Intravenous Polyethylene Glycol Alleviates Intestinal Ischemia-Reperfusion Injury in a Rodent Model
Abstract
1. Introduction
2. Results
2.1. Intravenous PEG Pretreatment Did Not Prevent Epithelial Intestinal Damage but Reduced Paracellular Permeability
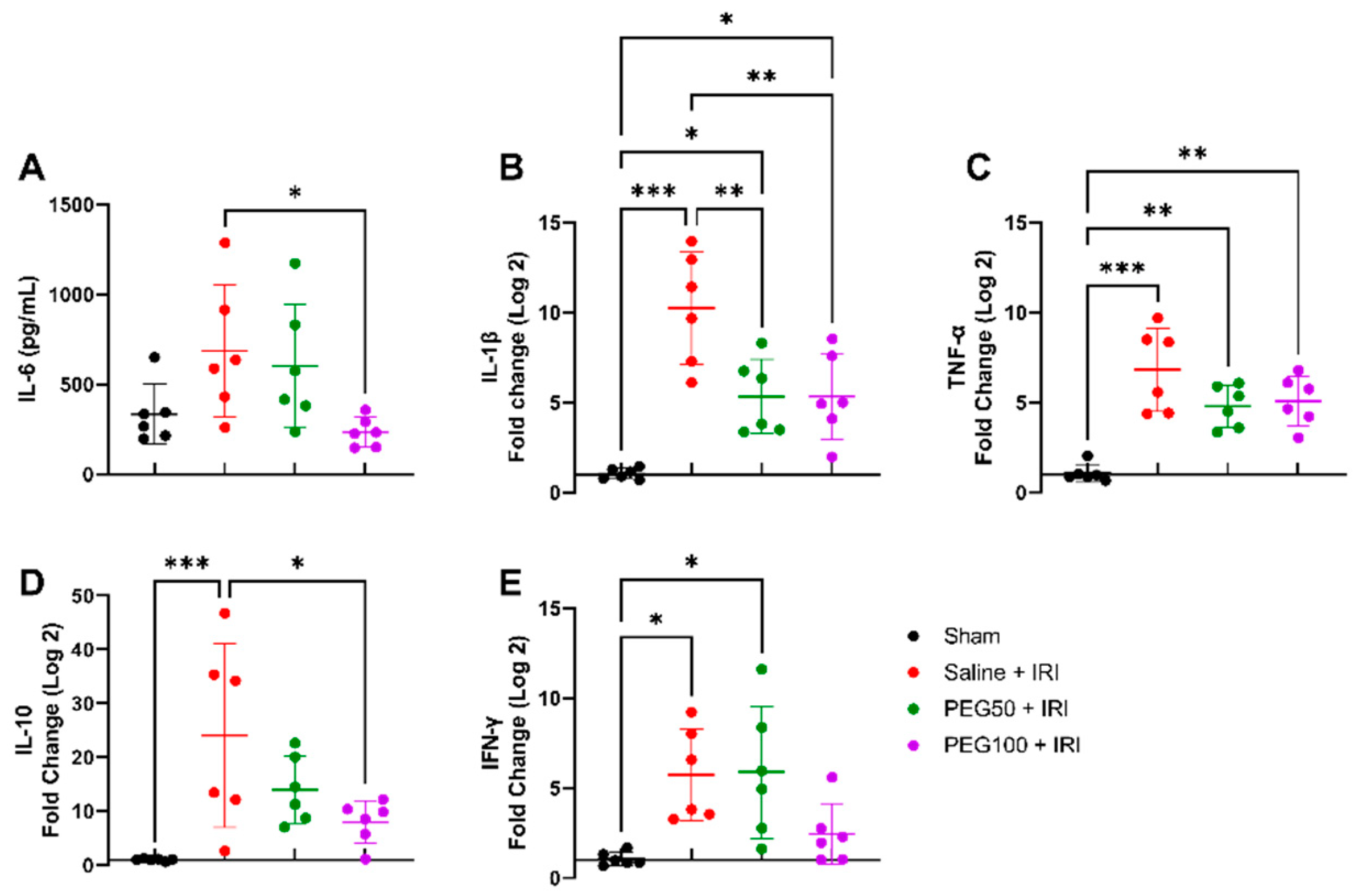
2.2. High-Dose PEG Pretreatment Reduced the Inflammatory Response
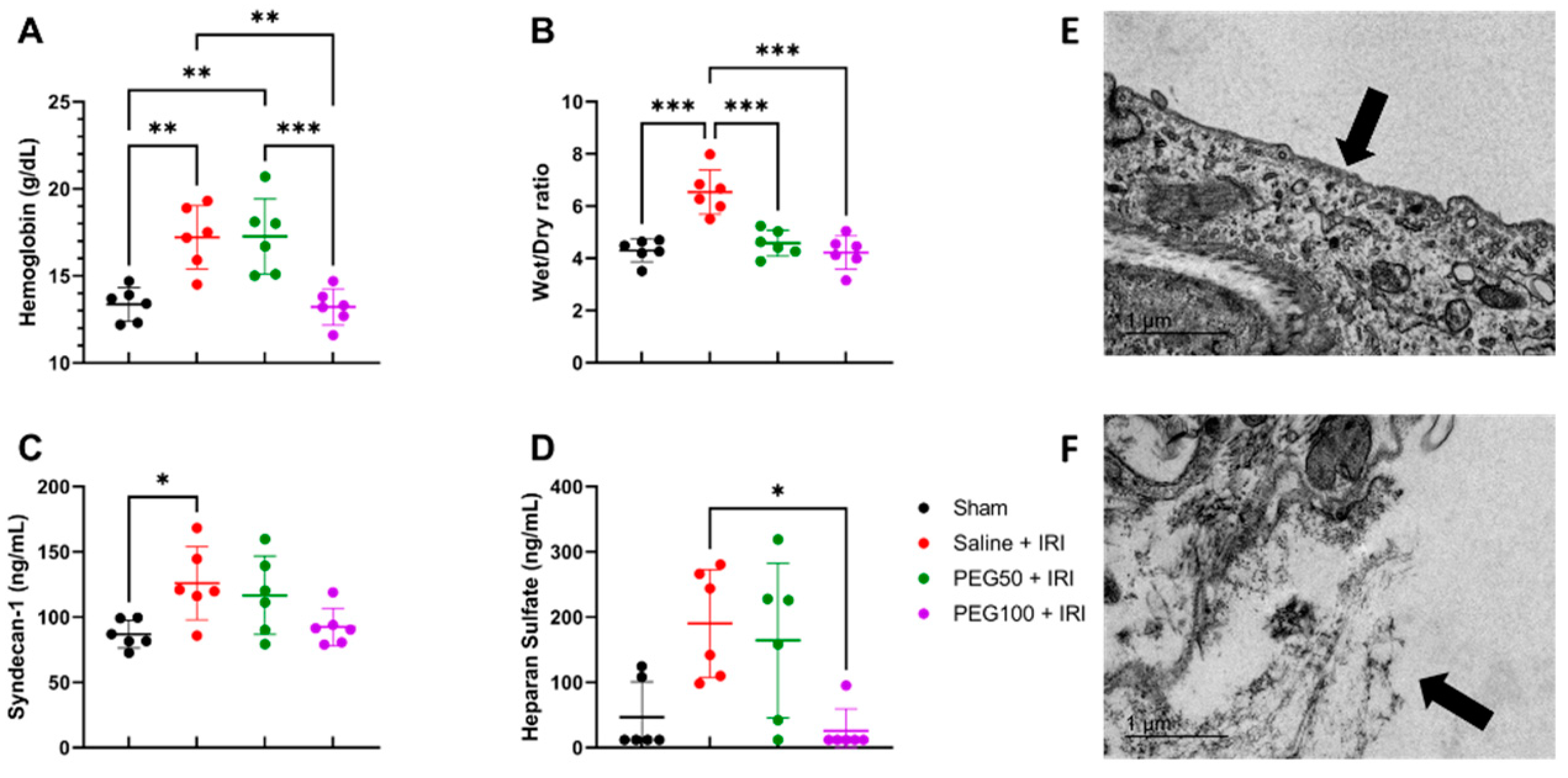
2.3. High-Dose PEG Pretreatment Preserved Vascular Permeability by Protecting the Endothelial Glycocalyx
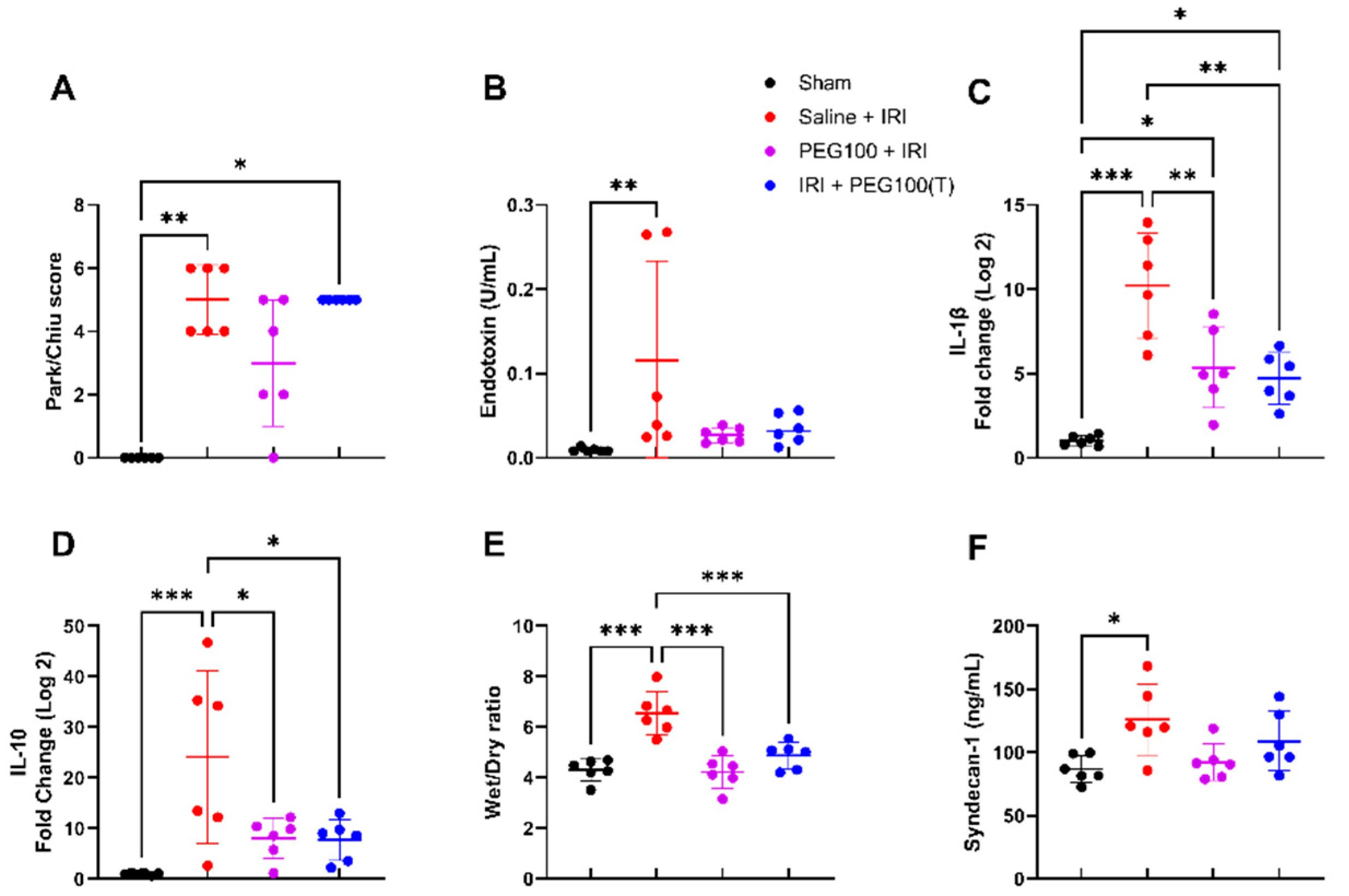
2.4. Treatment with High-Dose PEG Improved Outcome after Intestinal IRI
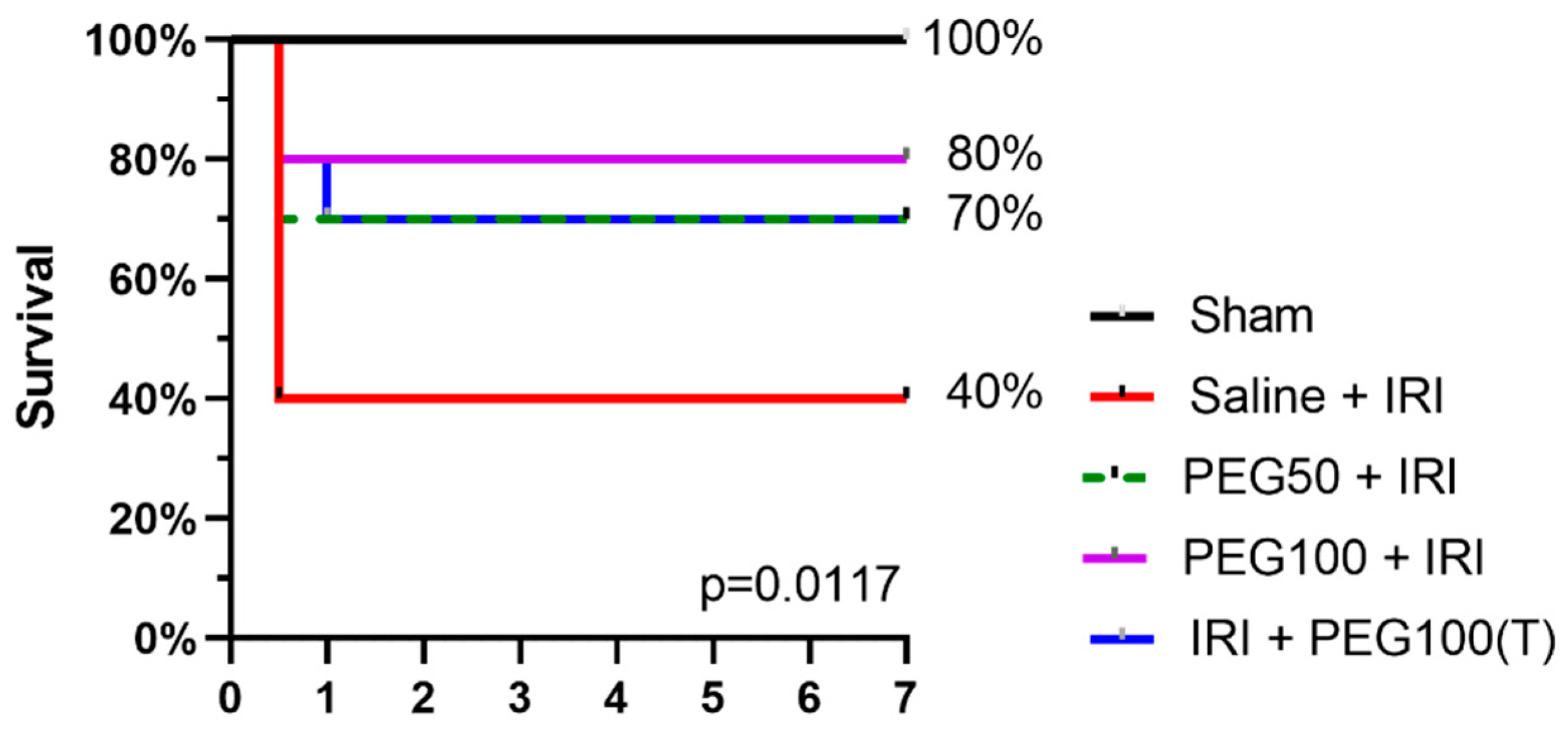
2.5. IV PEG Administration Improved Survival after Intestinal IRI
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Anesthesia
4.3. Surgery
4.4. Blood and Tissue Sampling
4.5. Arterial Blood Gas Analysis
4.6. Histological Evaluation
4.7. Ussing Chamber Experiments
Electrophysiological Parameters
4.8. Bacterial Translocation
4.9. Edema
4.10. Osmolality
4.11. ELISA
4.12. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.13. Transmission Electron Microscopy (TEM)
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carden, D.L.; Granger, D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Bala, M.; Catena, F.; Kashuk, J.; De Simone, B.; Gomes, C.A.; Weber, D.; Sartelli, M.; Coccolini, F.; Kluger, Y.; Abu-Zidan, F.M.; et al. Acute mesenteric ischemia: Updated guidelines of the World Society of Emergency Surgery. World J. Emerg. Surg. 2022, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Vollmar, B.; Menger, M.D. Intestinal ischemia/reperfusion: Microcirculatory pathology and functional consequences. Langenbeck’s Arch. Surg. 2011, 396, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Ischemia—Reperfusion Injury of the Intestine and Protective Strategies Against Injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, X.; Liu, X.; Li, Z.; Shi, A.; Tang, X.; Xia, P.; Zhang, J.; Yu, P. AMPK: The key to ischemia-reperfusion injury. J. Cell. Physiol. 2022, 237, 4079–4096. [Google Scholar] [CrossRef]
- Liao, S.; Luo, J.; Kadier, T.; Ding, K.; Chen, R.; Meng, Q. Mitochondrial DNA Release Contributes to Intestinal Ischemia/Reperfusion Injury. Front. Pharmacol. 2022, 13, 854994. [Google Scholar] [CrossRef]
- Swamy, M.; Jamora, C.; Havran, W.; Hayday, A. Epithelial decision makers: In search of the “epimmunome”. Nat. Immunol. 2010, 11, 656–665. [Google Scholar] [CrossRef]
- DiPalma, J.; DeRidder, P.; Orlando, R.; Kolts, B.; Cleveland, M. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am. J. Gastroenterol. 2000, 95, 446–450. [Google Scholar] [CrossRef]
- Pasut, G.; Panisello, A.; Folch-Puy, E.; Lopez, A.; Castro-Benítez, C.; Calvo, M.; Carbonell, T.; García-Gil, A.; Adam, R.; Roselló-Catafau, J. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 2016, 22, 6501. [Google Scholar] [CrossRef]
- Luo, J.; Borgens, R.; Shi, R. Polyethylene Glycol Improves Function and Reduces Oxidative Stress in Synaptosomal Preparations following Spinal Cord Injury. J. Neurotrauma 2004, 21, 994–1007. [Google Scholar] [CrossRef]
- Chen, H.; Quick, E.; Leung, G.; Hamann, K.; Fu, Y.; Cheng, J.-X.; Shi, R. Polyethylene Glycol Protects Injured Neuronal Mitochondria. Pathobiology 2009, 76, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Valuckaite, V.; Staron, M.L.; Theccanat, T.; D’Souza, K.M.; Alverdy, J.C.; Akhter, S.A. High-molecular-weight polyethylene glycol protects cardiac myocytes from hypoxia- and reoxygenation-induced cell death and preserves ventricular function. Am. J. Physiol. Circ. Physiol. 2011, 300, H1733–H1742. [Google Scholar] [CrossRef]
- Valuckaite, V.; Seal, J.; Zaborina, O.; Tretiakova, M.; Testa, G.; Alverdy, J.C. High molecular weight polyethylene glycol (PEG 15-20) maintains mucosal microbial barrier function during intestinal graft preservation. J. Surg. Res. 2013, 183, 869–875. [Google Scholar] [CrossRef]
- Yandza, T.; Tauc, M.; Canioni, D.; Rogel-Gaillard, C.; Bernard, G.; Bernard, A.; Gugenheim, J. Effect of Polyethylene Glycol in Pig Intestinal Allotransplantation Without Immunosuppression. J. Surg. Res. 2012, 176, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, R. Diffusive oxidative stress following acute spinal cord injury in guinea pigs and its inhibition by polyethylene glycol. Neurosci. Lett. 2004, 359, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Pantazi, E.; Calvo, M.; Folch-Puy, E.; Serafín, A.; Pasut, G.; Panisello, A.; Adam, R.; Roselló-Catafau, J. Polyethylene Glycol Preconditioning: An Effective Strategy to Prevent Liver Ischemia Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, 9096549. [Google Scholar] [CrossRef]
- Zaouali, M.A.; Bejaoui, M.; Calvo, M.; Folch-Puy, E.; Pantazi, E.; Pasut, G.; Rimola, A.; Abdennebi, H.B.; Adam, R.; Roselló-Catafau, J. Polyethylene glycol rinse solution: An effective way to prevent ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 16203. [Google Scholar] [CrossRef]
- Xu, X.; Philip, J.L.; Razzaque, M.A.; Lloyd, J.W.; Muller, C.M.; Akhter, S.A. High-molecular-weight polyethylene glycol inhibits myocardial ischemia–reperfusion injury in vivo. J. Thorac. Cardiovasc. Surg. 2015, 149, 588–593. [Google Scholar] [CrossRef]
- Bejaoui, M.; Pantazi, E.; Folch-Puy, E.; Panisello, A.; Calvo, M.; Pasut, G.; Rimola, A.; Navasa, M.; Adam, R.; Roselló-Catafau, J. Protective Effect of Intravenous High Molecular Weight Polyethylene Glycol on Fatty Liver Preservation. BioMed Res. Int. 2015, 2015, 794287. [Google Scholar] [CrossRef]
- Chiang, E.T.; Camp, S.M.; Dudek, S.M.; Brown, M.E.; Usatyuk, P.V.; Zaborina, O.; Alverdy, J.C.; Garcia, J.G.N. Protective effects of high-molecular weight Polyethylene Glycol (PEG) in human lung endothelial cell barrier regulation: Role of actin cytoskeletal rearrangement. Microvasc. Res. 2009, 77, 174–186. [Google Scholar] [CrossRef]
- Abassi, Z.; Armaly, Z.; Heyman, S.N. Glycocalyx Degradation in Ischemia-Reperfusion Injury. Am. J. Pathol. 2020, 190, 752–767. [Google Scholar] [CrossRef]
- Schiefer, J.; Faybik, P.; Koch, S.; Tudor, B.; Kollmann, D.; Kuessel, L.; Krenn, C.G.; Berlakovich, G.; Baron, D.M.; Baron-Stefaniak, J. Glycocalyx damage within human liver grafts correlates with graft injury and postoperative graft function after orthotopic liver transplantation. Transplantation 2019, 104, 72–78. [Google Scholar] [CrossRef]
- Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalyx Preservation and NO Production in Fatty Livers—The Protective Role of High Molecular Polyethylene Glycol in Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2375. [Google Scholar] [CrossRef]
- Rosello, A.P.; da Silva, R.T.; Castro, C.; Bardallo, R.G.; Calvo, M.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.; Catafau, J.R.; Adam, R. Polyethylene Glycol 35 as a Perfusate Additive for Mitochondrial and Glycocalyx Protection in HOPE Liver Preservation. Int. J. Mol. Sci. 2020, 21, 5703. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, L.; Verbeke, L.; Decuypere, J.-P.; Farré, R.; De Hertogh, G.; Lenaerts, K.; Jochmans, I.; Monbaliu, D.; Nevens, F.; Tack, J.; et al. Farnesoid X Receptor Activation Attenuates Intestinal Ischemia Reperfusion Injury in Rats. PLoS ONE 2017, 12, e0169331. [Google Scholar] [CrossRef] [PubMed]
- Parrish, D.; Plant, V.; Lindell, S.L.; Limkemann, A.; Reichstetter, H.; Aboutanos, M.; Mangino, M.J. New low-volume resuscitation solutions containing PEG-20k. J. Trauma Acute Care Surg. 2015, 79, 22–29. [Google Scholar] [CrossRef]
- Plant, V.; Limkemann, A.; Liebrecht, L.; Blocher, C.; Ferrada, P.; Aboutanos, M.; Mangino, M.J. Low-volume resuscitation using polyethylene glycol-20k in a preclinical porcine model of hemorrhagic shock. J. Trauma Acute Care Surg. 2016, 81, 1056–1061. [Google Scholar] [CrossRef]
- Canovai, E.; Oltean, M.; Herlenius, G.; Trentadue, G.; Dijkstra, G.; Haveman, J.; Pascher, A.; Monbaliu, D.; Pirenne, J.; Ceulemans, L. First clinical multi-center experience of IGL-1 for intestinal graft preservation. Transplantation 2019, 103, S118. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. Principles of solid-organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Belzer, F.O. The University of Wisconsin organ preservation solution: Components, comparisons, and modifications. Transpl. Rev. 1993, 7, 176–190. [Google Scholar] [CrossRef]
- Oltean, M.; Joshi, M.; Björkman, E.; Oltean, S.; Casselbrant, A.; Herlenius, G.; Olausson, M. Intraluminal Polyethylene Glycol Stabilizes Tight Junctions and Improves Intestinal Preservation in the Rat. Am. J. Transpl. 2012, 12, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M.; Hellström, M.; Ciuce, C.; Zhu, C.; Casselbrant, A. Luminal solutions protect mucosal barrier during extended preservation. J. Surg. Res. 2015, 194, 289–296. [Google Scholar] [CrossRef]
- Søfteland, J.M.; Bagge, J.; Padma, A.M.; Casselbrant, A.; Zhu, C.; Wang, Y.; Hellström, M.; Olausson, M.; Oltean, M. Luminal polyethylene glycol solution delays the onset of preservation injury in the human intestine. Am. J. Transpl. 2021, 21, 2220–2230. [Google Scholar] [CrossRef]
- Bjørnsdottir, I.; Sternebring, O.; Kappers, W.A.; Selvig, H.; Kornø, H.T.; Kristensen, J.B.; Bagger, M.A. Pharmacokinetics, tissue distribution and excretion of 40kDa PEG and PEGylated rFVIII (N8-GP) in rats. Eur. J. Pharm. Sci. 2016, 87, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Andrés, A.; Panisello-Roselló, A.; Serafín, A.; Roselló-Catafau, J.; Folch-Puy, E. Polyethylene glycol 35 (PEG35) protects against inflammation in experimental acute necrotizing pancreatitis and associated lung injury. Int. J. Mol. Sci. 2020, 21, 917. [Google Scholar] [CrossRef]
- Corcos, O.; Castier, Y.; Sibert, A.; Gaujoux, S.; Ronot, M.; Joly, F.; Paugam, C.; Bretagnol, F.; Abdel-Rehim, M.; Francis, F.; et al. Effects of a Multimodal Management Strategy for Acute Mesenteric Ischemia on Survival and Intestinal Failure. Clin. Gastroenterol. Hepatol. 2013, 11, 158–165. [Google Scholar] [CrossRef]
- Sert, N.P.d.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Clarysse, M.; Accarie, A.; Farré, R.; Canovai, E.; Monbaliu, D.; Gunst, J.; De Hertogh, G.; Vanuytsel, T.; Pirenne, J.; Ceulemans, L.J. Protective Effect of Oxygen and Isoflurane in Rodent Model of Intestinal Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2023, 24, 2587. [Google Scholar] [CrossRef]
- Chiu, C.-J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal Mucosal Lesion in Low-Flow States: I. A Morphological, Hemodynamic, and Metabolic Reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Haglund, U.; Bulkley, G.; Fält, K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 1990, 107, 574–580. [Google Scholar] [PubMed]
- Grootjans, J.; Thuijls, G.; Derikx, J.P.M.; Van Dam, R.M.; Dejong, C.H.C.; Buurman, W.A. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J. Pathol. 2011, 224, 411–419. [Google Scholar] [CrossRef] [PubMed]

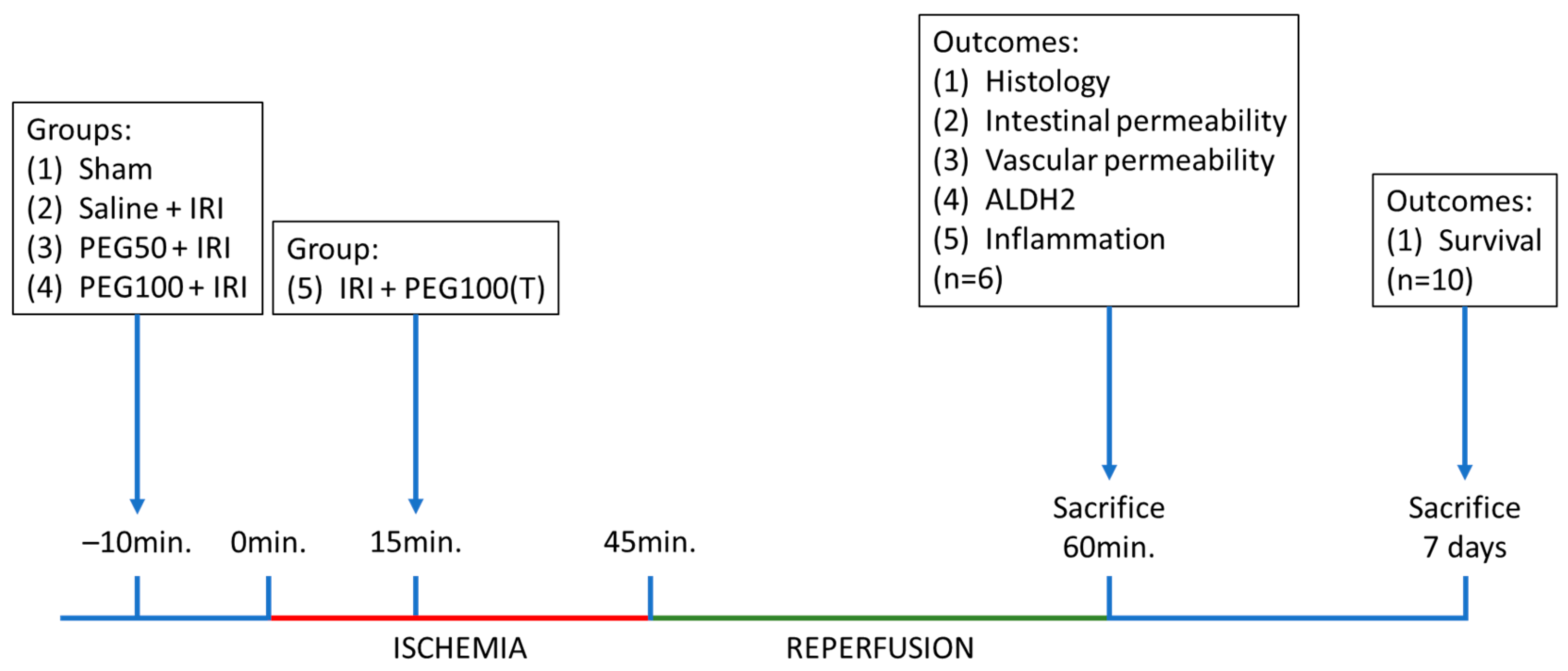
| Group | Product | Dosage (mg/kg) | Pretreatment/Treatment |
|---|---|---|---|
| Sham | Saline | 0 mg/kg | NA |
| Saline + IRI | Saline | 0 mg/kg | Pretreatment |
| PEG50 + IRI | PEG | 50 mg/kg | Pretreatment |
| PEG100 + IRI | PEG | 100 mg/kg | Pretreatment |
| IRI + PEG100(T) | PEG | 100 mg/kg | Treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarysse, M.; Accarie, A.; Panisello-Roselló, A.; Farré, R.; Canovai, E.; Monbaliu, D.; De Hertogh, G.; Vanuytsel, T.; Pirenne, J.; Ceulemans, L.J. Intravenous Polyethylene Glycol Alleviates Intestinal Ischemia-Reperfusion Injury in a Rodent Model. Int. J. Mol. Sci. 2023, 24, 10775. https://doi.org/10.3390/ijms241310775
Clarysse M, Accarie A, Panisello-Roselló A, Farré R, Canovai E, Monbaliu D, De Hertogh G, Vanuytsel T, Pirenne J, Ceulemans LJ. Intravenous Polyethylene Glycol Alleviates Intestinal Ischemia-Reperfusion Injury in a Rodent Model. International Journal of Molecular Sciences. 2023; 24(13):10775. https://doi.org/10.3390/ijms241310775
Chicago/Turabian StyleClarysse, Mathias, Alison Accarie, Arnau Panisello-Roselló, Ricard Farré, Emilio Canovai, Diethard Monbaliu, Gert De Hertogh, Tim Vanuytsel, Jacques Pirenne, and Laurens J. Ceulemans. 2023. "Intravenous Polyethylene Glycol Alleviates Intestinal Ischemia-Reperfusion Injury in a Rodent Model" International Journal of Molecular Sciences 24, no. 13: 10775. https://doi.org/10.3390/ijms241310775
APA StyleClarysse, M., Accarie, A., Panisello-Roselló, A., Farré, R., Canovai, E., Monbaliu, D., De Hertogh, G., Vanuytsel, T., Pirenne, J., & Ceulemans, L. J. (2023). Intravenous Polyethylene Glycol Alleviates Intestinal Ischemia-Reperfusion Injury in a Rodent Model. International Journal of Molecular Sciences, 24(13), 10775. https://doi.org/10.3390/ijms241310775







