Effects of Individual Circulating FFAs on Plasma and Hepatic FFA Epoxides, Diols, and Epoxide-Diol Ratios as Indices of Soluble Epoxide Hydrolase Activity
Abstract
1. Introduction
2. Results
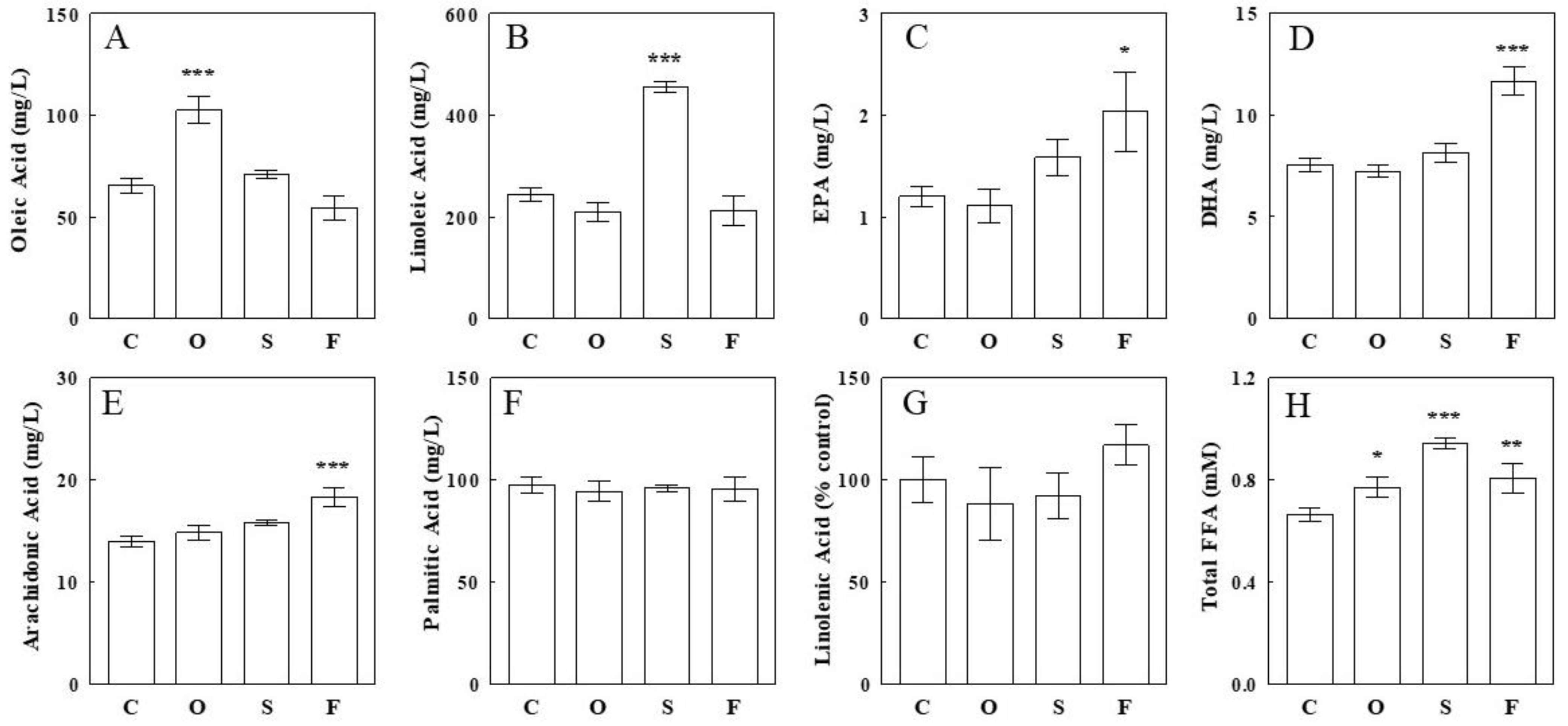
2.1. Effects of Oil Infusions on Individual Circulating FFA Levels
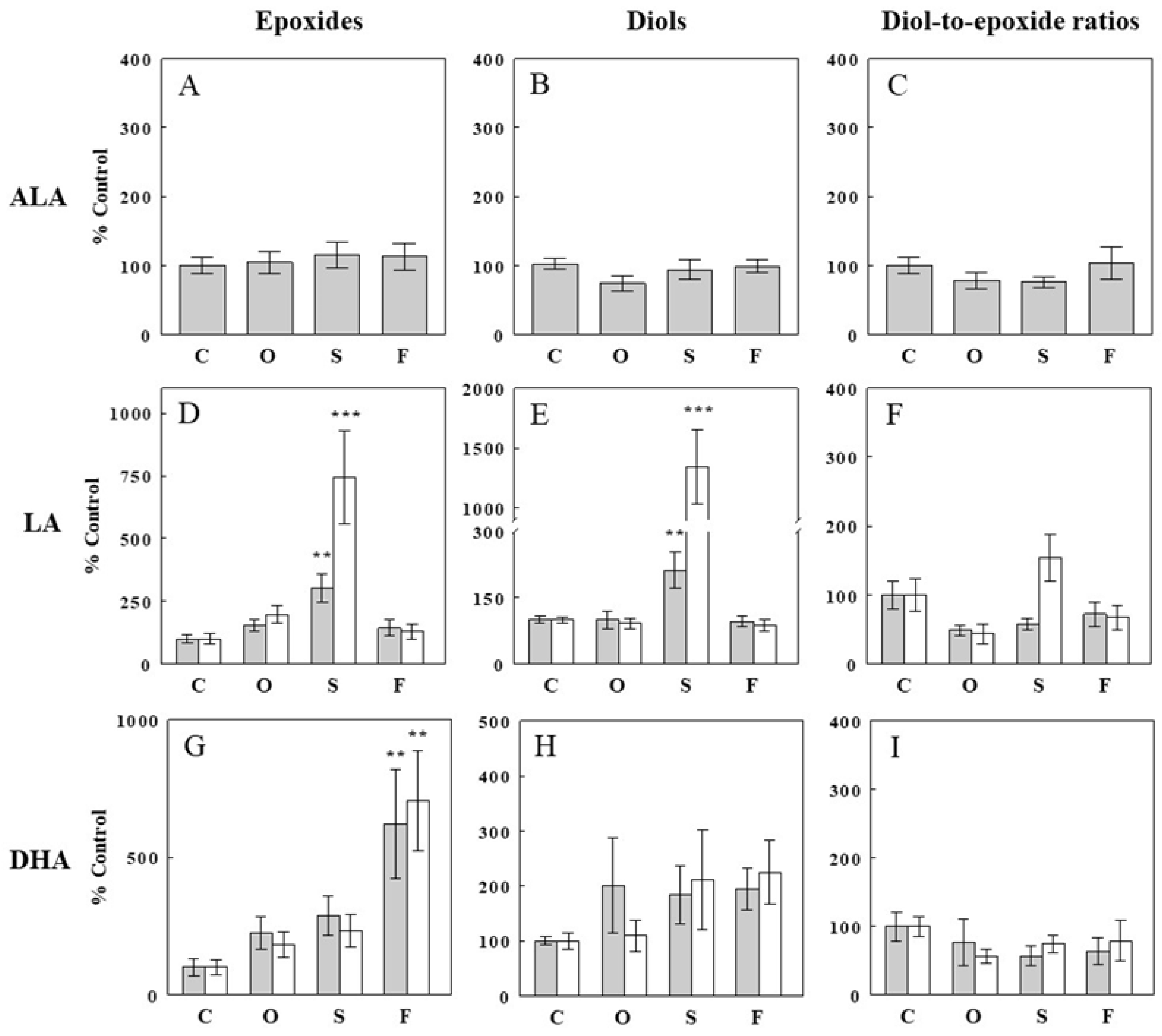
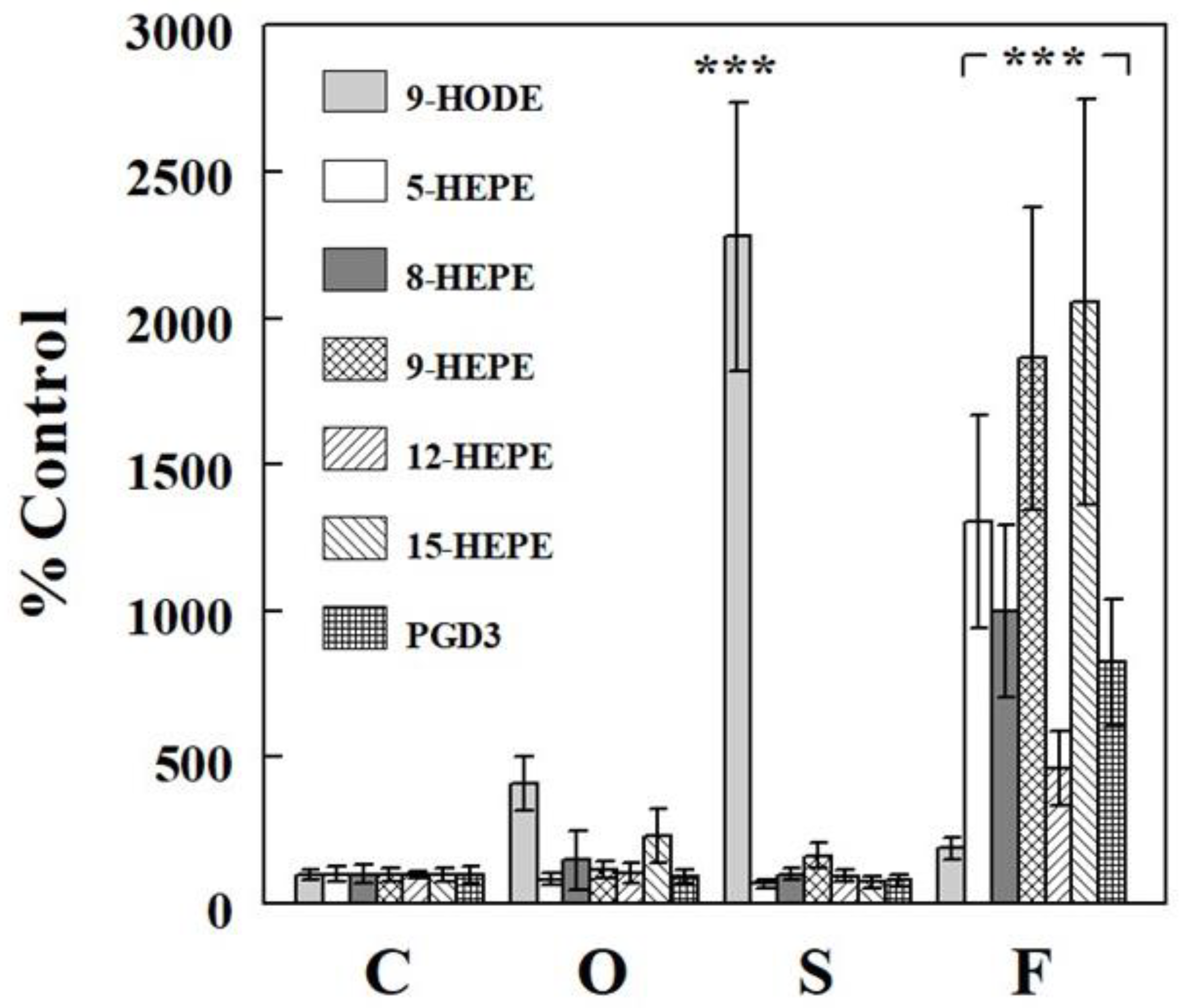
2.2. Effects of Oil Infusions on Hepatic Epoxide and Diol Levels and Epoxide-Diol Ratios
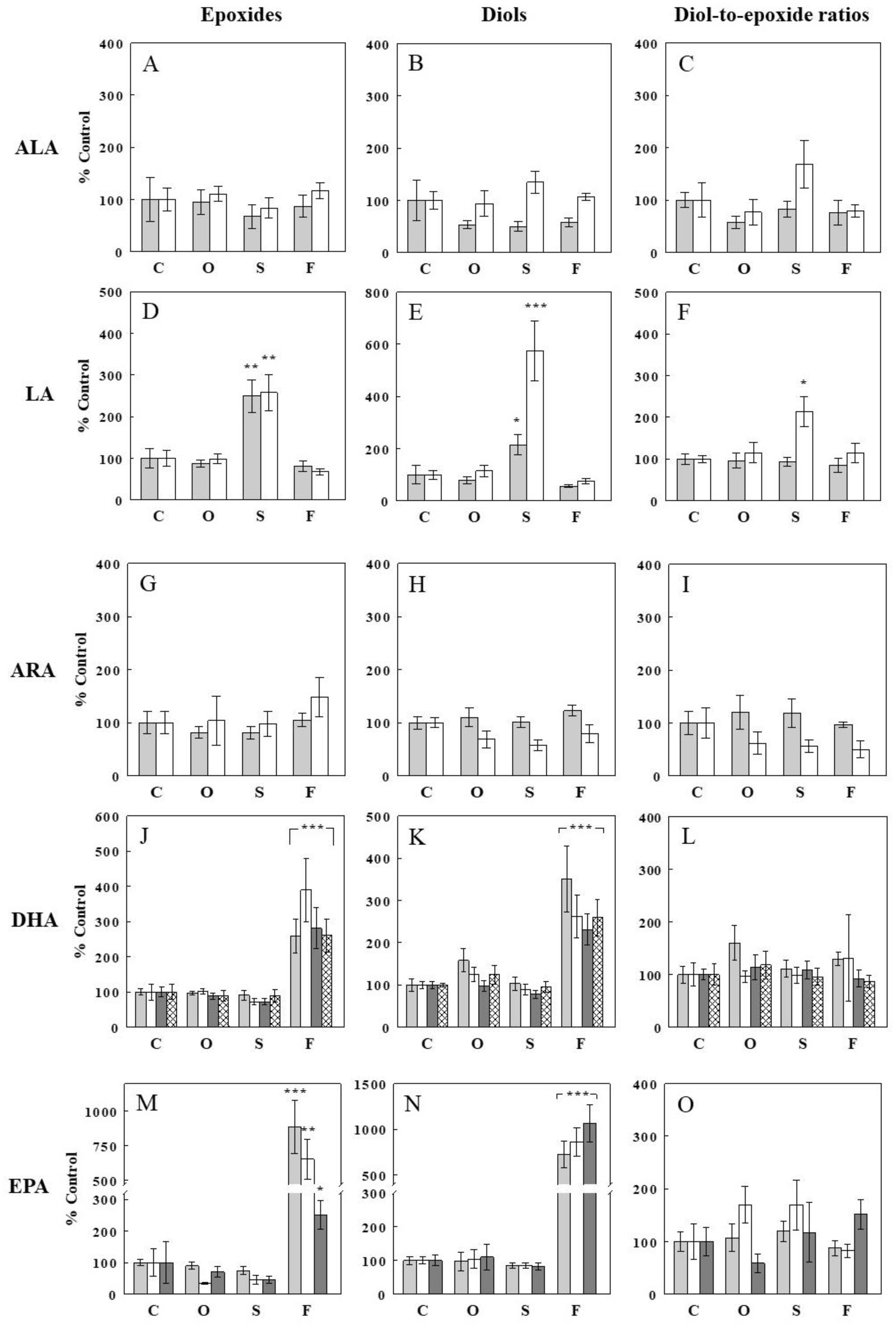
2.3. Effects of Oil Infusions on Plasma FFA Epoxide and Diol Levels and Epoxide-Diol Ratios
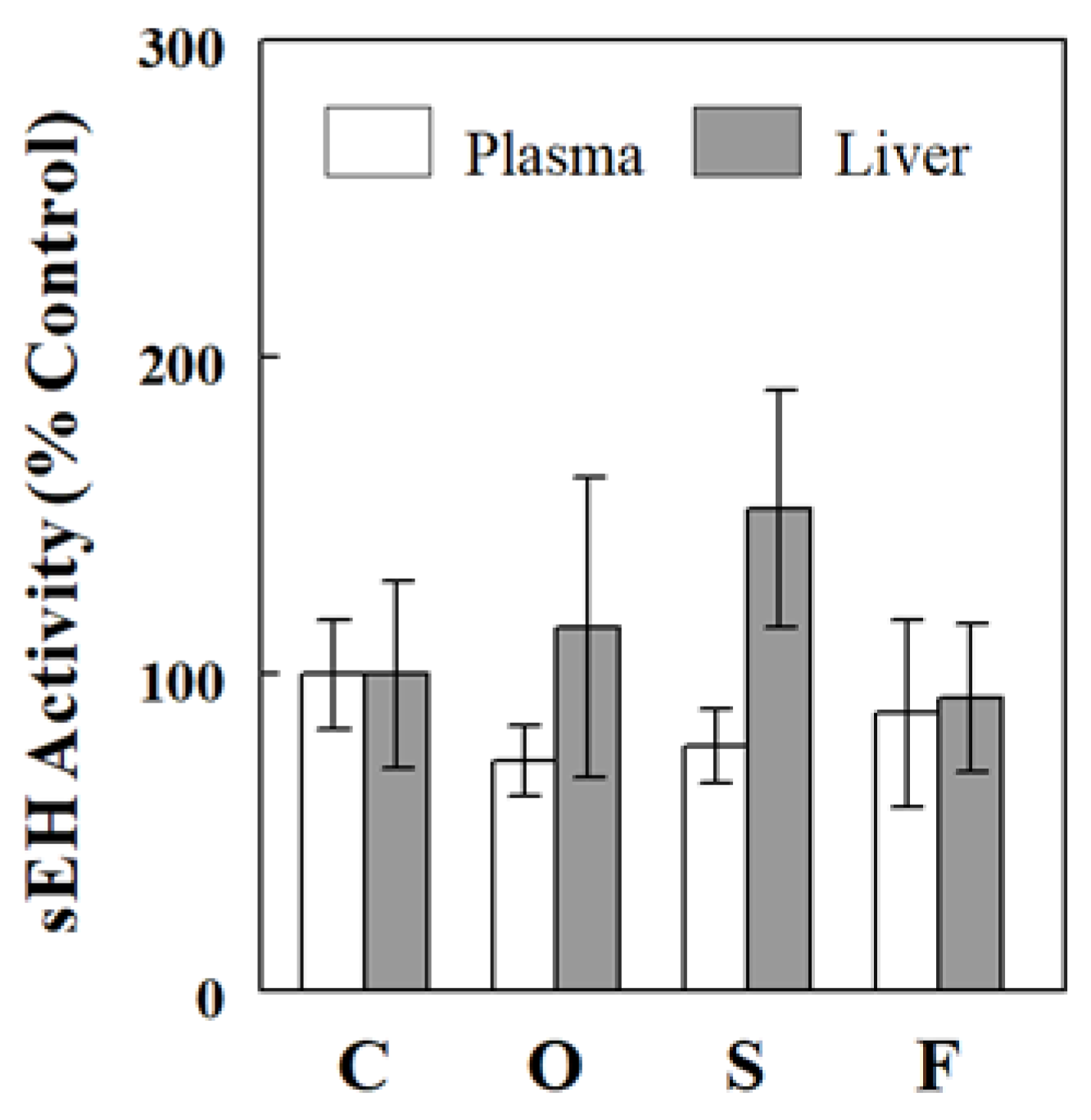
2.4. Effects of Oil Infusions on SAMI-Estimated and Directly Measured sEH Activity
2.5. Effects of Oil Infusions on Hepatic Oxylipins Produced by LOX and COX
3. Discussion
4. Materials and Methods
4.1. Animals and Catheterization
4.2. Animal Experiments
4.3. Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | α-linolenic acid |
| ARA | arachidonic acid |
| COX | cyclooxygenase |
| CYP | cytochrome P450 |
| DHA | docosahexaenoic acid |
| DiHDPE | dihydroxydocosa-pentaenoic acid |
| DiHETE | dihydroxyeicosatetraenoic acid |
| DiHETrE | dihydroxyeicosatrienoic acid |
| DiHODE | dihydroxyoctadecadienoic acid |
| DiHOME | dihydroxyoctamonoenoic acid |
| EPA | eicosapentaenoic acid |
| EpDPE | epoxydocosapen-taenoic acid |
| EpETE | epoxyeicosatetreaenoic acid |
| EpETrE | epoxyeicosatrienoic acid |
| EpODE | epoxyoctadecadienoic acid |
| EpOME | epoxyoctamonoenoic acid |
| FFA | free fatty acid |
| HEPE | hydroxyeicosapentaenoic acid |
| 9-HODE | 9-hydroxyoctadecadienoic acid |
| LA | linoleic acid |
| LOX | lipoxygenase |
| OA | oleic acid |
| PGD3 | prostaglandin D3 |
| sEH | soluble epoxide hydrolase |
References
- Huang, H.; Weng, J.; Wang, M.-H. EETs/sEH in diabetes and obesity-induced cardiovascular diseases. Prostaglandins Other Lipid Mediat. 2016, 125, 80–89. [Google Scholar] [CrossRef]
- Taha, A.Y.; Hennebelle, M.; Yang, J.; Zamora, D.; Rapoport, S.I.; Hammock, B.D.; Ramsden, C.E. Regulation of rat plasma and cerebral cortex oxylipin concentrations with increasing levels of dietary linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2016, 138, 71–80. [Google Scholar] [CrossRef]
- Mustafa, S.; Sharma, V.; McNeill, J.H. Insulin resistance and endothelial dysfunction: Are epoxyeicosatrienoic acids the link? Exp. Clin. Cardiol. 2009, 14, e41–e50. [Google Scholar] [PubMed]
- Luria, A.; Bettaieb, A.; Xi, Y.; Shieh, G.-J.; Liu, H.-C.; Inoue, H.; Tsai, H.-J.; Imig, J.D.; Haj, F.G.; Hammock, B.D. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, R.; Chen, G.; Hoopes, S.L.; Zeldin, D.C.; Wang, D.W. The Role of Cytochrome P450 Epoxygenases, Soluble Epoxide Hydrolase, and Epoxyeicosatrienoic Acids in Metabolic Diseases. Adv. Nutr. Int. Rev. J. 2016, 7, 1122–1128. [Google Scholar] [CrossRef]
- Xu, D.; Li, N.; He, Y.; Timofeyev, V.; Lu, L.; Tsai, H.-J.; Kim, I.-H.; Tuteja, D.; Mateo, R.K.P.; Singapuri, A.; et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci. USA 2006, 103, 18733–18738. [Google Scholar] [CrossRef] [PubMed]
- Chiamvimonvat, N.; Ho, C.M.; Tsai, H.J.; Hammock, B.D. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J. Cardiovasc. Pharmacol. 2007, 50, 225–237. [Google Scholar] [CrossRef]
- Lee, C.R.; Pretorius, M.; Schuck, R.N.; Burch, L.H.; Bartlett, J.; Williams, S.M.; Zeldin, D.C.; Brown, N.J. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with forearm vasodilator responses in humans. Hypertension 2011, 57, 116–122. [Google Scholar] [CrossRef]
- Lee, J.; Dahl, M.; Grande, P.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Genetically reduced soluble epoxide hydrolase activity and risk of stroke and other cardiovascular disease. Stroke 2010, 41, 27–33. [Google Scholar] [CrossRef]
- Gschwendtner, A.; Ripke, S.; Freilinger, T.; Lichtner, P.; Müller-Myhsok, B.; Wichmann, H.E.; Meitinger, T.; Dichgans, M. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white europeans. Stroke 2008, 39, 1593–1596. [Google Scholar] [CrossRef]
- Fornage, M.; Lee, C.R.; Doris, P.A.; Bray, M.S.; Heiss, G.; Zeldin, D.C.; Boerwinkle, E. The soluble epoxide hydrolase gene harbors se-quence variation associated with susceptibility to and protection from incident ischemic stroke. Hum. Mol. Genet. 2005, 14, 2829–2837. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, H.; Yan, J.; Hui, R.; Wang, W.; Kissling, G.E.; Zeldin, D.C.; Wang, D.W. Genetic variation in cytochrome P450 2J2 and soluble epoxide hydrolase and risk of ischemic stroke in a Chinese population. Pharm. Genom. 2008, 18, 45–51. [Google Scholar] [CrossRef]
- Yang, L.; Cheriyan, J.; Gutterman, D.D.; Mayer, R.J.; Ament, Z.; Griffin, J.L.; Lazaar, A.L.; Newby, D.E.; Tal-Singer, R.; Wilkinson, I.B. Mechanisms of Vascular Dysfunction in COPD and Effects of a Novel Soluble Epoxide Hydrolase Inhibitor in Smokers. Chest 2017, 151, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Hye Khan, M.A.; Hwang, S.H.; Sharma, A.; Corbett, J.A.; Hammock, B.D.; Imig, J.D. A dual COX-2/sEH inhibitor improves the metabolic profile and reduces kidney injury in Zucker diabetic fatty rat. Prostaglandins Other Lipid Mediat. 2016, 125, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Swardfager, W.; Hennebelle, M.; Yu, D.; Hammock, B.; Levitt, A.; Hashimoto, K.; Taha, A. Metabolic/inflammatory/vascular comorbidity in psychiatric disorders; soluble epoxide hydrolase (sEH) as a possible new target. Neurosci. Biobehav. Rev. 2018, 87, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.P.; Yang, S.H.; Kim, D.K.; Lee, H.; Kim, B.; Cho, J.-Y.; Yu, K.-S.; Paik, J.H.; Kim, M.; Lim, C.S.; et al. In vivo activity of epoxide hydrolase according to sequence variation affects the progression of human IgA nephropathy. Am. J. Physiol. Renal Physiol. 2011, 300, F1283–F1290. [Google Scholar] [CrossRef]
- Shih, P.B.; Yang, J.; Morisseau, C.; German, J.B.; Zeeland, A.A.S.-V.; Armando, A.M.; Quehenberger, O.; Bergen, A.; Magistretti, P.J.; Berrettini, W.; et al. Dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol. Psychiatry 2016, 21, 537–546. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, D.; Dou, X.; Niu, N.; Huang, W.; Bai, J.; Zhang, G. Elevated 14,15-epoxyeicosatrienoic acid by increasing of cytochrome P450 2C8, 2C9 and 2J2 and decreasing of soluble epoxide hydrolase associated with aggressiveness of human breast cancer. BMC Cancer 2014, 14, 841. [Google Scholar] [CrossRef]
- Theken, K.N.; Schuck, R.N.; Edin, M.L.; Tran, B.; Ellis, K.; Bass, A.; Lih, F.B.; Tomer, K.B.; Poloyac, S.M.; Wu, M.C.; et al. Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis 2012, 222, 530–536. [Google Scholar] [CrossRef]
- Kondo, K.; Morino, K.; Nishio, Y.; Kondo, M.; Nakao, K.; Nakagawa, F.; Ishikado, A.; Sekine, O.; Yoshizaki, T.; Kashiwagi, A.; et al. A fish-based diet intervention improves endothelial function in postmenopausal women with type 2 diabetes mellitus: A randomized crossover trial. Metabolism 2014, 63, 930–940. [Google Scholar] [CrossRef]
- Oh, Y.T.; Oh, H.H.; Nguyen, A.-K.; Choi, C.S.; Youn, J.H. Circulating free fatty acids inhibit food intake in an oleate-specific manner in rats. Physiol. Behav. 2016, 167, 194–201. [Google Scholar] [CrossRef]
- Oh, Y.T.; Kim, J.; Kang, I.; Youn, J.H. Regulation of hypothalamic-pituitary-adrenal Axis by circulating free fatty acids in male Wistar rats: Role of individual free fatty acids. Endocrinology 2014, 155, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Stefanovski, D.; Shih, P.-A.B.; Hammock, B.D.; Watanabe, R.M.; Youn, J.H. Assessment of soluble epoxide hydrolase activity in vivo: A metabolomic approach. Prostaglandins Other Lipid Mediat. 2020, 148, 106410. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Oh, Y.T.; Wan, D.; Watanabe, R.M.; Hammock, B.D.; Youn, J.H. Postprandial effect to decrease soluble epoxide hydrolase activity: Roles of insulin and gut microbiota. J. Nutr. Biochem. 2017, 49, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Edin, M.L.; Vendrov, K.C.; Schuck, R.N.; Lih, F.B.; Jat, J.L.; Bradbury, J.A.; DeGraff, L.M.; Hua, K.; Tomer, K.B.; et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J. Lipid Res. 2014, 55, 2124–2136. [Google Scholar] [CrossRef]
- Abraham, N.G.; Sodhi, K.; Silvis, A.M.; Vanella, L.; Favero, G.; Rezzani, R.; Lee, C.; Zeldin, D.C.; Schwartzman, M.L. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension 2014, 64, 1352–1361. [Google Scholar] [CrossRef]
- Bettaieb, A.; Nagata, N.; AbouBechara, D.; Chahed, S.; Morisseau, C.; Hammock, B.D.; Haj, F.G. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J. Biol. Chem. 2013, 288, 14189–14199. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, H.; Li, D.; Pang, W.; Hammock, B.D.; Zhu, Y. Inhibition of soluble epoxide hydrolase attenuates high-fat-diet–induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS ONE 2012, 7, e39165. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Massey, K.; Watson, M.A.; Hussain, T.; Volpe, G.; Buckley, C.D.; Nicolaou, A.; Badenhorst, P. Oxidised metabolites of the omega-6 fatty acid linoleic acid activate dFOXO. Life Sci. Alliance 2020, 3, e201900356. [Google Scholar] [CrossRef]
- Hamilton, J.S.; Klett, E.L. Linoleic acid and the regulation of glucose homeostasis: A review of the evidence. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102366. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Virk, R.; Van Dyke, T.E. Potential Mechanisms by Which Hydroxyeicosapentaenoic Acids Regulate Glucose Homeostasis in Obesity. Adv. Nutr. 2022, 13, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Yamada, H.; Hirose, M. Euphausia pacifica (North Pacific Krill): Review of Chemical Features and Potential Benefits of 8-HEPE against Metabolic Syndrome, Dyslipidemia, NAFLD, and Atherosclerosis. Nutrients 2021, 13, 3765. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.T.; Stevenson, B.E.; Chester, M.W.; Basit, M.; Daniels, M.B.; Turley, S.D.; McGarry, J.D. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Investig. 1997, 100, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Schmelzer, K.; Georgi, K.; Hammock, B.D. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Chem. 2009, 81, 8085–8093. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.M.; Yang, J.; Georgi, K.; Hegedus, C.; Nording, M.L.; O’Sullivan, A.; German, J.B.; Hogg, R.J.; Weiss, R.H.; Bay, C.; et al. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics 2012, 8, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Morisseau, C.; Hammock, B.D. Measurement of soluble epoxide hydrolase (sEH) activity. Curr. Protoc. Toxicol. 2007, 33, 4–23. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Morisseau, C.; Gee, S.J.; Bever, C.S.; Liu, X.; Wu, J.; Hammock, B.D.; Ying, Y. Nanobody Based Immunoassay for Human Soluble Epoxide Hydrolase Detection Using Polymeric Horseradish Peroxidase (PolyHRP) for Signal Enhancement: The Rediscovery of PolyHRP? Anal. Chem. 2017, 89, 6248–6256. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.T.; Yang, J.; Morisseau, C.; He, Q.; Hammock, B.; Youn, J.H. Effects of Individual Circulating FFAs on Plasma and Hepatic FFA Epoxides, Diols, and Epoxide-Diol Ratios as Indices of Soluble Epoxide Hydrolase Activity. Int. J. Mol. Sci. 2023, 24, 10760. https://doi.org/10.3390/ijms241310760
Oh YT, Yang J, Morisseau C, He Q, Hammock B, Youn JH. Effects of Individual Circulating FFAs on Plasma and Hepatic FFA Epoxides, Diols, and Epoxide-Diol Ratios as Indices of Soluble Epoxide Hydrolase Activity. International Journal of Molecular Sciences. 2023; 24(13):10760. https://doi.org/10.3390/ijms241310760
Chicago/Turabian StyleOh, Young Taek, Jun Yang, Christophe Morisseau, Qiyi He, Bruce Hammock, and Jang H. Youn. 2023. "Effects of Individual Circulating FFAs on Plasma and Hepatic FFA Epoxides, Diols, and Epoxide-Diol Ratios as Indices of Soluble Epoxide Hydrolase Activity" International Journal of Molecular Sciences 24, no. 13: 10760. https://doi.org/10.3390/ijms241310760
APA StyleOh, Y. T., Yang, J., Morisseau, C., He, Q., Hammock, B., & Youn, J. H. (2023). Effects of Individual Circulating FFAs on Plasma and Hepatic FFA Epoxides, Diols, and Epoxide-Diol Ratios as Indices of Soluble Epoxide Hydrolase Activity. International Journal of Molecular Sciences, 24(13), 10760. https://doi.org/10.3390/ijms241310760






