The Role of Sirtuins in the Pathogenesis of Psoriasis
Abstract
1. Introduction
1.1. Psoriasis—Epidemiology
1.2. Psoriasis—Aetiopathogenesis
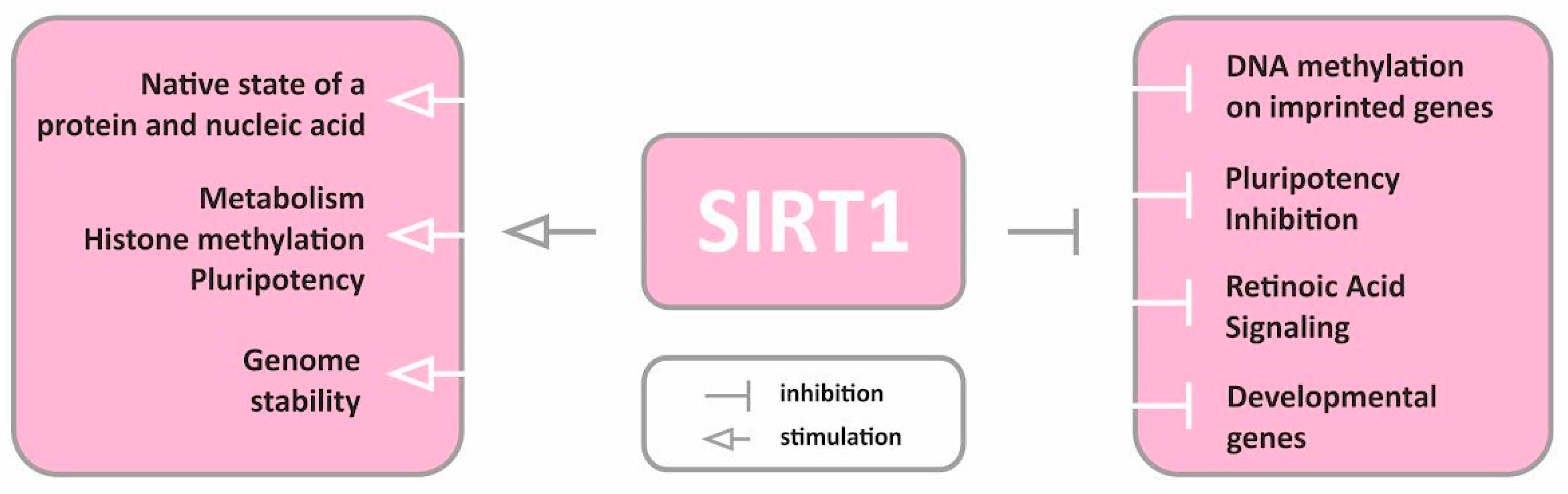
2. SIRTs
3. The Role of SIRTs in Psoriasis
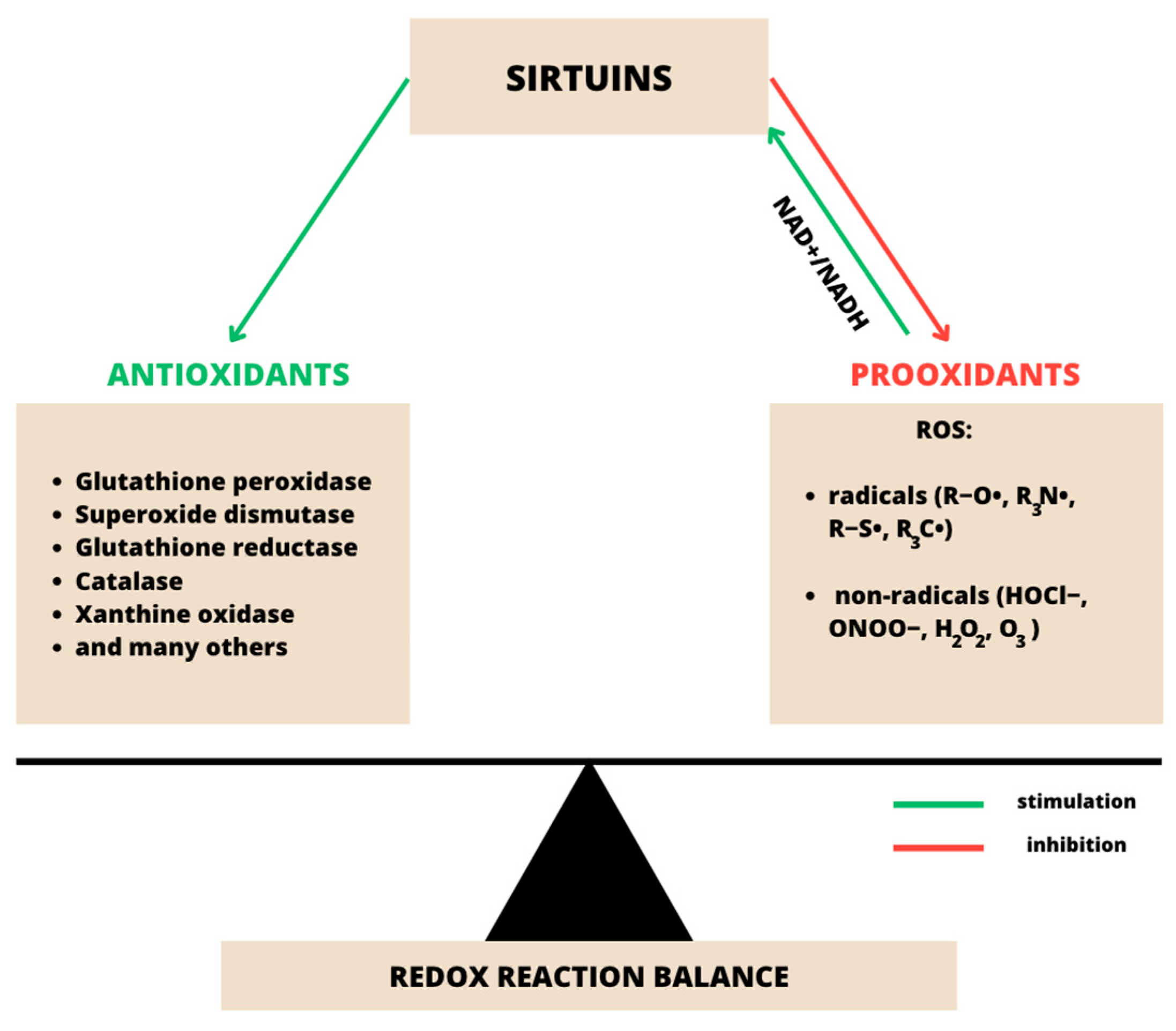
3.1. SIRT1 and Oxidative Stress in Psoriasis
3.2. SIRT1 and the MAPK Pathway
3.3. SIRT1 and the STAT3 Pathway
3.4. SIRT 1 and the NF-κB Pathway
3.5. SIRT1 and the AMPK Pathway
3.6. SIRT1 and the Nicotinamide Phosphoribosyltransferase (NAMPT) Pathway
3.7. SIRT2
3.8. SIRT3
3.9. SIRT5
4. SIRT-Activating Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Li, F.; Xu, Y.; Jin, X.; Dong, S.; Xia, J. Global trends in the incidence of psoriasis from 1990 to 2019. Eur. J. Dermatol. 2022, 32, 207–213. [Google Scholar] [CrossRef]
- Buja, A.; Miatton, A.; Cozzolino, C.; Monasta, L.; Grada, A.; Karimkhani, C.A.; Naghavi, M.; Damiani, G. The global, regional, and national burden of seborrheic dermatitis: Results and insights from the Global Burden of Disease 2019 Study. Arch. Dermatol. Res. 2022, 315, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Damiani, G.; Radaeli, A.; Olivini, A.; Calvara-Pinton, P.; Malerba, M. Increased airway inflammation in patients with psoriasis. Br. J. Dermatol. 2016, 175, 797–799. [Google Scholar] [CrossRef]
- Malerba, M.; Damiani, G.; Radaeli, A.; Ragnoli, B.; Olivini, A.; Calzavara-Pinton, P. Narrowband ultraviolet B phototherapy in psoriasis reduces proinflammatory cytokine levels and improves vitiligo and neutrophilic asthma. Br. J. Dermatol. 2015, 173, 1544–1545. [Google Scholar] [CrossRef]
- Damiani, G.; Cazzaniga, S.; Conic, R.R.; Naldi, L. Pruritus characteristics in large Italian cohort of psoriatic patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1316–1324. [Google Scholar] [CrossRef]
- Chen, Z.; Laurence, A.; O’Shea, J.J. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin. Immunol. 2007, 19, 400–408. [Google Scholar] [CrossRef]
- Damiani, G.; Pacifico, A.; Linder, D.M.; Pigatto, P.D.; Conic, R.; Grada, A.; Bragazzi, N.L. Nanodermatology-based solutions for psoriasis: State-of-the art and future prospects. Dermatol. Ther. 2019, 32, e13113. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Gertler, A.A.; Cohen, H.Y. SIRT6, a protein with many faces. Biogerontology 2013, 14, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.N.; Finkel, T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb. Perspect. Biol. 2012, 4, a013102. [Google Scholar] [CrossRef]
- Mathias, R.A.; Greco, T.M.; Cristea, I.M. Identification of Sirtuin4 (SIRT4) protein interactions: Uncovering candidate acyl-modified mitochondrial substrates and enzymatic regulators. Methods Mol. Biol. 2016, 1436, 213–239. [Google Scholar]
- Kiran, S.; Anwar, T.; Kiran, M.; Ramakrishna, G. Sirtuin 7 in cell proliferation, stress and disease: Rise of the seventh Sirtuin! Cell. Signal. 2015, 27, 673–682. [Google Scholar] [CrossRef]
- Vachharajani, V.T.; Liu, T.; Wang, X.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins link inflammation and metabolism. J. Immunol. Res. 2016, 2016, 8167273. [Google Scholar] [CrossRef]
- Blander, G.; Bhimavarapu, A.; Mammone, T.; Maes, D.; Elliston, K.; Reich, C.; Matsui, M.S.; Guarente, L.; Loureiro, J.J. SIRT1 Promotes Differentiation of Normal Human Keratinocytes. J. Investig. Dermatol. 2009, 129, 41–49. [Google Scholar] [CrossRef]
- Wu, Z.; Uchi, H.; Morino-Koga, S.; Shi, W.; Furue, M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J. Dermatol. Sci. 2014, 75, 16–23. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS ONE 2011, 6, e27081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, J.; Zhang, D.; Hu, G.; Zhang, Y. Chemerin/ChemR23 axis triggers an inflammatory response in keratinocytes through ROS-sirt1-NF-κB signaling. J. Cell. Biochem. 2019, 120, 6459–6470. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, F.; Galli, M.; Gueydan, C.; Kruys, V.; Prevot, P.P.; Bedalov, A.; Mostoslavsky, R.; Alt, F.W.; De Smedt, T.; Leo, O. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat. Med. 2009, 15, 206–210. [Google Scholar] [CrossRef]
- D’Amico, F.; Costantino, G.; Salvatorelli, L.; Ramondetta, A.; De Pasquale, R.; Sortino, M.A.; Merlo, S. Inverse correlation between the expression of AMPK/SIRT1 and NAMPT in psoriatic skin: A pilot study. Adv. Med Sci. 2022, 67, 262–268. [Google Scholar] [CrossRef]
- Fan, X.; Yan, K.; Meng, Q.; Sun, R.; Yang, X.; Yuan, D.; Li, F.; Deng, H. Abnormal expression of SIRTs in psoriasis: Decreased expression of SIRT 1-5 and increased expression of SIRT 6 and 7. Int. J. Mol. Med. 2019, 44, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Su, Z.; Zhang, B.; Ge, J.; Song, S.; Sun, G.; Sun, X.; Yi, L.; Wang, Y.; Sun, W.; et al. SIRT1 Activation Ameliorates Aldara-Induced Psoriasiform Phenotype and Histology in Mice. J. Investig. Dermatol. 2015, 135, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.G.; Suárez-Fariñas, M.; Cueto, I.; Khacherian, A.; Matheson, R.; Parish, L.C.; Leonardi, C.; Shortino, D.; Gupta, A.; Haddad, J.; et al. A randomized, placebo-controlled study of SRT2104, a SIRT1 activator, in patients with moderate to severe psoriasis. PLoS ONE 2015, 10, e0142081. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Harrison, D.G. Basic science: Pathophysiology: Oxidative stress. J. Am. Soc. Hypertens. 2014, 8, 601–603. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Péter, I.; Jagicza, A.; Ajtay, Z.; Kiss, I.; Németh, B. A psoriasis és az oxidatív stressz. Orv. Hetil. 2016, 157, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Barygina, V.; Mannucci, A.; Emmi, G.; Prisco, D.; Lotti, T.; Fiorillo, C.; Taddei, N. Sirt1 Protects against Oxidative Stress-Induced Apoptosis in Fibroblasts from Psoriatic Patients: A New Insight into the Pathogenetic Mechanisms of Psoriasis. Int. J. Mol. Sci. 2018, 19, 1572. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.-R.; Tampa, M.; Caruntu, C.; Sarbu, M.-I.; Mitran, C.-I.; Mitran, M.-I.; Matei, C.; Constantin, C.; Neagu, M. Advances in Understanding the Immunological Pathways in Psoriasis. Int. J. Mol. Sci. 2019, 20, 739. [Google Scholar] [CrossRef] [PubMed]
- Richarz, N.; Boada, A.; Carrascosa, J. Angiogenesis in Dermatology-Insights of Molecular Mechanisms and Latest Developments. Actas Dermo-Sifiliogr. 2017, 108, 515–523. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A 10-Year Update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Gu, X.; Cai, Z.; Cai, M.; Liu, K.; Liu, D.; Zhang, Q.; Tan, J.; Ma, Q. AMPK/SIRT1/p38 MAPK signaling pathway regulates alcohol-induced neurodegeneration by resveratrol. Mol. Med. Rep. 2018, 17, 5402–5408. [Google Scholar] [CrossRef]
- Wang, T.; Takikawa, Y. Carnosic acid protects normal mouse hepatocytes against H2O2-induced cytotoxicity via sirtuin 1-mediated signaling. Hepatol. Res. 2015, 46, 239–246. [Google Scholar] [CrossRef]
- Yu, X.-J.; Li, C.-Y.; Dai, H.-Y.; Cai, D.-X.; Wang, K.-Y.; Xu, Y.-H.; Chen, L.-M.; Zhou, C.-L. Expression and localization of the activated mitogen-activated protein kinase in lesional psoriatic skin. Exp. Mol. Pathol. 2007, 83, 413–418. [Google Scholar] [CrossRef]
- Sano, S.; Chan, K.S.; DiGiovanni, J. Impact of Stat3 activation upon skin biology: A dichotomy of its role between homeostasis and diseases. J. Dermatol. Sci. 2008, 50, 1–14. [Google Scholar] [CrossRef]
- Zhou, Q.; Mrowietz, U.; Rostami-Yazdi, M. Oxidative stress in the pathogenesis of psoriasis. Free. Radic. Biol. Med. 2009, 47, 891–905. [Google Scholar] [CrossRef]
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci. 2018, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Sestito, R.; Madonna, S.; Scarponi, C.; Cianfarani, F.; Failla, C.M.; Cavani, A.; Girolomoni, G.; Albanesi, C. STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J. 2010, 25, 916–927. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef] [PubMed]
- Garcin, G.; Guiraud, I.; Lacroix, M.; Genthon, C.; Rialle, S.; Joujoux, J.-M.; Meunier, L.; Lavabre-Bertrand, T.; Stoebner, P.-E.; Le Gallic, L. AMPK/HuR-Driven IL-20 Post-Transcriptional Regulation in Psoriatic Skin. J. Investig. Dermatol. 2015, 135, 2732–2741. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Zhang, X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp. Mol. Pathol. 2019, 107, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, T.; Merlo, S.; Spampinato, S.F.; De Pasquale, R.; Sortino, M.A. High mobility group box 1 contributes to wound healing induced by inhibition of dipeptidylpeptidase 4 in cultured keratinocytes. Front. Pharmacol. 2015, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.; Slama, J.T.; Sternglanz, R. Role of NAD+ in the Deacetylase Activity of the SIR2-like Proteins. Biochem. Biophys. Res. Commun. 2000, 278, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.-I. Nicotinamide Phosphoribosyltransferase (Nampt): A Link Between NAD Biology, Metabolism, and Diseases. Curr. Pharm. Des. 2009, 15, 20–28. [Google Scholar] [CrossRef]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD + Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Xie, S.; Chen, Z.; Wang, Q.; Song, X.; Zhang, L. Comparisons of gene expression in normal, lesional, and non-lesional psoriatic skin using DNA microarray techniques. Int. J. Dermatol. 2014, 53, 1213–1220. [Google Scholar] [CrossRef]
- Gao, L.; Shen, J.; Ren, Y.; Shi, J.; Wang, D.; Cao, J. Discovering novel hub genes and pathways associated with the pathogenesis of psoriasis. Dermatol. Ther. 2020, 33, e13993. [Google Scholar] [CrossRef]
- Koczan, D.; Guthke, R.; Thiesen, H.-J.; Ibrahim, S.M.; Kundt, G.; Krentz, H.; Gross, G.; Kunz, M. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur. J. Dermatol. 2005, 15, 251–257. [Google Scholar]
- Mercurio, L.; Morelli, M.; Scarponi, C.; Scaglione, G.L.; Pallotta, S.; Avitabile, D.; Albanesi, C.; Madonna, S. Enhanced NAMPT-Mediated NAD Salvage Pathway Contributes to Psoriasis Pathogenesis by Amplifying Epithelial Auto-Inflammatory Circuits. Int. J. Mol. Sci. 2021, 22, 6860. [Google Scholar] [CrossRef]
- Hao, L.; Park, J.; Jang, H.-Y.; Bae, E.J.; Park, B.-H. Inhibiting Protein Kinase Activity of Pyruvate Kinase M2 by SIRT2 Deacetylase Attenuates Psoriasis. J. Investig. Dermatol. 2020, 141, 355–363.e6. [Google Scholar] [CrossRef] [PubMed]
- Cirotti, C.; Rizza, S.; Giglio, P.; Poerio, N.; Allega, M.F.; Claps, G.; Pecorari, C.; Lee, J.; Benassi, B.; Barilŕ, D.; et al. Redox activation of ATM enhances GSNOR translation to sustain mitophagy and tolerance to oxidative stress. EMBO Rep. 2020, 22, e50500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nie, P.; Zhou, C.; Hu, Y.; Duan, S.; Gu, M.; Jiang, D.; Wang, Y.; Deng, Z.; Chen, J.; et al. Oxidative stress-induced mitophagy is suppressed by the miR-106b-93-25 cluster in a protective manner. Cell Death Dis. 2021, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, L.; Jiang, J.; Li, H.; Wu, Q.; Ooi, K.; Wang, J.; Feng, Y.; Zhu, D.; Xia, C. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J. Neuroinflamm. 2020, 17, 15. [Google Scholar] [CrossRef]
- Yanli, M.; Yu, W.; Yuzhen, L. Elevated SIRT3 Parkin-dependently activates cell mitophagy to ameliorate TNF-α-induced psoriasis-related phenotypes in HaCaT cells through deacetylating FOXO3a for its activation. Arch. Dermatol. Res. 2022, 315, 847–857. [Google Scholar] [CrossRef]
- Wang, C.; He, D.; Shi, C. SIRT5 reduces the inflammatory response and barrier dysfunction in IL-17A-induced epidermal keratinocytes. Allergol. Immunopathol. 2023, 51, 30–36. [Google Scholar] [CrossRef]
- Elias, P.M.; Wakefield, J.S. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 781–791.e1. [Google Scholar] [CrossRef]
- Sassa, T.; Kihara, A. Metabolism of Very Long-Chain Fatty Acids: Genes and Pathophysiology. Biomol. Ther. 2014, 22, 83–92. [Google Scholar] [CrossRef]
- Miao, X.; Xiang, Y.; Mao, W.; Chen, Y.; Li, Q.; Fan, B. TRIM27 promotes IL-6-induced proliferation and inflammation factor production by activating STAT3 signaling in HaCaT cells. Am. J. Physiol. Physiol. 2020, 318, C272–C281. [Google Scholar] [CrossRef]
- Chen, J.; Fan, H.; Wang, T.; Lin, L.; Cai, T. Silencing KRT16 inhibits keratinocyte proliferation and VEGF secretion in psoriasis via inhibition of ERK signaling pathway. Kaohsiung J. Med. Sci. 2019, 35, 284–296. [Google Scholar] [CrossRef]
- Tang, X.; Liu, Y.; Xiao, Q.; Yao, Q.; Allen, M.; Wang, Y.; Gao, L.; Qi, Y.; Zhang, P. Pathological cyclic strain promotes proliferation of vascular smooth muscle cells via the ACTH/ERK/STAT3 pathway. J. Cell. Biochem. 2018, 119, 8260. [Google Scholar] [CrossRef]
- Qin, K.; Han, C.; Zhang, H.; Li, T.; Li, N.; Cao, X. NAD + dependent deacetylase Sirtuin 5 rescues the innate inflammatory response of endotoxin tolerant macrophages by promoting acetylation of p65. J. Autoimmun. 2017, 81, 120–129. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Dyck, J.R. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1477–1489. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Alrafas, H.R.; Busbee, P.B.; Chitrala, K.N.; Nagarkatti, M.; Nagarkatti, P. Alterations in the Gut Microbiome and Suppression of Histone Deacetylases by Resveratrol Are Associated with Attenuation of Colonic Inflammation and Protection Against Colorectal Cancer. J. Clin. Med. 2020, 9, 1796. [Google Scholar] [CrossRef]
- Švajger, U.; Jeras, M. Anti-inflammatory Effects of Resveratrol and Its Potential Use in Therapy of Immune-mediated Diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef]
- Saqib, U.; Kelley, T.T.; Panguluri, S.K.; Liu, D.; Savai, R.; Baig, M.S.; Schürer, S.C. Polypharmacology or Promiscuity? Structural Interactions of Resveratrol with Its Bandwagon of Targets. Front. Pharmacol. 2018, 9, 1201. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Hänsel, A.; Günther, C.; Ingwersen, J.; Starke, J.; Schmitz, M.; Bachmann, M.; Meurer, M.; Rieber, E.P.; Schäkel, K. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong Th17/Th1 T-cell responses. J. Allergy Clin. Immunol. 2011, 127, 787–794.e9. [Google Scholar] [CrossRef] [PubMed]
- Lynde, C.W.; Poulin, Y.; Vender, R.; Bourcier, M.; Khalil, S. Interleukin 17A: Toward a new understanding of psoriasis pathogenesis. J. Am. Acad. Dermatol. 2014, 71, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, J.-S.; Park, S.-Y.; Lee, Y.-J. Resveratrol induces human keratinocyte damage via the activation of class III histone deacetylase, Sirt1. Oncol. Rep. 2015, 35, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Kjćr, T.N.; Thorsen, K.; Jessen, N.; Stenderup, K.; Pedersen, S.B. Resveratrol Ameliorates Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice. PLoS ONE 2015, 10, e0126599. [Google Scholar] [CrossRef]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of Ultraviolet B Exposure-Mediated Activation of NF-κB in Normal Human Keratinocytes by Resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Greene, R.S.; Downing, D.T.; Pochi, P.E.; Strauss, J.S. Anatomical Variation in the Amount and Composition of Human Skin Surface Lipid. J. Investig. Dermatol. 1970, 54, 240–247. [Google Scholar] [CrossRef]
- Lovászi, M.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermato Endocrinol. 2017, 9, e1375636. [Google Scholar] [CrossRef]
- Shi, V.Y.; Leo, M.; Hassoun, L.; Chahal, D.S.; Maibach, H.I.; Sivamani, R.K. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 2015, 73, 856–863. [Google Scholar] [CrossRef]
- Yang, Z.; Kahn, B.B.; Shi, H.; Xue, B.-Z. Macrophage α1 AMP-activated Protein Kinase (α1AMPK) Antagonizes Fatty Acid-induced Inflammation through SIRT1. J. Biol. Chem. 2010, 285, 19051–19059. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, G.; Hu, T.; Mo, X.; Hou, X.; Cao, K.; Wang, L.; Pan, Z.; Wu, Q.; Li, X.; et al. Resveratrol ameliorates lipid accumulation and inflammation in human SZ95 sebocytes via the AMPK signaling pathways in vitro. J. Dermatol. Sci. 2021, 103, 156–166. [Google Scholar] [CrossRef]
- Bai, X.; Yao, L.; Ma, X.; Xu, X. Small Molecules as SIRT Modulators. Mini-Rev. Med. Chem. 2018, 18, 1151–1157. [Google Scholar] [CrossRef]
- Bao, Q.; Shen, X.; Qian, L.; Gong, C.; Nie, M.; Dong, Y. Anti-diabetic activities of catalpol in db/db mice. Korean J. Physiol. Pharmacol. 2016, 20, 153–160. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zhan-Sheng, H. Catalpol inhibits migration and induces apoptosis in gastric cancer cells and in athymic nude mice. Biomed. Pharmacother. 2018, 103, 1708–1719. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, G.; Ma, S.; Li, F.; Yuan, M.; Xu, H.; Huang, K. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-κB pathways. Biochem. Biophys. Res. Commun. 2015, 467, 853–858. [Google Scholar] [CrossRef]
- Bi, J.; Jiang, B.; Liu, J.H.; Lei, C.; Zhang, X.L.; An, L.-J. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci. Lett. 2008, 442, 224–227. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, B.; Zhao, W.; Tu, Y.; Wang, Q.; Li, J. Catalpol ameliorates psoriasis-like phenotypes via SIRT1 mediated suppression of NF-κB and MAPKs signaling pathways. Bioengineered 2020, 12, 183–195. [Google Scholar] [CrossRef]
- Xiong, Y.; Shi, L.; Wang, L.; Zhou, Z.; Wang, C.; Lin, Y.; Luo, D.; Qiu, J.; Chen, D. Activation of sirtuin 1 by catalpol-induced down-regulation of microRNA-132 attenuates endoplasmic reticulum stress in colitis. Pharmacol. Res. 2017, 123, 73–82. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, R.; Meng, Q.; Wang, C.; Huo, X.; Liu, Z.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; et al. Catalpol alleviates adriamycin-induced nephropathy by activating the SIRT1 signalling pathway in vivo and in vitro. Br. J. Pharmacol. 2019, 176, 4558–4573. [Google Scholar] [CrossRef]
- Fei, B.; Dai, W.; Zhao, S. Efficacy, Safety, and Cost of Therapy of the Traditional Chinese Medicine, Catalpol, in Patients Following Surgical Resection for Locally Advanced Colon Cancer. Experiment 2018, 24, 3184–3192. [Google Scholar] [CrossRef]
- Elgewelly, M.A.; Elmasry, S.M.; El Sayed, N.S.; Abbas, H. Resveratrol-Loaded Vesicular Elastic Nanocarriers Gel in Imiquimod-Induced Psoriasis Treatment: In Vitro and In Vivo Evaluation. J. Pharm. Sci. 2021, 111, 417–431. [Google Scholar] [CrossRef]
- Khurana, B.; Arora, D.; Narang, R.K. QbD based exploration of resveratrol loaded polymeric micelles based carbomer gel for topical treatment of plaque psoriasis: In vitro, ex vivo and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 59, 101901. [Google Scholar] [CrossRef]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słuczanowska-Głabowska, S.; Salmanowicz, M.; Staniszewska, M.; Pawlik, A. The Role of Sirtuins in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2023, 24, 10782. https://doi.org/10.3390/ijms241310782
Słuczanowska-Głabowska S, Salmanowicz M, Staniszewska M, Pawlik A. The Role of Sirtuins in the Pathogenesis of Psoriasis. International Journal of Molecular Sciences. 2023; 24(13):10782. https://doi.org/10.3390/ijms241310782
Chicago/Turabian StyleSłuczanowska-Głabowska, Sylwia, Maria Salmanowicz, Marzena Staniszewska, and Andrzej Pawlik. 2023. "The Role of Sirtuins in the Pathogenesis of Psoriasis" International Journal of Molecular Sciences 24, no. 13: 10782. https://doi.org/10.3390/ijms241310782
APA StyleSłuczanowska-Głabowska, S., Salmanowicz, M., Staniszewska, M., & Pawlik, A. (2023). The Role of Sirtuins in the Pathogenesis of Psoriasis. International Journal of Molecular Sciences, 24(13), 10782. https://doi.org/10.3390/ijms241310782





