Integration of Transcriptomic and Metabolomic Profiles Provides Insights into the Influence of Nitrogen on Secondary Metabolism in Fusarium sacchari
Abstract
1. Introduction
2. Results
2.1. Transcriptome Analysis
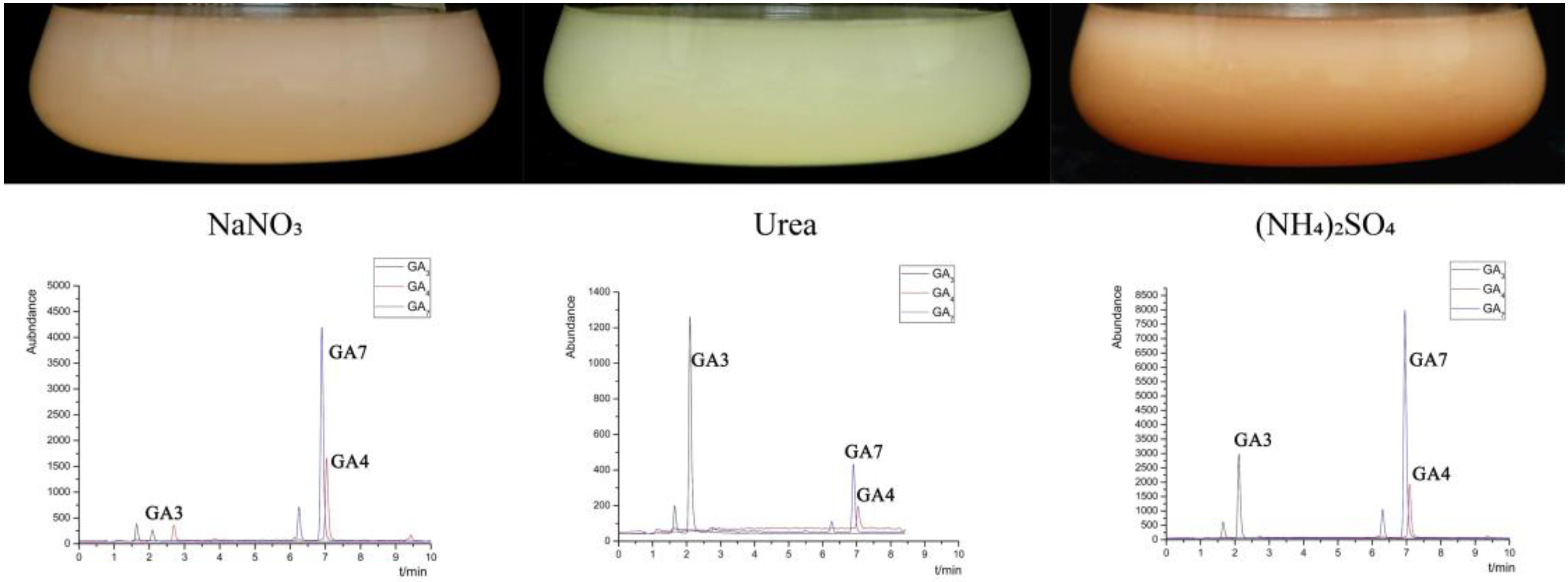
2.2. Gibberellins Production
2.3. Genes Associated with GA Production in FsCNO-1
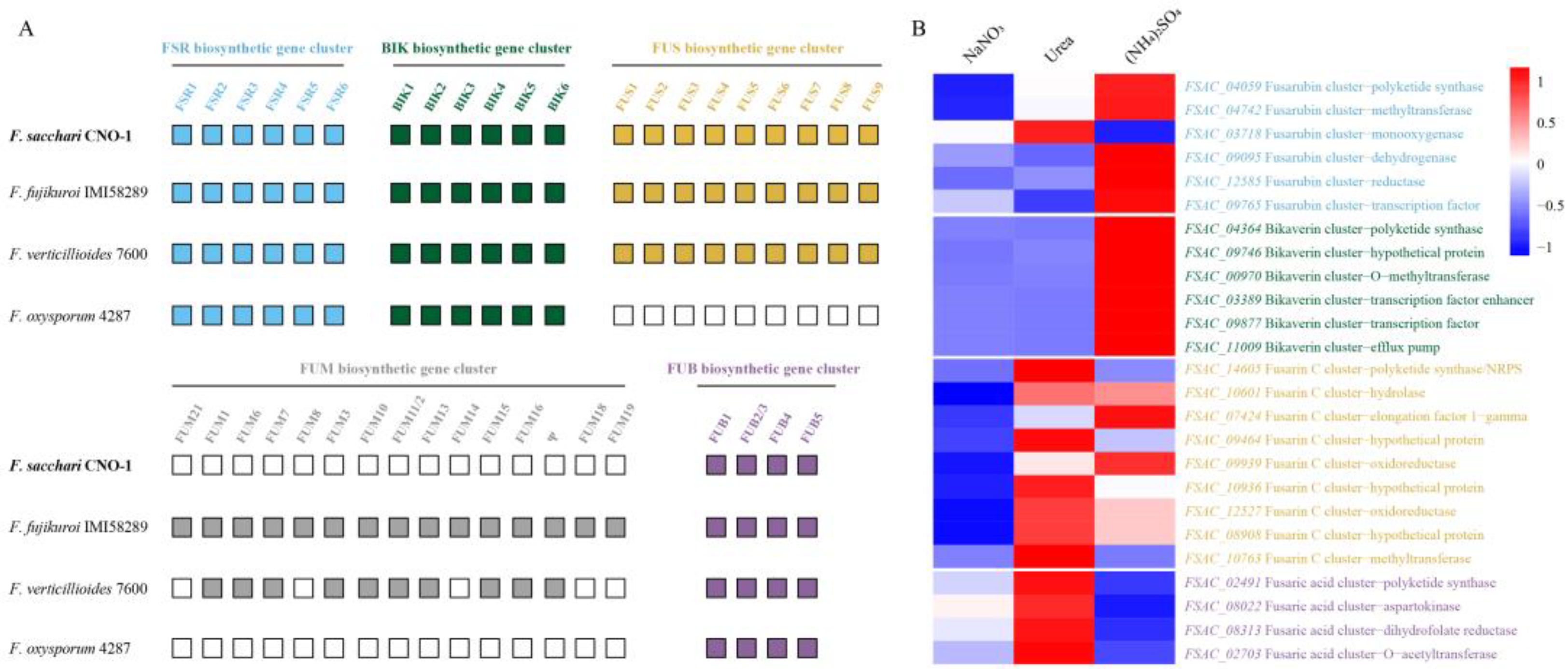
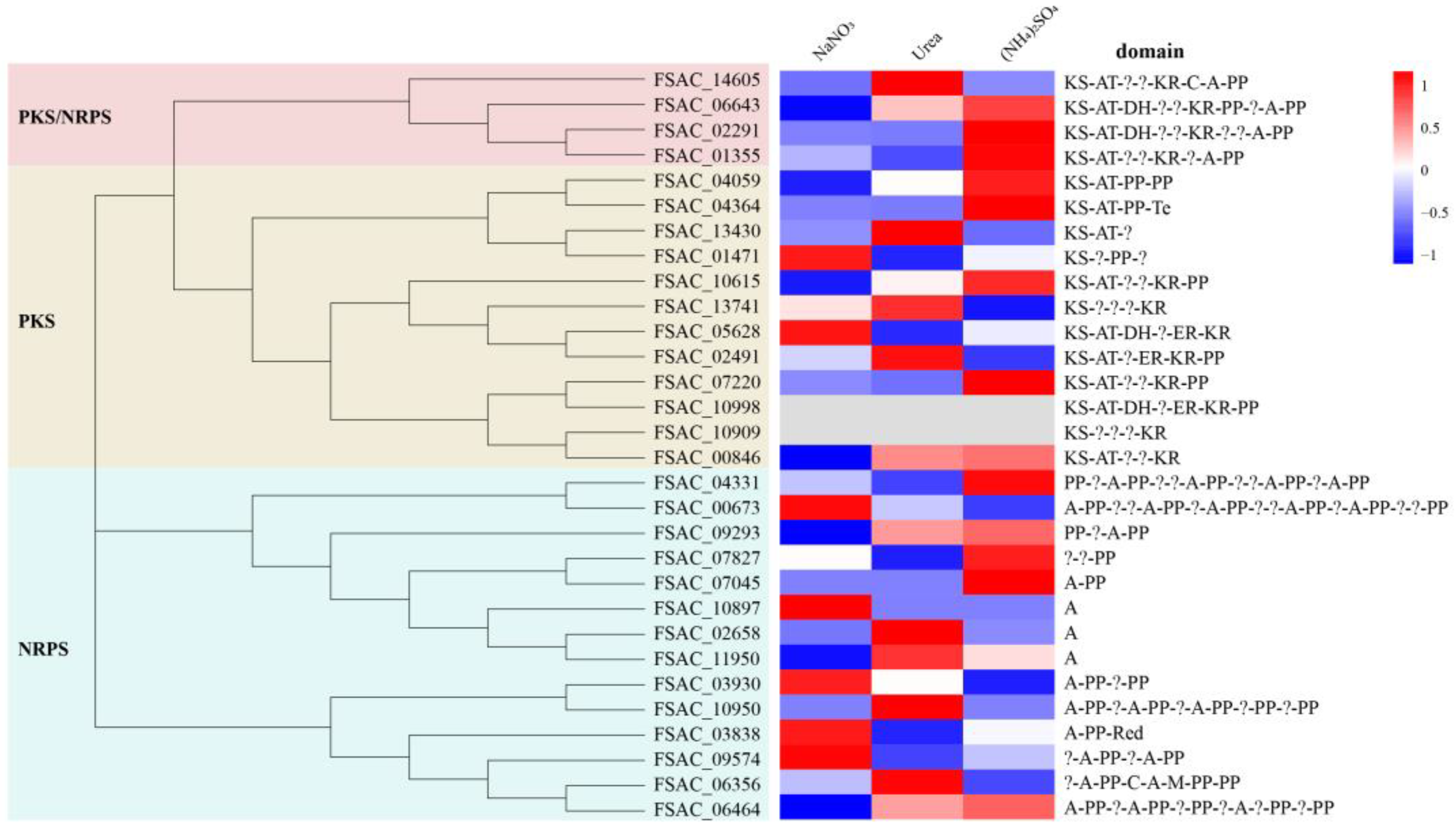
2.4. Genes Related to Secondary Metabolite Production in FsCNO-1
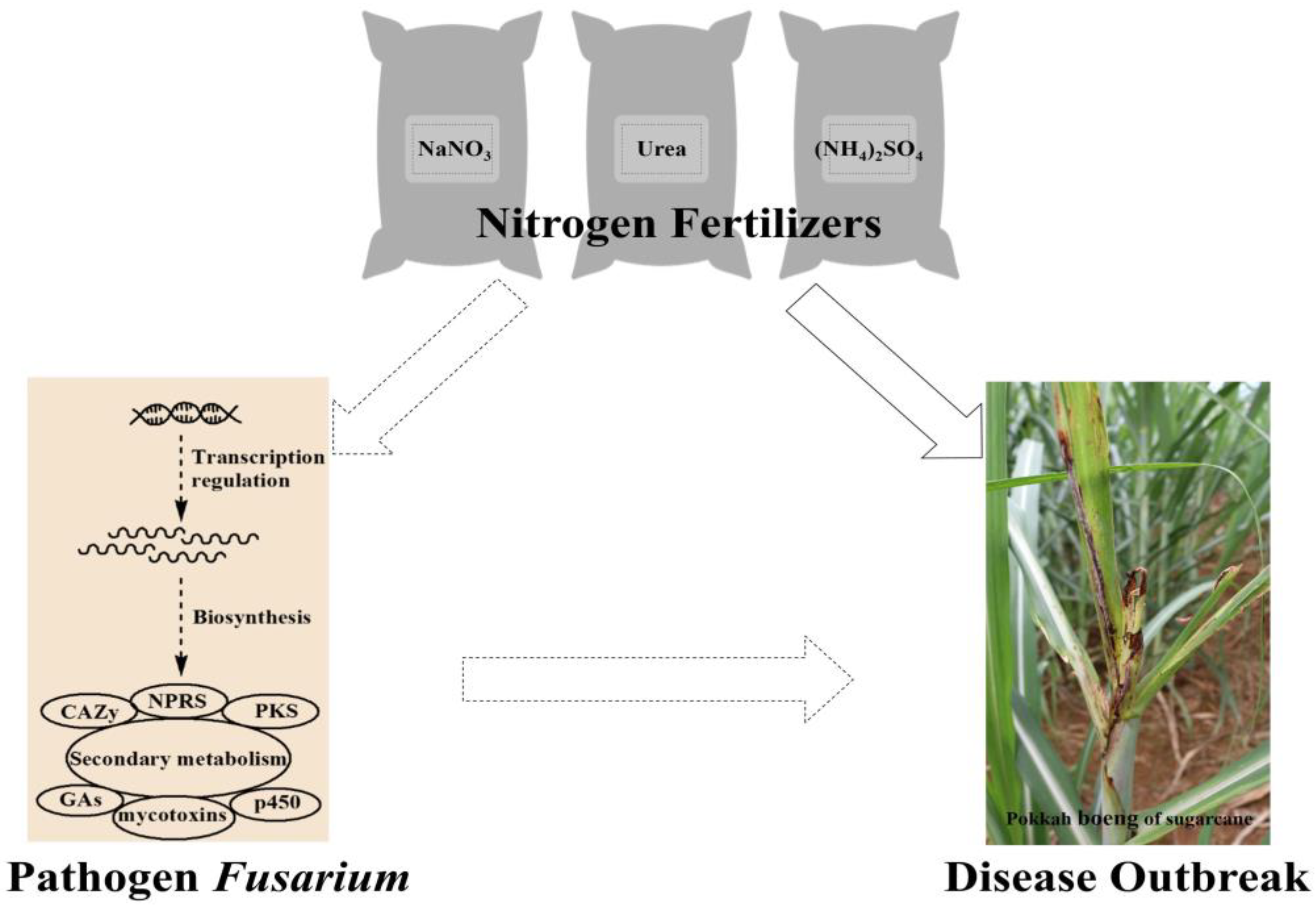
3. Discussion
4. Materials and Methods
4.1. Fungal Strain and Culture Conditions
4.2. Transcriptomic Analysis
4.3. In Silico Analysis of PKS/NRPS-, SM-, and CAZYme-Associated Genes
4.4. Quantitative RT-PCR
4.5. Extraction and Analysis of Gibberellins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hobbie, E.A.; Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New. Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Brotman, Y.; Segu, S.; Zeier, T.; Zeier, J.; Persijn, S.T.; Cristescu, S.M.; Harren, F.J.M.; Bauwe, H.; Fernie, A.R.; et al. The form of nitrogen nutrition affects resistance against Pseudomonas syringae pv. phaseolicola in tobacco. J. Exp. Bot. 2012, 64, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Sooväli, P.; Kangor, T.; Koppel, R.; Ingver, A.; Tamm, I. Spring Wheat Disease and Yield Responses to Nitrogen Fertilization and Chemical Treatments. J. Agr. Sci. Tech.-Iran. 2013, 3, 290–296. [Google Scholar]
- Obasa, K.; Fry, J.; Bremer, D.; John, R.S.; Kennelly, M.M. Effect of Cultivation and Timing of Nitrogen Fertilization on Large Patch Disease of Zoysiagrass. Plant. Dis. 2013, 97, 1075–1081. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Kumar, P.; Nigam, A.; Singh, A.; Kumar, A. Pokkah Boeng: An Emerging Disease of Sugarcane. J. Plant. Pathol. Microb. 2013, 4, 170. [Google Scholar] [CrossRef]
- Wang, Z.P.; Sun, H.J.; Guo, Q.; Xu, S.Q.; Wang, J.H.; Lin, S.H.; Zhang, M.Q. Artificial Inoculation Method of Pokkah Boeng Disease of Sugarcane and Screening of Resistant Germplasm Resources in Subtropical China. Sugar Tech. 2016, 19, 283–292. [Google Scholar] [CrossRef]
- Bao, Y.X.; Akbar, S.; Yao, W.; Xu, Y.Z.; Xu, J.L.; Powell, C.A.; Chen, B.S.; Zhang, M.Q. Genetic diversity and pathogenicity of Fusarium fujikuroi species complex (FFSC) causing sugarcane pokkah boeng disease in China. Plant. Dis. 2023, 107, 1299–1309. [Google Scholar] [CrossRef]
- Lysøe, E.; Seong, K.; Kistler, H.C. The Transcriptome of Fusarium graminearum During the Infection of Wheat. Mol. Plant-Microbe Interact. 2011, 24, 995–1000. [Google Scholar] [CrossRef]
- Wiemann, P.; Sieber, C.M.K.; Von Bargen, K.W.; Studt, L.; Niehaus, E.M.; Espino, J.J.; Huß, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the Cryptic Genome: Genome-wide Analyses of the Rice Pathogen Fusarium fujikuroi Reveal Complex Regulation of Secondary Metabolism and Novel Metabolites. PLoS Pathog. 2013, 9, e1003475. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Chen, M.L.; Dong, X.; Zheng, X.Q.; Huang, H.N.; Xu, X.; Chen, J.M. Transcriptome profiling of Galaxea fascicularis and its endosymbiont Symbiodinium reveals chronic eutrophication tolerance pathways and metabolic mutualism between partners. Sci. Rep. UK 2017, 7, 42100. [Google Scholar] [CrossRef]
- Carapito, R.; Vorwerk, S.; Jeltsch, J.; Phalip, V. Genome-wide transcriptional responses of Fusarium graminearum to plant cell wall substrates. FEMS Microbiol. Lett. 2013, 340, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Niño-Sánchez, J.; Tello, V.; del-Casado, V.C.; Thon, M.R.; Benito, E.P.; Díaz-Mínguez, J.M. Gene expression patterns, and dynamics of the colonization of common bean (Phaseolus vulgaris L.) by highly virulent and weakly virulent strains of Fusarium oxysporum. Front. Microbiol. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Wang, J.H.; Bao, Y.X.; Guo, Q.; Powell, C.A.; Xu, S.Q.; Chen, B.S.; Zhang, M.Q. Deciphering the transcriptomic response of Fusarium verticillioides in relation to nitrogen availability and the development of sugarcane pokkah boeng disease. Sci. Rep. 2016, 6, 29692. [Google Scholar] [CrossRef] [PubMed]
- Tudzynski, B.; Holter, K. Gibberellin biosynthetic pathway in Gibberella fujikuroi: Evidence for a gene cluster. Fungal Genet. Biol. 1998, 25, 157–170. [Google Scholar] [CrossRef]
- Howlett, B.J. Secondary metabolite toxins and nutrition of plant pathogenic fungi. Curr. Opin. Plant. Biol. 2006, 9, 371–375. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Choi, J.; Ahn, K.; Park, B.; Park, J.; Kang, S.; Lee, Y.H. Fungal cytochrome P450 database. BMC Genomics 2008, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Geisler, M.; Coutinho, P.M.; Segerman, B.; Nishikubo, N.; Takahashi, J.; Aspeborg, H.; Djerbi, S.; Master, E.; Andersson-Gunneras, S.; et al. Poplar Carbohydrate-Active Enzymes. Gene Identification and Expression Analyses. Plant. Physiol. 2006, 140, 946–962. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Xu, S.Q.; Que, Y.X.; Wang, J.H.; Comstock, J.C.; Wei, J.J.; McCord, P.H.; Chen, B.S.; Chen, R.K.; Zhang, M.Q. Species-Specific Detection and Identification of Fusarium Species Complex, the Causal Agent of Sugarcane Pokkah Boeng in China. PLoS ONE 2014, 9, e104195. [Google Scholar] [CrossRef]
- Macios, M.; Caddick, M.X.; Weglenski, P.; Scazzocchio, C.; Dzikowska, A. The GATA factors AREA and AREB together with the co-repressor NMRA, negatively regulate arginine catabolism in Aspergillus nidulans in response to nitrogen and carbon source. Fungal Genet. Biol. 2012, 49, 189–198. [Google Scholar] [CrossRef]
- Teichert, S.; Rutherford, J.C.; Wottawa, M.; Heitman, J.; Tudzynski, B. Impact of ammonium permeases MepA, MepB, and MepC on nitrogen-regulated secondary metabolism in Fusarium fujikuroi. Eukaryot. Cell 2008, 7, 187–201. [Google Scholar] [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Song, Q.; Liang, S.; Htun, R.; Malviya, M.K.; Li, Y.J.; Liang, Q.; Zhang, G.M.; Zhang, M.Q.; Zhou, F.J. Metabolic and proteomic analysis of nitrogen metabolism mechanisms involved in the sugarcane-Fusarium verticillioides interaction. J. Plant. Physiol. 2020, 251, 153207. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Keller, N. Regulation of Secondary Metabolism in Filamentous Fungi. Annu. Rev. Phytopathol. 2005, 43, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Subbarao, K.V.; Kang, S.; Veronese, P.; Gold, S.E.; Thomma, B.P.H.J.; Chen, Z.H.; Henrissat, B.; Lee, Y.H.; Park, J.; et al. Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens. PLoS Pathog. 2011, 7, e1002137. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.R.; Pan, H.Q.; et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.; Gardiner, D.M.; Lysøe, E.; Fuertes, P.R.; Tudzynski, B.; Wiemann, P.; Sondergaard, T.E.; Giese, H.; Brodersen, D.; Srensen, J.L. An update to polyketide synthase and nonribosomal synthetase genes and nomenclature in Fusarium. Fungal Genet. Biol. 2014, 75, 20–29. [Google Scholar] [CrossRef]
- Hansen, F.; Sørensen, J.L.; Giese, H.; Sondergaard, T.E.; Frandsen, R.J. Quick guide to polyketide synthase and nonribosomal synthetase genes in Fusarium. Int. J. Food Microbiol. 2012, 155, 128–136. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Kawaide, H. Biochemical and Molecular Analyses of Gibberellin Biosynthesis in Fungi. Biosci. Biotech. Bioch. 2006, 70, 583–590. [Google Scholar] [CrossRef]
- Bömke, C.; Tudzynski, B. Diversity, regulation, and evolution of the gibberellin biosynthetic pathway in fungi compared to plants and bacteria. Phytochemistry 2009, 70, 1876–1893. [Google Scholar] [CrossRef]
- Morrone, D.; Chambers, J.; Lowry, L.; Kim, G.; Anterola, A.; Bender, K.; Peters, R.J. Gibberellin biosynthesis in bacteria: Separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum. FEBS Lett. 2009, 583, 475–480. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2013, 35, 62–74. [Google Scholar] [CrossRef]
- Albermann, S.; Linnemannstöns, P.; Tudzynski, B. Strategies for strain improvement in Fusarium fujikuroi: Overexpression and localization of key enzymes of the isoprenoid pathway and their impact on gibberellin biosynthesis. Appl. Microbiol. Biot. 2013, 97, 2979–2995. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.A.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2012, 31, 46–54. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ginestet, C.E. ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. A Stat. 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Ziemert, N.; Podell, S.; Penn, K.; Badger, J.H.; Allen, E.E.; Jensen, P.R. The Natural Product Domain Seeker NaPDoS: A Phylogeny Based Bioinformatic Tool to Classify Secondary Metabolite Gene Diversity. PLoS ONE 2012, 7, e34064. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Ravel, J. Chapter 8 Methods for In Silico Prediction of Microbial Polyketide and Nonribosomal Peptide Biosynthetic Pathways from DNA Sequence Data. Method. Enzymol. 2009, 458, 181–217. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Ma, L.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Pietro, A.D.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The carbohydrate-active ENZYMES database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2008, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Kenneth, J.L.; Thomas, D.S. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar]
- Podojil, M.; Ševčík, V.; Kuhr, I.; Fuska, J. Isolation of gibberellic acid on ion-exchange resins. Folia Microbiol. 1961, 6, 273–276. [Google Scholar] [CrossRef]
- Flores, M.I.A.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. QuEChERS-based extraction procedure for multifamily analysis of phytohormones in vegetables by UHPLC-MS/MS. J. Sep. Sci. 2011, 34, 1517–1524. [Google Scholar] [CrossRef]


| Mycotoxin Synthesis | Gene_ID/Description | FPKM | log2FC | ||||
|---|---|---|---|---|---|---|---|
| NaNO3 | Urea | (NH4)2SO4 | Urea vs. NaNO3 | (NH4)2SO4 vs. NaNO3 | Urea vs. (NH4)2SO4 | ||
| Fusarubin | FSAC_04059 polyketide synthase | 1.66 | 3.62 | 5.57 | −1.12 | −1.75 | 0.62 |
| FSAC_04742 methyltransferase | 0.55 | 1.01 | 1.51 | −0.88 | −1.46 | 0.58 | |
| FSAC_03718 monooxygenase | 0.17 | 0.33 | 0.02 | −0.96 | 3.09 | −4.04 | |
| FSAC_09095 dehydrogenase | 0.20 | 0.15 | 0.54 | 0.42 | −1.43 | 1.85 | |
| FSAC_12585 reductase | 0.77 | 1.03 | 3.66 | −0.42 | −2.25 | 1.83 | |
| FSAC_09765 transcription factor | 0.12 | 0.00 | 0.37 | −3.06 | −1.62 | −1.43 | |
| Bikaverin | FSAC_04364 polyketide synthase | 0.01 | 0.01 | 0.96 | 0 | −6.58 | 6.58 |
| FSAC_09746 hypothetical protein | 0.37 | 0.66 | 8.22 | −0.83 | −4.47 | 3.64 | |
| FSAC_00970 o-methyltransferase | 0.08 | 0.19 | 9.72 | −1.25 | −6.92 | 5.68 | |
| FSAC_03389 transcription factor | 7.01 | 2.52 | 323.19 | 1.48 | −5.53 | 7.00 | |
| FSAC_09877 transcription factor | 1.30 | 1.02 | 22.08 | 0.35 | −4.09 | 4.44 | |
| FSAC_11009 efflux pump | 1.56 | 0.67 | 124.02 | 1.22 | −6.31 | 7.53 | |
| Fusaric_acid | FSAC_02491 polyketide synthase | 921.40 | 1699.88 | 496.25 | −0.88 | 0.89 | −1.78 |
| FSAC_08022 aspartokinase | 1066.35 | 1586.00 | 445.86 | −0.57 | 1.26 | −1.83 | |
| FSAC_08313 dihydrofolate reductase | 5615.34 | 10,397.00 | 2127.52 | −0.89 | 1.40 | −2.29 | |
| FSAC_02703 o-acetyltransferase | 1077.10 | 2977.85 | 406.74 | −1.47 | 1.40 | −2.87 | |
| Fusarin C | FSAC_14605 polyketide synthase/NRPS | 6.87 | 47.48 | 9.05 | −2.79 | −0.40 | −2.39 |
| FSAC_10601 hydrolase | 132.87 | 270.61 | 260.08 | −1.02 | −0.97 | −0.06 | |
| FSAC_07424 elongation factor 1-gamma | 46.03 | 102.39 | 199.67 | −1.15 | −2.12 | 0.96 | |
| FSAC_09464 hypothetical protein | 9.19 | 39.23 | 18.16 | −2.09 | −0.98 | −1.11 | |
| FSAC_09939 oxidoreductase | 22.35 | 74.63 | 111.84 | −1.74 | −2.32 | 0.58 | |
| FSAC_10936 hypothetical protein | 47.74 | 134.21 | 90.51 | −1.49 | −0.92 | −0.57 | |
| FSAC_12527 oxidoreductase | 14.15 | 52.27 | 40.10 | −1.89 | −1.50 | −0.38 | |
| FSAC_08908 hypothetical protein | 86.96 | 358.76 | 184.48 | −2.04 | −1.09 | −0.96 | |
| FSAC_10763 methyltransferase | 1.85 | 85.71 | 0.00 | −5.53 | 0.89 | −6.42 | |
| Gene ID | Gene Name | Function Class/Group | FPKM | log2FC | ||||
|---|---|---|---|---|---|---|---|---|
| NaNO3 | Urea | (NH4)2SO4 | Urea vs. NaNO3 | (NH4)2SO4 vs. NaNO3 | Urea vs. (NH4)2SO4 | |||
| FSAC_07416 | TRI13 | Secondary metabolism/ trichothecene biosynthesis | 1.04 | 0.03 | 2.96 | 5.12 | −1.51 | 6.62 |
| FSAC_07004 | TRI13 | Secondary metabolism/ trichothecene biosynthesis | 181.85 | 272.37 | 301.56 | −0.58 | −0.73 | 0.15 |
| FSAC_02438 | STCS | Secondary metabolism/ sterigmatocystin biosynthesis | 4.93 | 6.64 | 4.80 | −0.43 | 0.04 | −0.47 |
| FSAC_07378 | STCS | Secondary metabolism/ sterigmatocystin biosynthesis | 0.75 | 1.99 | 0.93 | −1.41 | −0.31 | −1.10 |
| FSAC_03140 | Bph | Xenobiotic metabolism/ benzoate para hydroxylase | 0.04 | 0.06 | 0.16 | −0.58 | −2.00 | 1.42 |
| FSAC_06106 | Bph | Xenobiotic metabolism/ benzoate para hydroxylase | 0.93 | 0.68 | 13.73 | 0.45 | −3.88 | 4.34 |
| FSAC_12735 | CYP52A3 | Xenobiotic metabolism/ alkane inducible P450 | 0.31 | 1.01 | 0.15 | −1.70 | 1.05 | −2.75 |
| FSAC_01874 | Pdm | Defense against host secreted factors/Pisatin demethylase | 1.16 | 2.73 | 3.24 | −1.23 | −1.48 | 0.25 |
| FSAC_10736 | Pdm | Defense against host secreted factors/Pisatin demethylase | 9.35 | 17.04 | 41.21 | −0.87 | −2.14 | 1.27 |
| FSAC_07317 | Pdm | Defense against host secreted factors/Pisatin demethylase | 2.97 | 6.25 | 15.65 | −1.07 | −2.40 | 1.32 |
| FSAC_13209 | Pdm | Defense against host secreted factors/Pisatin demethylase | 1.01 | 1.29 | 4.41 | −0.35 | −2.13 | 1.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Lin, Z.; Yao, W.; Akbar, S.; Lin, W.; Powell, C.A.; Xu, J.; Zhang, M. Integration of Transcriptomic and Metabolomic Profiles Provides Insights into the Influence of Nitrogen on Secondary Metabolism in Fusarium sacchari. Int. J. Mol. Sci. 2023, 24, 10832. https://doi.org/10.3390/ijms241310832
Bao Y, Lin Z, Yao W, Akbar S, Lin W, Powell CA, Xu J, Zhang M. Integration of Transcriptomic and Metabolomic Profiles Provides Insights into the Influence of Nitrogen on Secondary Metabolism in Fusarium sacchari. International Journal of Molecular Sciences. 2023; 24(13):10832. https://doi.org/10.3390/ijms241310832
Chicago/Turabian StyleBao, Yixue, Zhenyue Lin, Wei Yao, Sehrish Akbar, Wenfeng Lin, Charles A. Powell, Jianlong Xu, and Muqing Zhang. 2023. "Integration of Transcriptomic and Metabolomic Profiles Provides Insights into the Influence of Nitrogen on Secondary Metabolism in Fusarium sacchari" International Journal of Molecular Sciences 24, no. 13: 10832. https://doi.org/10.3390/ijms241310832
APA StyleBao, Y., Lin, Z., Yao, W., Akbar, S., Lin, W., Powell, C. A., Xu, J., & Zhang, M. (2023). Integration of Transcriptomic and Metabolomic Profiles Provides Insights into the Influence of Nitrogen on Secondary Metabolism in Fusarium sacchari. International Journal of Molecular Sciences, 24(13), 10832. https://doi.org/10.3390/ijms241310832






