Prenatal SAMe Treatment Changes via Epigenetic Mechanism/s USVs in Young Mice and Hippocampal Monoamines Turnover at Adulthood in a Mouse Model of Social Hierarchy and Depression
Abstract
1. Introduction
2. Results
2.1. SAMe Effect on Stress-Induced USVs in Sub Offspring
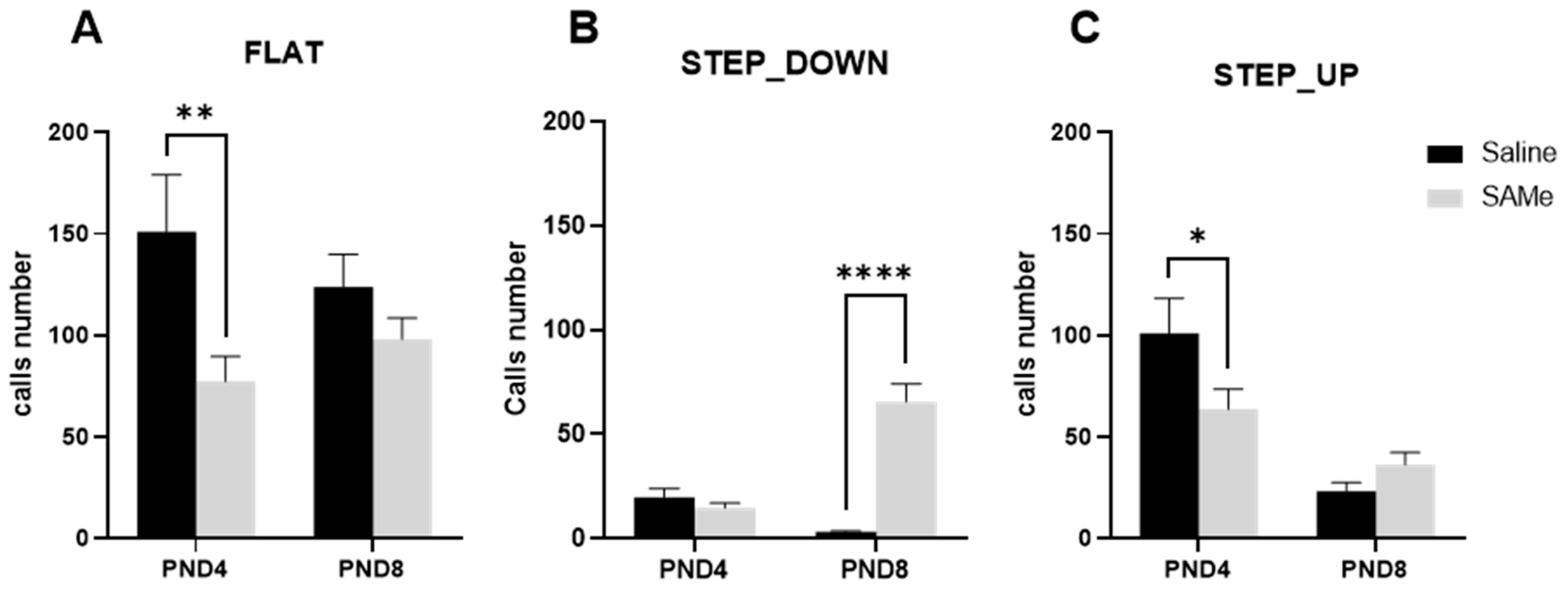
2.1.1. SAMe Effect on Total Number of Emission Calls in Offspring
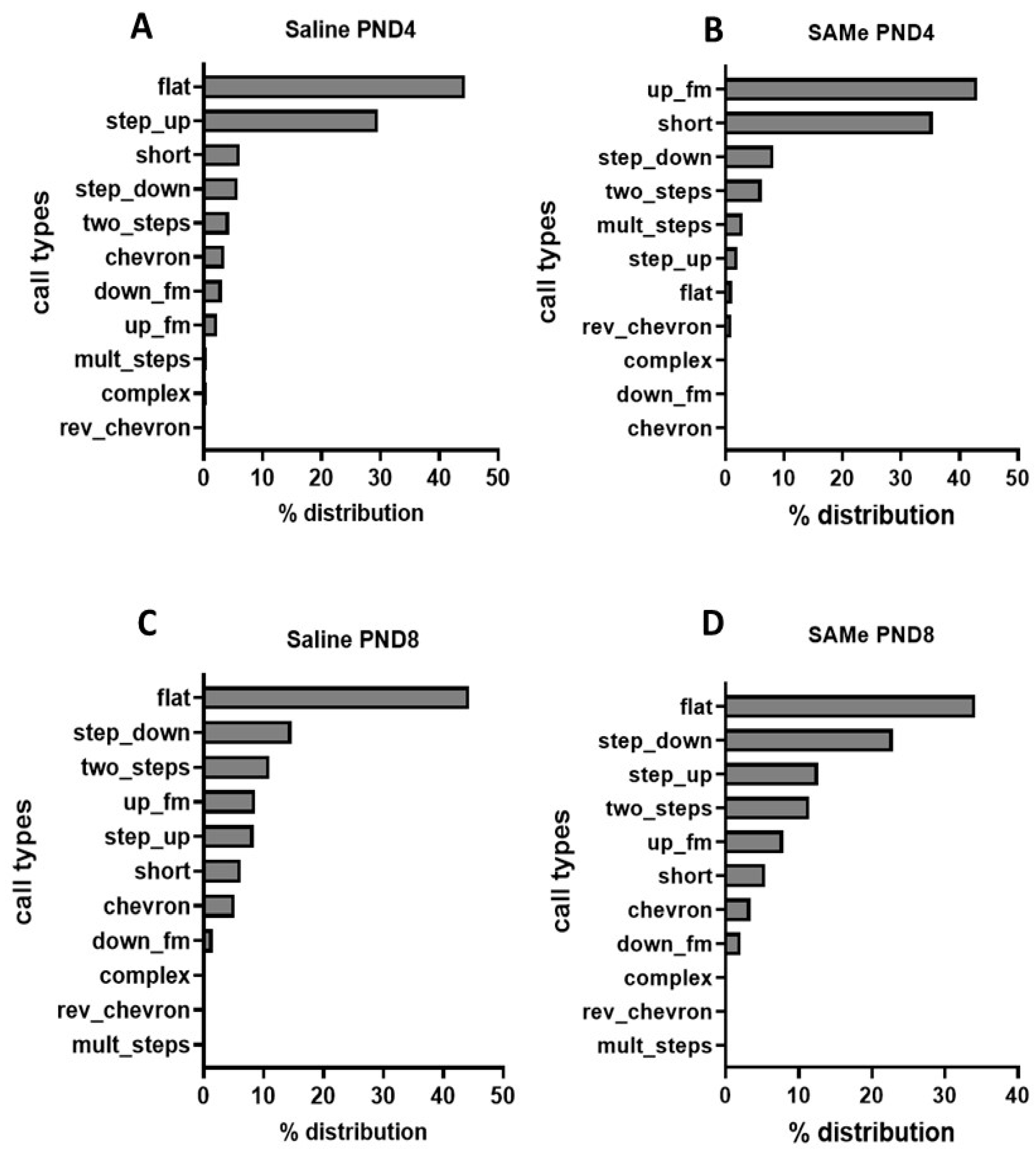
2.1.2. SAMe Effect on the Distribution of Call Types
2.2. SAMe Effect on Serotonin Metabolism in the Hippocampus
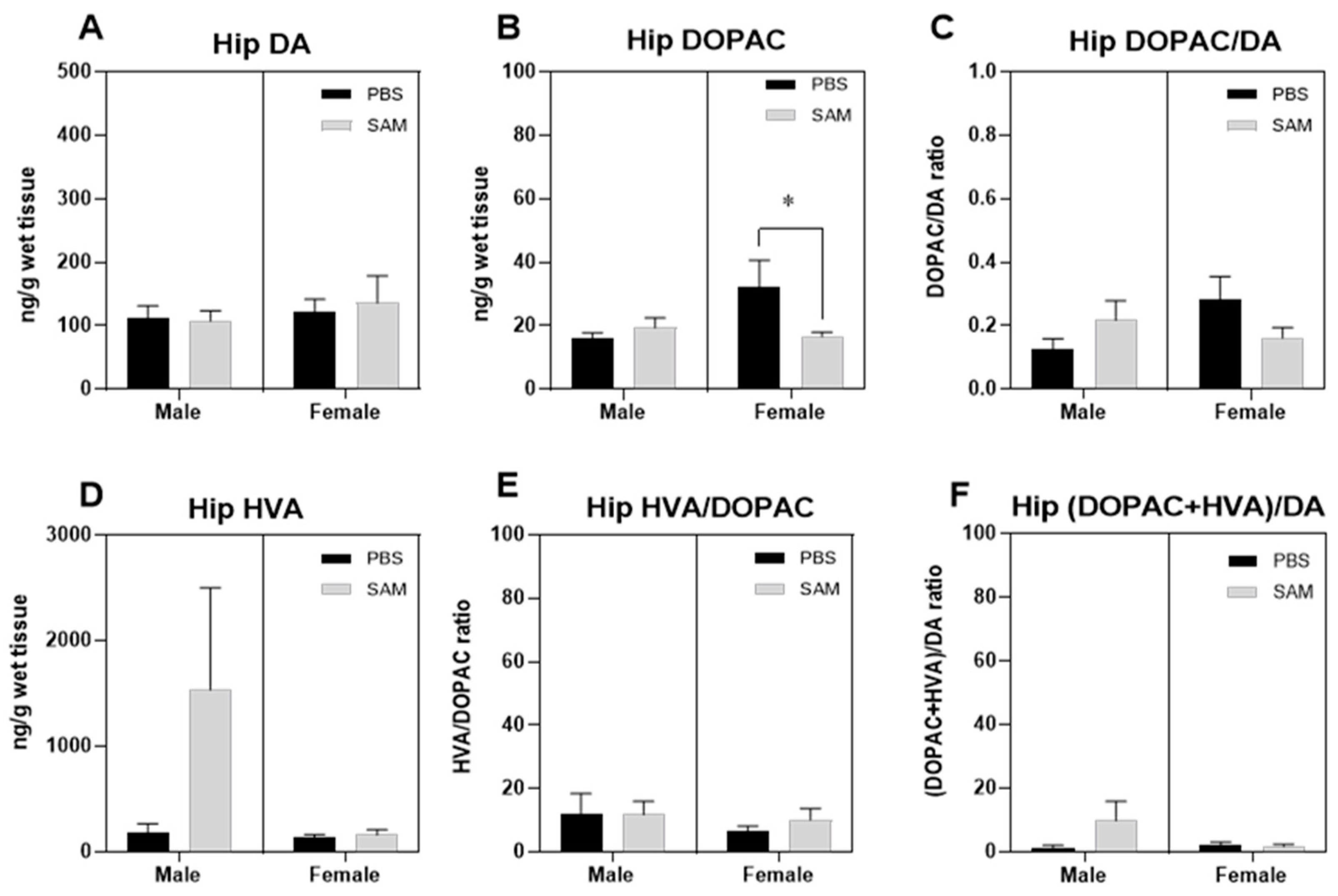
2.3. SAMe Effect on Dopamine, Dopamine Metabolites and of Metabolite/Dopamine Ratio in the Hippocampus
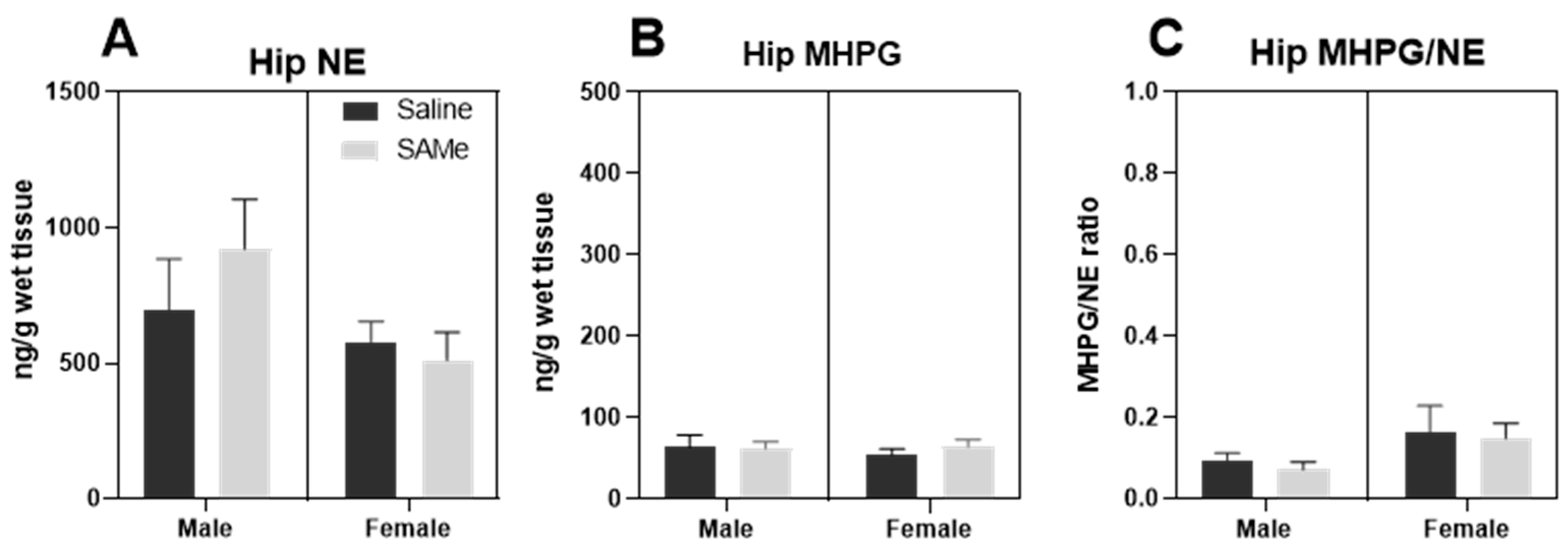
2.4. SAMe Effect on Norepinephrine Metabolism in the Hippocampus
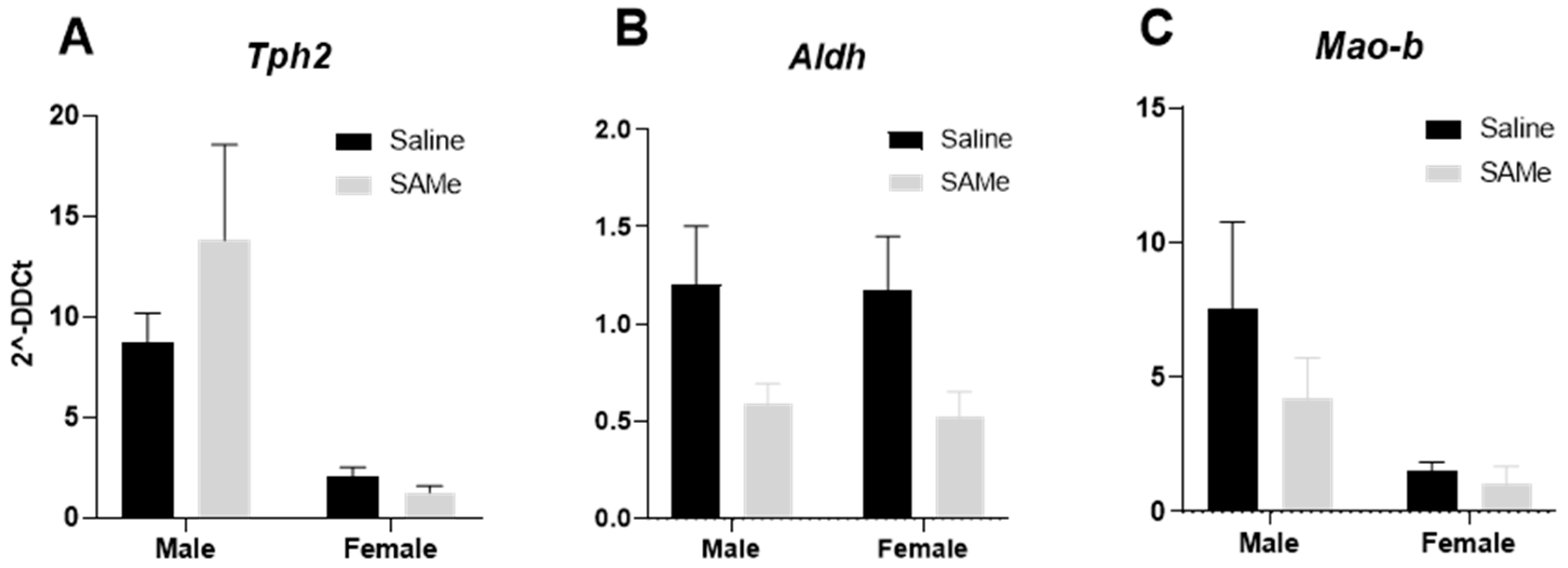
2.5. SAMe Effects on Tph2, Mao-b and Aldh Gene Expression in the in Hippocampus
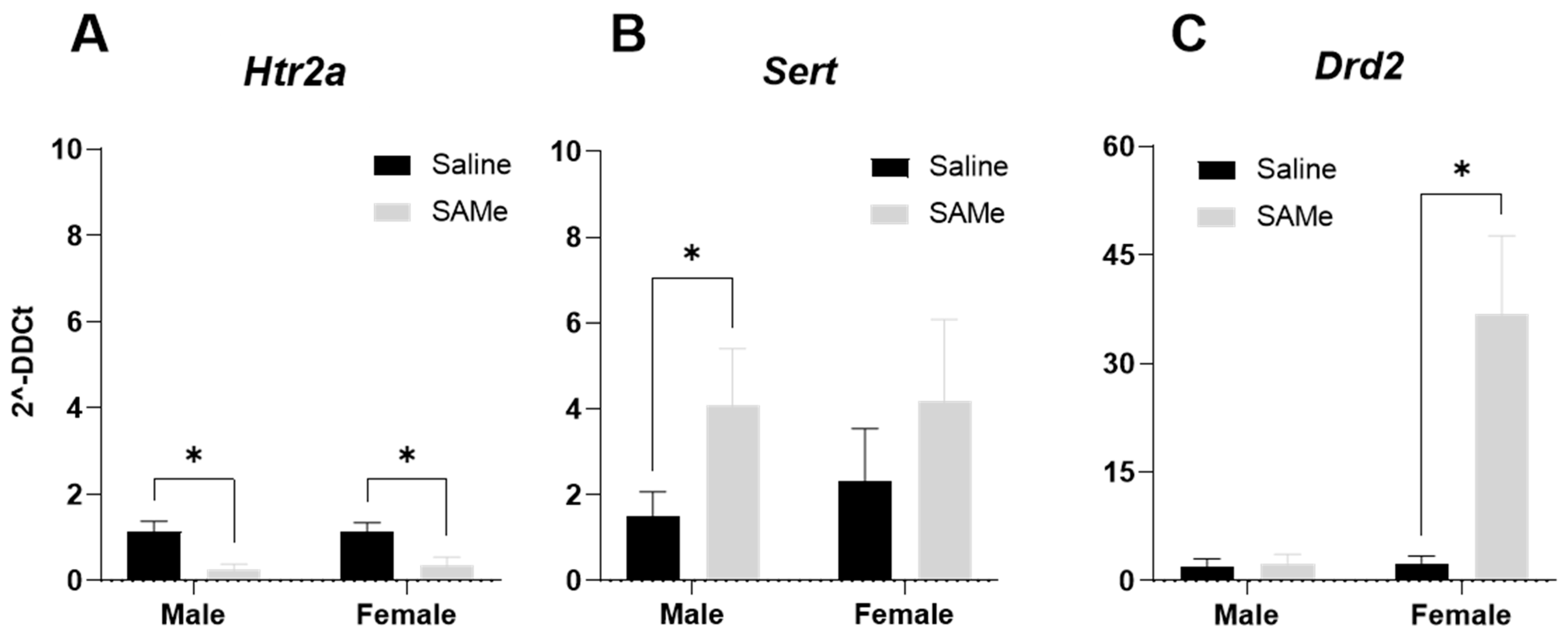
2.6. SAMe Effects on Htr2a, Sert, Drd2 Receptor Genes Expression in the Hippocampus of Sub Mice
3. Discussion
3.1. SAMe Effect on USVs Emitted by Stressed Pups in Relation to Depression and Sociability
3.2. Hippocampal Monoamines in Relation to Previously Described Changes in the Prefrontal Cortex
4. Materials and Methods
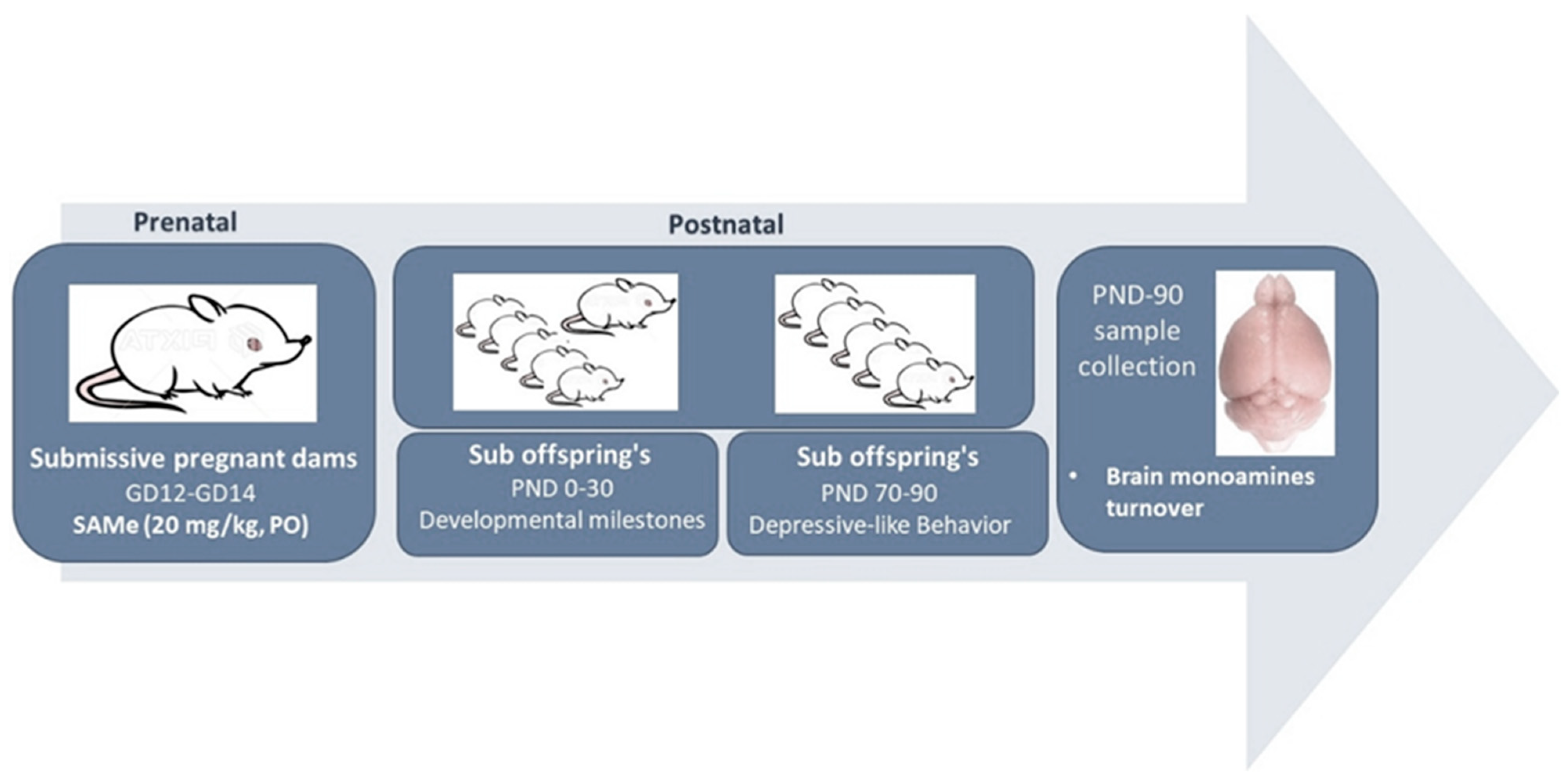
4.1. Animals
4.2. USV Recording and Analysis
- Short syllables—short vocalizations with durations shorter than 5 ms.
- Flat syllables—calls with small frequency modulations or without frequency modulations (less than 5 kHz).
- Upward syllables—the frequency modulation at the end of the call is ≥6 kHz than the frequency at the start.
- Downward syllables—the frequency modulation at the end of the call is ≤6 kHz than the frequency at the start.
- Complex—single syllables consisting of combined bidirectional frequency modulations greater than 6 kHz.
- One frequency step up—two-component vocalizations with no time difference between them and a second component where the emission frequency is higher than the emission frequency of the first by ≥10 kHz.
- One frequency step down—two-component vocalizations with no time difference between them and a second component where the emission frequency is lower than the emission frequency of the first by ≥10 kHz.
- Chevron syllables—a type of call with a reversed U shape, in which the difference between the highest point and the start and end points is ≥6 kHz.
- Reverse chevron—a type of call with a U-shaped syllable type, in which the difference between the lowest point and the start and end points is ≥6 kHz.
- Multiple frequency steps—a more than three-component vocalization, with differences between the frequencies of each component’s emission.
- Two frequency steps—a three-component vocalization with no time delay between the emission of each component, where second component differs from the first by ≥10 kHz, and the third one differs from the second by ≥10 kHz.
4.3. Monoamine Measurements
4.4. Gene Expression Studies
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine or serotonin |
| 5-HT2AR | 5-hydroxytryptamine receptor 2A |
| Aldh | Aldehyde dehydrogenase gene |
| ASD | Autism Spectrum Disorder |
| COMT | Catechol-O-methyl transferase |
| CSF | Cerebrospinal fluid |
| DA | Dopamine |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| DRD1 | Dopamine receptors D1 |
| DRD2 | Dopamine receptors D2 |
| EPM | Elevated Plus Maze |
| HPC | Hippocampus |
| HT1A | Gene 5-hydroxytryptamine receptor 1A gene |
| HT2A | Gene for 5-hydroxytryptamine receptor 2A |
| HVA | Homovanillic acid |
| MAO-A | Monoamine oxidase A |
| MAO-B | Monoamine oxidase B |
| MDD | Major Depressive Disorder |
| MHPG | 4-hydroxy-3-Methoxyphenylglycol |
| NE | Norepinephrine |
| OF | Open field |
| PFC | Prefrontal cortex |
| PND | Postnatal day |
| SAMe | S-adenosyl-methionine |
| SERT | Serotonin transporter |
| Sub mice | Submissive mice |
| TCA | Tricyclic antidepressants |
| Tph2 | Tryptophan hydroxylase enzyme 2 |
| USVs | Ultrasonic vocalizations |
References
- McCoy, C.R.; Jackson, N.L.; Day, J.; Clinton, S.M. Genetic predisposition to high anxiety- and depression-like behavior coincides with diminished DNA methylation in the adult rat amygdala. Behav. Brain Res. 2017, 320, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Darby, M.M.; Yolken, R.H.; Sabunciyan, S. Consistently altered expression of gene sets in postmortem brains of individuals with major psychiatric disorders. Transl. Psychiatry 2016, 6, e890. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. S-Adenosine Methionine (SAMe) and Valproic Acid (VPA) as Epigenetic Modulators: Special Emphasis on their Interactions Affecting Nervous Tissue during Pregnancy. Int. J. Mol. Sci. 2020, 21, 3721. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Weinstein-Fudim, L.; Becker, M. SAMe, Choline, and Valproic Acid as Possible Epigenetic Drugs: Their Effects in Pregnancy with a Special Emphasis on Animal Studies. Pharmaceuticals 2022, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Sghendo, L.; Mifsud, J. Understanding the molecular pharmacology of the serotonergic system: Using fluoxetine as a model. J. Pharm. Pharmacol. 2012, 64, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Bottiglieri, T.; Godfrey, P.; Flynn, T.; Carney, M.W.; Toone, B.K.; Reynolds, E.H. Cerebrospinal fluid S-adenosyl-methionine in depression and dementia: Effects of treatment with parenteral and oral S-adenosyl-methionine. J. Neurol. Neurosurg. Psychiatry 1990, 53, 1096–1098. [Google Scholar] [CrossRef]
- Wilson, A. S-adenosyl-methionine (SAMe) for Depression in Adults. Issues Ment. Health Nurs. 2019, 40, 725–772. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Fava, M. S-adenosyl-methionine (SAMe) Augmentation of Serotonin Reuptake Inhibitors for Antidepressant Nonresponders with Major Depressive Disorder: A Double-Blind, Randomized Clinical Trial. Am. J. Psychiatry 2010, 167, 942–948. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. Adjunctive S-adenosyl-methionine (SAMe) in treating non-remittent major depressive disorder: An 8-week double-blind, randomized, controlled trial. Eur. Neuropsychopharmacol. 2018, 28, 1126–1136. [Google Scholar] [CrossRef]
- Mischoulon, D.; Fava, M. Role of S-adenosyl-l-methionine in the treatment of depression: A review of the evidence. Am. J. Clin. Nutr. 2002, 76, 1158S–1161S. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hattori, K.; Ogawa, S.; Sasayama, D.; Ota, M.; Teraishi, T.; Kunugi, H. Relationships of Cerebrospinal Fluid Monoamine Metabolite Levels with Clinical Variables in Major Depressive Disorder. J. Clin. Psychiatry 2017, 78, e947–e956. [Google Scholar] [CrossRef]
- Gross, M.; Romi, H.; Miller, A.; Pinhasov, A. Social dominance predicts hippocampal glucocorticoid receptor recruitment and resilience to prenatal adversity. Sci. Rep. 2018, 8, 9595. [Google Scholar] [CrossRef]
- Nesher, E.; Gross, M.; Lisson, S.; Tikhonov, T.; Yadid, G.; Pinhasov, A. Differential responses to distinct psychotropic agents of selectively bred dominant and submissive animals. Behav. Brain Res. 2013, 236, 225–235. [Google Scholar] [CrossRef]
- Becker, M.; Abaev, K.; Shmerkin, E.; Weinstein-Fudim, L.; Pinhasov, A.; Ornoy, A. Prenatal SAMe Treatment Induces Changes in Brain Monoamines and in the Expression of Genes Related to Monoamine Metabolism in a Mouse Model of Social Hierarchy and Depression, Probably via an Epigenetic Mechanism. Int. J. Mol. Sci. 2022, 23, 11898. [Google Scholar] [CrossRef]
- Murlanova, K.; Michaelevski, I.; Kreinin, A.; Terrillion, C.; Pletnikov, M.; Pinhasov, A. Link between temperament traits, brain neurochemistry and response to SSRI: Insights from animal model of social behavior. J. Affect. Disord. 2020, 282, 1055–1066. [Google Scholar] [CrossRef]
- Salamone, J.D.; Yohn, S.E.; López-Cruz, L.; San Miguel, N.; Correa, M. Activational and effort-related aspects of motivation: Neural mechanisms and implications for psychopathology. Brain 2016, 139, 1325–1347. [Google Scholar] [CrossRef]
- Palmiter, R.D. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 2008, 1129, 35–46. [Google Scholar] [CrossRef]
- Becker, M.; Abaev, K.; Pinhasov, A.; Ornoy, A. S-adenosyl-methionine alleviates sociability aversion and reduces changes in gene expression in a mouse model of social hierarchy. Behav. Brain Res. 2022, 427, 113866. [Google Scholar] [CrossRef]
- Simola, N.; Granon, S. Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacol. Soc. Behav. Bench Bedside 2019, 159, 107420. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G. Infant rodent ultrasounds—A gate to the understanding of sound communication. Behav. Genet. 2005, 35, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Hammerschmidt, K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: Insights into the evolution of vocal communication. Genes Brain Behav. 2011, 10, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Schwarting, R.K.W. Affective communication in rodents: Ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013, 354, 81–97. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Crawley, J.; Ricceri, L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009, 33, 508–515. [Google Scholar] [CrossRef]
- Granon, S.; Faure, A.; Chauveau, F.; Cressant, A.; Ey, E. Chapter 40—Why Should My Mouse Call Me? Acoustic Communication in Mouse Models of Social Disorders: Ultrasonic Vocalizations as an Index of Emotional and Motivational States. In Handbook of Behavioral Neuroscience; Brudzynski, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 423–431. [Google Scholar]
- Jouda, J.; Wöhr, M.; del Rey, A. Immunity and ultrasonic vocalization in rodents. Ann. N. Y. Acad. Sci. 2019, 1437, 68–82. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Gandhy, S.U.; Ricceri, L.; Crawley, J.N. Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism. PLoS ONE 2008, 3, e3067. [Google Scholar] [CrossRef]
- Chabout, J.; Serreau, P.; Ey, E.; Bellier, L.; Aubin, T.; Bourgeron, T.; Granon, S. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS ONE 2012, 7, e29401. [Google Scholar] [CrossRef]
- Faure, A.; Pittaras, E.; Nosjean, A.; Chabout, J.; Cressant, A.; Granon, S. Social behaviors and acoustic vocalizations in different strains of mice. Behav. Brain Res. 2017, 320, 383–390. [Google Scholar] [CrossRef]
- Grimsley, J.M.S.; Monaghan, J.J.M.; Wenstrup, J.J. Development of Social Vocalizations in Mice. PLoS ONE 2011, 6, e17460. [Google Scholar] [CrossRef]
- Heun-Johnson, H.; Levitt, P. Early-Life Stress Paradigm Transiently Alters Maternal Behavior, Dam-Pup Interactions, and Offspring Vocalizations in Mice. Front. Behav. Neurosci. 2016, 10, 142. [Google Scholar] [CrossRef]
- Yin, X.; Chen, L.; Xia, Y.; Cheng, Q.; Yuan, J.; Yang, Y.; Wang, Z.; Wang, H.; Dong, J.; Ding, Y.; et al. Maternal Deprivation Influences Pup Ultrasonic Vocalizations of C57BL/6J Mice. PLoS ONE 2016, 11, e0160409. [Google Scholar] [CrossRef]
- Premoli, M.; Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Aria, F.; Paiardi, G.; Memo, M. Specific profile of ultrasonic communication in a mouse model of neurodevelopmental disorders. Sci. Rep. 2019, 9, 15912. [Google Scholar] [CrossRef]
- Lumley, L.A.; Sipos, M.L.; Charles, R.C.; Charles, R.F.; Meyerhoff, J.L. Social Stress Effects on Territorial Marking and Ultrasonic Vocalizations in Mice. Physiol. Behav. 1999, 67, 769–775. [Google Scholar] [CrossRef]
- Brunelli, S.A.; Curley, J.P.; Gudsnuk, K.; Champagne, F.A.; Myers, M.M.; Hofer, M.A.; Welch, M.G. Variations in maternal behavior in rats selected for infant ultrasonic vocalization in isolation. Horm. Behav. 2015, 75, 78–83. [Google Scholar] [CrossRef]
- Feifel, A.J.; Shair, H.N.; Schmauss, C. Lasting effects of early life stress in mice: Interaction of maternal environment and infant genes. Genes Brain Behav. 2017, 16, 768–780. [Google Scholar] [CrossRef]
- Moles, A.; Kieffer, B.L.; D’Amato, F.R. Deficit in attachment behavior in mice lacking the µ-opioid receptor gene. Science 2004, 304, 1983–1986. [Google Scholar] [CrossRef]
- McNamara, G.I.; Creeth, H.D.J.; Harrison, D.J.; Tansey, K.E.; Andrews, R.M.; Isles, A.R.; John, R.M. Loss of offspring Peg3 reduces neonatal ultrasonic vocalizations and increases maternal anxiety in wild-type mothers. Hum. Mol. Genet. 2018, 27, 440–450. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Felice, D.; Galimberti, S.; Savignac, H.M.; Bravo, J.A.; Crowley, T.; El Yacoubi, M.; Vaugeois, J.-M.; Gassmann, M.; Bettler, B.; et al. GABAB(1) receptor subunit isoforms differentially regulate stress resilience. Proc. Natl. Acad. Sci. USA 2014, 111, 15232–15237. [Google Scholar] [CrossRef]
- Fonseca, J.; Douzas, G.; Bacao, F. Increasing the Effectiveness of Active Learning: Introducing Artificial Data Generation in Active Learning for Land Use/Land Cover Classification. Remote Sens. 2021, 13, 2619. [Google Scholar] [CrossRef]
- Bach, H.; Huang, Y.-Y.; Underwood, M.D.; Dwork, A.J.; Mann, J.J.; Arango, V. Elevated serotonin and 5-HIAA in the brainstem and lower serotonin turnover in the prefrontal cortex of suicides. Synapse 2014, 68, 127–130. [Google Scholar] [CrossRef]
- Barton, D.A.; Esler, M.D.; Dawood, T.; Lambert, E.A.; Haikerwal, D.; Brenchley, C.; Socratous, F.; Hastings, J.; Guo, L.; Wiesner, G.; et al. Elevated Brain Serotonin Turnover in Patients with Depression: Effect of Genotype and Therapy. Arch. Gen. Psychiatry 2008, 65, 38–46. [Google Scholar] [CrossRef]
- Shannon, N.J.; Gunnet, J.W.; Moore, K.E. A comparison of biochemical indices of 5-hydroxytryptaminergic neuronal activity following electrical stimulation of the dorsal raphe nucleus. J. Neurochem. 1986, 47, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol. Rev. 2004, 56, 331. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; Ricceri, L.; Crawley, J.N. Unusual repertoire of vocalizations in adult BTBR T+ tf/J mice during three types of social encounters. Genes Brain Behav. 2011, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Watkins, N.; Heck, D. Comprehensive Analysis of Ultrasonic Vocalizations in a Mouse Model of Fragile X Syndrome Reveals Limited, Call Type Specific Deficits. PLoS ONE 2012, 7, e44816. [Google Scholar] [CrossRef]
- Nolan, S.O.; Hodges, S.L.; Lugo, J.N. High-throughput analysis of vocalizations reveals sex-specific changes in Fmr1 mutant pups. Genes Brain Behav. 2020, 19, e12611. [Google Scholar] [CrossRef]
- Grant, L.M.; Richter, F.; Miller, J.E.; White, S.A.; Fox, C.M.; Zhu, C.; Chesselet, M.-F.; Ciucci, M.R. Vocalization deficits in mice over-expressing alpha-synuclein, a model of pre-manifest Parkinson’s disease. Behav. Neurosci. 2014, 128, 110–121. [Google Scholar] [CrossRef]
- Premoli, M.; Pietropaolo, S.; Wöhr, M.; Simola, N.; Bonini, S.A. Mouse and rat ultrasonic vocalizations in neuroscience and neuropharmacology: State of the art and future applications. Eur. J. Neurosci. 2023. [Google Scholar] [CrossRef]
- Cagiano, R.; Sales, G.D.; Renna, G.; Racagni, G.; Cuomo, V. Ultrasonic vocalization in rat pups: Effects of early postnatal exposure to haloperidol. Life Sci. 1986, 38, 1417–1423. [Google Scholar] [CrossRef]
- Cuomo, V.; Cagiano, R.; Renna, G.; De Salvia, M.A.; Racagni, G. Ultrasonic vocalization in rat pups: Effects of early postnatal exposure to SCH 23390 (a DA1-receptor antagonist) and sulpiride (a DA2-receptor antagonist). Neuropharmacology 1987, 26, 701–705. [Google Scholar] [CrossRef]
- Curry, T.; Egeto, P.; Wang, H.; Podnos, A.; Wasserman, D.; Yeomans, J. Dopamine receptor D2 deficiency reduces mouse pup ultrasonic vocalizations and maternal responsiveness. Genes Brain Behav. 2013, 12, 397–404. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Burgdorf, J.; Wess, J.; Yeomans, J. Ultrasonic Vocalizations Induced by Sex and Amphetamine in M2, M4, M5 Muscarinic and D2 Dopamine Receptor Knockout Mice. PLoS ONE 2008, 3, e1893. [Google Scholar] [CrossRef]
- Budylin, T.; Guariglia, S.R.; Duran, L.I.; Behring, B.M.; Shaikh, Z.; Neuwirth, L.S.; Banerjee, P. Ultrasonic vocalization sex differences in 5-HT1A-R deficient mouse pups: Predictive phenotypes associated with later-life anxiety-like behaviors. Behav. Brain Res. 2019, 373, 112062. [Google Scholar] [CrossRef]
- Mosienko, V.; Beis, D.; Alenina, N.; Wöhr, M. Reduced isolation-induced pup ultrasonic communication in mouse pups lacking brain serotonin. Mol. Autism 2015, 6, 13. [Google Scholar] [CrossRef]
- Borovok, N.; Nesher, E.; Reichenstein, M.; Tikhonova, T.; Levin, Y.; Pinhasov, A.; Michaelevski, I. Effect of social interactions on hippocampal protein expression in animal dominant and submissive model of behavioral disorders. Proteom.—Clin. Appl. 2017, 11, 1700089. [Google Scholar] [CrossRef]
- Gross, M.; Romi, H.; Gilimovich, Y.; Drori, E.; Pinhasov, A. Placental glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase-2 recruitment indicates impact of prenatal adversity upon postnatal development in mice. Stress 2018, 21, 474–483. [Google Scholar] [CrossRef]
- Delva, N.C.; Stanwood, G.D. Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Cuzzocrea, S.; Iavicoli, I.; Rizzarelli, E.; Calabrese, E.J. Hormesis, cellular stress response and vitagenes as determinants in aging and longevity. Mol. Asp. Med. 2011, 901, 279–304. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J. Vitagenes, cellular stress response, and acetylcarnitine: Relevance to hormesis. BioFactors 2009, 35, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Weinstein-Fudim, L.; Tfilin, M.; Ergaz, Z.; Yanai, J.; Szyf, M.; Turgeman, G. S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicology Teratol. 2019, 71, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, R.; Deng, Q.; Wang, W.; Cao, C.; Yu, C.; Li, S.; Shi, L.; Tian, J. S-adenosyl-methionine improves cognitive impairment in D-galactose-induced brain aging by inhibiting oxidative stress and neuroinflammation. J. Chem. Neuroanat. 2023, 128, 102232. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Chen, K.; Shih, J.C. Monoamine oxidase inactivation: From pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Feder, Y.; Nesher, E.; Ogran, A.; Kreinin, A.; Malatynska, E.; Yadid, G.; Pinhasov, A. Selective breeding for dominant and submissive behavior in Sabra mice. J. Affect. Disord. 2010, 126, 214–222. [Google Scholar] [CrossRef]
- Malatynska, E.; Pinhasov, A.; Crooke, J.J.; Smith-Swintosky, V.L.; Brenneman, D.E. Reduction of dominant or submissive behaviors as models for antimanic or antidepressant drug testing: Technical considerations. J. Neurosci. Methods 2007, 165, 175–182. [Google Scholar] [CrossRef]
- Sueur, J.; Aubin, T.; Simonis, C. Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics 2008, 18, 213–226. [Google Scholar] [CrossRef]
- Ligges, U.; Krey, S.; Mersmann, O.; Schnackenberg, S. TuneR: Analysis of Music and Speech. 2023. Available online: https://CRAN.R-project.org/package=tuneR (accessed on 1 May 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Garnier, S.; Ross, N.; Bo, B.R.; Filipovic-Pierucci, A.; Galili, T.; Timelyportfolio; Greenwell, B.; Sievert, C.; Harris, D.J.; Chen, J.J. sjmgarnier/viridis: CRAN release v0.6.2, v0.6.0pre; Zenodo. 2021. Available online: https://zenodo.org/record/5579397 (accessed on 1 May 2023).
- Murrell, P.; Graphics, R. R Graphics, 1st ed.; Chapman and Hall: New York, NY, USA, 2005; p. 328. [Google Scholar]
- Auguie, B.; Antonov, A. gridExtra: Miscellaneous Functions for “Grid” Graphics. R Package Version 2.3. Computer Software. 2017. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 1 May 2023).
- Audacity® Software Is Copyright © 1999–2021 Audacity Team. It Is Free Software Distributed Under the Terms of the GNU General Public License. The Name Audacity® Is a registered Trademark. Available online: https://audacityteam.org/ (accessed on 1 May 2023).
- Wolf, W.A.; Youdim, M.B.H.; Kuhn, D.M. Does brain 5-HIAA indicate serotonin release or monoamine oxidase activity? Eur. J. Pharmacol. 1985, 109, 381–387. [Google Scholar] [CrossRef]
- Ogawa, N.; Tanaka, K.; Asanuma, M. Bromocriptine markedly suppresses levodopa-induced abnormal increase of dopamine turnover in the parkinsonian striatum. Neurochem. Res. 2000, 25, 755–758. [Google Scholar] [CrossRef]
- Megyeri, K.; Marko, B.; Sziray, N.; Gacsalyi, I.; Juranyi, Z.; Levay, G.; Harsing, L.G., Jr. Effects of 2,3-benzodiazepine AMPA receptor antagonists on dopamine turnover in the striatum of rats with experimental parkinsonism. Brain Res. Bull. 2007, 71, 501–507. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]


| Prefrontal Cortex | Hippocampus | |||
|---|---|---|---|---|
| Monoamines | Male | Female | Male | Female |
| Serotonin | no change | increase ↑ | increase ↑ | increase ↑ |
| 5 HIAA | no change | no change | no change | no change |
| DA | increase ↑ | increase ↑ | no change | no change |
| DOPAC | increase ↑ | increase ↑ | no change | increase ↑ |
| HVA | no change | increase ↑ | no change | no change |
| Norepinephrine | no change | no change | no change | no change |
| MHPG | no change | no change | no change | no change |
| Prefrontal Cortex | Hippocampus | |||
|---|---|---|---|---|
| Genes | Male | Female | Male | Female |
| Tph2 | no change | no change | no change | no change |
| Htr2a | Downregulated ↓ | no change | Downregulated ↓ | Downregulated ↓ |
| Sert | no change | no change | Upregulated ↑ | Trend to increase |
| Mao-b | Downregulated ↓ | Trend to decrease ↓ | no change | no change |
| Drd2 | no change | no change | no change | Upregulated ↑ |
| Oligo Name | Sequence 5′ to 3′ | |
|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase—Gapdh | F | GGGGCTCTCTGCTCCTCCCTGT |
| R | TGACCCTTTTGGCCCCACCCT | |
| Serotonin receptor—Htr2a | F | CCTGATGTCACTTGCCATAGCTG |
| R | CAGGTAAATCCAGACGGCACAG | |
| Tryptophan hydroxylase 2—Tph2 | F | GTTGATGCTGCGGCTCAGATCT |
| R | GAAGCTCGTCATGCAGTTCACC | |
| Serotonin Transporter—Sert | F | TCGCCTCCTACTACAACACC |
| R | ATGTTGTCCTGGGCGAAGTA | |
| Aldehyde dehydrogenase—Aldh | F | ACACAGAAGTGAAGACGGTTACTG |
| R | TCTTTGGAGGCGCAGTGTGT | |
| Monoamine oxidase B—Mao-b | F | GGGGGCGGCATCTCAGGTAT |
| R | TGCTTCCAGAACCACCACACT | |
| Dopamine receptor D2—Drd2 | F | TTCCCAGTGAACAGGCGGAGA |
| R | TTTGGCAGGACTGTCAGGGTT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, M.; Gorobets, D.; Shmerkin, E.; Weinstein-Fudim, L.; Pinhasov, A.; Ornoy, A. Prenatal SAMe Treatment Changes via Epigenetic Mechanism/s USVs in Young Mice and Hippocampal Monoamines Turnover at Adulthood in a Mouse Model of Social Hierarchy and Depression. Int. J. Mol. Sci. 2023, 24, 10721. https://doi.org/10.3390/ijms241310721
Becker M, Gorobets D, Shmerkin E, Weinstein-Fudim L, Pinhasov A, Ornoy A. Prenatal SAMe Treatment Changes via Epigenetic Mechanism/s USVs in Young Mice and Hippocampal Monoamines Turnover at Adulthood in a Mouse Model of Social Hierarchy and Depression. International Journal of Molecular Sciences. 2023; 24(13):10721. https://doi.org/10.3390/ijms241310721
Chicago/Turabian StyleBecker, Maria, Denis Gorobets, Elena Shmerkin, Liza Weinstein-Fudim, Albert Pinhasov, and Asher Ornoy. 2023. "Prenatal SAMe Treatment Changes via Epigenetic Mechanism/s USVs in Young Mice and Hippocampal Monoamines Turnover at Adulthood in a Mouse Model of Social Hierarchy and Depression" International Journal of Molecular Sciences 24, no. 13: 10721. https://doi.org/10.3390/ijms241310721
APA StyleBecker, M., Gorobets, D., Shmerkin, E., Weinstein-Fudim, L., Pinhasov, A., & Ornoy, A. (2023). Prenatal SAMe Treatment Changes via Epigenetic Mechanism/s USVs in Young Mice and Hippocampal Monoamines Turnover at Adulthood in a Mouse Model of Social Hierarchy and Depression. International Journal of Molecular Sciences, 24(13), 10721. https://doi.org/10.3390/ijms241310721






