Abstract
There is an increasing interest in understanding the connection between the immune and cardiovascular systems, which are highly integrated and communicate through finely regulated cross-talking mechanisms. Recent evidence has demonstrated that the immune system does indeed have a key role in the response to cardiac injury and in cardiac regeneration. Among the immune cells, macrophages appear to have a prominent role in this context, with different subtypes described so far that each have a specific influence on cardiac remodeling and repair. Similarly, there are significant differences in how the innate and adaptive immune systems affect the response to cardiac damage. Understanding all these mechanisms may have relevant clinical implications. Several studies have already demonstrated that stem cell-based therapies support myocardial repair. However, the exact role that cardiac macrophages and their modulation may have in this setting is still unclear. The current need to decipher the dual role of immunity in boosting both heart injury and repair is due, at least for a significant part, to unresolved questions related to the complexity of cardiac macrophage phenotypes. The aim of this review is to provide an overview on the role of the immune system, and of macrophages in particular, in the response to cardiac injury and to outline, through the modulation of the immune response, potential novel therapeutic strategies for cardiac regeneration.
1. Introduction
Cardiovascular diseases (CVDs) represent the leading cause of death worldwide, accounting for about 31% of all deaths [1]. The recent technological and therapeutical advances, along with a better understanding of the pathophysiological mechanisms involved in several CVDs, have led to the increased survival rates of these patients. This is however counterbalanced by an increased number of patients suffering from the sequalae of an acute cardiovascular event. For instance, patients with a previous acute coronary syndrome are still at risk of developing heart failure (HF) due to the presence of reparative fibrosis and consequent adverse cardiac remodeling with impaired function [2]. The adult heart harbors multiple heterogeneous cellular components, including cardiomyocytes (CMs), fibroblasts, smooth muscle cells, endothelial cells, cardiac stem cells, pericytes, and a plethora of immune cells [3], as shown in Figure 1. The latter are actively involved in the inflammatory response that follows cardiac injury in attempts to antagonize and repair myocardial damage and restore cardiac homeostasis. The goal is the clearance of the fibrotic tissue with the initiation of a reparative cascade preventing adverse myocardial tissue remodeling. Among all the innate immune cells, macrophages are specifically involved in the onset and resolution of inflammation. Their dysregulation is a primary contributor to tissue inflammaging, a pro-inflammatory status associated with high levels of pro-inflammatory markers. Tissue-resident C-C chemokine receptor 2 (CCR2−) and tissue-resident/systemically recruited CCR2+ cardiac macrophages differentially affect cardiac remodeling and repair following myocardial injury [4] (Figure 1). An exhaustive comprehension of the different responses induced by the two subsets of cells is key for the development of new therapeutic strategies to prevent fibrosis and adverse remodeling and promote the formation of new functional myocardium. The intrinsic regenerative potential of the adult heart after an injury is in fact significantly limited, at least in response to ischemic damage [5,6]. It has been demonstrated that this endogenous potential can be fostered after an ischemic injury using several approaches, from the administration of exogenous cell therapy to RNA therapeutics [7,8,9]. However, this reparative response may be further reduced by the concomitant presence of cardiovascular risk factors (i.e., aging or diabetes) that create an adverse cellular microenvironment halting regeneration [7,10,11,12,13,14,15,16]. In this scenario, cell therapy was intended as an alternative strategy to restore/replace the damaged and dysfunctional cardiac tissue to improve cardiac function [7,17,18]. The immune response and the endogenous cardiac repair system interact to modulate damage resulting from inflammatory response. It is still an open question however as to whether specific aspects of the immune/inflammatory response are responsible for a predominantly fibrotic and poorly regenerative response to injury in the adult heart and/or whether their modulation could positively affect the regenerative response. The aim of this review is to provide a comprehensive analysis about the interaction between the immune response and the cardiac reparative/regenerative process, focusing on the specific role of the cells involved in this tangle.
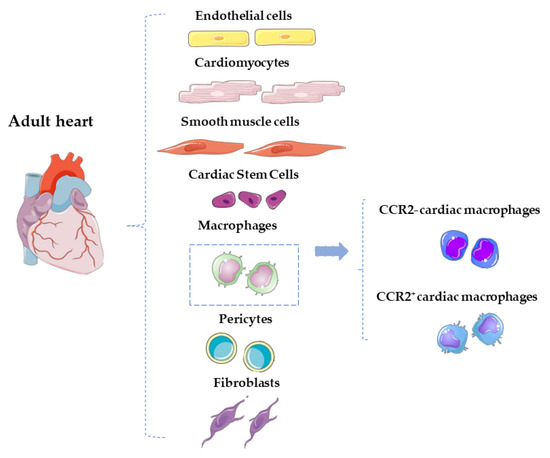
Figure 1.
Schematic representation of the main cardiac cellular component of the adult heart. Among the immune cells, macrophages can be distinguished in CCR2+ and CCR2– cardiac macrophages.
2. Innate Immunomodulation after Injury
Insults that induce cardiomyocyte death promote the activation of the immune response to restore tissue integrity [19]. The immune system is involved in damage-associated signaling, inflammation, revascularization, and fibrotic scar formation [19]. Macrophages play a key role in all the stages of the immune response, with distinct phenotypes performing specific functions at different time points [20]. It is known that macrophages derived from monocytes can be further classified into two main types: the pro-inflammatory M1 type and the resolving M2 type [21]. However, it is also widely accepted that the M1/M2 paradigm is just a simplification, and the exact sources and phenotypes of macrophages are yet to be fully clarified. In addition, it still remains unclear if M1 macrophages can switch to an M2 type, representing a mixed phenotype, or if these two subsets of macrophages necessarily originate from completely different sources [22]. Nevertheless, it has been established that the cellular response to heart damage can be divided into three distinct phases: the inflammatory, proliferative, and resolutive phases (Figure 2) [23].
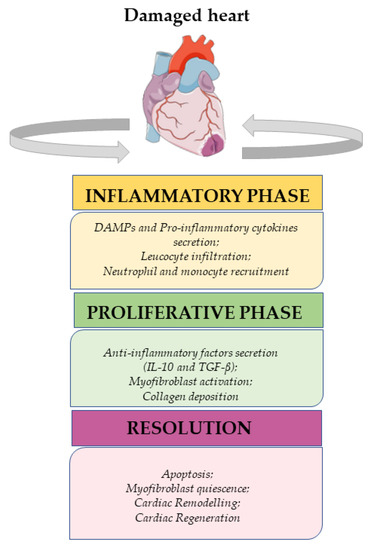
Figure 2.
From inflammation to resolution signaling after cardiac damage.
2.1. Inflammatory Phase
Cardiomyocyte death and the subsequent release of intracellular components into the extracellular compartment trigger the inflammatory response, attracting resident immune cells. These components, known also as Damage-Associated Molecular Patterns (DAMPs), include nucleic acid fragments, heat shock proteins, adenosine triphosphate (ATP), and fragmented extracellular matrix (ECM) components. They primarily activate the innate immune pathways and the inflammatory response through Toll-like receptors (TLRs) and NOD-like receptors (NLRs), which are expressed on both cardiomyocytes (CMs) and resident immune cells [24,25]. This leads to the release of specific pro-inflammatory cytokines, particularly Interleukin (IL)-1β and IL-18 [26].
Furthermore, DAMPs activate the complement system, which recognizes and subsequently destroys damaged cells through phagocytosis [27]. Uncontrolled activation and amplification of the complement cascade can be detrimental, resulting in significant tissue damage. However, the neutralization of complement can reduce myocardial injury and mortality in patients with myocardial infarction (MI) [28].
After an injury, vascular endothelial cells increase the expression of endothelial intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), while histamine released by mast cells increases vascular permeability. Both of these phenomena contribute to facilitating leucocyte infiltration. Consequently, multiple cellular effectors such as neutrophils and monocytes are attracted to the sites of damage. Neutrophils are the first to migrate, followed by monocytes, which subsequently differentiate into M1-type macrophages. The reactive oxygen species (ROS) generated by activated neutrophils [29] promote the infiltration and proliferation of monocytes, dendritic cells, natural killer (NK) cells, T helper cell type 1 (Th1), T helper cell type 17 (Th17), B-lymphocytes, and additional neutrophils [30]. Additionally, neutrophils serve as a source of matrix-degrading enzymes and are responsible for the phagocytosis of the degraded matrix components and cells coated in complement opsonin [31].
Neutrophil activity can also contribute to exacerbating injury. The secretion of myeloperoxidase (MPO) by neutrophils, in fact, leads to maladaptive cardiac remodeling after injury. For instance, deletion of MPO in adult wild-type (WT) mice results in decreased left ventricular (LV) dilatation and a significant improvement in LV function compared to the control group [32]. Furthermore, the depletion of endothelial Brahma-related gene 1 (Brg1), which mediates neutrophil–endothelium adhesion, results in decreased ventricular fibrosis, reduced infarct size, and better recovery of cardiac function [33]. Inhibition of certain neutrophil-derived enzymes and the reduction of neutrophil infiltration achieved through antibody-mediated blockage of specific adhesion molecules have been shown to decrease tissue damage following MI and reperfusion [34]. Monocytes represent the second type of immune cells involved in the inflammatory phase. Interferon (IFN)-γ, Tumor Necrosis Factor (TNF)-α, and DAMPs promote monocyte recruitment and their differentiation into M1-type macrophages, which are inflammatory macrophages. These M1 macrophages, in turn, release other pro-inflammatory factors such as TNF-α, IL-1β, chemokine C-X-C motif ligand 10 (CXCL10), IL-6, IL-12, and IL-23 [35,36]. As with neutrophils, monocytes also release matrix metallopeptidase 9 (MMP-9) that degrades basement membranes, thereby facilitating the recruitment of additional immune cells [37].
2.2. Proliferative Phase
The inflammatory phase ends when M1 macrophages phagocytose neutrophils [38]. At this stage, M1 macrophages decrease their production of pro-inflammatory factors and begin to increase the secretion of two anti-inflammatory factors, namely IL-10 and transforming growth factor beta (TGF-β). This represents a signal of a shift towards the M2 phenotype. It is important to note that macrophages likely exist along a spectrum of mixed phenotypes [39] and exhibit plasticity [40]; thus, the M1/M2 paradigm may be an oversimplified and inaccurate classification. Analysis using single-cell RNA sequencing (sc-RNA seq) has revealed the presence of distinct macrophage phenotypes associated with regenerative and fibrotic processes [41]. Regardless, macrophages operating at this stage promote angiogenesis by secreting IL-10, TGF-β, and vascular endothelial growth factor (VEGF) [42], as well as activate fibroblasts [43]. TGF-β stimulates newly formed myofibroblasts to secrete collagen (predominantly type III), fibronectin, and other extracellular components [44]. Additionally, macrophages regulate matrix turnover through the modulation of MMPs and tissue inhibitors of metallopeptidases (TIMPs) [45]. As a result of all these processes, a temporary collagenous matrix enriched in fibrin and fibronectin is formed [44,45].
2.3. Resolutive Phase
The final phase is the resolutive phase, during which a remodeling process occurs involving the replacement of newly laid type III collagen with type I collagen. Type I collagen becomes cross-linked, resulting in a stronger scar with increased tensile strength. The matrix becomes less populated with cells as most of the remaining leukocytes undergo apoptosis. However, some myofibroblasts may persist, and their presence can have a negative impact on cardiac function due to their distinct electrical properties compared to CMs [46].
The exact mechanisms that trigger this phase are not yet fully understood. Typically, the described events progress through a series of cascade steps involving the reduction of pro-inflammatory factors, cessation of granulocyte recruitment, and an increase in monocyte levels. Monocyte-derived macrophages play a crucial role in removing inflammatory cells and tissue debris, leading to the resolution of inflammation and promotion of the recovery of cardiac structure and contractile function [47,48].
3. Adaptive Immune Response
In addition to the innate immune response, the release of DAMPs triggers an adaptive immune response, which involves the activation of B-lymphocytes and T-lymphocytes. Moreover, in the presence of an inflammatory environment, cardiac self-antigens such as myosin and troponin can disrupt the tolerance mechanism, leading to the activation of long-lived antigen-specific adaptive immune cells [49,50]. While the innate immune response is regulated by negative feedback mechanisms aimed at resolving early inflammation, the adaptive immune response does not appear to be controlled in the same manner. The release of substantial amounts of self-antigens can ultimately result in autoimmune tissue damage, leading to the subsequent release of more self-antigens.
The precise role of B-lymphocytes in this phase is still not fully understood, although there is evidence suggesting their involvement in the immune response to cardiac injury. Mice with depleted B-lymphocytes have shown improved cardiac function after MI [51]. Furthermore, activated B-lymphocytes produce pro-inflammatory cytokines such as TNF-α which directly contribute to myocardial dysfunction by reducing contractility, inducing myocyte apoptosis [52], and promoting fibroblast differentiation into myofibroblasts [53]. Similarly, the exact function of T-lymphocytes in response to cardiac injury has not been completely elucidated. The rapid changes in the local environment following an insult lead to the emergence of a heterogeneous subpopulation of T-lymphocytes which, depending on the timing and type of cardiac damage, have varying effects that can either have a positive or detrimental impact on the healing process [54]. Specifically, regulatory T-lymphocytes appear to play a beneficial role in the immediate post-MI phase [55,56]. Conversely, it has been demonstrated that CD4+ T cells, but not CD8+ T cells, contribute to myocardial ischemia–reperfusion injury through the release of IFN-γ [54].
Indeed, in the context of cardiac injury, the adaptive immune response can have an overall negative effect, potentially leading to further tissue damage. The continuous exposure to self-antigens can trigger persistent immune autoreactivity that may eventually involve previously unaffected cardiac regions of the heart. This process can contribute to negative remodeling and LV dilation.
4. Distinct Cardiac Macrophage Subsets among the Adult Heart
The adult mammalian heart harbors heterogeneous populations of macrophages, each originating from distinct developmental pathways [57,58]. Macrophages serve as resident immune cells in the tissue and play a crucial role in maintaining tissue homeostasis [59]. Following an injury, the recruitment of specific macrophage subsets influences the cellular microenvironment and the resulting response to cardiac injury. These macrophage subsets can either contribute to maladaptive remodeling through a pro-inflammatory response [60] or promote reparative processes, including cardiac regeneration [21,61]. As mentioned earlier, macrophages are typically classified into two types: M1 and M2. M1-type macrophages are classically activated by factors such as IFN-γ and lipopolysaccharide (LPS), leading to a pro-inflammatory response [62]. On the other hand, M2-type macrophages are alternatively activated in response to cytokines such as IL-4 and IL-13, exhibiting a resolving phenotype in vitro [63].
However, recent experiments utilizing genetic lineage tracing and fate mapping have challenged the simplistic classification of macrophages into M1 and M2 types. These findings suggest that the polarization process of macrophages may be more complex and dynamic than previously thought, and the observed markers on in vitro-generated macrophages may not accurately reflect the phenotype of macrophages in vivo, particularly in classically activated mice models [64]. Furthermore, it is important to differentiate between macrophages derived from circulating monocytes and tissue-resident macrophages, which exhibit tissue-specific features [57,58]. Tissue-resident macrophages originate from the yolk sac or fetal monocyte progenitors [65], and they are ontogenetically older than macrophages derived from the bone marrow [66]. These tissue-resident macrophages are evolutionarily conserved throughout the lifespan [67]. In the murine heart, resident cardiac macrophages constitute around 5–10% of the non-myocyte population, and their percentage increases following cardiac damage [68]. Within cardiac tissue, at least two distinct macrophage subsets and one monocyte subset have been identified based on the presence or absence of the CCR2 receptor on their surface. CCR2 negative (CCR2−) cells originate from yolk sac progenitors and are detected in the cardiac tissue around embryonic day 12.5 (E12.5) [58]. These cells are primarily located within the myocardial wall and in close proximity to the coronary vasculature. On the other hand, CCR2 positive (CCR2+) cells are derived from fetal monocyte progenitors and can be found in the trabecular projection of the endocardium starting from E14.5 [58]. CCR2− cells, also known as a “resident macrophage population”, are self-regenerating macrophages that do not require prior monocyte recruitment. CCR2+ cells, derived from hematopoiesis, are maintained through monocyte recruitment and are referred to as the “non-resident macrophage” population derived from circulating monocytes [69]. Although cardiac macrophages can exhibit M1 or M2 phenotypes in response to various stimuli, it is important to note that this phenotypic expression may not be permanent. Studies have shown that cardiac macrophages, primarily exhibiting an M2-like phenotype, can transition to an M1-like phenotype in aged mice [70]. Moreover, tissue-specific gene expression in cardiac macrophages can be significantly altered in response to injuries such as MI, stroke, or sepsis [70].
In mice, the expression of CCR2, major histocompatibility complex (MHC) class II, and CD11c allows for further differentiation into three subtypes of cardiac macrophages [57]. There are two predominant CCR2− populations: MHCIIhigh/CD11clow and MHCIIlow/CD11clow. These subsets are derived from yolk sac progenitors and are renewed through in situ proliferation. Additionally, there is a CCR2+, MHCIIhigh, and CD11chigh subset, which is slowly replaced by circulating monocytes. Furthermore, there is one monocyte subset characterized by CCR2+/MHC-IIlow expression. Transcriptomic analysis of these subsets reveals both overlapping and non-overlapping functions. CCR2+ macrophages exhibit a significant number of genes involved in the inflammatory process, suggesting pro-inflammatory activity [57,58,71]. The MHCIIhigh subsets have genes involved in antigen presentation to T cells, indicating a potential role in immune surveillance [57,58,71]. The CCR2−/MHCIIlow subset has demonstrated uptake of apoptotic/necrotic cells, indicating a role in the clearance of dead cells and prevention of immune response [71]. It is important to note that these subsets represent only a part of the cardiac macrophages described in the literature. Additional studies have identified four distinct cardiac macrophage clusters with unique functions at steady state [72]. For example, CCR2−/TIMD4+/LYVE1+/MHCIIlow corresponds to the CCR2−/MHCIIlow subset and expresses genes involved in homeostasis and regeneration [58]. Similar CCR2 macrophage subsets have also been identified in the human heart, suggesting comparable functions [73]. In the human heart, distinct subsets of monocytes and macrophages can be identified based on the expression of CCR2 and HLA-DR [73]. Human cardiac macrophages are characterized by the co-expression of CD14, CD45, and CD64 markers. Within the CD14+/CD45+/CD64+ population, three subsets can be distinguished based on the expression of the human homologue of MHC-II (HLA-DR) and CCR2: CCR2+HLA-DRlow, CCR2+HLA-DRhigh, and CCR2−HLA-DRhigh cells [73]. It is important to note that there are differences between mouse and human macrophages. In mice, CCR2− macrophages are divided into MHCIIlow and MHCIIhigh subsets, while in humans they are predominantly HLA-DRhigh.
5. Cardiac Macrophage Recruitment following Myocardial Injury
In the response to cardiac injury, both cardiac and non-cardiac macrophages play a role. Following acute MI in mice, there is a significant reduction of approximately 60% in the number of resident CCR2− macrophages in the infarcted area within 2 days of the event [74]. These resident macrophages are replaced by inflammatory CCR2+ monocytes and monocyte-derived macrophages. The role of these monocyte-derived macrophages is to promote monocyte recruitment through the production of C-C motif ligand 2 (CCL2), overcoming the inhibitory effect exerted by resident CCR2− macrophages. While these monocyte-derived macrophages have a pro-inflammatory function, their production of inflammatory factors is generally lower than that of recruited CCR2+ macrophages [4]. To differentiate cardiac CCR2+ macrophages from circulating monocytes and monocytes-derived macrophages, the expression of type I IFN-stimulated genes can be used. This suggests that CCR2+ macrophages are responsive to type I IFN produced during myocardial infarction.
In murine models, circulating monocytes consist of two subsets: classical pro-inflammatory monocytes expressing high levels of lymphocyte antigen 6 complex (Ly6C) (or CD14high/CD16− in humans) that are recruited to sites of inflammation and non-classical Ly6Clow monocytes (or CD14low/CD16+ in humans) that survey the luminal surface of vascular endothelial cells [75,76,77].
Indeed, both Ly6Chigh and Ly6Clow monocytes participate in the immune response observed in the infarcted heart, representing distinct phases of monocytes recruitment [75,78]. During the initial inflammatory phase, there is a peak in the recruitment of Ly6Chigh monocytes observed at around 3 days post infarction, followed by a gradual decline. These Ly6Chigh monocytes express CCR2 and migrate into the injured site in response to the chemokine CCL2. Upon migration, they differentiate into recruited CCR2+ macrophages. CCL2 plays a crucial role in the recruitment of the Ly6Chigh monocyte into the infarcted area, and CCL2-deficient (CCL2−/−) mice have shown reduced monocyte infiltration, interstitial fibrosis, and ventricular dysfunction in response to myocardial ischemia compared to WT mice [79]. Once recruited, CCR2+ macrophages contribute to the immune response by releasing pro-inflammatory factors and matrix metalloproteinases that facilitate the degradation of the extracellular matrix and removal of debris and necrotic cells. Therefore, a reduction in circulating monocytes can result in the accumulation of uncleared debris, necrotic tissue, and myocardial fibrosis [75].
Ly6Clow monocytes appear later in the response to cardiac injury, with a peak observed at around day 7 post injury, and they represent a significant portion (approximately 75%) of the total macrophage population by day 16. These Ly6Clow monocytes give rise to reparative and non-inflammatory macrophages. Ly6Clow monocytes express high levels of growth factors such as VEGF [75], which are involved in promoting myofibroblast accumulation, angiogenesis, and collagen deposition [78]. Previous studies have proven that two different pathways are responsible for the accumulation of Ly6Clow macrophages in the damaged area [75]. One pathway is related to the expression of C-X3-C Motif Chemokine Receptor 1 (CX3CR1) in the injured zone. Additionally, it is possible that Ly6Chigh monocytes directly differentiate into proliferating Ly6Clow macrophages within the myocardium, driven by the induction of the orphan nuclear receptor Nr4a1 [78]. Depletion of Nr4a1 results in increased expression levels of CCR2 among cardiac Ly6Chigh monocytes, leading to the induction of macrophages with high pro-inflammatory activity. The lack of Nr4a1 has also been associated with impaired LV function after myocardial infarction, limited cardiac healing, enhanced myocardial scar size, and reduced collagen density [78]. Therefore, Nr4a1 plays a crucial role in regulating the differentiation and function of Ly6Clow macrophages in the context of cardiac injury.
6. Macrophages and Cardiac Tissue Regeneration
The debate regarding cardiac regeneration and the existence and role of endogenous resident cardiac stem cells (CSCs) is indeed ongoing. While the regenerative capacity of skeletal muscle mediated by satellite cells is well established, the presence and phenotypic characterization of CSCs in the heart are still subjects of investigation [15]. In skeletal muscle regeneration, the process begins with an inflammatory response triggered by an insult. This leads to the activation, differentiation, and fusion of satellite cells, including muscle stem cells. These activated satellite cells contribute to the growth and remodeling of newly formed myofibers. Interestingly, a small portion of myogenic precursor cells does not undergo terminal differentiation, instead remaining as a pool of stem cells that can be utilized for future regeneration if needed [80]. It has been demonstrated that the complete elimination of the satellite cell pool, specifically all Pax7+ cells, in adult skeletal muscle suppresses muscle regeneration entirely [81].
Indeed, macrophages have been implicated in the fate of skeletal muscle satellite stem cells during regeneration [82]. While the exact role of resident macrophages in this context is not yet fully understood, studies have shown their involvement in regulating tissue homeostasis under normal conditions. However, they appear to have limited phagocytic capacity during injury and instead act as sentinels, becoming activated in response to DAMPs and promoting the recruitment of circulating leukocytes [83]. Similar to the observations in cardiac injury, the recruitment of monocytes/macrophages in skeletal muscle regeneration follows a sequential pattern [84,85]. The pro-inflammatory Ly6Chigh monocytes are the first population recruited during the acute phase of inflammation, while the anti-inflammatory Ly6Clow subset appears later [86]. It has been demonstrated that Ly6Clow macrophages can arise from the Ly6Chigh subset [84,85], as evidenced by an in vivo experiment showing a nearly complete transition to the Ly6Clow phenotype by day 3 after acute injury [85]. The sequential presence of Ly6Chigh and Ly6Clow macrophages is associated with specific events in the regenerative process [87]. One study demonstrated numerous regenerating areas seven days after an injury, characterized by the presence of proliferating cells (ki67+/CD56+) and/or differentiating cells (myogenin+) [82]. These areas also exhibited a positive presence of both pro-inflammatory macrophages (identified by the expression of iNOS and COX2 in CD68pos cells) and anti-inflammatory macrophages (marked with CD206 and CD163). Specifically, pro-inflammatory markers were commonly expressed by macrophages in regenerating areas containing only myogenin− cells compared to those containing at least one myogenin+. Conversely, Arg1 macrophages (another anti-inflammatory marker) were more abundant in regenerating areas containing myogenin+-proliferating myogenic precursor cells (MPCs) compared to those lacking differentiating MPCs. Collectively, these findings suggest a preferential association between proliferating MPCs and macrophages expressing pro-inflammatory markers, while regenerating areas with differentiating myogenin+ MPCs tend to be associated with anti-inflammatory macrophages.
There is growing interest in research focused on promoting cardiac regeneration through stem cell transplantation or the induction of endogenous CSCs [88,89]. While pre-clinical and clinical trials have reported beneficial effects of stem cell therapy on infarcted hearts, there are currently limited data regarding stem cell survival after transplantation and their ability to generate new functional myocytes [90]. It has been suggested that the pro-inflammatory environment following cardiac injury can lead to damage to transplanted stem cells, primarily through the stimulation of apoptosis, necrosis, and autophagy cascades [91,92]. Additionally, the pro-inflammatory cytokines present in this environment may contribute to the failure of stem cells to commit to the cardiac lineage [93]. However, it appears that the functional benefits observed with injected cell therapy are related to an acute inflammation-based wound-healing response [94]. Vagnozzi et al. conducted experiments on healthy mice to assess the effects of two types of primary adult cells, both of which contained small fractions of true stem cells: fractionated bone marrow mononuclear cells (MNCs) and cardiac mesenchymal cells. These cells were administered either as living cells or as dead cells, previously killed through a freeze–thaw cycle. In their findings, Vagnozzi et al. observed a temporary and regional induction of CCR2+ and CX3CR1+ macrophages following the injection of cells, regardless of cell type or viability. This led to a shift in the composition of macrophage subsets, transitioning from a predominant population of CX3CR1+/CCR2− macrophages in the naive state to a mix of CCR2+ and CCR2+/CX3CR1+ macrophages. The experiment was also conducted on mice with induced myocardial infarction one week after ischemia–reperfusion (I-R) injury. Injection of mononuclear cells, cardiac mesenchymal cells, or zymosan (a yeast-derived protein–carbohydrate complex capable of inducing sterile inflammation) into the infarct border zone improved LV contractility and decreased end-systolic volume. These beneficial effects were associated with the selective activation of innate immune responses. Importantly, the positive effects of cell injection on infarcted mice were abolished when a broad spectrum of immunosuppressant agents or macrophage depletion was co-administered. However, the experimental design employed by Vagnozzi et al. did not allow for the detection of new cardiomyocyte formation [94]. However, other studies have demonstrated the generation of new cardiomyocytes following injury [23,95]. For instance, an acute cardiomyocyte loss caused by an isoproterenol overdose (ISO) activates the resident cardiac c-kit+ stem/progenitor cells (CSCs), leading to the generation of new cardiomyocytes that replace those lost after ISO-induced damage [23].
Similar to what is observed in skeletal muscle, macrophages are also known to be involved in cardiac regeneration in neonatal mice, which exhibit regenerative potential and can fully regenerate following MI [19,61,96]. Cardiac macrophage subsets in mice differ between adults and neonatal mice. Neonatal hearts host only one embryonal-derived macrophage subset of CCR2−/MHC-IIlow and one monocyte CCR2+ population. In response to injury, neonatal mice selectively expand CCR2−/MHC-IIlow macrophages without recruiting additional CCR2+ monocytes. These CCR2− macrophages isolated from injured neonatal hearts produce lower levels of pro-inflammatory mediators. In contrast, in adult mice hearts, the CCR2−/MHC-IIlow population is rapidly lost and replaced by pro-inflammatory monocytes and monocyte-derived macrophages expressing CCR2+/MHC-IIhigh, characterized by limited capacity to promote cardiac repair and to generate inflammation or oxidative stress [21] (Figure 3). Assessing changes in epigenetic regulation through cardiomyocyte development has been of interest because of the drastic change in cardiomyocyte proliferation ability after the first few days of life [97]. Changes in epigenetics have proven to vary from neonatal proliferative-competent to adult terminally differentiated cardiomyocytes [97]. Furthermore, loss of heart regenerative capacity in adult versus neonatal mammals is triggered by increasing thyroid hormones and may be a trade-off for the acquisition of endothermy [98].
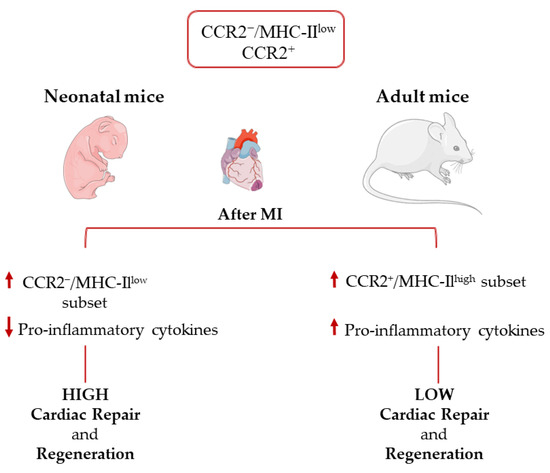
Figure 3.
Immune response in the neonatal and adult heart after myocardial infarction.
Finally, with aging, myocardial T cells undergo clonal expansion and exhibit an upregulated pro-inflammatory transcription signature, marked by increased IFN-γ production [99]. Physiological T-cell development or adoptive transfer of adult IFN-γ-producing T cells into neonatal infarcted mice shifted them toward an adult-like healing phenotype with monocyte-derived macrophage recruitment, contributing to impaired cardiac regeneration and promoting irreversible structural and functional cardiac damage [100]. These findings suggest a trade-off between myocardial regenerative potential and the development of T-cell competence and allow us to postulate that immunosenescence may account for the deficit in regenerative capability with age.
Aurora et al. conducted a study comparing the immune response of mice at different regenerative time points following MI and identified differences in the power and kinetics of the monocyte and macrophage response to injury [61]. Macrophages can secrete a plethora of soluble factors that may contribute to the formation of new cardiomyocytes [61]. Their depletion instead promotes fibrotic scar formation, resulting in reduced cardiac function and angiogenesis in neonatal mice.
Other experimental in vitro observations based on cell tracking strategies or tissue-specific gene depletion demonstrated the involvement of macrophages in skeletal muscle regeneration in both mice and human models [82]. It has been postulated that the recruitment of Ly6C+ monocytes/macrophages stimulates the quiescence niche of muscle stem cells (MuSCs), promoting their proliferation and preventing premature fusion of myogenic cells. It seems that during the resolutive phase, macrophages reduced inflammation levels while boosting stem cell angiogenesis, differentiation, and matrix remodeling [101].
Further studies are needed to determine whether the beneficial effects of cardiac macrophages on cardiac remodeling can contribute to enhancing the regenerative potential of CSCs. It is also important to investigate the potential link between macrophage activation/polarization and the fate of stem cells.
7. Macrophages and Tissue Degeneration during Aging
Aging is a complex phenomenon that involves several physiological changes as well as the immune system [102,103].
Cell senescence, although often used interchangeably with aging, is instead characterized by multiple hallmarks, including progressive accumulation of DNA damage, mitochondrial dysfunction, apoptosis, telomere shortening, oncogene activation or inactivation, epigenetic alterations, and ROS accumulation [104,105]. Senescent cells acquire a senescence-associated secretory phenotype (SASP) that involves the secretion of a wide range of soluble molecules, varying on the basis of the cell type and the triggering factor [10,16].
The combination of changes affecting the immune system during aging is known as immunosenescence and is characterized by the presence of a low-grade inflammation (inflammaging) that modulates macrophage activity and phenotype expression [106,107]. Interestingly, inflammaging is a common feature of different age-related diseases such as cardiovascular disease [16,108], type 2 diabetes mellitus, and diabetic cardiomyopathy [11,13,109], conditions all characterized by a loss of cardiac regenerative potential [10,11,13,14,16].
Aging can also negatively affect the ability to mitigate inflammation following a cardiac injury in murine models through the deregulation of certain metabolic pathways [110]. In aged mice, the clearance of senescent cells has been found to improve cardiac remodeling and function after myocardial infarction [7], which was also observed in animal models of diabetic cardiomyopathy [13]. Furthermore, aged hearts dramatically change the landscape of their leukocyte population with more monocyte-derived cardiac macrophages, though they are smaller in size and with larger granulocytes [111].
In addition, the SASP factors can shift macrophage polarization from an anti-inflammatory phenotype to a pro-inflammatory one [112]. This is in line with the linear increase in cardiac macrophages with a pro-inflammatory phenotype observed with aging [36], that may be a result of uncontrolled monocyte recruitment, alterations in monocyte fate determination, or changes in resident macrophage behavior [36].
Resident cardiac macrophages in fact exhibit a reduction in self-renewal ability that is maintained, at least in part, by the increased contribution of macrophages derived from circulating monocytes [111]. Similarly, macrophages that are implicated in skeletal muscle regeneration underwent significant changes during aging [113]. Their release of proliferative factors is in fact impaired, with consequences on satellite cell function and muscle regeneration [113]. Previously, Wang et al. demonstrated that the supernatant obtained from old bone marrow-derived macrophages (BMDMs), compared to that obtained from young BMDMs, has a reduced number of proliferative Ki67+ myoblasts [114]. However, the observed effects of aging on macrophages are widely influenced by the marker and the experimental model used, as well as by the subtype of population examined. For example, an increase in anti-inflammatory macrophages (marked as CD68+/CD163+) has been described in resting muscles of aged mice, correlating with an increase in skeletal muscle fibrosis [115,116]. Another study instead demonstrated a decrease in the number of both pro-inflammatory (CD11b+) and anti-inflammatory macrophages (CD163+) in old subjects compared to young controls (average 71.4 years vs. 31.9 years) [117].
8. Therapeutic Perspectives
All the evidence discussed so far seems to open new interesting therapeutical approaches based on the modulation of macrophage function. Several data indicate that a switch towards the cardioprotective anti-inflammatory phenotype can improve cardiac repair and function after injury [118,119,120].
In a rat model of acute MI, treatment with phosphatidylserine (PS)-presenting liposomes (mimicking the anti-inflammatory effects of apoptotic cells) induced increased release of anti-inflammatory cytokines such as TGFβ and IL-10, along with concomitant downregulation of the pro-inflammatory markers TNFα and CD86, in macrophages in both in vivo and in vitro models. This treatment supported angiogenesis and prevented ventricular dilatation and remodeling [118].
Additionally, some stem cell therapies also appear to have positive effects on recovery after myocardial damage through macrophage modulation. For example, bone marrow-derived mesenchymal stem cells (BM-MSCs) are able to modify their macrophage phenotype toward an M2-like status. Infarcted mice treated with BM-MSCs exhibited increased cardiac expression of F4/80 + CD206 + macrophages and demonstrated an improvement in cardiac function, as well as a reduction in pro-inflammatory factors and an increase in the expression of anti-inflammatory markers [119].
Similar results were obtained using cardiosphere-derived cells (CDCs) that are capable of secreting exosomes [120,121] enriched with specific small RNAs [122,123] and proteins [124] in response to cardiac injury. When delivered acutely post MI, these exosomes can polarize macrophages to a cardioprotective state, suppressing the expression of pro-inflammatory cytokines and promoting efferocytosis. In this context, at least under in vitro conditions, miR-181b seems to be the key mediator of this process [125].
Another promising therapeutic strategy may involve the use of human embryonic stem cell-derived cardiovascular progenitor cells (hESC-CVPCs). Recent data show that their use can induce a reparative phenotype in cardiac macrophages in infarcted hearts through a pathway involving signal transducer and activator of transcription 6 (STAT6). Injection of hESC-CVPCs into acutely infarcted myocardium significantly improves cardiac function and scar formation, reducing inflammatory response and cardiomyocyte apoptosis [114].
As already mentioned, the challenge when using stem cells for therapeutic purposes is mainly related to their limited survival after injection due to failed engraftment, necrosis, and apoptosis [94]. However, even these ungrafted cells may have a role in cardiac regeneration. It has been postulated that the apoptotic transplanted cells can inhibit macrophages and dendritic cells and stimulate regulatory T cells, resulting in the downregulation of both innate and adaptive immunity. The result is reduced fibrosis and an overall improved cardiac outcome [126].
Recent studies have also shown positive effects on cardiac function due to drugs commonly used in clinical practice that are mediated by the modulation of macrophage pro-inflammatory activity. Among these drugs, statins, inhibitors of the liver enzyme β-hydroxy β-methylglutaryl-coenzyme A (HMG-CoA) reductase commonly used in the treatment of hypercholesterolemia, have been found. This pharmacological class has also demonstrated anti-thrombotic and anti-inflammatory properties [127] and can stimulate new myocyte formation after MI [128]. Pravastatin inhibits IFN-γ-induced macrophage activation, while simvastatin interrupts MHC class II interactions between macrophages and the adaptive immune complex [129,130]. In addition, angiotension-converting enzyme inhibitors (ACEi) such as Enalapril have been shown to reduce angiotensin II (ATII)-stimulated monocyte recruitment from splenic reservoirs in a murine model, resulting in a 14% improvement in the ejection fraction following MI [131].
9. Conclusions
The available evidence suggests that specific immunity may significantly contribute to the different endogenous regenerative responses to injury in regenerative tissues, as opposed to the non-regenerative response of the adult mammalian heart. Nowadays, a great number of different immune cell types have been investigated in cardiac repair and myocardial remodeling after damage, and the main features of their phenotypes are summarized in Table 1. It remains to be established whether cardiac macrophages (recruited vs. resident) overall modulate cardiac pathologic remodeling after injury by preventing an effective regenerative response. Furthermore, it is unknown whether altering the type of cardiac macrophage response (switching from CCR2+ to CCR2− by eliminating CCR2+ macrophages) promotes effective myocardial regeneration after injury. These missing data could form the conceptual basis for therapeutic strategies that enhance cardiac tissue-resident CCR2−, inhibit CCR2+ macrophages, or achieve both; these strategies may also have the potential to achieve anatomical and functional myocardial regeneration after injury. Additionally, investing in this research topic will also help to clarify whether cardiac macrophage-dependent immunity activates the formation of new cardiomyocytes through CSC myogenic differentiation or through the unexpected duplication of pre-existing cardiomyocytes.

Table 1.
Immune cell systems involved in cardiac repair.
Author Contributions
Conceptualization, C.M., D.T. and F.M.; methodology, M.S., E.C., L.S. and J.S.; writing—original draft preparation, C.M., M.S., E.C., L.S. and N.S.; writing—review and editing, I.L., S.D.R., D.T. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Italian Ministry of University and Research (2017NKB2N4_005; 20203YAYGB_005; 2020L45ZWA_005), PON-AIM—1829805-2, PNRR-National Center for Gene Therapy and Drugs based on RNA Technology (CN00000041), and from the Italian Ministry of Health (PSC SALUTE 2014-2020, POS4 “Cal-Hub-Ria”, T4-AN-09).
Data Availability Statement
All data are available within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Smolina, K.; Wright, F.L.; Rayner, M.; Goldacre, M.J. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2019, 65, 70–99. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, G.; Bredemeyer, A.; Li, W.; Zaitsev, K.; Koenig, A.L.; Lokshina, I.; Mohan, J.; Ivey, B.; Hsiao, H.M.; Weinheimer, C.; et al. Tissue Resident CCR2− and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res. 2019, 124, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Nadal-Ginard, B.; Ellison, G.M.; Torella, D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Res. 2014, 13, 615–630. [Google Scholar] [CrossRef]
- Salerno, N.; Marino, F.; Scalise, M.; Salerno, L.; Molinaro, C.; Filardo, A.; Chiefalo, A.; Panuccio, G.; De Angelis, A.; Urbanek, K.; et al. Pharmacological clearance of senescent cells improves cardiac remodeling and function after myocardial infarction in female aged mice. Mech. Ageing Dev. 2022, 208, 111740. [Google Scholar] [CrossRef]
- Scalise, M.; Marino, F.; Salerno, L.; Mancuso, T.; Cappetta, D.; Barone, A.; Parrotta, E.I.; Torella, A.; Palumbo, D.; Veltri, P.; et al. In vitro CSC-derived cardiomyocytes exhibit the typical microRNA-mRNA blueprint of endogenous cardiomyocytes. Commun. Biol. 2021, 4, 1146. [Google Scholar] [CrossRef]
- Cianflone, E.; Scalise, M.; Marino, F.; Salerno, L.; Salerno, N.; Urbanek, K.; Torella, D. The negative regulation of gene expression by microRNAs as key driver of inducers and repressors of cardiomyocyte differentiation. Clin. Sci. 2022, 136, 1179–1203. [Google Scholar] [CrossRef]
- Marino, F.; Scalise, M.; Salerno, N.; Salerno, L.; Molinaro, C.; Cappetta, D.; Torella, M.; Greco, M.; Foti, D.; Sasso, F.C.; et al. Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age. Diabetes 2022, 71, 1081–1098. [Google Scholar] [CrossRef]
- Molinaro, C.; Salerno, L.; Marino, F.; Scalise, M.; Salerno, N.; Pagano, L.; De Angelis, A.; Cianflone, E.; Torella, D.; Urbanek, K. Unraveling and Targeting Myocardial Regeneration Deficit in Diabetes. Antioxidants 2022, 11, 208. [Google Scholar] [CrossRef]
- Torella, D.; Iaconetti, C.; Tarallo, R.; Marino, F.; Giurato, G.; Veneziano, C.; Aquila, I.; Scalise, M.; Mancuso, T.; Cianflone, E.; et al. miRNA Regulation of the Hyperproliferative Phenotype of Vascular Smooth Muscle Cells in Diabetes. Diabetes 2018, 67, 2554–2568. [Google Scholar] [CrossRef]
- Marino, F.; Salerno, N.; Scalise, M.; Salerno, L.; Torella, A.; Molinaro, C.; Chiefalo, A.; Filardo, A.; Siracusa, C.; Panuccio, G.; et al. Streptozotocin-Induced Type 1 and 2 Diabetes Mellitus Mouse Models Show Different Functional, Cellular and Molecular Patterns of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 1132. [Google Scholar] [CrossRef]
- Cianflone, E.; Torella, M.; Chimenti, C.; De Angelis, A.; Beltrami, A.P.; Urbanek, K.; Rota, M.; Torella, D. Adult Cardiac Stem Cell Aging: A Reversible Stochastic Phenomenon? Oxid. Med. Cell. Longev. 2019, 2019, 5813147. [Google Scholar] [CrossRef]
- Costamagna, D.; Berardi, E.; Ceccarelli, G.; Sampaolesi, M. Adult Stem Cells and Skeletal Muscle Regeneration. Curr. Gene Ther. 2015, 15, 348–363. [Google Scholar] [CrossRef]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting Cardiac Stem Cell Senescence to Treat Cardiac Aging and Disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef]
- Salerno, N.; Salerno, L.; Marino, F.; Scalise, M.; Chiefalo, A.; Panuccio, G.; De Angelis, A.; Cianflone, E.; Urbanek, K.; Torella, D. Myocardial regeneration protocols towards the routine clinical scenario: An unseemly path from bench to bedside. eClinicalMedicine 2022, 50, 101530. [Google Scholar] [CrossRef]
- Mancuso, A.; Cianflone, E.; Cristiano, M.C.; Salerno, N.; Tarsitano, M.; Marino, F.; Molinaro, C.; Fresta, M.; Torella, D.; Paolino, D. Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application. Pharmaceutics 2022, 14, 452. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Filardo, A.; Smith, A.J.; Cappetta, D.; De Angelis, A.; Urbanek, K.; et al. c-kit Haploinsufficiency impairs adult cardiac stem cell growth, myogenicity and myocardial regeneration. Cell Death Dis. 2019, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Farache Trajano, L.; Smart, N. Immunomodulation for optimal cardiac regeneration: Insights from comparative analyses. NPJ Regen. Med. 2021, 6, 8. [Google Scholar] [CrossRef]
- Relja, B.; Land, W.G. Damage-associated molecular patterns in trauma. Eur. J. Trauma Emerg. Surg. 2020, 46, 751–775. [Google Scholar] [CrossRef]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef]
- Carter, A.M. Complement activation: An emerging player in the pathogenesis of cardiovascular disease. Scientifica 2012, 2012, 402783. [Google Scholar] [CrossRef]
- Granger, C.B.; Mahaffey, K.W.; Weaver, W.D.; Theroux, P.; Hochman, J.S.; Filloon, T.G.; Rollins, S.; Todaro, T.G.; Nicolau, J.C.; Ruzyllo, W.; et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction—The COMplement inhibition in myocardial infarction treated with angioplasty (COMMA) trial. Circulation 2003, 108, 1184–1190. [Google Scholar] [CrossRef]
- Yan, X.; Anzai, A.; Katsumata, Y.; Matsuhashi, T.; Ito, K.; Endo, J.; Yamamoto, T.; Takeshima, A.; Shinmura, K.; Shen, W.; et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 2013, 62, 24–35. [Google Scholar] [CrossRef]
- Cassatella, M.A. On the production of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2L) by human neutrophils. J. Leukoc. Biol. 2006, 79, 1140–1149. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Vasilyev, N.; Williams, T.; Brennan, M.L.; Unzek, S.; Zhou, X.; Heinecke, J.W.; Spitz, D.R.; Topol, E.J.; Hazen, S.L.; Penn, M.S. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation 2005, 112, 2812–2820. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Weng, X.; Zeng, S.; Yu, L.; Guo, J.; Xu, Y. Brg1 deficiency in vascular endothelial cells blocks neutrophil recruitment and ameliorates cardiac ischemia-reperfusion injury in mice. Int. J. Cardiol. 2018, 269, 250–258. [Google Scholar] [CrossRef]
- García-Prieto, J.; Villena-Gutiérrez, R.; Gómez, M.; Bernardo, E.; Pun-García, A.; García-Lunar, I.; Crainiciuc, G.; Fernández-Jiménez, R.; Sreeramkumar, V.; Bourio-Martínez, R.; et al. Neutrophil stunning by metoprolol reduces infarct size. Nat. Commun. 2017, 8, 14780. [Google Scholar] [CrossRef]
- Ma, Y.; Chiao, Y.A.; Clark, R.; Flynn, E.R.; Yabluchanskiy, A.; Ghasemi, O.; Zouein, F.; Lindsey, M.L.; Jin, Y.F. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc. Res. 2015, 106, 421–431. [Google Scholar] [CrossRef]
- Ma, Y.; Mouton, A.J.; Lindsey, M.L. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl. Res. 2018, 191, 15–28. [Google Scholar] [CrossRef]
- Kiczak, L.; Tomaszek, A.; Bania, J.; Paslawska, U.; Zacharski, M.; Noszczyk-Nowak, A.; Janiszewski, A.; Skrzypczak, P.; Ardehali, H.; Jankowska, E.A.; et al. Expression and complex formation of MMP9, MMP2, NGAL, and TIMP1 in porcine myocardium but not in skeletal muscles in male pigs with tachycardia-induced systolic heart failure. BioMed Res. Int. 2013, 2013, 283856. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Results Probl. Cell Differ. 2017, 62, 353–364. [Google Scholar] [CrossRef]
- Gordon, S.; Plüddemann, A.; Martinez Estrada, F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Sommerfeld, S.D.; Cherry, C.; Schwab, R.M.; Chung, L.; Maestas, D.R., Jr.; Laffont, P.; Stein, J.E.; Tam, A.; Ganguly, S.; Housseau, F.; et al. Interleukin-36γ-producing macrophages drive IL-17-mediated fibrosis. Sci. Immunol. 2019, 4, eaax4783. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.D.; Wyroba, B.; Chen, W.; Lockwood, R.A.; Tran, K.B.; Shepherd, P.R.; Hall, C.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. Dis. Model. Mech. 2018, 11, dmm035998. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef] [PubMed]
- Ignotz, R.A.; Massague, J. Transforming Growth-Factor-Beta Stimulates the Expression of Fibronectin and Collagen and Their Incorporation into the Extracellular-Matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Czubryt, M.P. Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair 2012, 5, 19. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Henson, P.M. Dampening inflammation. Nat. Immunol. 2005, 6, 1179–1181. [Google Scholar] [CrossRef]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Vanderlugt, C.L.; Miller, S.D. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guérin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef]
- Nian, M.; Lee, P.; Khaper, N.; Liu, P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004, 94, 1543–1553. [Google Scholar] [CrossRef]
- Porter, K.E.; Turner, N.A.; O’Regan, D.J.; Ball, S.G. Tumor necrosis factor alpha induces human atrial myofibroblast proliferation, invasion and MMP-9 secretion: Inhibition by simvastatin. Cardiovasc. Res. 2004, 64, 507–515. [Google Scholar] [CrossRef]
- Hofmann, U.; Beyersdorf, N.; Weirather, J.; Podolskaya, A.; Bauersachs, J.; Ertl, G.; Kerkau, T.; Frantz, S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 2012, 125, 1652–1663. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ogawa, M.; Suzuki, J.; Hirata, Y.; Nagai, R.; Isobe, M. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int. Heart J. 2011, 52, 382–387. [Google Scholar] [CrossRef]
- Tang, T.T.; Yuan, J.; Zhu, Z.F.; Zhang, W.C.; Xiao, H.; Xia, N.; Yan, X.X.; Nie, S.F.; Liu, J.; Zhou, S.F.; et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 2012, 107, 232. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014, 40, 91–104. [Google Scholar] [CrossRef]
- Motwani, M.P.; Gilroy, D.W. Macrophage development and polarization in chronic inflammation. Semin. Immunol. 2015, 27, 257–266. [Google Scholar] [CrossRef]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS−) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef]
- Edholm, E.S.; Rhoo, K.H.; Robert, J. Evolutionary Aspects of Macrophages Polarization. Results Probl. Cell Differ. 2017, 62, 3–22. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Leid, J.; Carrelha, J.; Boukarabila, H.; Epelman, S.; Jacobsen, S.E.; Lavine, K.J. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ. Res. 2016, 118, 1498–1511. [Google Scholar] [CrossRef]
- Hoyer, F.F.; Naxerova, K.; Schloss, M.J.; Hulsmans, M.; Nair, A.V.; Dutta, P.; Calcagno, D.M.; Herisson, F.; Anzai, A.; Sun, Y.; et al. Tissue-Specific Macrophage Responses to Remote Injury Impact the Outcome of Subsequent Local Immune Challenge. Immunity 2019, 51, 899–914.e7. [Google Scholar] [CrossRef]
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef]
- Bajpai, G.; Schneider, C.; Wong, N.; Bredemeyer, A.; Hulsmans, M.; Nahrendorf, M.; Epelman, S.; Kreisel, D.; Liu, Y.; Itoh, A.; et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 2018, 24, 1234–1245. [Google Scholar] [CrossRef]
- Heidt, T.; Courties, G.; Dutta, P.; Sager, H.B.; Sebas, M.; Iwamoto, Y.; Sun, Y.; Da Silva, N.; Panizzi, P.; van der Laan, A.M.; et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 2014, 115, 284–295. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef]
- Ingersoll, M.A.; Spanbroek, R.; Lottaz, C.; Gautier, E.L.; Frankenberger, M.; Hoffmann, R.; Lang, R.; Haniffa, M.; Collin, M.; Tacke, F.; et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010, 115, e10–e19. [Google Scholar] [CrossRef]
- Hilgendorf, I.; Gerhardt, L.M.; Tan, T.C.; Winter, C.; Holderried, T.A.; Chousterman, B.G.; Iwamoto, Y.; Liao, R.; Zirlik, A.; Scherer-Crosbie, M.; et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 2014, 114, 1611–1622. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Dewald, O.; Xia, Y.; Ren, G.; Haudek, S.; Leucker, T.; Kraemer, D.; Taffet, G.; Rollins, B.J.; Entman, M.L. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 2007, 115, 584–592. [Google Scholar] [CrossRef]
- Le Grand, F.; Rudnicki, M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef]
- Lepper, C.; Partridge, T.A.; Fan, C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011, 138, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef] [PubMed]
- McLennan, I.S. Resident macrophages (ED2- and ED3-positive) do not phagocytose degenerating rat skeletal muscle fibres. Cell Tissue Res. 1993, 272, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Poliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- St Pierre, B.A.; Tidball, J.G. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J. Appl. Physiol. 1994, 77, 290–297. [Google Scholar] [CrossRef]
- Duelen, R.; Sampaolesi, M. Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise. eBioMedicine 2017, 16, 30–40. [Google Scholar] [CrossRef]
- Scalise, M.; Marino, F.; Cianflone, E.; Mancuso, T.; Marotta, P.; Aquila, I.; Torella, M.; Nadal-Ginard, B.; Torella, D. Heterogeneity of Adult Cardiac Stem Cells. Adv. Exp. Med. Biol. 2019, 1169, 141–178. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Bolli, R. A realistic appraisal of the use of embryonic stem cell-based therapies for cardiac repair. Eur. Heart J. 2020, 41, 2397–2404. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Venkatesan, B.; Valente, A.J.; Melby, P.C.; Nandish, S.; Reusch, J.E.; Clark, R.A.; Chandrasekar, B. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J. Biol. Chem. 2009, 284, 14414–14427. [Google Scholar] [CrossRef]
- Suzuki, K.; Murtuza, B.; Beauchamp, J.R.; Brand, N.J.; Barton, P.J.; Varela-Carver, A.; Fukushima, S.; Coppen, S.R.; Partridge, T.A.; Yacoub, M.H. Role of interleukin-1beta in acute inflammation and graft death after cell transplantation to the heart. Circulation 2004, 110, II219–II224. [Google Scholar] [CrossRef]
- Hamid, T.; Xu, Y.; Ismahil, M.A.; Li, Q.; Jones, S.P.; Bhatnagar, A.; Bolli, R.; Prabhu, S.D. TNF receptor signaling inhibits cardiomyogenic differentiation of cardiac stem cells and promotes a neuroadrenergic-like fate. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1189–H1201. [Google Scholar] [CrossRef]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.Z.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020, 577, 405–409. [Google Scholar] [CrossRef]
- Marino, F.; Scalise, M.; Cianflone, E.; Salerno, L.; Cappetta, D.; Salerno, N.; De Angelis, A.; Torella, D.; Urbanek, K. Physical Exercise and Cardiac Repair: The Potential Role of Nitric Oxide in Boosting Stem Cell Regenerative Biology. Antioxidants 2021, 10, 1002. [Google Scholar] [CrossRef]
- Simões, F.C.; Cahill, T.J.; Kenyon, A.; Gavriouchkina, D.; Vieira, J.M.; Sun, X.; Pezzolla, D.; Ravaud, C.; Masmanian, E.; Weinberger, M.; et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 2020, 11, 600. [Google Scholar] [CrossRef]
- Sadek, H.; Olson, E.N. Toward the Goal of Human Heart Regeneration. Cell Stem Cell 2020, 26, 7–16. [Google Scholar] [CrossRef]
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188. [Google Scholar] [CrossRef]
- Ashour, D.; Rebs, S.; Arampatzi, P.; Saliba, A.E.; Dudek, J.; Schulz, R.; Hofmann, U.; Frantz, S.; Cochain, C.; Streckfuß-Bömeke, K.; et al. An interferon gamma response signature links myocardial aging and immunosenescence. Cardiovasc. Res. 2023, cvad068. [Google Scholar] [CrossRef]
- Dolejsi, T.; Delgobo, M.; Schuetz, T.; Tortola, L.; Heinze, K.G.; Hofmann, U.; Frantz, S.; Bauer, A.; Ruschitzka, F.; Penninger, J.M.; et al. Adult T-cells impair neonatal cardiac regeneration. Eur. Heart J. 2022, 43, 2698–2709. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ponnappan, S.; Ponnappan, U. Aging and immune function: Molecular mechanisms to interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.A.; Broughton, K.M.; Firouzi, F.; Sussman, M.A. Cardiac ageing: Extrinsic and intrinsic factors in cellular renewal and senescence. Nat. Rev. Cardiol. 2018, 15, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.B.; Sinclair, D.A. When stem cells grow old: Phenotypes and mechanisms of stem cell aging. Development 2016, 143, 3–14. [Google Scholar] [CrossRef]
- Martínez-Zamudio, R.I.; Robinson, L.; Roux, P.F.; Bischof, O. SnapShot: Cellular Senescence Pathways. Cell 2017, 170, 816–816.e1. [Google Scholar] [CrossRef]
- Jackaman, C.; Radley-Crabb, H.G.; Soffe, Z.; Shavlakadze, T.; Grounds, M.D.; Nelson, D.J. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 2013, 12, 345–357. [Google Scholar] [CrossRef]
- Mahbub, S.; Deburghgraeve, C.R.; Kovacs, E.J. Advanced age impairs macrophage polarization. J. Interferon Cytokine Res. 2012, 32, 18–26. [Google Scholar] [CrossRef]
- Savoia, C.; Schiffrin, E.L. Inflammation in hypertension. Curr. Opin. Nephrol. Hypertens. 2006, 15, 152–158. [Google Scholar] [CrossRef]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Halade, G.V.; Kain, V.; Black, L.M.; Prabhu, S.D.; Ingle, K.A. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging 2016, 8, 2611–2634. [Google Scholar] [CrossRef]
- Molawi, K.; Wolf, Y.; Kandalla, P.K.; Favret, J.; Hagemeyer, N.; Frenzel, K.; Pinto, A.R.; Klapproth, K.; Henri, S.; Malissen, B.; et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014, 211, 2151–2158. [Google Scholar] [CrossRef]
- Lujambio, A.; Akkari, L.; Simon, J.; Grace, D.; Tschaharganeh, D.F.; Bolden, J.E.; Zhao, Z.; Thapar, V.; Joyce, J.A.; Krizhanovsky, V.; et al. Non-cell-autonomous tumor suppression by p53. Cell 2013, 153, 449–460. [Google Scholar] [CrossRef]
- Lin, I.H.; Chang, J.L.; Hua, K.; Huang, W.C.; Hsu, M.T.; Chen, Y.F. Skeletal muscle in aged mice reveals extensive transformation of muscle gene expression. BMC Genet. 2018, 19, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Wehling-Henricks, M.; Welc, S.S.; Fisher, A.L.; Zuo, Q.; Tidball, J.G. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB J. 2019, 33, 1415–1427. [Google Scholar] [CrossRef]
- Wang, M.; Shah, A.M. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J. Mol. Cell. Cardiol. 2015, 83, 101–111. [Google Scholar] [CrossRef]
- Wang, Y.; Wehling-Henricks, M.; Samengo, G.; Tidball, J.G. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 2015, 14, 678–688. [Google Scholar] [CrossRef]
- Przybyla, B.; Gurley, C.; Harvey, J.F.; Bearden, E.; Kortebein, P.; Evans, W.J.; Sullivan, D.H.; Peterson, C.A.; Dennis, R.A. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp. Gerontol. 2006, 41, 320–327. [Google Scholar] [CrossRef]
- Harel-Adar, T.; Ben Mordechai, T.; Amsalem, Y.; Feinberg, M.S.; Leor, J.; Cohen, S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc. Natl. Acad. Sci. USA 2011, 108, 1827–1832. [Google Scholar] [CrossRef]
- Ben-Mordechai, T.; Holbova, R.; Landa-Rouben, N.; Harel-Adar, T.; Feinberg, M.S.; Abd Elrahman, I.; Blum, G.; Epstein, F.H.; Silman, Z.; Cohen, S.; et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 2013, 62, 1890–1901. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Cheng, K.; Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marbán, L.; Marbán, E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, T.; Barone, A.; Salatino, A.; Molinaro, C.; Marino, F.; Scalise, M.; Torella, M.; De Angelis, A.; Urbanek, K.; Torella, D.; et al. Unravelling the Biology of Adult Cardiac Stem Cell-Derived Exosomes to Foster Endogenous Cardiac Regeneration and Repair. Int. J. Mol. Sci. 2020, 21, 3725. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- de Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marbán, E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Bauersachs, J.; Poole-Wilson, P.A.; Volk, H.D.; Anker, S.D. The dying stem cell hypothesis: Immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J. Am. Coll. Cardiol. 2005, 46, 1799–1802. [Google Scholar] [CrossRef]
- Ludman, A.; Venugopal, V.; Yellon, D.M.; Hausenloy, D.J. Statins and cardioprotection--more than just lipid lowering? Pharmacol. Ther. 2009, 122, 30–43. [Google Scholar] [CrossRef]
- Cianflone, E.; Cappetta, D.; Mancuso, T.; Sabatino, J.; Marino, F.; Scalise, M.; Albanese, M.; Salatino, A.; Parrotta, E.I.; Cuda, G.; et al. Statins Stimulate New Myocyte Formation After Myocardial Infarction by Activating Growth and Differentiation of the Endogenous Cardiac Stem Cells. Int. J. Mol. Sci. 2020, 21, 7927. [Google Scholar] [CrossRef]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef]
- Ghittoni, R.; Napolitani, G.; Benati, D.; Ulivieri, C.; Patrussi, L.; Laghi Pasini, F.; Lanzavecchia, A.; Baldari, C.T. Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. Eur. J. Immunol. 2006, 36, 2885–2893. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Ong, F.S.; Blackwell, W.L.; Shah, K.H.; Giani, J.F.; Gonzalez-Villalobos, R.A.; Shen, X.Z.; Fuchs, S.; Touyz, R.M. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 2013, 65, 1–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).