Quantitative Proteomic Analysis on the Slightly Acidic Electrolyzed Water Triggered Viable but Non-Culturable Listeria monocytogenes
Abstract
1. Introduction
2. Results
2.1. The Culturability of L. monocytogenes after Treatment with Slightly Acidic Electrolyzed Water (SAEW)
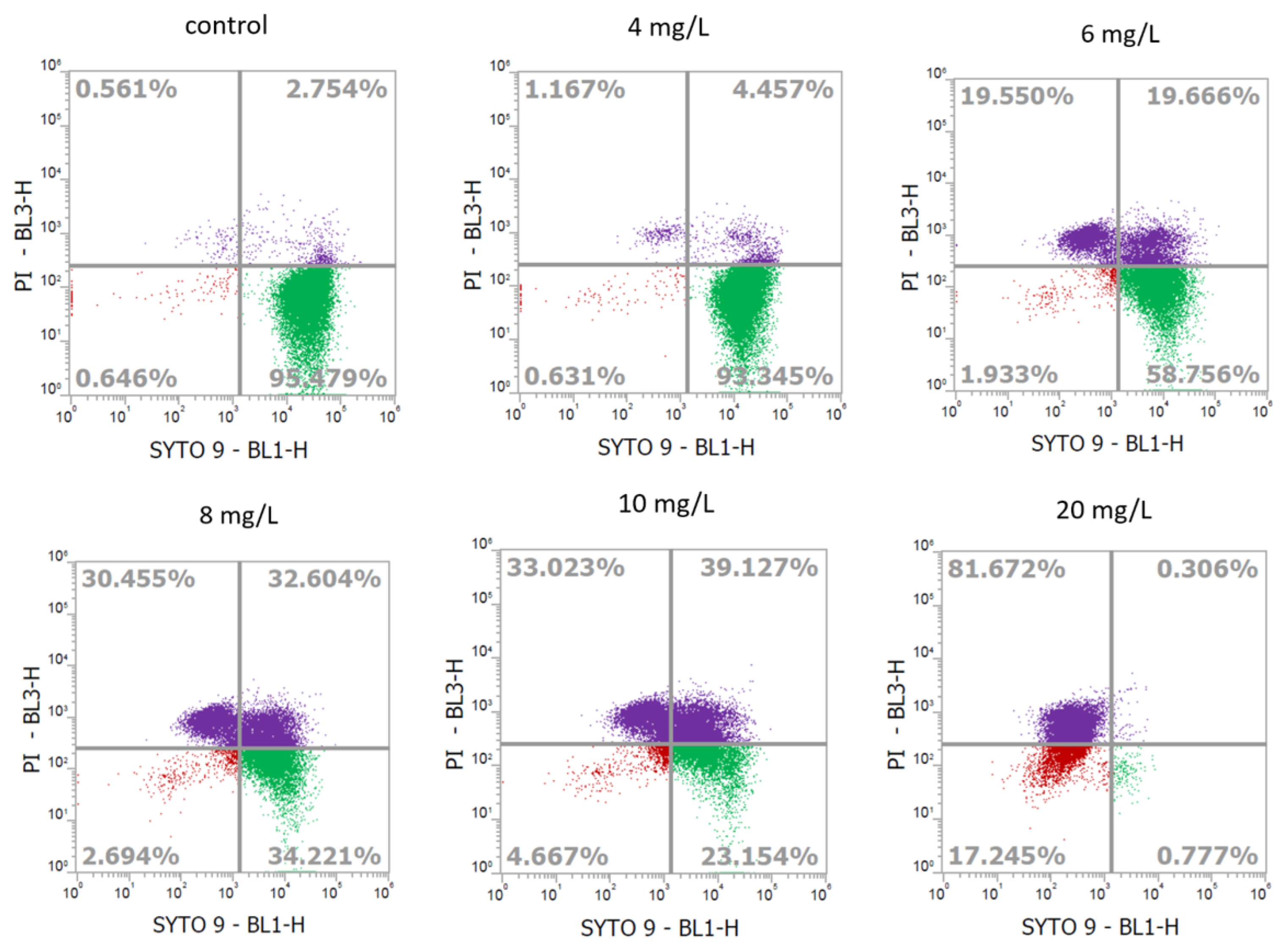
2.2. Determination of Cell Viability of L. monocytogenes Using Flow Cytometry
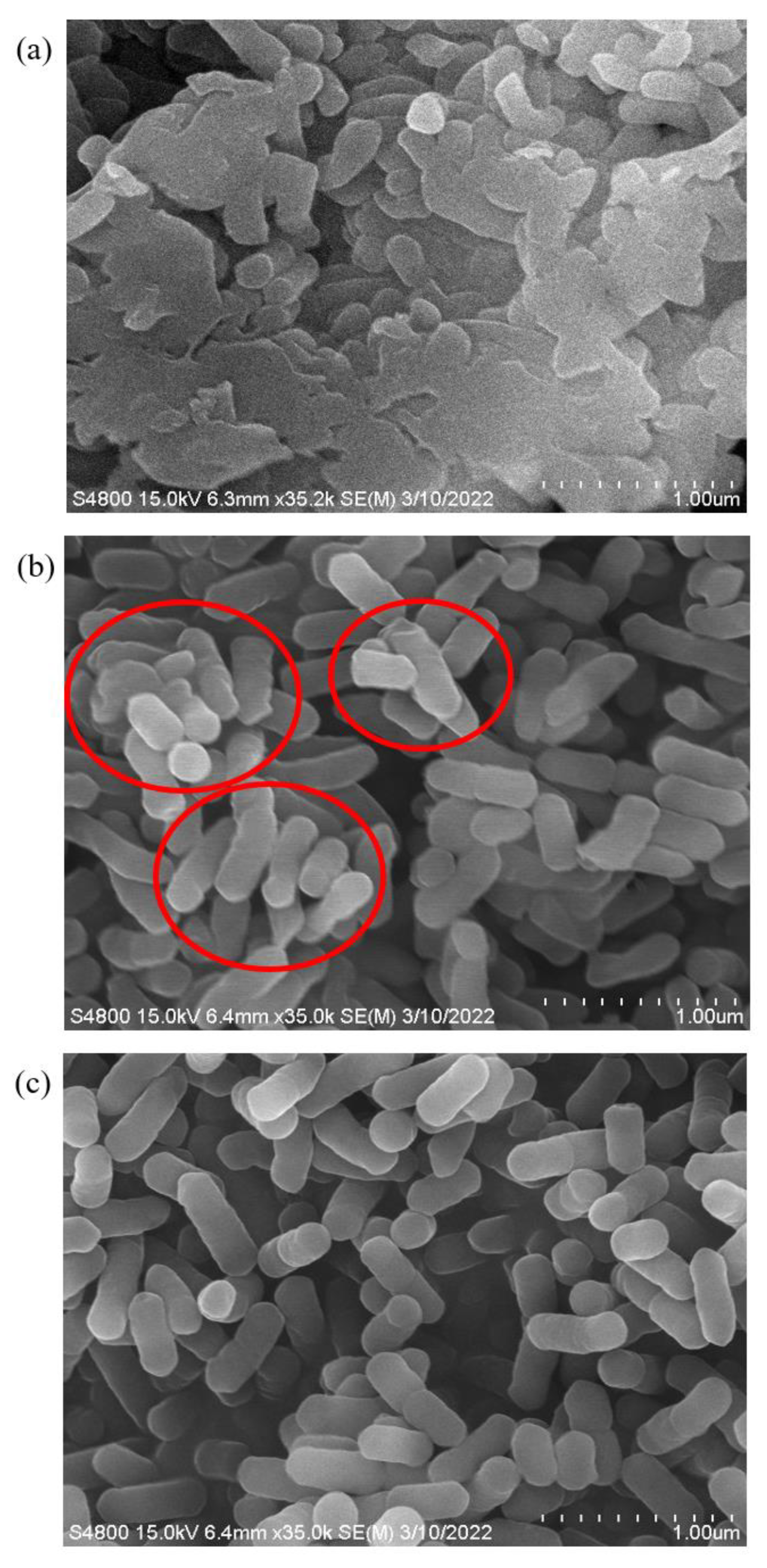
2.3. Morphological Changes of VBNC L. monocytogenes Using Scanning Electron Microscopy
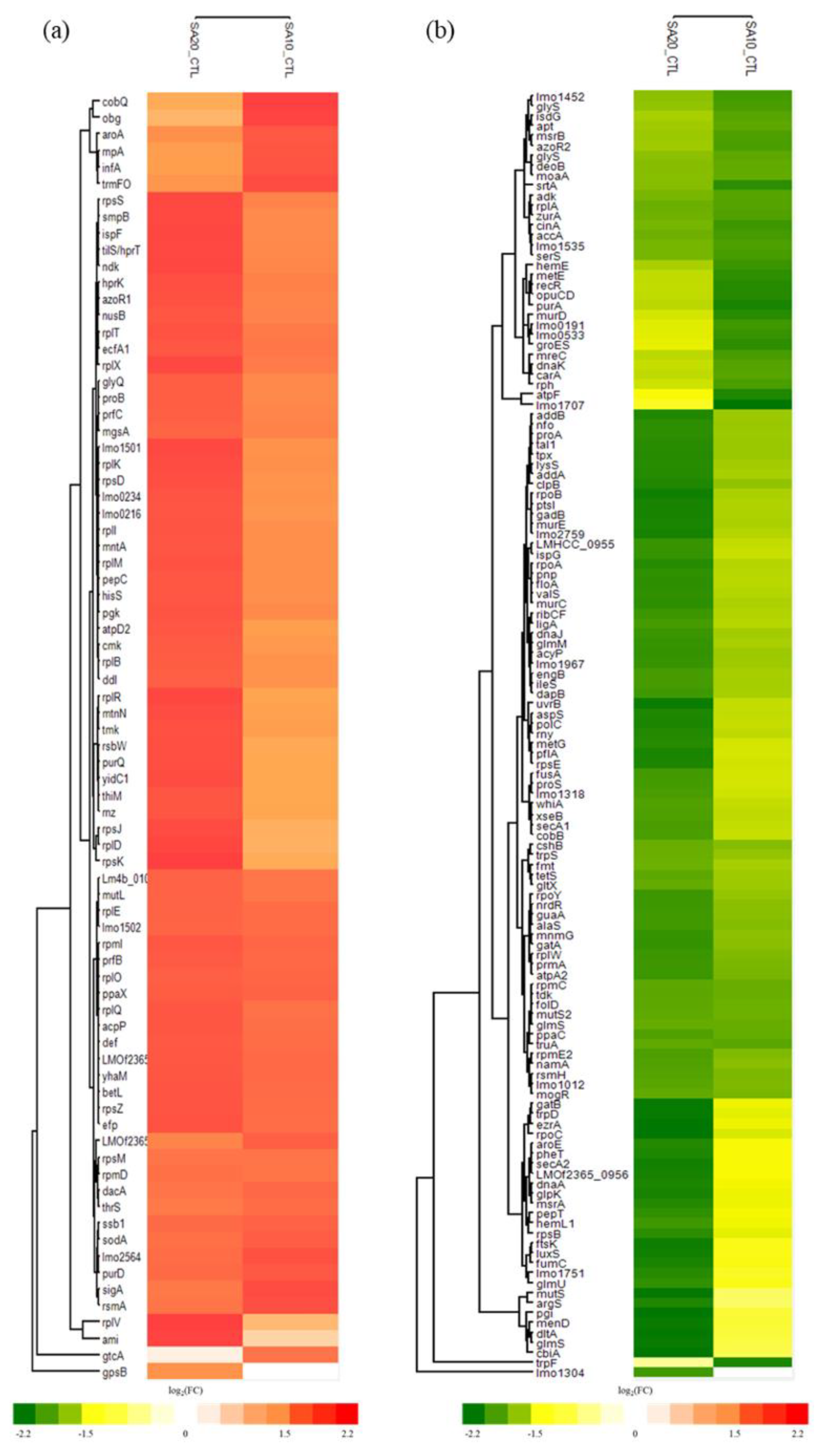
2.4. Differential Expressed Proteins (DEPs) of L. monocytogenes after Treating with SAEW
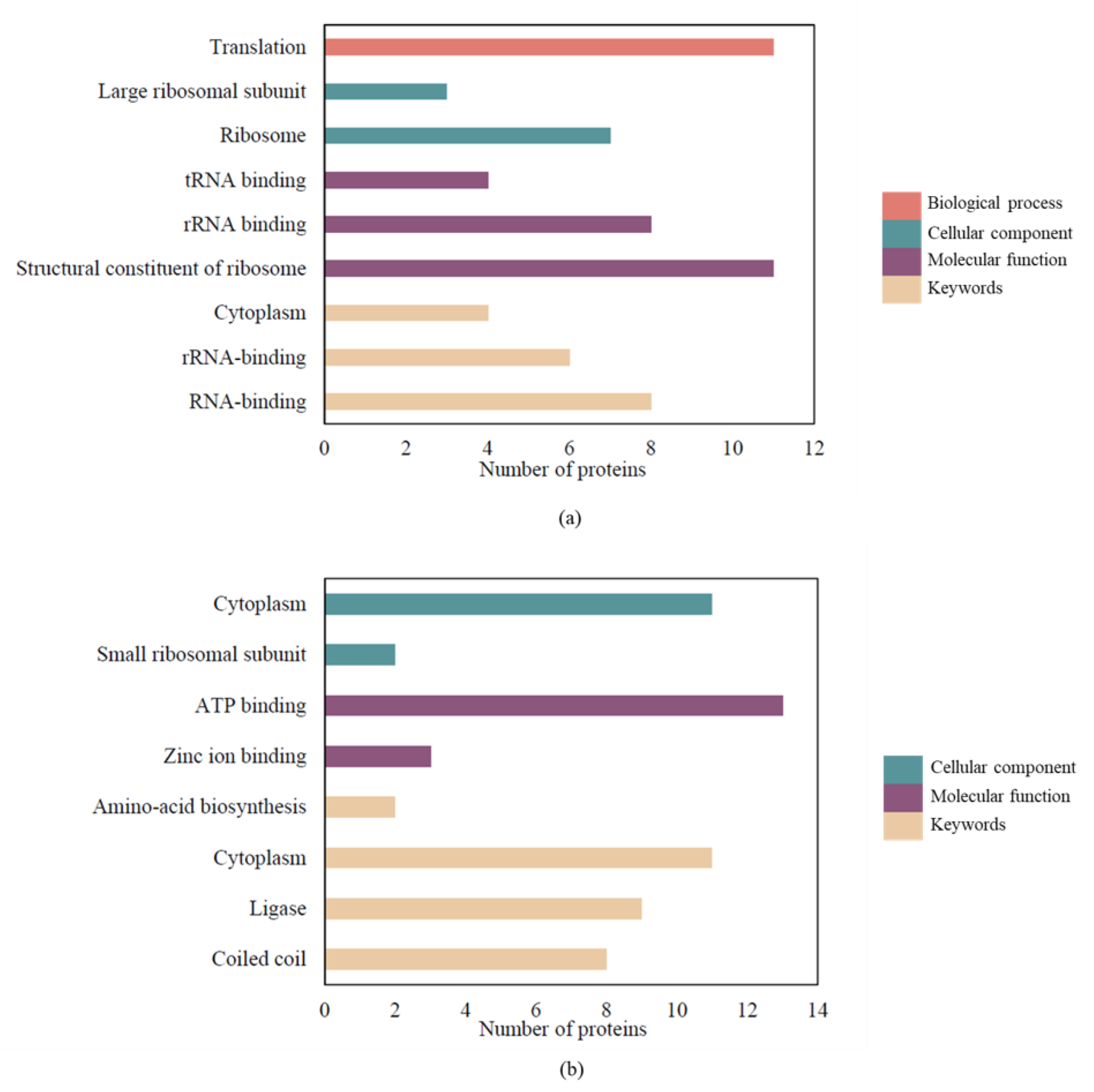
2.5. GO and KEGG Functional Enrichment Analysis of SAEW-Regulated Proteins
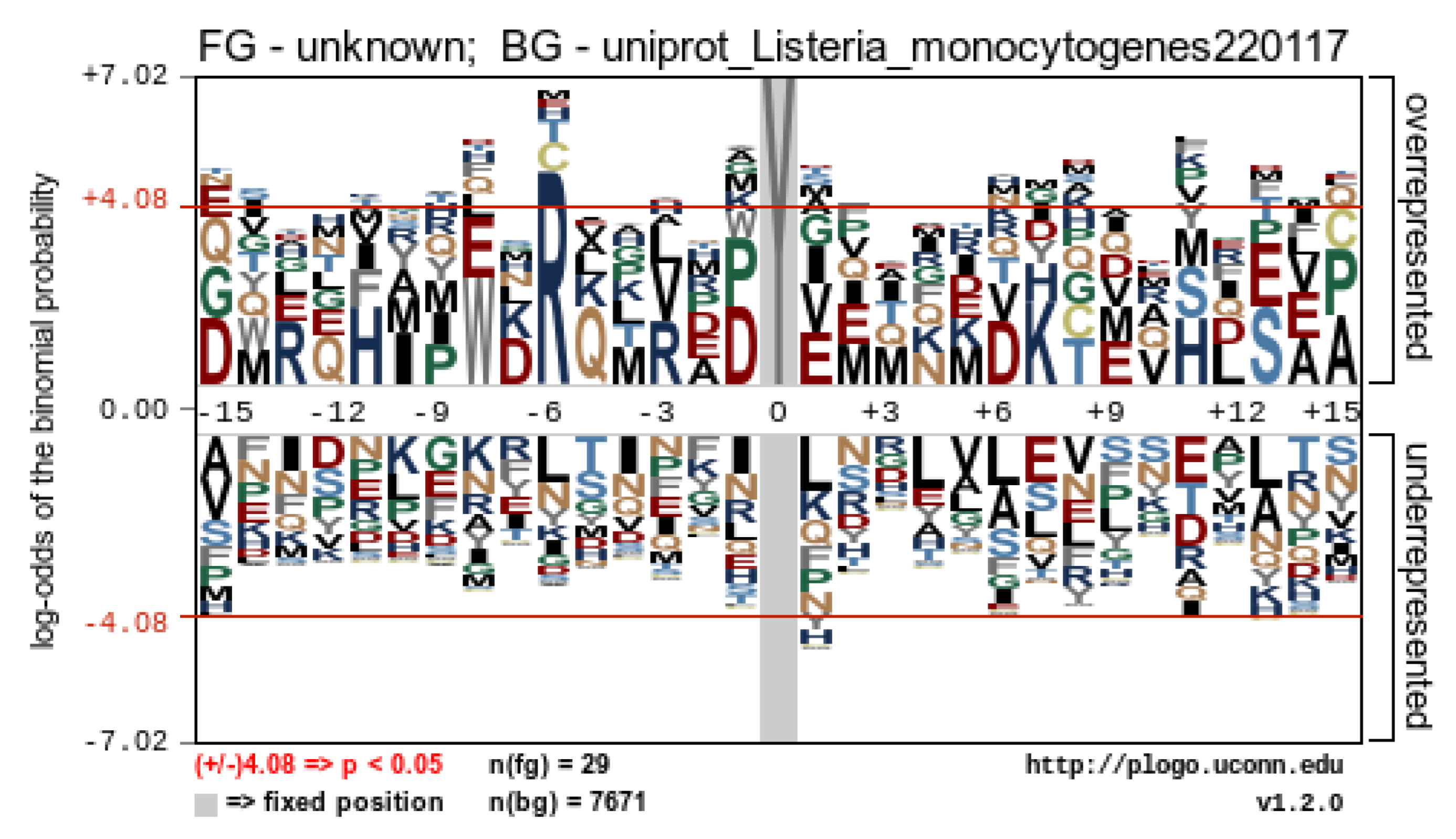
2.6. SAEW-Mediated Protein Chlorination in VBNC L. monocytogenes
3. Discussion
4. Materials and Methods
4.1. Bacterial Cultures and Preparations
4.2. Preparation of Slightly Acidic Electrolyzed Water (SAEW)
4.3. Sample Treatment
4.4. Flow Cytometry Analysis of Treated Samples
4.5. Scanning Electron Microscopy (SEM) Analysis
4.6. Whole-Protein Extraction for Proteomic Analysis
4.7. Mass Spectrometric Analysis
4.7.1. In Solution Digestion
4.7.2. Tandem Mass Tags (TMT) Labeling
4.7.3. SDB Fractionation and Desalted
4.7.4. LC-MS/MS
4.7.5. Data Analysis and Protein Network Construction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ray, L.C.; Collins, J.P.; Griffin, P.M.; Shah, H.J.; Boyle, M.M.; Cieslak, P.R.; Dunn, J.; Lathrop, S.; McGuire, S.; Rissman, T.; et al. Decreased Incidence of Infections Caused by Pathogens Transmitted Commonly through Food during the COVID-19 Pandemic—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2017–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhao, D.; Hao, J. Efficacy of the two-step disinfection with slightly acidic electrolyzed water for reduction of Listeria monocytogenes contamination on food raw materials. LWT—Food Sci. Technol. 2021, 140, 110699. [Google Scholar] [CrossRef]
- Dong, K.; Pan, H.; Yang, D.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Induction, detection, formation, and resuscitation of viable but non-culturable state microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 149–183. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Liao, X.; Zhao, X.; Liu, D.; Ding, T. The diagnostic tools for viable but nonculturable pathogens in the food industry: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2146–2175. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Yang, H. Recent advances on research of electrolyzed water and its applications. Curr. Opin. Food Sci. 2021, 41, 180–188. [Google Scholar] [CrossRef]
- Yan, P.; Chelliah, R.; Jo, K.; Oh, D.H. Research trends on the application of electrolyzed water in food preservation and sanitation. Processes 2021, 9, 2240. [Google Scholar] [CrossRef]
- Afari, G.K.; Hung, Y.C. Detection and verification of the viable but nonculturable (VBNC) state of Escherichia coli O157: H7 and Listeria monocytogenes using flow cytometry and standard plating. J. Food Sci. 2018, 83, 1913–1920. [Google Scholar] [CrossRef]
- Han, D.; Hung, Y.C.; Wang, L. Evaluation of the antimicrobial efficacy of neutral electrolyzed water on pork products and the formation of viable but nonculturable (VBNC) pathogens. Food Microbiol. 2018, 73, 227–236. [Google Scholar] [CrossRef]
- Schottroff, F.; Fröhling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schlüter, O.; Jäger, H. Sublethal injury and viable but non-culturable (VBNC) state in microorganisms during preservation of food and biological materials by non-thermal processes. Front. Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef]
- Chen, G.W.; Chen, Y.A.; Chang, H.Y.; Huang, T.C.; Chen, T.Y. Combined impact of high-pressure processing and slightly acidic electrolysed water on Listeria monocytogenes proteomes. Food Res. Int. 2021, 147, 110494. [Google Scholar] [CrossRef]
- Chen, T.Y.; Kuo, S.H.; Chen, S.T.; Hwang, D.F. Differential proteomics to explore the inhibitory effects of acidic, slightly acidic electrolysed water and sodium hypochlorite solution on Vibrio parahaemolyticus. Food Chem. 2016, 194, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Schubert, O.; Röst, H.; Collins, B.C.; Rosenberger, G.; Aebersold, R. Quantitative proteomics: Challenges and opportunities in basic and applied research. Nat. Protoc. 2017, 12, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Sinitcyn, P.; Rudolph, J.D.; Cox, J. Computational methods for understanding mass spectrometry–based shotgun proteomics data. Ann. Rev. Biomed. Data Sci. 2018, 1, 207–234. [Google Scholar] [CrossRef]
- Li, J.; Ding, T.; Liao, X.; Chen, S.; Ye, X.; Liu, D. Synergetic effects of ultrasound and slightly acidic electrolyzed water against Staphylococcus aureus evaluated by flow cytometry and electron microscopy. Ultrason. Sonochem. 2017, 38, 711–719. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Xia, X.; Li, B.; Hung, Y.C. Viability assay of E. coli O157: H7 treated with electrolyzed oxidizing water using flow cytometry. Food Control 2018, 88, 47–53. [Google Scholar] [CrossRef]
- Highmore, C.J.; Warner, J.C.; Rothwell, S.D.; Wilks, S.A.; Keevil, C.W. Viable-but-nonculturable Listeria monocytogenes and Salmonella enterica serovar Thompson induced by chlorine stress remain infectious. mBio 2018, 9, e00540-18. [Google Scholar] [CrossRef]
- Li, H.; Liang, D.; Huang, J.; Cui, C.; Rao, H.; Zhao, D.; Hao, J. The bactericidal efficacy and the mechanism of action of slightly acidic electrolyzed water on Listeria monocytogenes’ survival. Foods 2021, 10, 2671. [Google Scholar] [CrossRef]
- Debnath, A.; Mizuno, T.; Miyoshi, S.I. Comparative proteomic analysis to characterize temperature-induced viable but non-culturable and resuscitation states in Vibrio cholerae. Microbiology 2019, 165, 737–746. [Google Scholar] [CrossRef]
- Zhong, Q.; Wang, B.; Wang, J.; Liu, Y.; Fang, X.; Liao, Z. Global proteomic analysis of the resuscitation state of Vibrio parahaemolyticus compared with the normal and viable but non-culturable state. Front. Microbiol. 2019, 10, 1045–1060. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, X. Transcriptomic analysis of viable but non-culturable Escherichia coli O157: H7 formation induced by low temperature. Microorganisms 2019, 7, 634. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Lin, H.; Zhang, M.; Chen, S.; Yu, X. Characterization and potential mechanisms of highly antibiotic tolerant VBNC Escherichia coli induced by low level chlorination. Sci. Rep. 2020, 10, 1957. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Lapointe, J. The Bacterial Heterotrimeric Amidotransferase GatCAB: Functions, structures and mechanism-based inhibitors. Arch. Biotechnol. Biomed. 2017, 1, 21–32. [Google Scholar] [CrossRef]
- Li, Y.Y.; Cai, R.J.; Yang, J.Y.; Hendrickson, T.L.; Xiang, Y.; Javid, B. Clinically relevant mutations of mycobacterial GatCAB inform regulation of translational fidelity. mBio 2021, 12, e01100-21. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhao, X. Induction of viable but nonculturable Escherichia coli O157: H7 by low temperature and its resuscitation. Front. Microbiol. 2018, 9, 2728–2737. [Google Scholar] [CrossRef]
- Su, X.; Sun, F.; Wang, Y.; Hashmi, M.Z.; Guo, L.; Ding, L.; Shen, C. Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci. Rep. 2015, 5, 18590. [Google Scholar] [CrossRef]
- Ultee, E.; Ramijan, K.; Dame, R.T.; Briegel, A.; Claessen, D. Stress-induced adaptive morphogenesis in bacteria. Adv. Microb. Physiol. 2019, 74, 97–141. [Google Scholar]
- Smith, C.A. Structure, function and dynamics in the mur family of bacterial cell wall ligases. J. Mol. Biol. 2006, 362, 640–655. [Google Scholar] [CrossRef]
- Giagnoni, L.; Arenella, M.; Galardi, E.; Nannipieri, P.; Renella, G. Bacterial culturability and the viable but non-culturable (VBNC) state studied by a proteomic approach using an artificial soil. Soil Biol. Biochem. 2018, 118, 51–58. [Google Scholar] [CrossRef]
- Defeu Soufo, H.J.; Reimold, C.; Linne, U.; Knust, T.; Gescher, J.; Graumann, P.L. Bacterial translation elongation factor EF-Tu interacts and colocalizes with actin-like MreB protein. Proc. Natl. Acad. Sci. USA 2010, 107, 3163–3168. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship between quorum sensing and secretion systems. Front. Microbiol. 2019, 10, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Grenier, D.; Yi, L. Regulatory mechanisms of the LuxS/AI-2 system and bacterial resistance. Antimicrob. Agents Chemother. 2019, 63, e01186-19. [Google Scholar] [CrossRef] [PubMed]
- Algburi, A.; Zehm, S.; Netrebov, V.; Bren, A.B.; Chistyakov, V.; Chikindas, M.L. Subtilosin prevents biofilm formation by inhibiting bacterial quorum sensing. Probiotics Antimicrob. Proteins 2017, 9, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Abdellatif, G.R.; Elshishtawy, H.M.; Ashour, H.M. First-time characterization of viable but non-culturable Proteus mirabilis: Induction and resuscitation. J. Cell. Mol. Med. 2020, 24, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L. Hypochlorous acid-mediated modification of proteins and its consequences. Essays Biochem. 2020, 64, 75–86. [Google Scholar] [CrossRef]
- Prus, G.; Hoegl, A.; Weinert, B.T.; Choudhary, C. Analysis and interpretation of protein post-translational modification site stoichiometry. Trends Biochem. Sci. 2019, 44, 943–960. [Google Scholar] [CrossRef]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef]
- Winardhi, R.S.; Tang, Q.; You, H.; Sheetz, M.; Yan, J. The holdase function of Escherichia coli Hsp70 (DnaK) chaperone. bioRxiv 2018. [Google Scholar] [CrossRef]
- Laplace, J.M.; Thuault, M.; Hartke, A.; Boutibonnes, P.; Auffray, Y. Sodium hypochlorite stress in Enterococcus faecalis: Influence of antecedent growth conditions and induced proteins. Curr. Microbiol. 1997, 34, 284–289. [Google Scholar] [CrossRef]
- Alam, A.; Bröms, J.E.; Kumar, R.; Sjöstedt, A. The role of ClpB in bacterial stress responses and virulence. Front. Mol. Biosci. 2021, 8, 668910. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; Lin, S.Y.; Chen, Z.; Li, B.; Yao, G.; et al. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol. Cell 2019, 73, 143–156.e4. [Google Scholar] [CrossRef] [PubMed]
- Dallo, S.F.; Kannan, T.R.; Blaylock, M.W.; Baseman, J.B. Elongation factor Tu and E1 β subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol. Microbiol. 2002, 46, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Jarocki, V.M.; Charles, I.G.; Djordjevic, S.P. The diverse functional roles of elongation factor Tu (EF-Tu) in microbial pathogenesis. Front. Microbiol. 2019, 10, 2351–2370. [Google Scholar] [CrossRef] [PubMed]

| Chlorine Concentration (mg/L) | Recovery on BHIA (log CFU/mL) | Reduction on BHIA (log CFU/mL) |
|---|---|---|
| Control | 8.26 ± 0.09 | - |
| 4 | 7.95 ± 0.17 | 0.31 |
| 6 | 7.54 ± 0.44 | 0.72 |
| 8 | ND | 8.26 |
| 10 | ND | 8.26 |
| 20 | ND | 8.26 |
| Protein IDs | Identified Sequence | Gene Symbol Cl Residue | Fold Change (log2) | |
|---|---|---|---|---|
| SA20_CTL | SA10_CTL | |||
| Q8Y5V1 | EFVPLLVYSPR | deoB, Y350 | 1.038 | 1.102 |
| P0DJM2 | IINEPTAAALAYGMDK | dnaK, Y153 | 1.180 | 1.155 |
| Q8Y8G1 | MDDLYSEFGEQMDEVAER | dps, Y50 | 0.884 | 0.590 |
| P64074 | TSEEMVTWYEEMITK | eno, W273 | 1.809 | 0.564 |
| P64074 | TSEEMVTWYEEMITK | eno, Y274 | 1.500 | 1.024 |
| Q8Y7G7 | LSVDYYGAATPVNQMASISVPEAR | frr, Y45 | 0.869 | 1.217 |
| Q8Y421 | GVYTMQFDHYEEVPK | fusA, Y666 | 2.352 | 2.508 |
| Q8Y6D3 | INDLGLPAYDAMVLTLTK | gatB, Y313 | 1.455 | 1.589 |
| Q8Y571 | VIPYWMDTIAPEIK | gpmA, W162 | 1.282 | 1.615 |
| B8DH59 | HEAVGVGFNAANGEWINMIDAGIVDPTK | groEL, W482 | 1.543 | 1.041 |
| B8DH59 | GYTSPYMVTDSDK | groEL, Y201 | 1.044 | 1.056 |
| A0A0H3GEZ8 | YGLIETN | hpf, Y181 | 0.882 | 0.951 |
| A0A0H3GEZ8 | GENIEVTEPIRDYVEK | hpf, Y20 | 1.310 | 0.927 |
| C1KV98 | WDVADLMDYIK | ispH, W175; Y183 | 0.334 | 0.587 |
| Q8YAE0 | LAEKYGVSIR | lmo0191, Y143 | −0.053 | 0.076 |
| Q8Y8D7 | KYMITSK | nadK1, Y3 | 0.262 | 0.292 |
| Q8Y6C8 | VTAEGYVILNK | pcrB, Y112 | 2.267 | 2.457 |
| Q71XR3 | NFGAPYELIK | pdxS, Y194 | 1.158 | 1.349 |
| P58641 | ANDPVAAYNQVLKEWNA | pyrF, Y224; W231 | −0.504 | −0.326 |
| Q927L9 | EQLIFPEIDYDQVSK | rplE, Y143 | −0.272 | −0.532 |
| Q8Y6T6 | RPYAPGQHGPTQR | rpsD, Y31 | 1.469 | 0.732 |
| Q8Y6T6 | HMYGLTER | rpsD, Y61 | 1.781 | 1.652 |
| Q71WF0 | HVPVYVQEDMVGHK | rpsS, Y61 | 0.790 | 1.068 |
| Q8Y6U0 | YSGQVQMHIVPFTEIQEVIK | thiI, Y233 | 0.446 | 0.549 |
| Q8Y6M7 | TEMPNGQTVQDYITEAITK | tsf, Y125 | 1.771 | 1.791 |
| Q8Y422 | QVGVPYIVVFMNK | tuf, Y130 | 3.229 | 2.687 |
| Q8Y422 | DLLTEYEFPGDDIPVIK | tuf, Y161 | 1.010 | 0.921 |
| Q8Y422 | IDELMEAVDSYIPTPER | tuf, Y199 | 1.353 | 1.216 |
| Q8Y422 | LLDYAEAGDNIGALLR | tuf, Y269 | 0.157 | 0.229 |
| Q8Y422 | GYADAQAYDQIDGAPEER | tuf, Y41 | 1.229 | 1.230 |
| Q8Y422 | HYAHVDCPGHADYVK | tuf, Y88 | 0.538 | 1.220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-Y.; Gui, C.-Y.; Huang, T.-C.; Hung, Y.-C.; Chen, T.-Y. Quantitative Proteomic Analysis on the Slightly Acidic Electrolyzed Water Triggered Viable but Non-Culturable Listeria monocytogenes. Int. J. Mol. Sci. 2023, 24, 10616. https://doi.org/10.3390/ijms241310616
Chang H-Y, Gui C-Y, Huang T-C, Hung Y-C, Chen T-Y. Quantitative Proteomic Analysis on the Slightly Acidic Electrolyzed Water Triggered Viable but Non-Culturable Listeria monocytogenes. International Journal of Molecular Sciences. 2023; 24(13):10616. https://doi.org/10.3390/ijms241310616
Chicago/Turabian StyleChang, Hsin-Yi, Chin-Ying Gui, Tsui-Chin Huang, Yen-Con Hung, and Tai-Yuan Chen. 2023. "Quantitative Proteomic Analysis on the Slightly Acidic Electrolyzed Water Triggered Viable but Non-Culturable Listeria monocytogenes" International Journal of Molecular Sciences 24, no. 13: 10616. https://doi.org/10.3390/ijms241310616
APA StyleChang, H.-Y., Gui, C.-Y., Huang, T.-C., Hung, Y.-C., & Chen, T.-Y. (2023). Quantitative Proteomic Analysis on the Slightly Acidic Electrolyzed Water Triggered Viable but Non-Culturable Listeria monocytogenes. International Journal of Molecular Sciences, 24(13), 10616. https://doi.org/10.3390/ijms241310616






