Molecular Aspects of a Diet as a New Pathway in the Prevention and Treatment of Alzheimer’s Disease
Abstract
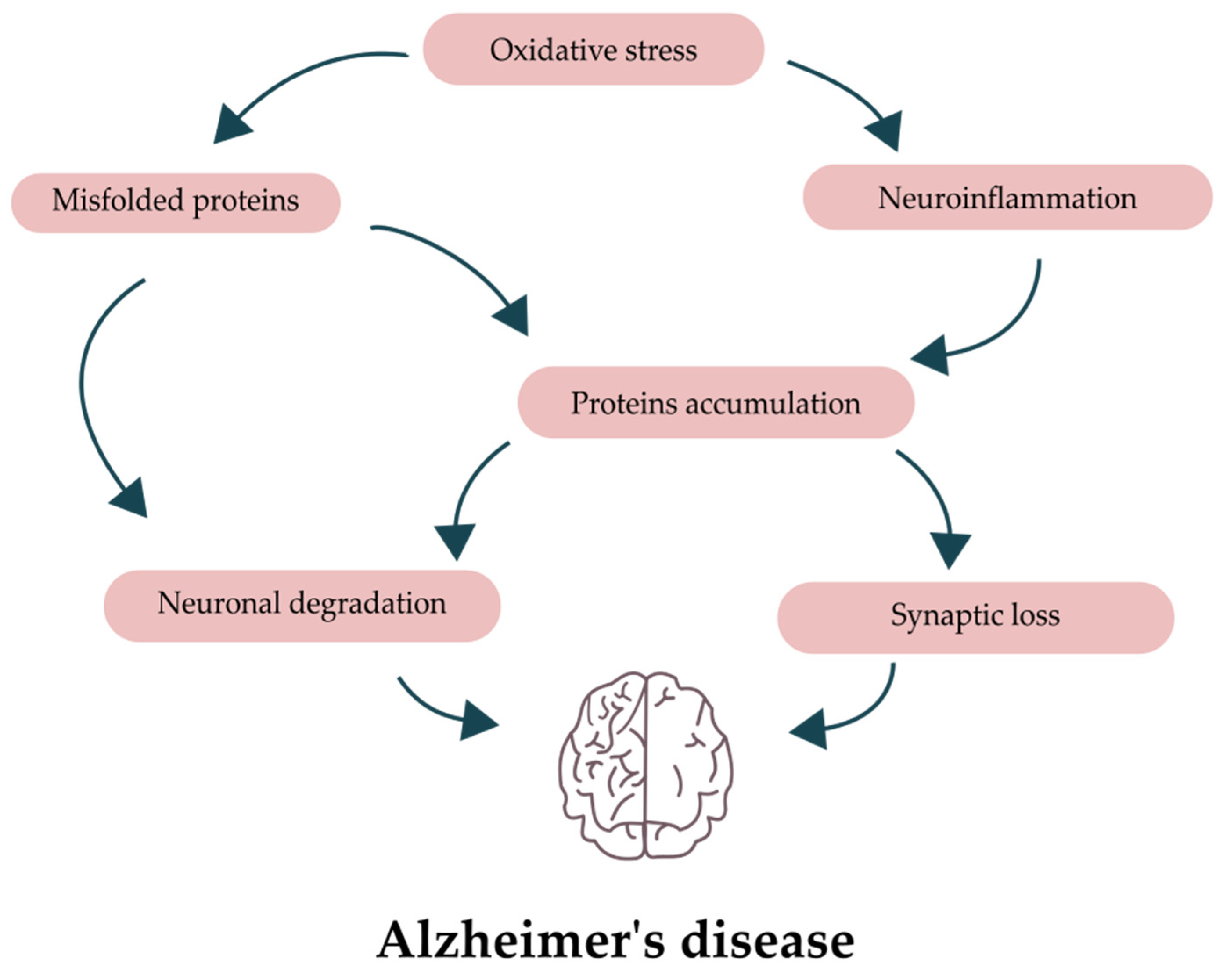
1. Alzheimer’s Disease
2. Pathology of Alzheimer’s Disease
3. Diets and Life Situations Negatively Influencing the Disease
3.1. Obesity and Malnutrition
3.2. Diet High in Fats
3.3. Western Diet
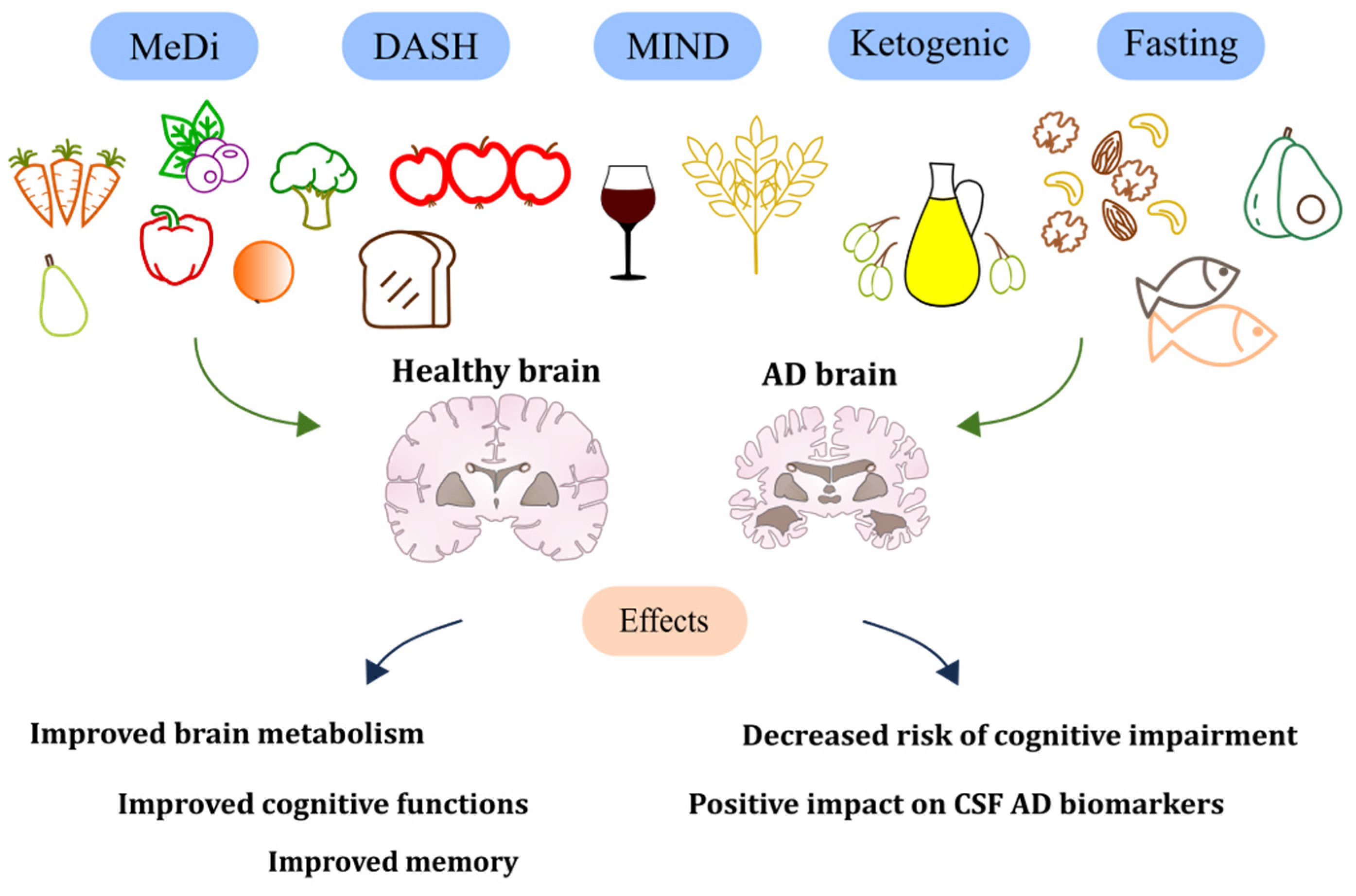
4. Diets with Positive Influence
4.1. Mediterranean Diet
4.2. DASH Diet
4.3. MIND Diet
4.4. Other Diets
4.4.1. Ketogenic Diet
4.4.2. Fasting
5. Beneficial Foods and Nutrients
5.1. Curcumin
5.2. Coffee and Tea
5.3. Cocoa
5.4. Vitamins
5.5. Foods Rising BDNF
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mendez, M.F. Early-Onset Alzheimer’s Disease. Neurol. Clin. 2017, 35, 263. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid Toxicity in Alzheimer’s Disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The Complexity of Tau in Alzheimer’s Disease. Neurosci. Lett. 2019, 705, 183. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terro, F. Tau Protein Kinases: Involvement in Alzheimer’s Disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Huang, H.C.; Jiang, Z.F. Accumulated Amyloid-Beta Peptide and Hyperphosphorylated Tau Protein: Relationship and Links in Alzheimer’s Disease. J. Alzheimers Dis. 2009, 16, 15–27. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Lou, Y.; Xu, J.; Feng, Z.; Chen, Y.; Tang, Q.; Zheng, G.; Zhang, Z.; Wu, Y.; et al. Salidroside Attenuates Neuroinflammation and Improves Functional Recovery after Spinal Cord Injury through Microglia Polarization Regulation. J. Cell Mol. Med. 2018, 22, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2015, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, M.-Y.Y.; Zhang, D.-F.F.; Zhong, X.; Du, K.; Qian, P.; Gao, H.; Wei, M.-J.J. Natural Products as a Potential Modulator of Microglial Polarization in Neurodegenerative Diseases. Pharmacol. Res. 2019, 145, 104253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Zhang, Z.Z.; Lu, H.; Yang, Q.; Wu, H.; Wang, J. Microglial Polarization and Inflammatory Mediators After Intracerebral Hemorrhage. Mol. Neurobiol. 2017, 54, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Burbach, G.J.; Bonin, M.; Walter, M.; Streit, W.; Bechmann, I.; Deller, T. TREM2 Is Upregulated in Amyloid Plaque-Associated Microglia in Aged APP23 Transgenic Mice. Glia 2008, 56, 1438–1447. [Google Scholar] [CrossRef]
- Hickman, S.E.; El Khoury, J. TREM2 and the Neuroimmunology of Alzheimer’s Disease. Biochem. Pharmacol. 2014, 88, 495–498. [Google Scholar] [CrossRef]
- Nasrolahi, A.; Javaherforooshzadeh, F.; Jafarzadeh-Gharehziaaddin, M.; Mahmoudi, J.; Asl, K.D.; Shabani, Z. Therapeutic Potential of Neurotrophic Factors in Alzheimer’s Disease. Mol. Biol. Rep. 2022, 49, 2345–2357. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease and Its Pharmaceutical Potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Edelmann, E.; Cepeda-Prado, E.; Franck, M.; Lichtenecker, P.; Brigadski, T.; Leßmann, V. Theta Burst Firing Recruits BDNF Release and Signaling in Postsynaptic CA1 Neurons in Spike-Timing-Dependent LTP. Neuron 2015, 86, 1041–1054. [Google Scholar] [CrossRef]
- Schinder, A.F.; Poo, M. ming The Neurotrophin Hypothesis for Synaptic Plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Atlas, R.; Lange, A.; Ginzburg, I. Brain-Derived Neurotrophic Factor Induces a Rapid Dephosphorylation of Tau Protein through a PI-3 Kinase Signalling Mechanism. Eur. J. Neurosci. 2005, 22, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Tanqueiro, S.R.; Ramalho, R.M.; Rodrigues, T.M.; Lopes, L.V.; Sebastião, A.M.; Diógenes, M.J. Inhibition of NMDA Receptors Prevents the Loss of BDNF Function Induced by Amyloid β. Front. Pharmacol. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; He, Z.; Xing, D. Low-Level Laser Therapy Rescues Dendrite Atrophy via Upregulating BDNF Expression: Implications for Alzheimer’s Disease. J. Neurosci. 2013, 33, 13505. [Google Scholar] [CrossRef] [PubMed]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and Brain Excitability. Front. Neuroendocrinol. 2012, 33, 116. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor Form of Brain-Derived Neurotrophic Factor and Mature Brain-Derived Neurotrophic Factor Are Decreased in the Pre-Clinical Stages of Alzheimer’s Disease. J Neurochem 2005, 93, 1412–1421. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Diniz, B.S.; Teixeira, A.L.; Radanovic, M.; Talib, L.L.; Rocha, N.P.; Gattaz, W.F. Lower Cerebrospinal Fluid Concentration of Brain-Derived Neurotrophic Factor Predicts Progression from Mild Cognitive Impairment to Alzheimer’s Disease. Neuromolecular. Med. 2015, 17, 326–332. [Google Scholar] [CrossRef]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum Brain-Derived Neurotrophic Factor and the Risk for Dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55. [Google Scholar] [CrossRef]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes Mellitus and Risk of Dementia: A Meta-Analysis of Prospective Observational Studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef]
- Picone, P.; di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s Disease: Molecular Bases. Eur. J. Neurosci. 2020, 52, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive Impairment Following High Fat Diet Consumption Is Associated with Brain Inflammation. J. Neuroimmunol. 2010, 219, 25. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Greenwood, C.E. Studies of the Effects of High Fat Diets on Cognitive Function in a Rat Model. Neurobiol. Aging 2005, 26 (Suppl. 1), 46–49. [Google Scholar] [CrossRef] [PubMed]
- Sobesky, J.L.; Barrientos, R.M.; De May, H.S.; Thompson, B.M.; Weber, M.D.; Watkins, L.R.; Maier, S.F. High-Fat Diet Consumption Disrupts Memory and Primes Elevations in Hippocampal IL-1β, an Effect That Can Be Prevented with Dietary Reversal or IL-1 Receptor Antagonism. Brain Behav. Immun. 2014, 42, 22. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary Fats and the Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Martin, J.M.; Maple, R.L.; Tharp, W.G.; Pratley, R.E. Plasma Amyloid-Beta Peptide Levels Correlate with Adipocyte Amyloid Precursor Protein Gene Expression in Obese Individuals. Neuroendocrinology 2009, 90, 383–390. [Google Scholar] [CrossRef]
- Grant, R.W.; Dixit, V.D. Adipose Tissue as an Immunological Organ. Obesity 2015, 23, 512–518. [Google Scholar] [CrossRef]
- Arnold, S.E.; Lucki, I.; Brookshire, B.R.; Carlson, G.C.; Browne, C.A.; Kazi, H.; Bang, S.; Choi, B.R.; Chen, Y.; McMullen, M.F.; et al. High Fat Diet Produces Brain Insulin Resistance, Synaptodendritic Abnormalities and Altered Behavior in Mice. Neurobiol. Dis. 2014, 67, 79–87. [Google Scholar] [CrossRef]
- de la Monte, S.M. Brain Insulin Resistance and Deficiency as Therapeutic Targets in Alzheimer’s Disease. Curr. Alzheimer Res. 2012, 9, 35. [Google Scholar] [CrossRef]
- Soto, M.E.; Secher, M.; Gillette-Guyonnet, S.; Van Kan, G.A.; Andrieu, S.; Nourhashemi, F.; Rolland, Y.; Vellas, B. Weight Loss and Rapid Cognitive Decline in Community-Dwelling Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2012, 28, 647–654. [Google Scholar] [CrossRef]
- Kimura, A.; Sugimoto, T.; Kitamori, K.; Saji, N.; Niida, S.; Toba, K.; Sakurai, T. Malnutrition Is Associated with Behavioral and Psychiatric Symptoms of Dementia in Older Women with Mild Cognitive Impairment and Early-Stage Alzheimer’s Disease. Nutrients 2019, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Suma, S.; Watanabe, Y.; Hirano, H.; Kimura, A.; Edahiro, A.; Awata, S.; Yamashita, Y.; Matsushita, K.; Arai, H.; Sakurai, T. Factors Affecting the Appetites of Persons with Alzheimer’s Disease and Mild Cognitive Impairment. Geriatr. Gerontol. Int. 2018, 18, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Ono, R.; Murata, S.; Saji, N.; Matsui, Y.; Niida, S.; Toba, K.; Sakurai, T. Prevalence and Associated Factors of Sarcopenia in Elderly Subjects with Amnestic Mild Cognitive Impairment or Alzheimer Disease. Curr. Alzheimer. Res. 2016, 13, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.S.; Jang, E.Y.; Kim, S.Y.; Kim, E.J.; Park, S.A.; Lee, Y.; Hong, C.H.; Choi, S.H.; Yoon, B.; Yoon, S.J.; et al. Unstable Body Mass Index and Progression to Probable Alzheimer’s Disease Dementia in Patients with Amnestic Mild Cognitive Impairment. J. Alzheimers Dis. 2016, 49, 483–491. [Google Scholar] [CrossRef]
- Hanson, L.C.; Ersek, M.; Lin, F.C.; Carey, T.S. Outcomes of Feeding Problems in Advanced Dementia in a Nursing Home Population. J. Am. Geriatr. Soc. 2013, 61, 1692–1697. [Google Scholar] [CrossRef]
- Shimizu, T.; Nagaoka, U.; Nakayama, Y.; Kawata, A.; Kugimoto, C.; Kuroiwa, Y.; Kawai, M.; Shimohata, T.; Nishizawa, M.; Mihara, B.; et al. Reduction Rate of Body Mass Index Predicts Prognosis for Survival in Amyotrophic Lateral Sclerosis: A Multicenter Study in Japan. Amyotroph. Lateral Scler. 2012, 13, 363–366. [Google Scholar] [CrossRef]
- Ousset, P.J.; Nourhashemi, F.; Reynish, E.; Vellas, B. Nutritional Status Is Associated with Disease Progression in Very Mild Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 66–71. [Google Scholar] [CrossRef]
- Yu, J.T.; Xu, W.; Tan, C.C.; Andrieu, S.; Suckling, J.; Evangelou, E.; Pan, A.; Zhang, C.; Jia, J.; Feng, L.; et al. Review: Evidence-Based Prevention of Alzheimer’s Disease: Systematic Review and Meta-Analysis of 243 Observational Prospective Studies and 153 Randomised Controlled Trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary Fat: From Foe to Friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef]
- Frausto, D.M.; Forsyth, C.B.; Keshavarzian, A.; Voigt, R.M. Dietary Regulation of Gut-Brain Axis in Alzheimer’s Disease: Importance of Microbiota Metabolites. Front. Neurosci. 2021, 15, 736814. [Google Scholar] [CrossRef]
- Lin, B.; Hasegawa, Y.; Takane, K.; Koibuchi, N.; Cao, C.; Kim-Mitsuyama, S. High-Fat-Diet Intake Enhances Cerebral Amyloid Angiopathy and Cognitive Impairment in a Mouse Model of Alzheimer’s Disease, Independently of Metabolic Disorders. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e003154. [Google Scholar] [CrossRef] [PubMed]
- Bracko, O.; Vinarcsik, L.K.; Cruz Hernández, J.C.; Ruiz-Uribe, N.E.; Haft-Javaherian, M.; Falkenhain, K.; Ramanauskaite, E.M.; Ali, M.; Mohapatra, A.; Swallow, M.A.; et al. High Fat Diet Worsens Alzheimer’s Disease-Related Behavioral Abnormalities and Neuropathology in APP/PS1 Mice, but Not by Synergistically Decreasing Cerebral Blood Flow. Sci. Rep. 2020, 10, 9884. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Bienias, J.L.; Evans, D.A.; Wilson, R.S. Validity and Reproducibility of a Food Frequency Questionnaire by Cognition in an Older Biracial Sample. Am. J. Epidemiol. 2003, 158, 1213–1217. [Google Scholar] [CrossRef]
- Laitinen, M.H.; Ngandu, T.; Rovio, S.; Helkala, E.L.; Uusitalo, U.; Viitanen, M.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Fat Intake at Midlife and Risk of Dementia and Alzheimer’s Disease: A Population-Based Study. Dement. Geriatr. Cogn. Disord. 2006, 22, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Tang, J.; Guo, X.; Li, K.; Li, D. Dietary Fat Intake and Risk of Alzheimer’s Disease and Dementia: A Meta-Analysis of Cohort Studies. Curr. Alzheimer Res. 2018, 15, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s Dementia—Nutritional Approach to Modulate Inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef] [PubMed]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Lustig, R.H. Ultraprocessed Food: Addictive, Toxic, and Ready for Regulation. Nutrients 2020, 12, 3401. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Ke, X.; Fu, Q.; Sterrett, J.; Hillard, C.J.; Lane, R.H.; Majnik, A. Adverse Maternal Environment and Western Diet Impairs Cognitive Function and Alters Hippocampal Glucocorticoid Receptor Promoter Methylation in Male Mice. Physiol. Rep. 2020, 8, e14407. [Google Scholar] [CrossRef]
- Leigh, S.J.; Morris, M.J. Diet, Inflammation and the Gut Microbiome: Mechanisms for Obesity-Associated Cognitive Impairment. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165767. [Google Scholar] [CrossRef] [PubMed]
- Leigh Gibson, E.; Barr, S.; Jeanes, Y.M. Habitual Fat Intake Predicts Memory Function in Younger Women. Front. Hum. Neurosci. 2013, 7, 838. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Francis, H.M.; Attuquayefio, T.; Gupta, D.; Yeomans, M.R.; Oaten, M.J.; Davidson, T. Hippocampal-Dependent Appetitive Control Is Impaired by Experimental Exposure to a Western-Style Diet. R. Soc. Open Sci. 2020, 7, 191338. [Google Scholar] [CrossRef]
- Rebai, R.; Jasmin, L.; Boudah, A. Agomelatine Effects on Fat-Enriched Diet Induced Neuroinflammation and Depression-like Behavior in Rats. Biomed. Pharmacother. 2021, 135, 111246. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.S. The Effect of Short-Term Feeding of a High-Coconut Oil or High-Fat Diet on Neuroinflammation and the Performance of an Object–Place Task in Rats. Neurochem. Res. 2021, 46, 287–298. [Google Scholar] [CrossRef]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A Four-Day Western-Style Dietary Intervention Causes Reductions in Hippocampal-Dependent Learning and Memory and Interoceptive Sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western Diet Is Associated with a Smaller Hippocampus: A Longitudinal Investigation. BMC Med. 2015, 13, 215. [Google Scholar] [CrossRef]
- Ullrich, C.; Pirchl, M.; Humpel, C. Hypercholesterolemia in Rats Impairs the Cholinergic System and Leads to Memory Deficits. Mol. Cell. Neurosci. 2010, 45, 408–417. [Google Scholar] [CrossRef]
- Selvi, Y.; Gergerlioglu, H.S.; Akbaba, N.; Oz, M.; Kandeger, A.; Demir, E.A.; Yerlikaya, F.H.; Nurullahoglu-Atalik, K.E. Impact of Enriched Environment on Production of Tau, Amyloid Precursor Protein and, Amyloid-β Peptide in High-Fat and High-Sucrose-Fed Rats. Acta Neuropsychiatr. 2017, 29, 291–298. [Google Scholar] [CrossRef]
- Hohsfield, L.A.; Daschil, N.; Orädd, G.; Strömberg, I.; Humpel, C. Vascular Pathology of 20-Month-Old Hypercholesterolemia Mice in Comparison to Triple-Transgenic and APPSwDI Alzheimer’s Disease Mouse Models. Mol. Cell. Neurosci. 2014, 63, 83–95. [Google Scholar] [CrossRef]
- Kang, J.; Wang, Z.; Oteiza, P.I. (−)-Epicatechin Mitigates High Fat Diet-Induced Neuroinflammation and Altered Behavior in Mice. Food. Funct. 2020, 11, 5065–5076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.; Lan, N.; Li, S.; Zhang, J.; Wang, S.; Li, C.; Shang, Y.; Huang, T.; Zhang, L. Luteolin Protects against High Fat Diet-Induced Cognitive Deficits in Obesity Mice. Behav. Brain Res. 2014, 267, 178–188. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts around the World. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, A.L.; Caffa, I.; Cea, M.; Nencioni, A.; Odetti, P.; Monacelli, F. Nutrients in the Prevention of Alzheimer’s Disease. Oxid. Med. Cell Longev. 2019, 2019, 9874159. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Bryan, J.; Murphy, K. Is the Mediterranean Diet a Feasible Approach to Preserving Cognitive Function and Reducing Risk of Dementia for Older Adults in Western Countries? New Insights and Future Directions. Ageing Res. Rev. 2016, 25, 85–101. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Guyer, H.; Langa, K.M.; Yaffe, K. Neuroprotective Diets Are Associated with Better Cognitive Function: The Health and Retirement Study. J. Am. Geriatr. Soc. 2017, 65, 1857. [Google Scholar] [CrossRef]
- Zbeida, M.; Goldsmith, R.; Shimony, T.; Yardi, H.; Naggan, L.; Shahar, D.R. Mediterranean Diet and Functional Indicators among Older Adults in Non-Mediterranean and Mediterranean Countries. J. Nutr. Health Aging 2014, 18, 411–418. [Google Scholar] [CrossRef]
- Anastasiou, C.A.; Yannakoulia, M.; Kontogianni, M.D.; Kosmidis, M.H.; Mamalaki, E.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Tsapanou, A.; Lykou, A.; et al. Mediterranean Lifestyle in Relation to Cognitive Health: Results from the HELIAD Study. Nutrients 2018, 10, 1557. [Google Scholar] [CrossRef]
- Galbete, C.; Toledo, E.; Toledo, J.B.; Bes-Rastrollo, M.; Buil-Cosiales, P.; Marti, A.; Guillén-Grima, F.; Martínez-González, M.A. Mediterranean Diet and Cognitive Function: The SUN Project. J. Nutr. Health Aging 2015, 19, 305–312. [Google Scholar] [CrossRef]
- Katsiardanis, K.; Diamantaras, A.A.; Dessypris, N.; Michelakos, T.; Anastasiou, A.; Katsiardani, K.P.; Kanavidis, P.; Papadopoulos, F.C.; Stefanadis, C.; Panagiotakos, D.B.; et al. Cognitive Impairment and Dietary Habits among Elders: The Velestino Study. J. Med. Food 2013, 16, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Judd, S.; Letter, A.J.; Alexandrov, A.V.; Howard, G.; Nahab, F.; Unverzagt, F.W.; Moy, C.; Howard, V.J.; Kissela, B.; et al. Adherence to a Mediterranean Diet and Risk of Incident Cognitive Impairment. Neurology 2013, 80, 1684. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean Diet and Risk for Alzheimer’s Disease. Ann. Neurol. 2006, 59, 912. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53. [Google Scholar] [CrossRef]
- Anastasiou, C.A.; Yannakoulia, M.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Arampatzi, X.; Bougea, A.; Labropoulos, I.; Scarmeas, N. Mediterranean Diet and Cognitive Health: Initial Results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS ONE 2017, 12, e018204. [Google Scholar] [CrossRef]
- Ballarini, T.; van Lent, D.M.; Brunner, J.; Schröder, A.; Wolfsgruber, S.; Altenstein, S.; Brosseron, F.; Buerger, K.; Dechent, P.; Dobisch, L.; et al. Mediterranean Diet, Alzheimer Disease Biomarkers, and Brain Atrophy in Old Age. Neurology 2021, 96, e2920. [Google Scholar] [CrossRef]
- Hoscheidt, S.; Sanderlin, A.H.; Baker, L.D.; Jung, Y.; Lockhart, S.; Kellar, D.; Whitlow, C.T.; Hanson, A.J.; Friedman, S.; Register, T.; et al. Mediterranean and Western Diet Effects on Alzheimer’s Disease Biomarkers, Cerebral Perfusion, and Cognition in Mid-life: A Randomized Trial. Alzheimer’s Dement. 2022, 18, 457. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) Diet on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef]
- Van Den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; Van De Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Smith, P.J.; Mabe, S.; Hinderliter, A.; Welsh-Bohmer, K.; Browndyke, J.N.; Lin, P.H.; Kraus, W.; Doraiswamy, P.M.; Burke, J.; et al. Lifestyle and Neurocognition in Older Adults with Cardiovascular Risk Factors and Cognitive Impairment. Psychosom. Med. 2017, 79, 719. [Google Scholar] [CrossRef]
- Haring, B.; Wu, C.; Mossavar-Rahmani, Y.; Snetselaar, L.; Brunner, R.; Wallace, R.B.; Neuhouser, M.L.; Wassertheil-Smoller, S. No Association between Dietary Patterns and Risk for Cognitive Decline in Older Women with Nine-Year Follow-up: Data from the Women’s Health Initiative Memory Study. J. Acad. Nutr. Diet. 2016, 116, 921. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A.; Babyak, M.A.; Craighead, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Strauman, T.A.; Sherwood, A. Effects of the Dietary Approaches to Stop Hypertension Diet, Exercise, and Caloric Restriction on Neurocognition in Overweight Adults with High Blood Pressure. Hypertension 2010, 55, 1331. [Google Scholar] [CrossRef] [PubMed]
- Shakersain, B.; Rizzuto, D.; Larsson, S.C.; Faxén-Irving, G.; Fratiglioni, L.; Xu, W.L. The Nordic Prudent Diet Reduces Risk of Cognitive Decline in the Swedish Older Adults: A Population-Based Cohort Study. Nutrients 2018, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Cherian, L.; Wang, Y.; Fakuda, K.; Leurgans, S.; Aggarwal, N.; Morris, M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diet Slows Cognitive Decline after Stroke. J. Prev. Alzheimers Dis. 2019, 6, 267. [Google Scholar] [CrossRef] [PubMed]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.A.T.; Norton, M.C.; Welsh-Bohmer, K.A. Prospective Study of Dietary Approaches to Stop Hypertension– and Mediterranean-Style Dietary Patterns and Age-Related Cognitive Change: The Cache County Study on Memory, Health and Aging. Am. J. Clin. Nutr. 2013, 98, 1263. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of Vegetable and Fruit Consumption with Age-Related Cognitive Change. Neurology 2006, 67, 1370. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary Intake of Berries and Flavonoids in Relation to Cognitive Decline. Ann. Neurol. 2012, 72, 135. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Fish Consumption and Cognitive Decline with Age in a Large Community Study. Arch. Neurol. 2005, 62, 1849–1853. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin Olive Oil and Nuts as Key Foods of the Mediterranean Diet Effects on Inflammatory Biomakers Related to Atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julián, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.Á. Mediterranean Diet Improves Cognition: The PREDIMED-NAVARRA Randomised Trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Nutritional Factors and Risk of Incident Dementia in the PAQUID Longitudinal Cohort. Available online: https://psycnet.apa.org/record/2004-14797-001 (accessed on 21 March 2023).
- Chen, X.; Huang, Y.; Cheng, H.G. Lower Intake of Vegetables and Legumes Associated with Cognitive Decline among Illiterate Elderly Chinese: A 3-Year Cohort Study. J. Nutr. Health Aging 2012, 16, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Hosking, D.E.; Eramudugolla, R.; Cherbuin, N.; Anstey, K.J. MIND Not Mediterranean Diet Related to 12-Year Incidence of Cognitive Impairment in an Australian Longitudinal Cohort Study. Alzheimers Dement. 2019, 15, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Leng, S.X.; Zhang, H. Ketogenic Diet: An Effective Treatment Approach for Neurodegenerative Diseases. Curr. Neuropharmacol. 2022, 20, 2303–2319. [Google Scholar] [CrossRef] [PubMed]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef]
- Hersant, H.; Grossberg, G. The Ketogenic Diet and Alzheimer’s Disease. J. Nutr. Health Aging 2022, 26, 606–614. [Google Scholar] [CrossRef]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022, 13, 1146–1165. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Xiao, A.; Han, J.; Wang, Z.; Wen, M. Ketogenic Diet Prevents Chronic Sleep Deprivation-Induced Alzheimer’s Disease by Inhibiting Iron Dyshomeostasis and Promoting Repair via Sirt1/Nrf2 Pathway. Front. Aging Neurosci. 2022, 14, 1013. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, C.; Wu, J.; Liu, P.; Deng, X.; Zhang, Y.; Peng, B.; Zhu, Y. Ketogenic Diet Ameliorates Cognitive Impairment and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. CNS Neurosci. Ther. 2022, 28, 580–592. [Google Scholar] [CrossRef]
- Neth, B.J.; Mintz, A.; Whitlow, C.; Jung, Y.; Solingapuram Sai, K.; Register, T.C.; Kellar, D.; Lockhart, S.N.; Hoscheidt, S.; Maldjian, J.; et al. Modified Ketogenic Diet Is Associated with Improved Cerebrospinal Fluid Biomarker Profile, Cerebral Perfusion, and Cerebral Ketone Body Uptake in Older Adults at Risk for Alzheimer’s Disease: A Pilot Study. Neurobiol. Aging 2020, 86, 54–63. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kasperek, K.; Rękawek, P.; Piątkowska-Chmiel, I. The Therapeutic Role of Ketogenic Diet in Neurological Disorders. Nutrients 2022, 14, 1952. [Google Scholar] [CrossRef]
- Sridharan, B.; Lee, M.-J. Ketogenic Diet: A Promising Neuroprotective Composition for Managing Alzheimer’s Diseases and Its Pathological Mechanisms. Curr. Mol. Med. 2021, 22, 640–656. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, M.; Chen, W.; Liu, Z. The Neuroprotective Effects of Intermittent Fasting on Brain Aging and Neurodegenerative Diseases via Regulating Mitochondrial Function. Free Radic. Biol. Med. 2022, 182, 206–218. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent Metabolic Switching, Neuroplasticity and Brain Health. Nat. Rev. Neurosci. 2018, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of Intermittent Fasting on Body Composition and Clinical Health Markers in Humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A Randomized Pilot Study Comparing Zero-Calorie Alternate-Day Fasting to Daily Caloric Restriction in Adults with Obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate Day Fasting for Weight Loss in Normal Weight and Overweight Subjects: A Randomized Controlled Trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate Day Fasting and Endurance Exercise Combine to Reduce Body Weight and Favorably Alter Plasma Lipids in Obese Humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lakhanpal, D.; Kumar, S.; Sharma, S.; Kataria, H.; Kaur, M.; Kaur, G. Late-Onset Intermittent Fasting Dietary Restriction as a Potential Intervention to Retard Age-Associated Brain Function Impairments in Male Rats. Age 2012, 34, 917–933. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, Z.; Li, X.; Xing, A.; Jiang, C.; Chen, Y.; Shi, W.; An, L. Intermittent Fasting Protects against Alzheimer’s Disease Possible through Restoring Aquaporin-4 Polarity. Front. Mol. Neurosci. 2017, 10, 395. [Google Scholar] [CrossRef]
- Halagappa, V.K.M.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; LaFerla, F.M.; Mattson, M.P. Intermittent Fasting and Caloric Restriction Ameliorate Age-Related Behavioral Deficits in the Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Lazic, D.; Tesic, V.; Jovanovic, M.; Brkic, M.; Milanovic, D.; Zlokovic, B.V.; Kanazir, S.; Perovic, M. Every-Other-Day Feeding Exacerbates Inflammation and Neuronal Deficits in 5XFAD Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2020, 136, 104745. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, A.; Li, Y.J.; Yang, Y.; Kishimoto, Y.; Zhang, S.; Wang, Y.; Wan, R.; Raefsky, S.M.; Lu, D.; et al. SIRT3 Mediates Hippocampal Synaptic Adaptations to Intermittent Fasting and Ameliorates Deficits in APP Mutant Mice. Nat. Commun. 2019, 10, 1886. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Zuo, Z. Chronic Intermittent Fasting Improves Cognitive Functions and Brain Structures in Mice. PLoS ONE 2013, 8, 66069. [Google Scholar] [CrossRef]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and Amino Acid Restriction, Aging and Disease: From Yeast to Humans. Trends Endocrinol. Metab. 2014, 25, 558. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Fobker, M.; Gellner, R.; Knecht, S.; Flöel, A. From the Cover: Caloric Restriction Improves Memory in Elderly Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 1255. [Google Scholar] [CrossRef]
- Bayer-Carter, J.L.; Green, P.S.; Montine, T.J.; Van-Fossen, B.; Baker, L.D.; Watson, G.S.; Bonner, L.M.; Callaghan, M.; Leverenz, J.B.; Walter, B.K.; et al. Diet Intervention and Cerebrospinal Fluid Biomarkers in Amnestic Mild Cognitive Impairment. Arch. Neurol. 2011, 68, 743. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid Beta Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin Labels Amyloid Pathology in Vivo, Disrupts Existing Plaques, and Partially Restores Distorted Neurites in an Alzheimer Mouse Model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid Beta in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 843. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, D.S.H.L. Discovery of Natural Products from Curcuma Longa That Protect Cells from Beta-Amyloid Insult: A Drug Discovery Effort against Alzheimer’s Disease. J. Nat. Prod. 2002, 65, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Lahiri, D.K. Neuroinflammation in Alzheimer’s Disease: Different Molecular Targets and Potential Therapeutic Agents Including Curcumin. Curr. Opin. Pharmacol. 2009, 9, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Endo, H.; Ise, S.; Miyazaki, K.; Aoki, H.; Sanjoh, A.; Kobayashi, K.; Hattori, Y.; Akaji, K. Synthesis and Evaluation of Curcumin Derivatives toward an Inhibitor of Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1. Bioorg. Med. Chem. Lett. 2014, 24, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Doroszkiewicz, J.; Mroczko, P.; Kulczyńska-Przybik, A. Inflammation in the CNS: Understanding Various Aspects of the Pathogenesis of Alzheimer’s Disease. Curr. Alzheimer Res. 2021, 19, 16–31. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and Cognition: A Randomised, Placebo-Controlled, Double-Blind Study of Community-Dwelling Older Adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Hoppe, J.B.; Coradini, K.; Frozza, R.L.; Oliveira, C.M.; Meneghetti, A.B.; Bernardi, A.; Pires, E.S.; Beck, R.C.R.; Salbego, C.G. Free and Nanoencapsulated Curcumin Suppress β-Amyloid-Induced Cognitive Impairments in Rats: Involvement of BDNF and Akt/GSK-3β Signaling Pathway. Neurobiol. Learn Mem. 2013, 106, 134–144. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral Curcumin for Alzheimer’s Disease: Tolerability and Efficacy in a 24-Week Randomized, Double Blind, Placebo-Controlled Study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Santos, C.; Lunet, N.; Azevedo, A.; De Mendonça, A.; Ritchie, K.; Barros, H. Caffeine Intake Is Associated with a Lower Risk of Cognitive Decline: A Cohort Study from Portugal. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S175–S185. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Khan, F.; Lam, H. Epidemiologic Evidence of a Relationship between Tea, Coffee, or Caffeine Consumption and Cognitive Decline. Adv. Nutr. 2013, 4, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.H.; Boia, R.; Ambrósio, A.F.; Santiago, A.R. Having a Coffee Break: The Impact of Caffeine Consumption on Microglia-Mediated Inflammation in Neurodegenerative Diseases. Mediators Inflamm. 2017, 2017, 4761081. [Google Scholar] [CrossRef]
- Arendash, G.W.; Cao, C. Caffeine and Coffee as Therapeutics Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, S117–S126. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D.; He, Y. Coffee Intake and the Incident Risk of Cognitive Disorders: A Dose–Response Meta-Analysis of Nine Prospective Cohort Studies. Clin. Nutr. 2017, 36, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, Tea, and Caffeine Consumption and Prevention of Late-Life Cognitive Decline and Dementia: A Systematic Review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef]
- Zhou, A.; Taylor, A.E.; Karhunen, V.; Zhan, Y.; Rovio, S.P.; Lahti, J.; Sjögren, P.; Byberg, L.; Lyall, D.M.; Auvinen, J.; et al. Habitual Coffee Consumption and Cognitive Function: A Mendelian Randomization Meta-Analysis in up to 415,530 Participants. Sci. Rep. 2018, 8, 7526. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1297. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-Gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Chieng, D.; Kistler, P.M. Coffee and Tea on Cardiovascular Disease (CVD) Prevention. Trends Cardiovasc. Med. 2022, 32, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, S.; Watanabe, H.; Murayama, N. Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review. Nutrients 2019, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Luca, A.; Calandra, C. The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxid. Med. Cell Longev. 2015, 2015, 504678. [Google Scholar] [CrossRef]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in Dementia: From Molecular Basis to Clinical Trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-Inflammatory Effects of Flavonoids in Neurodegenerative Disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Afzal, M.; Safer, A.M.; Menon, M. Green Tea Polyphenols and Their Potential Role in Health and Disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia Sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Green Tea Consumption and the Risk of Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. Am. J. Geriatr. Psychiatry 2016, 24, 881–889. [Google Scholar] [CrossRef]
- Schimidt, H.L.; Garcia, A.; Martins, A.; Mello-Carpes, P.B.; Carpes, F.P. Green Tea Supplementation Produces Better Neuroprotective Effects than Red and Black Tea in Alzheimer-like Rat Model. Food Res. Int. 2017, 100, 442–448. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Zeli, C.; Lombardo, M.; Storz, M.A.; Ottaviani, M.; Rizzo, G. Chocolate and Cocoa-Derived Biomolecules for Brain Cognition during Ageing. Antioxidants 2022, 11, 1353. [Google Scholar] [CrossRef]
- Septianti, E.; Salengke; Langkong, J. Profile of Bioactive Compounds, Antioxidant and Aromatic Component from Several Clones of Cocoa Beans during Fermentation. IOP Conf. Ser. Earth Environ. Sci. 2020, 575, 012009. [Google Scholar] [CrossRef]
- Cooper, K.A.; Donovan, J.L.; Waterhouse, A.I.; Williamson, G. Cocoa and Health: A Decade of Research. Br. J. Nutr. 2008, 99, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, S.A.; Hammerstone, J.F.; Schmitz, H.H. Chocolate Contains Additional Flavonoids Not Found in Tea. Lancet 1999, 354, 1825. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood Pressure Is Reduced and Insulin Sensitivity Increased in Glucose-Intolerant, Hypertensive Subjects after 15 Days of Consuming High-Polyphenol Dark Chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa Flavanol Consumption Improves Cognitive Function, Blood Pressure Control, and Metabolic Profile in Elderly Subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 538. [Google Scholar] [CrossRef]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D.L. Cerebral Blood Flow Response to Flavanol-Rich Cocoa in Healthy Elderly Humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433. [Google Scholar] [CrossRef]
- Jin Heo, H.; Lee, C.Y. Epicatechin and Catechin in Cocoa Inhibit Amyloid Beta Protein Induced Apoptosis. J. Agric. Food Chem. 2005, 53, 1445–1448. [Google Scholar] [CrossRef]
- Cimini, A.; Gentile, R.; D’Angelo, B.; Benedetti, E.; Cristiano, L.; Avantaggiati, M.L.; Giordano, A.; Ferri, C.; Desideri, G. Cocoa Powder Triggers Neuroprotective and Preventive Effects in a Human Alzheimer’s Disease Model by Modulating BDNF Signaling Pathway. J. Cell Biochem. 2013, 114, 2209. [Google Scholar] [CrossRef]
- Wang, J.; Varghese, M.; Ono, K.; Yamada, M.; Levine, S.; Tzavaras, N.; Gong, B.; Hurst, W.J.; Blitzer, R.D.; Pasinetti, G.M. Cocoa Extracts Reduce Oligomerization of Amyloid-β: Implications for Cognitive Improvement in Alzheimer’s Disease. J. Alzheimers Dis. 2014, 41, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance through Cocoa Flavanol Consumption in Elderly Subjects with Mild Cognitive Impairment: The Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Pupi, A.; De Leon, M.J. Brain Glucose Hypometabolism and Oxidative Stress in Preclinical Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2008, 1147, 180. [Google Scholar] [CrossRef] [PubMed]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-żukowska, R.; Socha, K. Vitamins in Alzheimer’s Disease—Review of the Latest Reports. Nutrients 2020, 12, 3458. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A. Dietary Factors and Cognitive Decline. J. Prev. Alzheimers Dis. 2016, 3, 53. [Google Scholar] [CrossRef]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and Clinical Outcomes of Homocysteine-Lowering B-Vitamin Treatment in Mild Cognitive Impairment: A Randomized Controlled Trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: A Randomized Controlled Trial. Mediators Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A Randomized Placebo-Controlled Trial of Using B Vitamins to Prevent Cognitive Decline in Older Mild Cognitive Impairment Patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef]
- Van Der Zwaluw, N.L.; Dhonukshe-Rutten, R.A.M.; Van Wijngaarden, J.P.V.; Brouwer-Brolsma, E.M.; Van De Rest, O.V.D.; In’t Veld, P.H.; Enneman, A.W.; Van Dijk, S.C.; Ham, A.C.; Swart, K.M.A.; et al. Results of 2-Year Vitamin B Treatment on Cognitive Performance: Secondary Data from an RCT. Neurology 2014, 83, 2158–2166. [Google Scholar] [CrossRef]
- Thiel, A.; Hermanns, C.; Lauer, A.A.; Reichrath, J.; Erhardt, T.; Hartmann, T.; Grimm, M.O.W.; Grimm, H.S. Vitamin D and Its Analogues: From Differences in Molecular Mechanisms to Potential Benefits of Adapted Use in the Treatment of Alzheimer’s Disease. Nutrients 2023, 15, 1684. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498. [Google Scholar] [CrossRef]
- Kiderman, D.; Ben-Shabat, N.; Tsur, A.M.; Anis, S.; Watad, A.; Cohen, A.D.; Paz, Z.; Amital, H. Vitamin D Insufficiency Is Associated with Higher Incidence of Dementia, a Large Community-Based Retrospective Cohort Study. J. Geriatr. Psychiatry Neurol. online ahead of print. 2023. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D Deficiency as a Risk Factor for Dementia and Alzheimer’s Disease: An Updated Meta-Analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Vitamin D Deficiency Is Associated with Increased Risk of Alzheimer’s Disease and Dementia: Evidence from Meta-Analysis. Nutr. J. 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, M.; Smith, E.E.; Chen, H.; Creese, B.; Goodarzi, Z.; Ismail, Z. Vitamin D Supplementation and Incident Dementia: Effects of Sex, APOE, and Baseline Cognitive Status. Alzheimers Dement. 2023, 15, 12404. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Whisner, C.M.; Johnston, C.S. Vitamin D Supplementation Appears to Increase Plasma Aβ40 in Vitamin D Insufficient Older Adults: A Pilot Randomized Controlled Trial. J. Alzheimers Dis. 2016, 52, 843–847. [Google Scholar] [CrossRef]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of Vitamin D Supplementation on Cognitive Function and Blood Aβ-Related Biomarkers in Older Adults with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-Inflammatory Properties of α- and γ-Tocopherol. Mol. Aspects Med. 2007, 28, 668. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant and Anti-Inflammatory Activities and the Role in Disease Prevention and Therapy. Free Radic. Biol. Med. 2014, 72, 76. [Google Scholar] [CrossRef]
- Lopes Da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma Nutrient Status of Patients with Alzheimer’s Disease: Systematic Review and Meta-Analysis. Alzheimers Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef]
- Mullan, K.; Cardwell, C.R.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Plasma Antioxidant Status in Patients with Alzheimer’s Disease and Cognitively Intact Elderly: A Meta-Analysis of Case-Control Studies. J. Alzheimers Dis. 2018, 62, 305–317. [Google Scholar] [CrossRef]
- Mangialasche, F.; Kivipelto, M.; Mecocci, P.; Rizzuto, D.; Palmer, K.; Winblad, B.; Fratiglioni, L. High Plasma Levels of Vitamin E Forms and Reduced Alzheimer’s Disease Risk in Advanced Age. J. Alzheimers Dis. 2010, 20, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Solomon, A.; Kåreholt, I.; Hooshmand, B.; Cecchetti, R.; Fratiglioni, L.; Soininen, H.; Laatikainen, T.; Mecocci, P.; Kivipelto, M. Serum Levels of Vitamin E Forms and Risk of Cognitive Impairment in a Finnish Cohort of Older Adults. Exp. Gerontol. 2013, 48, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Wilson, R.S.; Scherr, P.A. Dietary Intake of Antioxidant Nutrients and the Risk of Incident Alzheimer Disease in a Biracial Community Study. JAMA 2002, 287, 3230–3237. [Google Scholar] [CrossRef]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s Disease: What Do We Know so Far? Clin. Interv. Aging 2019, 14, 1303. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of Vitamin E and Memantine on Functional Decline in Alzheimer Disease: The TEAM-AD VA Cooperative Randomized Trial. J. Am. Med. Assoc. 2014, 311, 33. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol. 2017, 74, 567. [Google Scholar] [CrossRef] [PubMed]
- Von Arnim, C.A.F.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G. Dietary Antioxidants and Dementia in a Population-Based Case-Control Study among Older People in South Germany. J. Alzheimers Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef]
- Gravesteijn, E.; Mensink, R.P.; Plat, J. Effects of Nutritional Interventions on BDNF Concentrations in Humans: A Systematic Review. Nutr. Neurosci. 2022, 25, 1425–1436. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Galbete, C.; Martinez-González, M.A.; Martinez, J.A.; Razquin, C.; Salas-Salvadó, J.; Estruch, R.; Buil-Cosiales, P.; Martí, A. The Effect of the Mediterranean Diet on Plasma Brain-Derived Neurotrophic Factor (BDNF) Levels: The PREDIMED-NAVARRA Randomized Trial. Nutr. Neurosci. 2013, 14, 195–201. [Google Scholar] [CrossRef]
- Suzuki, T.; Kojima, N.; Osuka, Y.; Tokui, Y.; Takasugi, S.; Kawashima, A.; Yamaji, T.; Hosoi, E.; Won, C.W.; Kim, H. The Effects of Mold-Fermented Cheese on Brain-Derived Neurotrophic Factor in Community-Dwelling Older Japanese Women with Mild Cognitive Impairment: A Randomized, Controlled, Crossover Trial. J. Am. Med. Dir. Assoc. 2019, 20, 1509–1514. [Google Scholar] [CrossRef]
- Sandberg, J.C.; Björck, I.M.E.; Nilsson, A.C. Increased Plasma Brain-Derived Neurotrophic Factor 10.5 h after Intake of Whole Grain Rye-Based Products in Healthy Subjects. Nutrients 2018, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Amani, R.; Tarrahi, M.J. Effect of Zinc Supplementation on Physical and Psychological Symptoms, Biomarkers of Inflammation, Oxidative Stress, and Brain-Derived Neurotrophic Factor in Young Women with Premenstrual Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2020, 194, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Solati, Z.; Jazayeri, S.; Tehrani-Doost, M.; Mahmoodianfard, S.; Gohari, M.R. Zinc Monotherapy Increases Serum Brain-Derived Neurotrophic Factor (BDNF) Levels and Decreases Depressive Symptoms in Overweight or Obese Subjects: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutr. Neurosci. 2014, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015, 35, 406–410. [Google Scholar] [CrossRef] [PubMed]
| Authors | Type of Diet/Life Situation | Study Design | Study Population | Results | Reference |
|---|---|---|---|---|---|
| Lee et al. | Obesity | Pilot study | 10 obese patients before and after weight loss intervention | Changes in adipocyte APP expression correlated with changes in plasma Aβ40 levels (R = 0.74, p = 0.01). | [36] |
| Ye et al. | Malnutrition | Longitudinal study | 747 patients with aMCI | The underweight group had a higher risk of probable Alzheimer’s disease dementia (pADD), and the decreased-BMI (HR: 2.29, 95% CI: 1.41–3.72) groups were at increased risk of progression to pADD. | [44] |
| Hanson et al. | Malnutrition | Prospective cohort | 256 nursing home residents with advanced dementia and feeding problems | Significant mortality risk in patients with feeding problems | [45] |
| Laitinen et al. | High-fat diet | Population-based study | 1449 patients with 117 who had dementia | Moderate intake of polyunsaturated fats in mid-life decreased the risk of dementia even after adjustment for demographic variables, especially among the ApoE epsilon4 carriers. | [54] |
| Ruan et al. | High-fat diet | Meta-analysis of cohort studies | 8630 participants and 633 cases | A higher dietary saturated fat intake was significantly associated with increased risks of 39% and 105% for AD and dementia. | [55] |
| Jacka et al. | Western diet | Longitudinal study | 255 persons from the Personality and Total Health Through Life Study | Higher consumption of an unhealthy “Western” dietary pattern was associated with smaller left hippocampal volume. | [67] |
| Gibson et al. | Western diet | Observational study | 23 women with polycystic ovary syndrome from 25 to 45 years old | Greater intakes of saturated and trans fats, and higher saturated-to-unsaturated fat ratio (Sat:UFA) were associated with more errors in the visuospatial task and poorer word recall and recognition. | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroszkiewicz, J.; Mroczko, J.; Rutkowski, P.; Mroczko, B. Molecular Aspects of a Diet as a New Pathway in the Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 10751. https://doi.org/10.3390/ijms241310751
Doroszkiewicz J, Mroczko J, Rutkowski P, Mroczko B. Molecular Aspects of a Diet as a New Pathway in the Prevention and Treatment of Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(13):10751. https://doi.org/10.3390/ijms241310751
Chicago/Turabian StyleDoroszkiewicz, Julia, Jan Mroczko, Piotr Rutkowski, and Barbara Mroczko. 2023. "Molecular Aspects of a Diet as a New Pathway in the Prevention and Treatment of Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 13: 10751. https://doi.org/10.3390/ijms241310751
APA StyleDoroszkiewicz, J., Mroczko, J., Rutkowski, P., & Mroczko, B. (2023). Molecular Aspects of a Diet as a New Pathway in the Prevention and Treatment of Alzheimer’s Disease. International Journal of Molecular Sciences, 24(13), 10751. https://doi.org/10.3390/ijms241310751







