosan nanostructures. These enter fungal cells and induce the synthesis of highly reactive hydroxyl radicals, which destroy biological molecules [35]. Conventional nanoparticle synthesis routes using chemical and physical methods, such as chemical reduction, hydrothermal, and sol–gel, methods are considered harmful to the environment, due to the use of toxic chemical products, and are also costly. In response, many aqueous extracts from plants such as Portulaca oleracea and Piper nigrum have recently been used to biosynthesize promising, safe, cheap, and eco-friendly Cu-NPs [21,36,37]. Moreover, recent studies have shown that copper nanoparticles in combination with conventional fungicides can provide an environmentally safe and sustainable resistance management strategy through reducing the use of fungicides [38].

Table 1.
Various types of CBACs with advantages and disadvantages.
Table 1.
Various types of CBACs with advantages and disadvantages.
| Type | Name | Active Constituent | Advantages | Disadvantages |
|---|---|---|---|---|
| Inorganic copper fungicides | Copper sulphate | CuSO4 | Anti-microbial [8] | Phytotoxicity, Short-lasting [8] |
| Copper oxychloride | 3Cu(OH)2CuCl2 | Anti-microbial, Stable [27] | Short-lasting [39] | |
| Copper oxide | CuO | Low toxicity, Stable, Anti-microbial [8] | Low efficiency | |
| Copper hydroxide | Cu(OH)2 | Low toxicity, Stable, Anti-microbial [27] | Phytotoxicity [40] | |
| Organic copper fungicides | Oxine–copper | C18H12CuN2O2 | Low toxicity, Anti-microbial, Long-lasting [41] | Drug resistance, Environmental pollution, Phytotoxicity |
| Thiodiazole–copper | C4H4N6S4Cu | Low toxicity, Stable, Anti-microbe [42] | ||
| Copper abietate | C40H58CuO4 | Low toxicity, Stable, Anti-microbial | ||
| Copper-based nanoparticles | CuS nanoparticles | Cu and S | Slow-release, Stable, Low toxicity, High-efficiency [43] | Drug resistance, Phytotoxicity [44] |
| CuO nanoparticles | CuO | |||
| CuAlO2 nanoparticles | Cu and Al |
3. Construction of a Physical Barrier by Covering Plants with Slightly Soluble CBACs
A long historical practice is the application of CBACs as slightly soluble protective reagents, which cover the surface of plant tissues before diseases emerge and form a film to prevent direct contact between pathogens and plants. Moreover, they are absorbed into the tissue surface, making them difficult to be washed away by rain and dew, thus maintaining a long-term residual effect. In 1882, Millardet proposed that the actual treatment of mold with a mixture of CuSO4 and lime should not aim to kill the parasites in the leaves but, instead, should aim to prevent their development by covering the surface of leaves with various substances [45]. In the Bordeaux mixture, the generated calcium sulfate is thought to be necessary for tightly adhering the CBACs to the leaves. In other microsoluble CBACs, specific chemical additives facilitate this attachment [8,46]. In addition to separating the host plant from pathogens, the microsoluble film has two additional benefits: On one hand, the forms of CBACs that mainly coat the plant surface are soluble but complexed, thus only allowing a few free copper ions to be released and control plant diseases [47]. In fact, the concentration of copper ions on a leaf depends on the equilibrium established with complex and soluble copper forms [48]. This prevents the release of an excessive amount of Cu2+, which would lead to plant phytotoxicity. On the other hand, the slow and continuous release of Cu2+ on the tissue surface provides long-term prevention for plants; however, this kind of protection is not stable. Long-term rainwater scouring can break through the film of the Bordeaux mixture, reducing the protective effect and efficacy and allowing invasion by pathogens. Moreover, the weak acid substances secreted by plants and micro-organisms can also generate an acidic environment, leading to the inappropriate high-frequency release of Cu2+, which may have a negative phytotoxic effect [49]. High concentrations of Cu2+ can create visible corky damage on the surface of young fruit, reducing the aesthetic value of the fruits and compromising their marketability [8].
To reduce the environmental pollution and phytotoxicity caused by excess Cu2+, it is particularly important to develop novel CBACs. In fact, the release rate of Cu2+ affects the availability and persistence of conventional CBACs, such as the Bordeaux mixture. The rapid release of Cu2+ can have a good effect in terms of disease management, but the pesticide effect will be short and the security poor. Due to the large particle size and water solubility, CBACs can only form discontinuous deposits on the surface of plants, allowing for only partial blocking of direct contact between pathogenic microorganisms and the plants. In contrast, thicker deposits can increase the risk of excessive release of copper ions, causing plant toxicity [8]. Therefore, developing organic copper agents to reduce the excessive release of active copper ions and/or reducing the particle size of CBACs to promote the formation of a continuous film on plant surfaces are effective strategies to prevent bacterial and fungal spore invasion. Microscopically, oxine–copper is composed of copper ions and oxine rings: two oxine rings tightly grip the copper ions, which can gradually and safely release free copper ions [41]. The small size of these particles leads to a high surface-area-to-volume ratio, meaning more uniform coverage and better protection. Additionally, smaller particles are more tightly adsorbed on the plant surface and are more tolerant to rain wash than larger particles, giving longer effective protection. Studies using SEM have shown that foliar application of MoS2-CuNPs allowed for the formation of a protective film and increased the density of trichomes on the surface of rice leaves, thus preventing infection by Xanthomonas oryzae pv. oryzae cells [50]. Furthermore, NiO:Cu thin films observed by SEM presented antifungal activity against Aspergillus niger (which affects various fruits) and Macrophomina phaseolina (which is a soil-borne fungus responsible for root and lower stem infections in several plants) [51]. In addition to the smaller particle size, the diversity of forms of CBACs and their additives is another method to ensure even spraying, promoting adherence and stronger fixation on plant tissues. At present, various forms of CBACs, including aqueous solutions, wettable powders, and suspending agents, are broadly utilized. These have good efficacy but often a poor retention period, being greatly affected by rain wash. Some researchers have developed mineral oil emulsions for CBACs. The addition of mineral oil to fungicide spray mixtures is a frequently used strategy for the control of citrus black spot and potato pests [52,53], as mineral oil can significantly improve the diffusion, adhesion, and retention of copper ions; increase the deposition amount of effective components; and improve the ability to resist rain wash after mixing with CBACs [54]. Overall, it should be emphasized that mineral oil can highly improve the prevention effect of CBACs.
In addition, adjuvants are the key factors for improving the stability and efficacy of CBACs. During the processing and application of pesticides, surfactants can help them to distribute over, adhere to, and penetrate the surfaces of plants, directly or indirectly improving the effective pesticide usage rate. Agricultural organosilicon adjuvants, such as Silwet L-77 or siloxane, are often used as adjuvants for CBACs for the control of citrus canker due to their good wettability, ductility, and permeability. When mixed with CBACs, adjuvants can improve the ductility and adsorption properties of copper agents on the leaves, increasing the tolerance to rain acidification and plant disease resistance [46]. Alternatively, bamboo vinegar—which contains organic acids, ketones, and alcohols—is a good solubilizer, co-solvent, and penetrant. Researchers have boiled bamboo vinegar and CuSO4 to form a preparation which can enhance the control effect of CuSO4 on tobacco brown spot disease and black shank disease, as well as improving its inhibition of the growth of green algae, while the copper ion concentration remains unchanged [55]. Additionally, ethoxy-modified polysiloxane, polyoxyethylene monolau-rate β pine terpene polymer, ammonium salt, and other adjuvants play supporting roles to CBACs, helping them to attach to plant tissues more evenly and stably [46]. However, recent studies have also revealed that Cu2+ released from CuSO4 and nanomaterials is rapidly absorbed into the leaf cuticle [56]. Interestingly, copper-based nanoparticles can pass quickly through the cuticle, while CuSO4 can stay longer in the leaf cuticle, which appears to strengthen the alternative physical barrier [56].
4. The Second Tier of Protection and Copper-Resistant Strains
Generally, copper is a necessary metal ion for bacterial growth and development, and bacteria uptake Cu2+ into the cytoplasm through copper uptake transporters such as CcoA and YcnJ-like proteins [57,58,59,60]. Then, the copper reductase cbb3-type cytochrome c oxidase (cbb3-Cox) assembly factor CcoG is present on the cell membrane, where the incoming Cu2+ is assembled into the cysteine conservative motif of CcoG and converted into Cu+ through transferring electrons to the [4Fe-4S] cluster (Figure 1a). Furthermore, Cu+ binds to the active center of enzymes to maintain its vital role in bacteria [61,62]. However, excess free copper ions also lead to toxic or antimicrobial activity in bacteria, forming the second-tier protection of CBACs. The antimicrobial activity of CBACs can be further divided into two parts: first, deposited CBACs can react with water and oxygen to produce OH−, causing bacterial cell membranes to suffer from oxidative damage, leading to protein denaturation and increased membrane permeability. This damage to cell membranes further results in the leaking out of some bacterial essential nutrients and proteins. In addition, excessive Cu2+ or Cu+ entering the cytoplasm will cause bacterial oxidative stress and even cell death. Under an anoxic environment, Cu+ replaces iron in the iron–sulfur clusters of dehydratases, resulting in the degradation of those crucial enzymes. Furthermore, the released iron may subsequently initiate the Fenton/Haber–Weiss reaction, while the transformation between Cu+ and Cu2+ leads to a Fenton-like reaction, all of which generate OH− and ROS, consequently causing lipid peroxidation, protein oxidation, and nucleic acid damage [63,64,65,66].
Bacteria also depend on two systems to overcome excess copper: copper homeostasis and copper resistance protein (cop). As shown in Figure 1, in order to maintain the cytoplasmic copper concentration, bacteria have developed three strategies: First (I), the chaperone protein CopZ loads Cu+ and transfers it to P1B-type Cu-exporting ATPase CcoI and P-type ATPase family CopA, which respond to the efflux of excessive Cu+ [58,67,68,69,70,71]. Second (II), metallothionein (MT) is a super-family of cysteine-rich small proteins which bind Cu+ (as well as other heavy metal ions) through metal–sulfur bonds in order to neutralize their toxicity [72,73,74,75]. Third (III), excess Cu+ is bound with the elevated cytosolic copper storage protein (Ccsp), which consists of a homotetramer assembly capable of binding Cu+ with the help of a CopZ-like copper chaperone [76].
Over-use of CBACs has resulted in long-term exposure of plant pathogenic bacteria to high concentrations of copper ions, resulting in the selection of copper-resistant strains, such as Xanthomonas, Pseudomonas, and Erwinia spp. [77,78,79,80,81,82,83], which have direct and indirect impacts on agricultural production [84,85]. Through the isolation and identification of copper-resistant strains from copper-rich soil, more than 95% of the copper-resistant isolates were identified as Gram-negative bacteria [86]. Unlike Gram-positive bacteria, Gram-negative bacteria have an outer membrane, a periplasmic space, and an inner membrane which endow them with a special structural basis for copper resistance. To date, most copper-resistant strains have developed from horizontal transfer of the cop system or pco system in response to excessive copper, with representative examples being Pseudomonas syringae pv. tomato (Pst) and Escherichia coli, respectively [87].
The cop system is a conserved copper-resistant system in P. syringae pv. tomato, which is encoded by an operon containing up to six genes (copABCDRS) on the plasmid pPT23D [87]. CopA is a periplasmic protein that contains methionine, histidine, and aspartic-acid-rich motifs. Each CopA protein can combine up to eleven copper ions [88,89]. This high binding capacity restricts excess copper from entering the bacterial cytoplasm. As an outer membrane protein, CopB also contains repetitive amino acid sequences (Asp-His-X2-Met-X2-Met). Although there is no direct evidence that CopB can combine with copper [90,91,92], it may be assumed that the role of CopB is to fix extracellular copper ions. CopC is a periplasmic chaperone protein that contains two copper ion binding sites for binding either Cu+ or Cu2+. It has been proposed that CopC transfers Cu+ to different interactors, such as CopA, CopB, CopD, and CopS, in order to balance the Cu+ concentration in cells [92]. On one hand, CopD, the interaction protein of CopC, is a plasma membrane protein that transports essential CopC-delivered copper through the inner membrane into the cytoplasm [89,90]. On the other hand, CopC interacts with CopA and CopB to deliver the carried Cu+ for fixing, which can reduce the associated toxicity [90]. The CopS located on the plasma membrane serves as a copper sensor, which may interact with CopA or CopC to transmit copper signals to CopR, thus continuously regulating the expression of the cop operon activated by CopR [91]. To summarize the copper resistance mechanism of Pst, the cop operon located on plasmid pPT23D can chelate excessive copper ions through a group of proteins, particularly CopA and CopB. The copper resistance mechanism in E. coli is the pco system containing pcoABCD, which is located on the plasmid pRJ1004 and corresponds to the cop operon in Pst [93,94,95,96]. To date, all identified copper-resistant Pseudomonas strains have homologs of the cop operon in their chromosomes [97]; for example, Cupriavidus metallidurans CH34 contains the complete copABCDRS [98,99], while X. citri pv. citri contains only copABCD [100]. Some Xanthomonas copper-resistant strains only contain copLAB, conferring resistance to copper ions [101,102,103]. The mechanisms of copper-resistant fungi have been reviewed in [104], which were generally indicated to enhance Cu+ exporting and homeostasis. Yarrowia lipolytica is an inherently copper-resistant yeast in which Cu2+ significantly promotes the yeast-to-hypha transition, allowing for the better survival of hyphae than yeast-form cells in the presence of CuSO4 [105].
Some pathogens could alternatively develop new weapons to overcome their copper sensitivity. Xanthomonas oryzae PXO99A is more sensitive to copper than other wild strains caused by the copA mutation [106]. It seems not to back mutate for a copper-resistant strategy but to develop a novel TAL effector of PthXo1 to upregulate the expression of OsSWEET11/Xa13 in rice [107], which interacts with plant copper-uptake complex components of OsCOPT1 and OsCOPT5 to reduce the copper concentration in vascular tissue [108]. Moreover, the upregulated OsSWEET11 protein has additional susceptible functions as the sucrose efflux from the phloem parenchyma cells for bacterial proliferation [109]. Consistent with the conclusion, PXO99A introduced into the copAB could restore copper resistance but fail to overcome the xa13-mediated resistance [106].
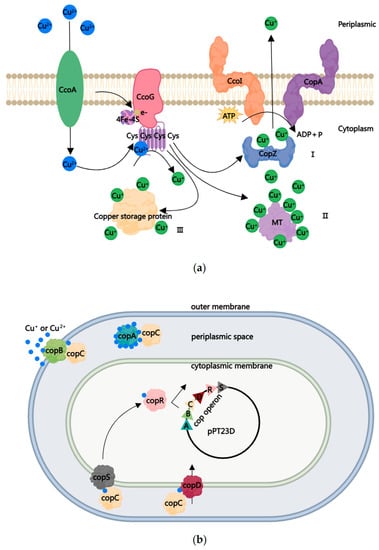
Figure 1.
Two strategies used to manipulate the excessive copper in Gram-negative bacteria. (a) Three strategies for copper homeostasis: (I) the chaperone protein CopZ loads Cu+ and transfers it to P1B-type Cu-exporting ATPase CcoI and P-type ATPase family CopA, which respond to the efflux of excessive Cu+; (II) each metallothionein (MT) protein binds seven Cu+ ions to neutralize the toxicity; and (III) copper storage protein binds excessive Cu+. (b) Cop systems to resist copper. P. syringae pv. tomato strains encode an operon of copABCDRS containing up to six genes on the plasmid pPT23D. The periplasmic protein CopA combines eleven copper ions to restrict the excess copper in the cytoplasm. The outer membrane protein copB may play a role in fixing extracellular copper ions, as does CopA. The periplasmic chaperone protein CopC contains two copper ion binding sites, for binding either Cu+ or Cu2+. CopC delivers Cu+ to different interactors—such as CopA, CopB, CopD, and CopS—either for uptake or to fix Cu+ to balance the concentration in cells. The plasma membrane protein CopS acts as a copper sensor. It may interact with CopA or CopC to transmit copper signals to CopR, thus continuously regulating the expression of cop operon activated by copR (modified by references [87] and [91]). The picture was drawn using the MedPeer software (https://user.medpeer.cn/ (2 February 2023)).
5. Enhanced Plant Resistance to Pathogens and Activation of Defense-like Responses
At present, the application of copper preparations is the only way to control many plant diseases. However, an over-reliance on and over-use of CBACs have resulted in the evolution of many Cu-resistant strains, reducing their effectiveness in controlling plant diseases [110,111]. Developing new types of CBACs or mixing them with other fungicides provides an effective way to control plant diseases caused by copper-resistant strains. Copper used with EBDCs (Ethylene bis-dithiocarbamates) can provide control of bacterial speck and spot diseases, even when copper-tolerant populations are present [112,113,114]. Recent studies have demonstrated that advanced copper composites and nano-magnesium oxide materials are effective against copper-tolerant Xanthomonas spp., increasing the control of bacterial spot in tomato under field conditions [115,116,117]. Furthermore, although the emergence of copper-resistant strains has rapidly increased, CBACs are still effective in controlling some of the diseases caused by those pathogens [118], implying that the protection imparted by CBACs does not only rely on their antimicrobial activity and the formation of a physical film. Previous reports have demonstrated that copper stress could also activate a series of defense-like responses in plants. In alfalfa (Medicago sativa), the mitogen-activated protein kinases (MAPKs) SIMK and SAMK are involved in the response to pathogen-associated stimulation. Excessive copper specifically activates the MAPK SIMKK, which can activate SIMK and SAMK in Medicago sativa [119]. Additionally, excess copper activated MAPK2, MAPK3, and MAPK4 in rice roots [120,121,122]. ROS burst is one of the earliest events in plants under heavy metal stress, as the ROS act as signaling molecules that regulate the plant’s response to abiotic and biotic stresses [123,124,125]. Excess copper promotes ROS synthesis through Fenton and Haber–Weiss reactions [126]. Copper-elevated ROS accumulation has already been identified in Arabidopsis thaliana [127], Pisum sativum L. [128], Medicago sativa [129], and Oryza sativa L. [130]. Previous reports have demonstrated that copper stress enhanced plant resistance to pathogens through copper-binding proteins. In cotton, the blue copper-binding protein GhUMC1 has been shown to be involved in resistance to Verticillium dahlia through regulating the jasmonic acid signaling pathway and lignin metabolism [131]. In barley, Mla and Rom1 negatively regulate miR398, which elevates the transcription level of SOD1 and enhances resistance against powdery mildew [132], indicating the important role of the miR398–SOD module in regulating plant resistance against pathogens. Interestingly, the foliar application of two copper nanomaterials enhanced resistance to Fusarium oxysporum f. sp. lycopersici, a pathogen that causes the root fungal disease Fusarium wilt, as well as enhancing phenylalanine ammonia-lyase (PAL) and peroxidase (POD) activities in tomato roots [30,56].
6. Eliciting Plant Immunity to Strengthen the Third-Tier Barrier
The above observations—that is, that CBACs can manage copper-resistant strains and excessive copper can trigger defense-like responses in addition to being toxic to plants [118,119,120,121,122,123,124,125,126,127,128,129,130]—indicate that copper may directly trigger plant immunity. Indeed, Liu et al. found that a concentration of 10 nM CuSO4 was sufficient to enhance the resistance of Arabidopsis plants against Pst DC3000 [133]. In addition, spraying potato with CuSO4 (100 nM) enhanced resistance to late blight [134]; however, in in vitro co-culture experiments, these concentrations of CuSO4 had no inhibitory effect on microbial growth [133,134]. Moreover, they found that copper ions triggered a series of immune responses, including ethylene (ET) and salicylic acid (SA) biosynthesis pathways, ROS burst, Ca2+ signaling, MAPK activation, callose deposition, and up-regulation of the expression of pathogenesis-related (PR) genes [133], which are similar to the responses induced by flg22, a conserved short peptide of flagellin from Pst DC3000 [135,136]. In contrast with flg22-triggered immunity, Cu2+ treatment rapidly activated the synthesis of ET by specifically inducing the expression of AtACS8 dependent on the CuRE cis-element in the promoter region [137]. Downstream of the ET signaling pathway, Cu2+-mediated callose deposition required both AtMYB51 and AtMYB122, while it mainly required AtMYB51 for flg22 in Arabidopsis [138]. A nuclear copper chaperone CCP containing the classical copper-binding site may interact with and recruit the transcription factor TGA2 to induce the expression of PR1 and enhance the resistance to Pst DC3000 [139]. In potato (Solanum tuberosum), Cu2+ activated ET biosynthesis to induce resistance to potato late blight, as well as inhibiting the biosynthesis of abscisic acid (ABA) by activating the transcription factor StEIN3 (ethylene insensitive 3), thus directly repressing the expression of StNCED1 (9-cis-epoxycarotenoid dioxygenase) and the ABA biosynthesis gene StABA1 by targeting their promoters [134]. Yao et al. have recently found that copper ion transporters and copper ion binding proteins, such as HMA5, were significantly induced and played a broad-spectrum role in virus–rice interactions. Most of the copper ions entered rice cells from the intercellular space, increasing the copper ion content in the leaves. Copper-orchestrated virus resistance was promoted through inhibiting the accumulation of the SPL9 protein, thus reducing the expression of SPL9 target gene miR528 and enhancing the transcription level of ascorbate oxidase (AO) and ROS levels [140]. On the other hand, copper ions could directly activate the AO enzyme activity to enhance viral resistance in rice [140]. Without a doubt, a low concentration of copper ions can trigger plant immune responses, thus participating in the construction of a third-tier barrier to protect plants against pathogens. Similarly, the induction of plant immunity was observed when using Cu2O-NPs to control cucumber root rot disease [141]. However, over thirteen decades, a considerable number of studies have shown that CBACs cannot effectively control the plant diseases caused by copper-resistant strains compared with copper-sensitive strains [142,143]. Inappropriate timing of applications, with respect to wounding and infection events, is an alternative explanation; that the activated PTI-like immunity may not be able to control all pathogens is also an alternative explanation. However, such results suggest that more research is needed to fully explain the specific mechanisms by which copper ions regulate plant immune responses, as well as the need for further research on whether copper can trigger immune responses in different kinds of plants.
7. Summary and Future Prospects
As a metal ion, copper is the main component of commercial CBACs. At present, the mechanisms of CBACs can be summarized into two- or three-tiered protection (Figure 2), detailed as follows. First, the slightly soluble CBACs form a dense protective film on the plant surface, which acts as a physical barrier to prevent contact between the invasive pathogenic microorganisms and the host; second, the released ionic copper destroys the cell membrane of the pathogenic bacteria, leading to the leakage of nutrients, denaturation of various proteins, and inactivation of enzymes, thereby killing the microorganisms; and third, copper ions can also stimulate plant immune responses to further strengthen the immunity of the host plant. Such a three-tiered protection provides a perfect design for broad spatial disease resistance, supporting the application of CBACs for more than thirteen decades.
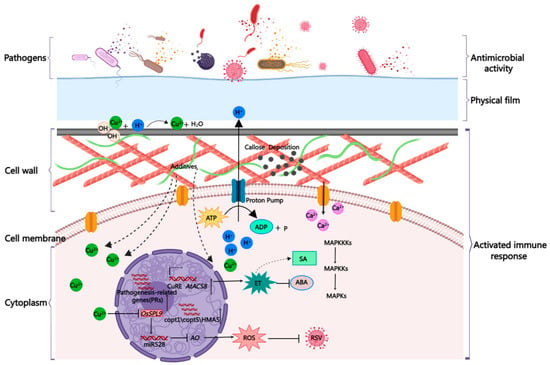
Figure 2.
Illustration of the three tiers of protection provided by copper-based antimicrobial compounds. The spatial defense network consists of a physical film, antimicrobial activity, and activation of immune responses. ABA, abscisic acid; AO, ascorbate oxidase; ET, ethylene; ROS, reactive oxygen species; SA, salicylic acid. The picture was drawn using the MedPeer software (https://user.medpeer.cn/ (6 February 2023)).
However, with increases in rain acidification and copper-resistant strains, it is necessary to constantly innovate relevant methods and technologies in order to optimize the application of CBACs. Based on our knowledge, prospective studies can be carried out at the following three levels. First, combinations of systemic fungicides and/or plant stimulants need to be broadly investigated. For example, thiodiazole mixed with copper to produce commercial thiodiazole–copper can recover the pathogen inhibition activity while reducing the usage of ionic copper [42]. Various plant stimulants have been widely used in agricultural production, some of which have been shown to possess novel bioactivities [49,144]; however, they normally have lower efficacy in reducing disease incidence and severity compared to CBACs [144]. Therefore, the combination of such stimulants with CBACs is worth investigating in future research.
Second, the long-term and excessive use of CBACs has caused the deposition of copper in the soil and environmental pollution [21,144]. Therefore, in order to ensure the safe use of CBACs for another 13 decades (or even longer), it is imperative to further reduce their usage, together with their tolerance to scouring by rain. As an advanced fungicide, Cu-NPs have smaller particle size and higher surface-area-to-volume ratio, and they can pass more quickly through the cuticle than traditional CBACs [56]. Therefore, Cu-NPs have attracted extensive attention in agricultural applications. Scientific researchers have revealed the positive effective roles of Cu-NPs in controlling diseases, reducing toxicity, promoting growth, and increasing ion content in rice seeds [29,30,31,32,145]. In addition, with technological improvement and development, the cost of Cu-NPs and plant-based CuO-NPs can be expected to gradually decrease [21,36,145], laying the foundation for the long-term use of CBACs. Along with the innovation of advanced production technologies, traditional CBACs are improving in a more stable, low-toxicity, and environmentally friendly manner. Scientists may develop novel adjuvants to increase the ductility, adhesion, and permeability of CBACs, allowing for a reduction in the content of ionic copper in the CBACs. In general, reducing the cost of Cu-NPs and novel additives may help CBACs to achieve better development and applications in the future.
Finally, although copper-triggered plant immunity has been reported, the signal transduction pathway(s) associated with such induced resistance remains unclear. Further detailed studies on the specific mechanisms underlying copper-triggered plant immunity should be conducted in order to better utilize CBACs, including the development of pesticide application techniques and the cultivation of ideal crop varieties that are more rapidly and strongly responsive to ionic copper than current versions. In general, once the above three problems are effectively solved, CBACs can be expected to serve humanity’s agricultural purposes for another thirteen decades.
Author Contributions
Y.Y. and H.X. collected the literature; Y.Y. and Z.C. wrote the manuscript; H.L. provided suggestions and proofread the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Program of Hubei Province (2022BFE003) and the Science and Technology Innovation Team of Hubei Province for Z.C. and the Shandong Modern Agricultural Technology & Industry system (SDAIT-17-06) for Z.C. and H.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- West, P.C.; Gerber, J.S.; Engstrom, P.M.; Mueller, N.D.; Brauman, K.A.; Carlson, K.M.; Cassidy, E.S.; Johnston, M.; MacDonald, G.K.; Ray, D.K.; et al. Leverage points for improving global food security and the environment. Science 2014, 345, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Change 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Jiang, F.B.; Ou, J.F. Global pesticide consumption and pollution: With China as a focus. Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 125–144. [Google Scholar]
- Jay, R.L.; Ebrahim, O.; Franklin, B.; Jürgen, K.; Jeffrey, B.; Jones, J.A. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar]
- Torre, A.L.; Chiranjib, M.; Federica, C.; Valerio, B. Natural alternatives to copper and low-rate copper formulations to control grape downy mildew in organic farming. Hell. Plant Prot. J. 2012, 5, 13–21. [Google Scholar]
- Schutte, G.C.; Beeton, K.V.; Kotzé, J.M. Rind stippling on valencia oranges by copper fungicides used for control of citrus black spot in South Africa. Plant Dis. 1997, 81, 851–854. [Google Scholar] [CrossRef]
- Elkins, R.B.; Temple, T.N.; Shaffer, C.A.; Ingels, C.A.; Lindow, S.B.; Zoller, B.G.; Johnson, K.B. Evaluation of dormant-stage inoculum sanitation as a component of a fire blight management program for fresh-market bartlett pear. Plant Dis. 2015, 99, 1147–1152. [Google Scholar] [CrossRef]
- Lee, Y.A.; Milton, N.; Schroth, M.H.; Steven, E.L.; Wang, X.L.; Bill, O.; Richard, B.; Beth, L.T. Increased toxicity of iron-amended copper-containing bactericides to the walnut blight pathogen Xanthomonas campestris pv. juglandis. Phytopathology 1993, 83, 1460–1465. [Google Scholar] [CrossRef]
- Ninot, A.; Aletà, N.; Moragrega, C.; Montesinos, E. Evaluation of a reduced copper spraying program to control bacterial blight of walnut. Plant Dis. 2002, 86, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Large, E.C.; Beer, W.J. Field trials of copper fungicides for the control of potato blight; low-copper fungicides. Ann. Appl. Biol. 1946, 33, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Wimalajeewa, D.L.S.; Robert, S.; Cahill, G.H.; Hg, S.; Washbourne, J.R. Chemical control of bacterial canker (Pseudomonas syringae pv. syringae) of apricot and cherry in Victoria. Aust. J. Exp. Agric. 1991, 31, 705–708. [Google Scholar] [CrossRef]
- Furtado, I. Effect of copper fungicides on the occurrence of the pathogenic form of Colletotrichum coffeanum. Trans. Br. Mycol. Soc. 1969, 53, 325–328. [Google Scholar] [CrossRef]
- Obanor, F.O.; Walter, M.; Jones, E.E.; Jaspers, M.V. Efficacy of systemic acquired resistance inducers in olive leaf spot management. Australas. Plant Pathol. 2013, 42, 163–168. [Google Scholar] [CrossRef]
- Graham, R.D. Susceptibility to powdery mildew of wheat plants deficient in copper. Plant Soil 1980, 56, 181–185. [Google Scholar] [CrossRef]
- Karl, H.S. The influence of boron and copper deficiency upon infection by Erysiphe graminis D.C. the powdery mildew, in wheat var. kenya. Plant Soil 1967, 27, 450–452. [Google Scholar]
- Beresford, R.M.; James, T.S.; Walker, M.J.; Spink, R.R.; Marshall; White, V. Copper and slaked lime for the control of black spot and powdery mildew in apples. Fruit Crops 1995, 48, 83–88. [Google Scholar] [CrossRef]
- Mesquita, A.F.; Gonçalves, F.J.M.; Gonçalves, A.M.M. The lethal and sub-lethal effects of fluorinated and copper-based pesticides—A review. Int. J. Environ. Res. Public Health 2023, 20, 3706. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hall, J.G. Diseases of economic plants. Science 1915, 33, 621–622. [Google Scholar]
- Tratman, E.E.R. Report on the Use of Metal Railroad Ties and on Preservative Processes and Metal Tie-Plates for Wooden Ties; Deptartment of Agriculture, Forestry Division: Washington, DC, USA, 1894; Bulletin 9; p. 269.
- Johnson, G.F. The early history of copper fungicides. Agric. His. 1935, 9, 67–79. [Google Scholar]
- Plant Diseases; Vermont Agricultural Experiment Station Bulletin: Burlington, VT, USA, 1892; Volume 28, p. 17.
- Mark, L. Dormant Sprays for Disease Control; Michigan State University Extension: East Lansing, MI, USA, 2008. [Google Scholar]
- Vicent, A.; Armengol, J.; García-Jiménez, J. Rain fastness and persistence of fungicides for control of alternaria brown spot of citrus. Plant Dis. 2007, 91, 393–399. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, H.; Liu, W.; Qin, Y.; Xing, R.; Li, P. Integrated proteomics and metabolomics analysis reveals the antifungal mechanism of the C-coordinated O-carboxymethyl chitosan Cu (II) complex. Int. J. Biol. Macromol. 2020, 155, 1491–1509. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Montero, L.A.; Pariona, N.; Mtz-Enriquez, A.I.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Aqueous-phase synthesis of nanoparticles of copper/copper oxides and their antifungal effect against Fusarium oxysporum. J. Hazard. Mater. 2019, 380, 120850. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Application of copper-chitosan nanoparticles stimulate growth and induce resistance in finger millet (Eleusine coracana Gaertn.) plants against blast disease. J. Agric. Food Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef]
- Gaba, S.; Rai, A.K.; Varma, A.; Prasad, R.; Goel, A. Biocontrol potential of mycogenic copper oxide nanoparticles against Alternaria brassicae. Front. Chem. 2022, 10, 966396. [Google Scholar] [CrossRef]
- Tarakanov, R.; Shagdarova, B.; Lyalina, T.; Zhuikova, Y.; Il’ina, A.; Dzhalilov, F.; Varlamov, V. Protective properties of copper-loaded chitosan nanoparticles against soybean pathogens Pseudomonas savastanoi pv. glycinea and Curtobacterium flaccumfaciens pv. flaccumfaciens. Polymers 2023, 15, 1100. [Google Scholar]
- Sadek, M.E.; Shabana, Y.M.; Sayed-Ahmed, K.; Abou Tabl, A.H. Antifungal activities of sulfur and copper nanoparticles against cucumber postharvest diseases caused by Botrytis cinerea and Sclerotinia sclerotiorum. J. Fungi 2022, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.G.; Sanada, K.; Pieretti, J.C.; Shigueoka, L.H.; Sera, G.H.; Seabra, A.B.; Oliveira, H.C. Nanoencapsulation boosts the copper-induced defense responses of a susceptible Coffea arabica cultivar against Hemileia vastatrix. Antibiotics 2023, 12, 249. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Hassan, S.E.-D.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-based copper oxide nanoparticles; biosynthesis, characterization, antibacterial activity, tanning wastewater treatment, and heavy metals sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Aien, J.; Khan, A.A.; Haq, S.; Khan, A.R.; Elmnasri, K.; Ben Ali, M.; AL-Harbi, M.S.; Alghonaim, M.I.; Alsalamah, S.A.; Qurtam, A.A.; et al. Antibacterial, antioxidant and physicochemical properties of Pipper nigram aided copper oxide nanoparticles. Crystals 2023, 13, 330. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Synergy between Cu-NPs and fungicides against Botrytis cinerea. Sci. Total Environ. 2020, 703, 135557. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, P.; Paradelo, M.; Rodríguez-Salgado, I.; Fernández-Calviño, D.; López-Periago, J.E. Modeling the influence of raindrop size on the wash-off losses of copper-based fungicides sprayed on potato (Solanum tuberosum L.) leaves. J. Environ. Sci. Health A 2013, 48, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Montag, J.; Schreiber, L.; Schönherr, J. An in vitro study on the postinfection activities of copper hydroxide and copper sulfate against conidia of Venturia inaequalis. J. Agric. Food Chem. 2006, 54, 893–899. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Chen, Y.; Zhang, Q.; Lu, P.; Hu, D. Dissipation, residues and risk assessment of oxine-copper and pyraclostrobin in citrus. Food Addit. Contam. 2019, 36, 1538–1550. [Google Scholar] [CrossRef]
- Tang, P.; Xiong, X.P.; Du, J.S. Control effect of 20% Longkejun (thiodiazole-copper) on crop bacterial diseases. Agric. Sci. Tech. Equip. 2008, 3, 35–36. [Google Scholar]
- Ahmad, H.; Venugopal, K.; Bhat, A.H.; Kavitha, K.; Ramanan, A.; Rajagopal, K.; Srinivasan, R.; Manikandan, E. Enhanced biosynthesis synthesis of copper oxide nanoparticles (CuO-NPs) for their antifungal activity toxicity against major phyto-pathogens of apple orchards. Pharmacol. Res. 2020, 37, 246. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 2015, 24, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Millardet, A. Traitement du Mildiou et du Rot. Agric. Prat. 1885, 49, 513–516. [Google Scholar]
- Li, J.H.; Zhang, H.Q.; Li, N.; Shu, G.P.; Gu, Y.H.; Deng, Z.N. Influences of five surface active agents on control effect of copper fungicides to citrus canker disease of bingtang sweet orange. Hunan Agric. Sci. 2013, 5, 71–73. [Google Scholar]
- Menkissoglu, O. Relationship of free ionic copper and toxicity to bacteria in solutions of organic compounds. Phytopathology 1991, 81, 1258–1263. [Google Scholar] [CrossRef]
- Menkissoglu, O.; Lindow, S.E. Chemical forms of copper on leaves in relation to the bactericidal activity of cupric hydroxide deposits on plants. Phytopathology 1991, 81, 1263–1270. [Google Scholar] [CrossRef]
- Arman, P.; Wain, R.L. The role of leaf exudates in the solution of copper from Bordeaux mixture. Ann. Appl. Biol. 1958, 46, 366–374. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yang, D.; Jin, Q.; Wu, C.; Cui, J. Multifunctional molybdenum disulfide-copper nanocomposite that enhances the antibacterial activity, promotes rice growth and induces rice resistance. J. Hazard 2020, 394, 122551. [Google Scholar] [CrossRef]
- Aftab, M.; Butt, M.Z.; Ali, D.; Aftab, Z.H.; Tanveer, M.U.; Fayyaz, B. Investigation of antifungal response of NiO and copper-doped NiO thin films against Aspergillus niger and Macrophomina phaseolina fungi. ESPR 2022, 29, 3840–3852. [Google Scholar] [CrossRef]
- Galimberti, A.; Alyokhin, A. Lethal and sublethal effects of mineral oil on potato pests. J. Econ. Entomol. 2018, 111, 1261–1267. [Google Scholar] [CrossRef]
- Silva Junior, G.J.; Moraes, M.R.; Moreira, R.R.; Behlau, F. Tree age and cultivar-oriented use of mineral oil added to fungicide tank mixture for the control of citrus black spot in sweet orange orchards. Pest Manag. Sci. 2022, 78, 488–498. [Google Scholar] [CrossRef]
- Lu, H.L.; OuYang, G.C.; Tan, B.L.; Hou, B.H.; Meng, X.; Fang, X.R. Synergistic effect of mineral spray oil on inorganic copper fungicides to control Elsinoe fawcettii and Panonychus citri. Agrochemicals 2018, 57, 383–386. [Google Scholar]
- Xu, L.J.; Fu, J.G.; Yuan, Q.H.; Zou, G.J.; Wang, Y.Q.; Chen, J. A Bamboo Vinegar Copper Preparation and Its Application in Tobacco Disease Control and Preparation Method. Patent CN106857666A, 2 June 2020. [Google Scholar]
- Shen, Y.; Borgatta, J.; Ma, C.; Elmer, W.; Hamers, R.J.; White, J.C. Copper nanomaterial morphology and composition control foliar transfer through the cuticle and mediate resistance to root fungal disease in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2020, 68, 11327–11338. [Google Scholar] [CrossRef] [PubMed]
- Ekici, S.; Yang, H.; Koch, H.G.; Daldal, F. Novel transporter required for biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. mBio 2012, 3, e00293-11. [Google Scholar] [CrossRef]
- Ekici, S.; Turkarslan, S.; Pawlik, G.; Dancis, A.; Baliga, N.S.; Koch, H.G.; Daldal, F. Intracytoplasmic copper homeostasis controls cytochrome c oxidase production. mBio 2014, 5, e01055-13. [Google Scholar] [CrossRef]
- Khalfaoui-Hassani, B.; Wu, H.; Blaby-Haas, C.E.; Zhang, Y.; Sandri, F.; Verissimo, A.F.; Koch, H.G.; Daldal, F. Widespread distribution and functional specificity of the copper importer CcoA: Distinct Cu uptake routes for bacterial cytochrome c oxidases. mBio 2018, 9, e00065-18. [Google Scholar] [CrossRef] [PubMed]
- Chillappagari, S.; Miethke, M.; Trip, H.; Kuipers, O.P.; Marahiel, M.A. Copper acquisition is mediated by YcnJ and regulated by YcnK and CsoR in Bacillus subtilis. J. Bacteriol. 2009, 191, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Marckmann, D.; Trasnea, P.I.; Schimpf, J.; Winterstein, C.; Andrei, A.; Schmollinger, S.; Blaby-Haas, C.E.; Friedrich, T.; Daldal, F.; Koch, H.G. The cbb3-type cytochrome oxidase assembly factor CcoG is a widely distributed cupric reductase. Proc. Natl. Acad. Sci. USA 2019, 116, 21166–21175. [Google Scholar] [CrossRef]
- Koch, H.G.; Winterstein, C.; Saribas, A.S.; Alben, J.O.; Daldal, F. Roles of the ccoGHIS gene products in the biogenesis of the cbb3-type cytochrome c oxidase. J. Mol. Biol. 2000, 297, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, C.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen-toxicity, oxygen radicals, transition-metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper toxicity and the origin of bacterial resistance-new insights and applications. Metallomics 2011, 3, 1109–1118. [Google Scholar] [CrossRef]
- Singleton, C.; Hearnshaw, S.; Zhou, L.; Le Brun, N.E.; Hemmings, A.M. Mechanistic insights into Cu(I) cluster transfer between the chaperone CopZ and its cognate Cu(I)-transporting P-type ATPase, CopA. Biochem. J. 2009, 424, 347–356. [Google Scholar] [CrossRef]
- Meydan, S.; Klepacki, D.; Karthikeyan, S.; Margus, T.; Thomas, P.; Jones, J.E.; Khan, Y.; Briggs, J.; Dinman, J.D.; Vázquez-Laslop, N.; et al. Programmed ribosomal frameshifting generates a copper transporter and a copper chaperone from the same gene. Mol. Cell 2017, 65, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Utz, M.; Andrei, A.; Milanov, M.; Trasnea, P.I.; Marckmann, D.; Daldal, F.; Koch, H.G. The Cu chaperone CopZ is required for Cu homeostasis in Rhodobacter capsulatus and influences cytochrome cbb3 oxidase assembly. Mol. Microbiol. 2019, 111, 764–783. [Google Scholar] [CrossRef]
- Kulajta, C.; Thumfart, J.O.; Haid, S.; Daldal, F.; Koch, H.G. Multi-step assembly pathway of the cbb3-type cytochrome c oxidase complex. J. Mol. Biol. 2006, 355, 989–1004. [Google Scholar] [CrossRef]
- Peters, A.; Kulajta, C.; Pawlik, G.; Daldal, F.; Koch, H.G. Stability of the cbb3-type cytochrome oxidase requires specific CcoQ-CcoP interactions. J. Bacteriol. 2008, 190, 5576–5586. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Bremner, I. Oxygen free radicals and metallothionein. Free Radic. Biol. Med. 1993, 14, 325–337. [Google Scholar] [CrossRef]
- Sutherland, D.E.; Stillman, M.J. The „magic numbers” of metallothionein. Metallomics 2011, 3, 444–463. [Google Scholar] [CrossRef]
- Olafson, R.W.; Abel, K.; Sim, R.G. Prokaryotic metallothionein: Preliminary characterization of a blue-green alga heavy metal-binding protein. Biochem. Biophys. Res. Commun. 1979, 89, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Jung, H.; Meloni, G. Copper metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef]
- Straw, M.L.; Chaplin, A.K.; Hough, M.A.; Paps, J.; Bavro, V.N.; Wilson, M.T.; Vijgenboom, E.; Worrall, J. A cytosolic copper storage protein provides a second level of copper tolerance in Streptomyces lividans. Metallomics 2018, 10, 180–193. [Google Scholar] [CrossRef]
- Masami, N.; Masao, G.; Katsumi, A.; Tadaaki, H. Nucleotide sequence and organization of copper resistance genes from Pseudomonas syringae pv. actinidiae. Eur. J. Plant Pathol. 2004, 110, 223–226. [Google Scholar] [CrossRef]
- Richard, D.; Tribot, N.; Boyer, M.; Terville, K.; Boyer, S.; Javegny, M.; Roux-Cuvelier, O.; Pruvost, A.; Chabirand, M.A.; Vernière, C. First report of copper-resistant Xanthomonas citri pv. citri pathotype A causing Asiatic citrus canker in Réunion, France. Plant Dis. 2017, 101, 503. [Google Scholar] [CrossRef]
- Sholberg, P.L.; Bedford, K.E.; Haag, P.; Randall, P. Survey of Erwinia amylovora isolates from British Columbia for resistance to bactericides and virulence on apple. Can. J. Plant Pathol. 2001, 23, 60–67. [Google Scholar] [CrossRef]
- Behlau, F.; Hong, J.C.; Jones, J.B.; Graham, J.H. Evidence for acquisition of copper resistance genes from different sources in citrus-associated xanthomonads. Phytopathology 2013, 103, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Basim, H.; Minsavage, G.V.; Stall, R.E.; Wang, J.F.; Shanker, S.; Jones, J.B. Characterization of a unique chromosomal copper resistance gene cluster from Xanthomonas campestris pv. vesicatoria. Appl. Environ. Microb. 2005, 71, 8284–8291. [Google Scholar] [CrossRef]
- Cooksey, D.A. Genetics of bactericide resistance in plant pathogenic bacteria. Annu. Rev. Phytopathol. 1990, 28, 201–219. [Google Scholar] [CrossRef]
- Voloudakis, A.E.; Reignier, T.M.; Cooksey, D.A. Regulation of resistance to copper in Xanthomonas axonopodis pv. vesicatoria. Appl. Environ. Microbiol. 2005, 71, 782–789. [Google Scholar] [CrossRef]
- Graham, J.H.; Gottwald, T.R.; Cubero, J.; Achor, D.S. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004, 5, 1–15. [Google Scholar] [PubMed]
- Gottwald, T.R.; Bassanezi, R.B.; Amorim, L.; Bergamin-Filho, A. Spatial pattern analysis of citrus canker-infected plantings in são paulo, Brazil, and augmentation of infection elicited by the asian leafminer. Phytopathology 2007, 97, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Tom-Petersen, A.; Nybroe, O. Copper amendment of agricultural soil selects for bacterial antibiotic resistance in the field. Lett. Appl. Microbiol. 2005, 40, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bondarczuk, K.; Piotrowska-Seget, Z. Molecular basis of active copper resistance mechanisms in Gram-negative bacteria. Cell Biol. Toxicol. 2013, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Cooksey, D.A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, D.A. Copper uptake and resistance in bacteria. Mol. Microbiol. 1993, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arnesano, F.; Banci, L.; Bertini, I.; Thompsett, A.R. Solution structure of CopC: A cupredoxin-like protein involved in copper homeostasis. Structure 2002, 10, 1337–1347. [Google Scholar] [CrossRef]
- Puig, S.; Rees, E.M.; Thiele, D.J. The ABCDs of periplasmic copper trafficking. Structure 2002, 10, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Koay, M.; Maher, M.J.; Xiao, Z.; Wedd, A.G. Intermolecular transfer of copper ions from the CopC protein of Pseudomonas syringae. Crystal structures of fully loaded CuI CuII forms. J. Am. Chem. Soc. 2006, 128, 5834–5850. [Google Scholar] [CrossRef]
- Brown, N.L.; Barrett, S.R.; Camakaris, J.; Lee, B.T.; Rouch, D.A. Molecular genetics and transport analysis of the copperresistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol. Microbiol. 1995, 17, 1153–1166. [Google Scholar] [CrossRef]
- Lee, S.M.; Grass, G.; Rensing, C.; Barrett, S.R.; Yates, C.J.; Stoyanov, J.V.; Brown, N.L. The Pco proteins are involved in periplasmic copper handling in Escherichia coli. Biochem. Biophys. Res. Commun. 2002, 295, 616–620. [Google Scholar] [CrossRef]
- Rouch, D.A.; Brown, N.L. Copper-inducible transcriptional regulation at two promoters in the Escherichia coli copper resistance determinant pco. Microbiology 1997, 143, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Huffman, D.L.; Huyett, J.; Outten, F.W.; Doan, P.E.; Finney, L.A.; Hoffman, B.M.; O’Halloran, T.V. Spectroscopy of Cu (II)-PcoC and the multicopper oxidase function of PcoA, two essential components of Escherichia coli pco copper resistance operon. Biochemistry 2002, 41, 10046–10055. [Google Scholar] [CrossRef]
- Cooksey, D.A.; Azad, H.R.; Cha, J.R.; Lim, C.K. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl. Environ. Microbe 1990, 56, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Monchy, S.; Benotmane, M.A.; Wattiez, R.; van Aelst, S.; Auquier, V.; Borremans, B.; Mergeay, M.; Taghavi, S.; van der Lelie, D.; Vallaeys, T. Transcriptomic and proteomic analyses of the pMOL30-encoded copper resistance in Cupriavidus metallidurans strain CH34. Microbiology 2006, 152, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Rensing, C.; Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 2003, 27, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Ravigné, V.; Rieux, A.; Facon, B.; Boyer, C.; Boyer, K.; Grygiel, P.; Javegny, S.; Terville, M.; Canteros, B.I.; et al. Adaptation of genetically monomorphic bacteria: Evolution of copper resistance through multiple horizontal gene transfers of complex and versatile mobile genetic elements. Mol. Ecol. 2017, 26, 2131–2149. [Google Scholar] [CrossRef]
- Behlau, F.; Canteros, B.I.; Minsavage, G.V.; Jones, J.B.; Graham, J.H. Molecular characterization of copper resistance genes from Xanthomonas citri subsp. citri and Xanthomonas alfalfae subsp. citrumelonis. Appl. Environ. Microb. 2011, 77, 4089–4096. [Google Scholar] [CrossRef]
- Behlau, F.; Canteros, B.I.; Jones, J.B.; Graham, J.H. Copper resistance genes from different xanthomonads and citrus epiphytic bacteria confer resistance to Xanthomonas citri subsp citri. Eur. J. Plant Pathol. 2012, 133, 949–963. [Google Scholar] [CrossRef]
- Pruvost, O.; Richard, D.; Boyer, K.; Javegny, S.; Boyer, C.; Chiroleu, F.; Grygiel, P.; Parvedy, E.; Robène, I.; Maillot-Lebon, V.; et al. Diversity and geographical structure of Xanthomonas citri pv. citri on citrus in the south west Indian ocean region. Microorganisms 2021, 9, 945. [Google Scholar]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal-metal interactions: A review of toxicity and homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef]
- Ran, M.; Zhao, G.; Jiao, L.; Gu, Z.; Yang, K.; Wang, L.; Cao, X.; Xu, L.; Yan, J.; Yan, Y.; et al. Copper ion mediates yeast-to-hypha transition in Yarrowia lipolytica. J. Fungi 2023, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ju, Y.; Dong, R.; Ding, X.; Li, N.; Chu, Z. The mutation of CopAB results in the sensitivity to copper and the reduction of pathogenicity for PXO99. Acta Phytopathol. Sin. 2018, 42, 176–186. (In Chinese) [Google Scholar]
- Yang, B.; Sugio, A.; White, F.F. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA 2006, 103, 10503–10508. [Google Scholar] [CrossRef]
- Yuan, M.; Chu, Z.; Li, X.; Xu, C.; Wang, S. The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 2010, 22, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hou, B.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Adaskaveg, J.E.; Hine, R.B. Copper tolerance and zinc sensitivity of Mexican strains of Xanthomonas campestris pv. vesicatoria, causal agent of bacterial spot of pepper. Plant Dis. 1985, 69, 993–996. [Google Scholar]
- Andersen, G.L.; Menkissoglou, O.; Lindow, S.E. Occurrence and properties of copper-tolerant strains of Pseudomonas syringae isolated from fruit trees in California. Phytopathology 1991, 81, 648–656. [Google Scholar] [CrossRef]
- Marco, G.M.; Stall, R.E. Control of bacterial spot of pepper initiated by strains of Xanthomonas campestris pv. vesicatoria that differ in sensitivity to copper. Plant Dis. 1983, 67, 779–781. [Google Scholar]
- Huang, C.H.; Vallad, G.E.; Zhang, S.; Wen, A.; Balogh, B.; Figueiredo, J.F.L.; Behlau, F.; Jones, J.B.; Momol, M.T.; Olson, S.M. Effect of application frequency and reduced rates of acibenzolar-s-methyl on the field efficacy of induced resistance against bacterial spot on tomato. Plant Dis. 2012, 96, 221–227. [Google Scholar] [CrossRef]
- Jones, J.B.; Jones, J.P. The effect of bactericides, tank mixing time and spray schedule on bacterial leaf spot of tomato. Proc. Fla. State Hort. Soc. 1985, 244, 247–298. [Google Scholar]
- Liao, Y.Y.; Strayer-Scherer, A.L.; White, J.; Mukherjee, A.; De La Torre-Roche, R.; Ritchie, L.; Colee, J.; Vallad, G.E.; Freeman, J.H.; Jones, J.B.; et al. Nano-Magnesium Oxide: A novel bactericide against copper-tolerant Xanthomonas perforans causing tomato bacterial spot. Phytopathology 2019, 109, 52–62. [Google Scholar] [CrossRef]
- Carvalho, R.; Duman, K.; Jones, J.B.; Paret, M.L. Bactericidal activity of copper-zinc hybrid nanoparticles on copper-tolerant Xanthomonas perforans. Sci. Rep. 2019, 9, 20124. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, L.A.; Kimura, O.; Castilho, A.M.C.; Castilho, K.S.C.; Ribeiro, R.L.D.; Akiba, F.; Carmo, M.G.F. Effect of copper formulations on resident Xanthomonas campestris pv. vesicatoria populations on sweet pepper leaf surfaces. Hortic. Bras. 2003, 21, 44–50. [Google Scholar]
- Strayer-Scherer, A.; Liao, Y.Y.; Young, M.; Ritchie, L.; Vallad, G.E.; Santra, S.; Freeman, J.H.; Clark, D.; Jones, J.B.; Paret, M.L. Advanced copper composites against copper-tolerant Xanthomonas perforans and tomato bacterial spot. Phytopathology 2018, 108, 196–205. [Google Scholar] [CrossRef]
- Jonak, C.; Nakagami, H.; Hirt, H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004, 136, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Lee, K.T.; Chi, W.C.; Hirt, H.; Chang, C.C.; Huang, H.J. Possible involvement of MAP kinase pathways in acquired metal-tolerance induced by heat in plants. Planta 2008, 228, 499–509. [Google Scholar] [CrossRef]
- Yeh, C.M.; Chien, P.S.; Huang, H.J. Distinct signalling pathways for induction of MAP kinase activities by cadmium and copper in rice roots. J. Exp. Bot. 2007, 58, 659–671. [Google Scholar] [CrossRef]
- Hung, W.C.; Huang, D.D.; Yeh, C.M.; Huang, H.J. Reactive oxygen species, calcium and serine/threonine phosphatase are required for copper-induced map kinase gene osmapk2, expression in rice. Plant Growth Regul. 2005, 45, 233–241. [Google Scholar] [CrossRef]
- Dietz, K.J.; Baier, M.; Krämer, U. Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 73–97. [Google Scholar]
- Stoiber, T.L.; Shafer, M.M.; Armstrong, D.E. Induction of reactive oxygen species in chlamydomonas reinhardtii in response to contrasting trace metal exposures. Environ. Toxicol. 2013, 28, 516–523. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Cheng, R.; Li, G.; Fan, L.; Jiang, J.; Zhao, Y. Therapeutic iminoboronate-based polymersomes with a Cu(ii)-mediated Fenton reaction-enhanced ROS-response. Chem. Commun. 2020, 56, 12246–12249. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef]
- Raklami, A.; Oufdou, K.; Tahiri, A.I.; Mateos-Naranjo, E.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Meddich, A.; Redondo-Gómez, S.; Pajuelo, E. Safe cultivation of Medicago sativa in metal-polluted soils from semi-arid regions assisted by heat- and metallo-resistant PGPR. Microorganisms 2019, 7, 212. [Google Scholar] [CrossRef]
- Bianka, S. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar]
- Wan, Z.; Erlin, G.; Muhammad, S.; Yujing, W.; Honglei, W.; Xinhui, N.; Longfu, Z. Ghumc1, a blue copper-binding protein, regulates lignin synthesis and cotton immune response. Biochem. Biophys. Res. Commun. 2018, 504, 75–81. [Google Scholar]
- Xu, W.; Meng, Y.; Wise, R.P. Mla- and Rom1-mediated control of microRNA398 and chloroplast copper/zinc superoxide dismutase regulates cell death in response to the barley powdery mildew fungus. New Phytol. 2014, 201, 1396–1412. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Wu, T.; Ding, Y.; Ding, X.; Chu, Z. Copper ion elicits defense response in Arabidopsis thaliana by activating salicylate- and ethylene-dependent signaling pathways. Mol. Plant 2015, 8, 1550–1553. [Google Scholar] [CrossRef]
- Liu, H.; Xue, X.; Yu, Y.; Xu, M.; Lu, C.; Meng, X.; Zhang, B.; Ding, X.; Chu, Z. Copper ions suppress abscisic acid biosynthesis to enhance defence against Phytophthora infestans in potato. Mol. Plant Pathol. 2020, 21, 636–651. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, C.; Wang, M.; Wan, D.; Huang, F.; Jiang, Z.; Crawford, B.M.; Vo-Dinh, T.; Yuan, F.; Wu, F.; et al. Flg22-induced Ca2+ increases undergo desensitization and resensitization. Plant Cell Environ. 2021, 44, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Ding, X.; Qiu, J.; Zhang, M.; Chu, Z. Arabidopsis thaliana ACS8 plays a crucial role in the early biosynthesis of ethylene elicited by Cu2+ ions. J. Cell Sci. 2018, 131, jcs202424. [Google Scholar] [PubMed]
- Yu, Y.; Xu, M.; Ding, X.; Chu, Z.; Liu, H. Activating the MYB51 and MYB122 to upregulate the transcription of glucosinolates biosynthesis genes by copper ions in Arabidopsis. Plant Physiol. Biochem. 2021, 162, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Dong, K.; Liu, S.; Zhang, Z.; Zhang, X.; Tong, X.; Zhu, F.; Zou, J.; Wang, X. A putative nuclear copper chaperone promoters plant immunity in Arabidopsis. J. Exp. Bot. 2020, 71, 6684–6696. [Google Scholar] [CrossRef]
- Yao, S.; Kang, J.; Guo, G.; Yang, Z.; Huang, Y.; Lan, Y.; Zhou, T.; Wang, L.; Wei, C.; Xu, Z.; et al. The key micronutrient copper orchestrates broad-spectrum virus resistance in rice. Sci. Adv. 2022, 8, eabm0660. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal activity of copper oxide nanoparticles against root rot disease in cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.L.; Lopes, C.A. Pseudomonas syringae pv. tomato resistant to copper in copper-sprayed tomato fields. Fitopatol. Bras. 1995, 20, 85–89. [Google Scholar]
- Pernezny, K.; Kudela, V.; Kokoskova, B.; Hladka, I. Bacterial disease of tomato in the Czech and Slovak republics and lack of streptomycin resistance among copper-tolerant bacterial strains. Crop Prot. 1995, 14, 267–270. [Google Scholar] [CrossRef]
- Lombardo, M.F.; Panebianco, S.; Azzaro, A.; Catara, V.; Cirvilleri, G. Assessing copper-alternative products for the control of pre- and postharvest citrus Anthracnose. Plants 2023, 12, 904. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Q.; Lin, X.; Shang, Y.; Cui, X.; Guo, L.; Huang, Y.; Wu, M.; Song, K. Potential effects of metal oxides on agricultural production of rice: A mini review. Plants 2023, 12, 778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).