A New Method to Detect Variants of SARS-CoV-2 Using Reverse Transcription Loop-Mediated Isothermal Amplification Combined with a Bioluminescent Assay in Real Time (RT-LAMP-BART)
Abstract
1. Introduction
2. Results
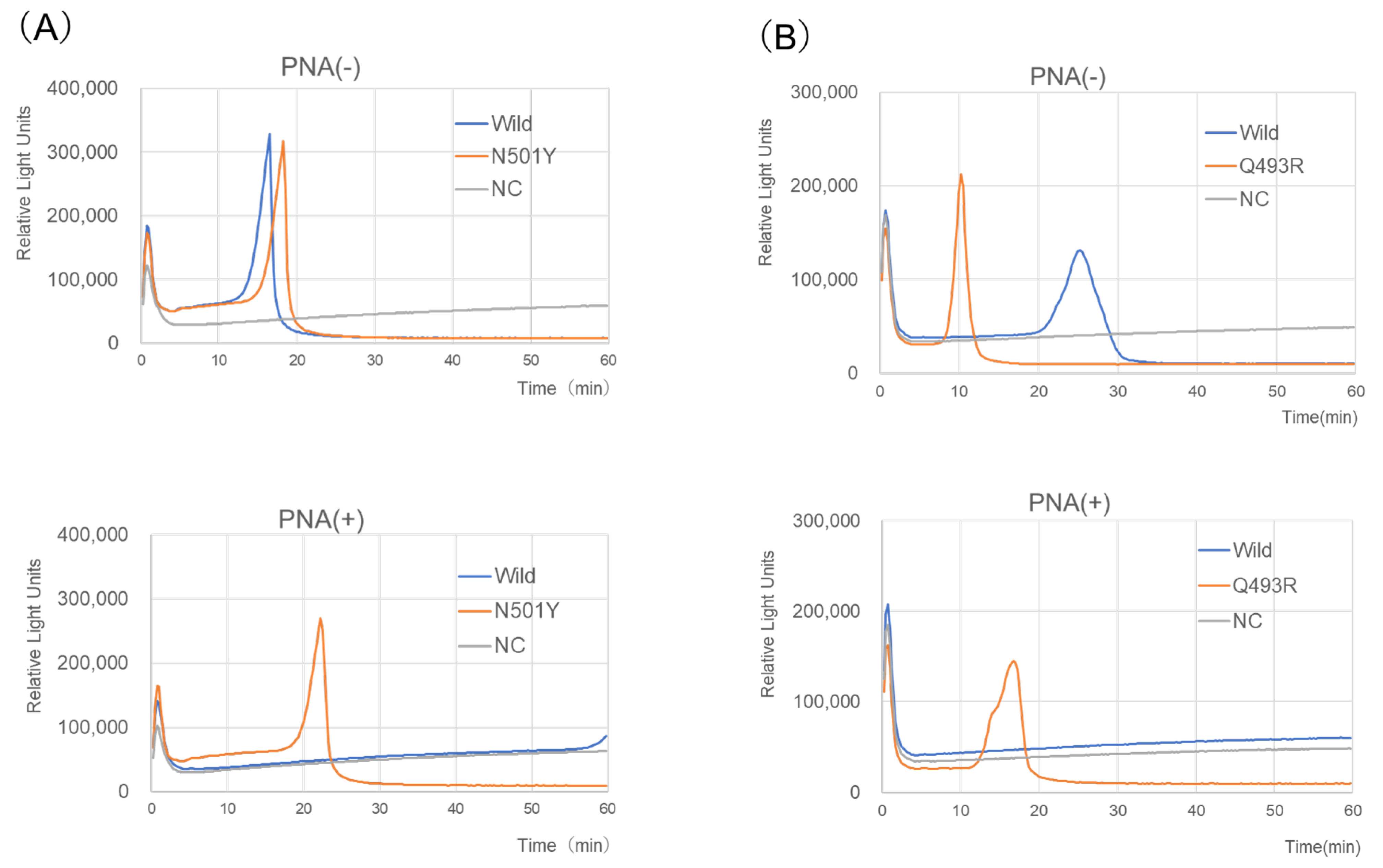
2.1. Analytical Reactivity and Specificity
2.2. Reaction Time and Analytical Sensitivity
2.3. RT-LAMP-BART Assays of RNA-Spiked Specimens
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Viral and Bacterial Strains
5.2. Preparation of Genomic DNA/RNA
5.3. Synthetic SARS-CoV-2 RNA and Analytical Sensitivity
5.4. RNA-Spiked Clinical Specimens
5.5. LAMP Primer Design
5.6. RT-LAMP-BART Assay
5.7. Analysis of RT-LAMP-BART Products
5.8. In Silico Analysis
5.9. Real-Time RT-PCR
5.10. Ethics Statement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Um, J.; Antigua, K.J.C.; Park, J.H.; Kim, Y.; Oh, S.; Kim, Y.I.; Choi, W.S.; Kim, S.G.; Jeong, J.H.; et al. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Hodcroft, E. Shared Mutations. Available online: https://covariants.org/shared-mutations (accessed on 3 November 2022).
- TAKARA about Detection of Omicron Strain (B.1.1.529+BA.* Strain) Using this Product Series. Available online: https://catalog.takara-bio.co.jp/product/basic_info.php?unitid=U100009522 (accessed on 1 April 2023).
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): Expansion of its practical application as a tool to achieve universal health coverage. J. Infect. Chemother. 2020, 26, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.C.; Mai, Q.L.; Cuong, D.V.; Parida, M.; Minekawa, H.; Notomi, T.; Hasebe, F.; Morita, K. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004, 42, 1956–1961. [Google Scholar] [PubMed]
- Shirato, K.; Yano, T.; Senba, S.; Akachi, S.; Kobayashi, T.; Nishinaka, T.; Notomi, T.; Matsuyama, S. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP). Virol. J. 2014, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Gandelman, O.A.; Church, V.L.; Moore, C.A.; Kiddle, G.; Carne, C.A.; Parmar, S.; Jalal, H.; Tisi, L.C.; Murray, J.A. Novel bioluminescent quantitative detection of nucleic acid amplification in real-time. PLoS ONE 2010, 5, e14155. [Google Scholar] [CrossRef] [PubMed]
- Iijima, T.; Ando, S.; Kanamori, D.; Kuroda, K.; Nomura, T.; Tisi, L.; Kilgore, P.E.; Percy, N.; Kohase, H.; Hayakawa, S.; et al. Detection of SARS-CoV-2 and the L452R spike mutation using reverse transcription loop-mediated isothermal amplification plus bioluminescent assay in real-time (RT-LAMP-BART). PLoS ONE 2022, 17, e0265748. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, H.; Tamura, Y.; Yoshida, H.; Kinoshita, H.; Katsuta, H.; Matsui, C.; Matsushita, A.; Arai, T.; Hashimoto, S.; Iuchi, A.; et al. Clinical COVID-19 diagnostic methods: Comparison of reverse transcription loop-mediated isothermal amplification (RT-LAMP) and quantitative RT-PCR (qRT-PCR). J. Clin. Virol. 2021, 139, 104813. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.; Lim, B.; Hsu, C.C.; Xiong, D.; Wu, W.; Yu, Y.; Jia, H.; Wang, Y.; Zeng, Y.; Ji, M.; et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020, 13, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020, 26, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Orihara, Y.; Kawamura, R.; Imai, K.; Sakai, J.; Tarumoto, N.; Matsuoka, M.; Takeuchi, S.; Maesaki, S.; Maeda, T. Evaluation of rapid diagnosis of novel coronavirus disease (COVID-19) using loop-mediated isothermal amplification. J. Clin. Virol. 2020, 129, 104446. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, Y.; Mori, A.; Miyamoto, S.; Ito, E.; Arikawa, K.; Iwamoto, T. Comparison of RT-PCR, RT-LAMP, and Antigen Quantification Assays for the Detection of SARS-CoV-2. Jpn. J. Infect. Dis. 2022, 75, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Wei, R.; Cheng, C.; Xiao, P. A Novel Approach to the Bioluminescent Detection of the SARS-CoV-2 ORF1ab Gene by Coupling Isothermal RNA Reverse Transcription Amplification with a Digital PCR Approach. Int. J. Mol. Sci. 2021, 22, 1017. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, E.J.; Kilgore, P.E.; Kim, S.A.; Takahashi, H.; Ohnishi, M.; Anh, D.D.; Dong, B.Q.; Kim, J.S.; Tomono, J.; et al. Clinical evaluation of a loop-mediated isothermal amplification (LAMP) assay for rapid detection of Neisseria meningitidis in cerebrospinal fluid. PLoS ONE 2015, 10, e0122922. [Google Scholar] [CrossRef] [PubMed]
- Takano, C.; Kuramochi, Y.; Seki, M.; Kim, D.W.; Omagari, D.; Sasano, M.; Chang, B.; Ohnishi, M.; Kim, E.J.; Fuwa, K.; et al. Molecular serotype-specific identification of Streptococcus pneumoniae using loop-mediated isothermal amplification. Sci. Rep. 2019, 9, 19823. [Google Scholar] [CrossRef] [PubMed]
- Takano, C.; Seki, M.; Kim, D.W.; Gardner, H.; McLaughlin, R.E.; Kilgore, P.E.; Kumasaka, K.; Hayakawa, S. Development of a Novel Loop-Mediated Isothermal Amplification Method to Detect Guiana Extended-Spectrum (GES) beta-Lactamase Genes in Pseudomonas aeruginosa. Front. Microbiol. 2019, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Pellestor, F.; Paulasova, P. The peptide nucleic acids (PNAs), powerful tools for molecular genetics and cytogenetics. Eur. J. Hum. Genet. 2004, 12, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Nao, N.; Katano, H.; Takayama, I.; Saito, S.; Kato, F.; Katoh, H.; Sakata, M.; Nakatsu, Y.; Mori, Y.; et al. Development of Genetic Diagnostic Methods for Detection for Novel Coronavirus 2019(nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020, 73, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jean, S.; Eltringham, R.; Madison, J.; Snyder, P.; Tu, H.; Jones, D.M.; Leber, A.L. Mutation-Specific SARS-CoV-2 PCR Screen: Rapid and Accurate Detection of Variants of Concern and the Identification of a Newly Emerging Variant with Spike L452R Mutation. J. Clin. Microbiol. 2021, 59, e0092621. [Google Scholar] [CrossRef] [PubMed]
- Takemae, N.; Doan, Y.H.; Momose, F.; Saito, T.; Kageyama, T. Development of New SNP Genotyping Assays to Discriminate the Omicron Variant of SARS-CoV-2. Jpn. J. Infect. Dis. 2022, 75, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Fujitsu, L. PrimerExplorer V5 2016 [Updated 2016/10/11; Cited 2019]. V5. Available online: http://primerexplorer.jp/e/ (accessed on 16 April 2020).
- PrimerDigital. FastPCR Is an Integrated Tool for PCR Primers or Probe Design, in Silico PCR, Oligonucleotide Assembly and Analyses, Alignment and Repeat Searching. Available online: https://primerdigital.com/fastpcr.html (accessed on 9 June 2022).
| Virus/Bacteria | Results of Two Assays | |||||
|---|---|---|---|---|---|---|
| RT-LAMP-BART | Real-Time PCR | |||||
| N501Y | L452R | Q493R | N501Y | L452R | G339D | |
| SARS-CoV-2 | ||||||
| JPN/AI/1-004 b | − a | − | − | − | − | − |
| JPN/TY/WK-521 b | − | − | − | − | − | − |
| B.1.1.7 (ALPHA) hCoV-19/Spain/AN-HUSC_24581802/2020 c | + | − | − | + | − | − |
| B.1.351 (BETA) hCoV-19/Spain/GA-CHUVI-19118872/2020 c | + | − | − | + | − | − |
| P.1 (GAMMA) hCoV-19/Spain/GA-CHUVI-19250962/2021 c | + | − | − | + | − | − |
| B.1.617.2 (DELTA) hCoV-19/Spain/GA-CHUVI-33984566/2021 c | − | + | − | − | + | − |
| BA.1 (Omicron) hCoV-19/Hong Kong/HKU-211129-001/2021 d | + | − | + | − | − | + |
| BA.2 (Omicron) hCoV-19/Australia/QLD2568/2021 d | + | − | + | − | − | + |
| BA.2 (Omicron) hCoV-19/England/MILK-2DF642C/2021 d | + | − | + | − | − | + |
| SARS-CoV c | − | − | − | − | − | − |
| MERS-CoV Betacoronavirus England 1 c | − | − | − | − | − | − |
| Human CoV OC43 e | − | − | − | − | − | − |
| Human CoV 229E e | − | − | − | − | − | − |
| Influenza A (H1N1) PR8 f | − | − | − | − | − | − |
| Influenza A (H1N1) Pdm2009 f | − | − | − | − | − | − |
| RSV Long f | − | − | − | − | − | − |
| RSV subgroups A f | − | − | − | − | − | − |
| RSV subgroups B f | − | − | − | − | − | − |
| Haemophilus influenzae Rd KW20 | − | − | − | − | − | − |
| Streptococcus pneumoniae 6305 | − | − | − | − | − | − |
| Neisseria meningitidis HY0001 g | − | − | − | − | − | − |
| Escherichia coli DH5a | − | − | − | − | − | − |
| Analytical Sensitivity | ||
|---|---|---|
| RT-LAMP-BART (Reaction Time, 30 min) | Real-Time RT-PCR (Reaction Time, 105–130 min) | |
| Purified RNA | ||
| N501Y | 200 copies/reaction a | 3000 |
| L452R | 100 | 3000 |
| Omicron: Q493R, LAMP; G339D, PCR | 100 | 500 |
| RNA-spiked specimens | ||
| Nasopharyngeal swab b | ||
| N501Y | 500 | 5000 |
| L452R | 500 | 5000 |
| Omicron: Q493R, LAMP; G339D, PCR | 500 | 5000 |
| saliva b | ||
| N501Y | 5000 | 50,000 |
| L452R | 5000 | 50,000 |
| Omicron: Q493R, LAMP; G339D, PCR | 5000 | 50,000 |
| Name | Sequence (5′ to 3′) |
|---|---|
| N501Y-RT-LAMP | |
| N501Y_F3 | CTA ATC TCA AAC CTT TTG AGA GAG A |
| N501Y_B3 | AAT TAG TAG ACT TTT TAG GTC CAC AA |
| N501Y_FIP | GGA AAC CAT ATG ATT GTA AAG GAA AGT ACT GAA ATC TAT CAG GCC GGT AG |
| N501Y_BIP | TAT GGT GT GGT TAC CAA CCA TAA CAG TTG CTG GTG CAT GT |
| N501Y_LF | ACC TTT AAC ACC ATT ACA AGG TGT |
| N501Y_LB | AGA GTA GTA GTA CTT TCT TTT GAA CTT CT |
| N501Y_PNA probe | CCA ACA CCA TT a A GTG GG |
| L452R-RT-LAMP [9] | |
| L452R_F3 | GCA AAC TGG AAA GAT TGC TGA T |
| L452R_B3 | TTG GAA ACC ATA TGA TTG TAA AGG A |
| L452R_FIP | CGG TAA TTA TAA TTA CCA CCA ACC TAG ATG ATT TTA CAG GCT GCG T |
| L452R_BIP | GTT TAG GAA GTC TAA TCT CAA ACC TAA CAC CAT TAC AAG GTG TGC TA |
| L452R_LF | TCA AGA TTG TTA GAA TTC CAA GCT AT |
| L452R_LB | AGA GAG ATA TTT CAA CTG AAA TCT ATC AG |
| L452R_PNA probe | CCT AAA CAA TCT ATA CA b G G |
| Q493R-RT-LAMP | |
| Q493R_F3 | CTA ATC TCA AAC CTT TTG AGA GAG A |
| Q493R_B3 | AGACTT TTT AGG TCC ACA AAC AGT |
| Q493R_FIP | CGT AAA GGA AAG TAA CAA TTA AAA CCT TTT CAA CTG AAA TCT ATC AGG CC |
| Q493R_BIP | GTT TCC GAC CCA CTT ATG GTG TGT GGT GCA TGT AGA AGT TC |
| Q493R_LF | GCA ACA CCA TTA CAA GGT |
| Q493R_LB | TTG GTC ACC AAC CAT ACA GAG |
| Q493R/Q498R_PNA probe | TT c G GAA ACC A c TA TGA TT cG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iijima, T.; Sakai, J.; Kanamori, D.; Ando, S.; Nomura, T.; Tisi, L.; Kilgore, P.E.; Percy, N.; Kohase, H.; Hayakawa, S.; et al. A New Method to Detect Variants of SARS-CoV-2 Using Reverse Transcription Loop-Mediated Isothermal Amplification Combined with a Bioluminescent Assay in Real Time (RT-LAMP-BART). Int. J. Mol. Sci. 2023, 24, 10698. https://doi.org/10.3390/ijms241310698
Iijima T, Sakai J, Kanamori D, Ando S, Nomura T, Tisi L, Kilgore PE, Percy N, Kohase H, Hayakawa S, et al. A New Method to Detect Variants of SARS-CoV-2 Using Reverse Transcription Loop-Mediated Isothermal Amplification Combined with a Bioluminescent Assay in Real Time (RT-LAMP-BART). International Journal of Molecular Sciences. 2023; 24(13):10698. https://doi.org/10.3390/ijms241310698
Chicago/Turabian StyleIijima, Takahiro, Jun Sakai, Dai Kanamori, Shinnosuke Ando, Tsutomu Nomura, Laurence Tisi, Paul E. Kilgore, Neil Percy, Hikaru Kohase, Satoshi Hayakawa, and et al. 2023. "A New Method to Detect Variants of SARS-CoV-2 Using Reverse Transcription Loop-Mediated Isothermal Amplification Combined with a Bioluminescent Assay in Real Time (RT-LAMP-BART)" International Journal of Molecular Sciences 24, no. 13: 10698. https://doi.org/10.3390/ijms241310698
APA StyleIijima, T., Sakai, J., Kanamori, D., Ando, S., Nomura, T., Tisi, L., Kilgore, P. E., Percy, N., Kohase, H., Hayakawa, S., Maesaki, S., Hoshino, T., & Seki, M. (2023). A New Method to Detect Variants of SARS-CoV-2 Using Reverse Transcription Loop-Mediated Isothermal Amplification Combined with a Bioluminescent Assay in Real Time (RT-LAMP-BART). International Journal of Molecular Sciences, 24(13), 10698. https://doi.org/10.3390/ijms241310698







