Energy Landscapes and Heat Capacity Signatures for Monomers and Dimers of Amyloid-Forming Hexapeptides
Abstract
1. Introduction
2. Results and Discussion
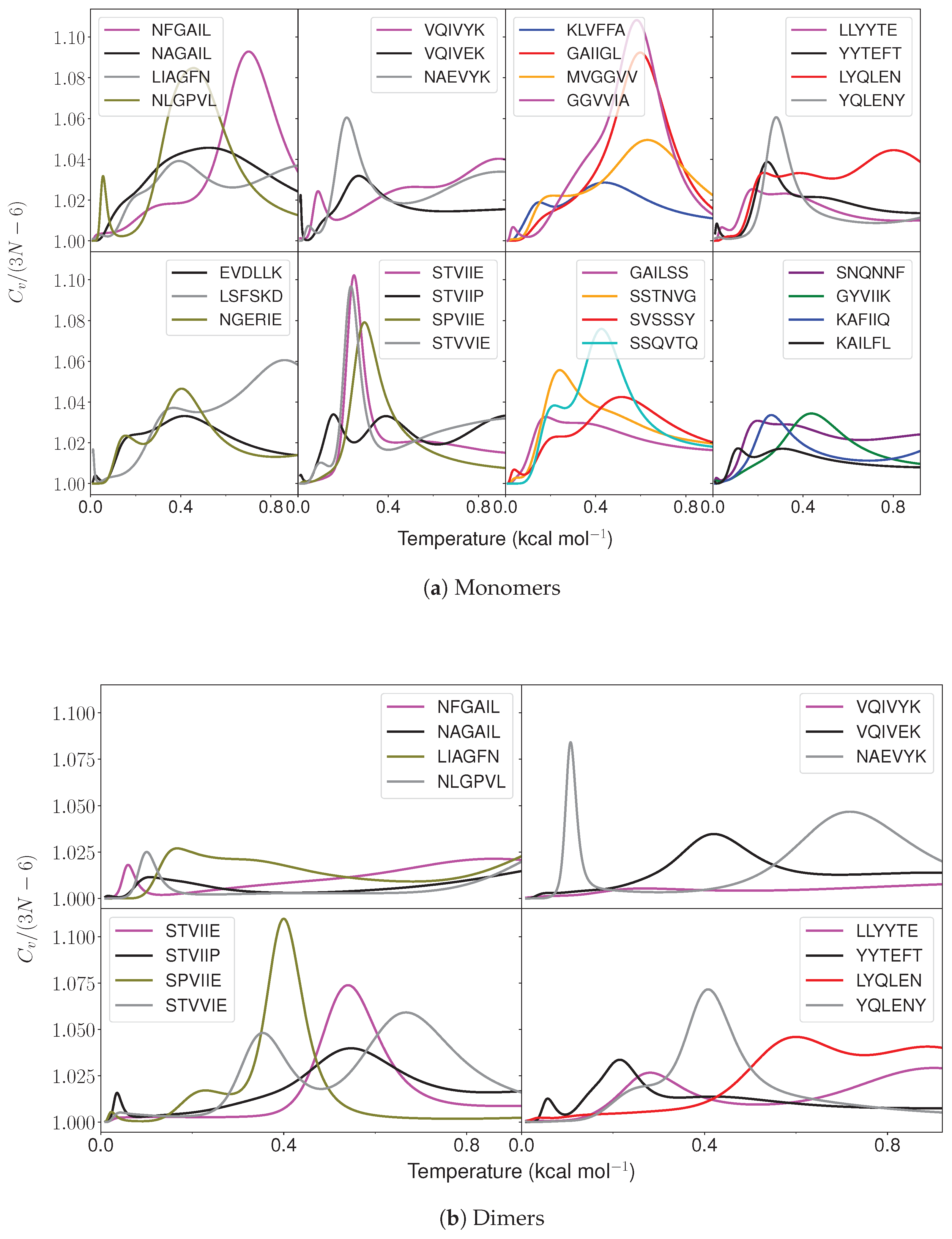

2.1. Monomer Heat Capacity
2.2. Dimer Heat Capacity

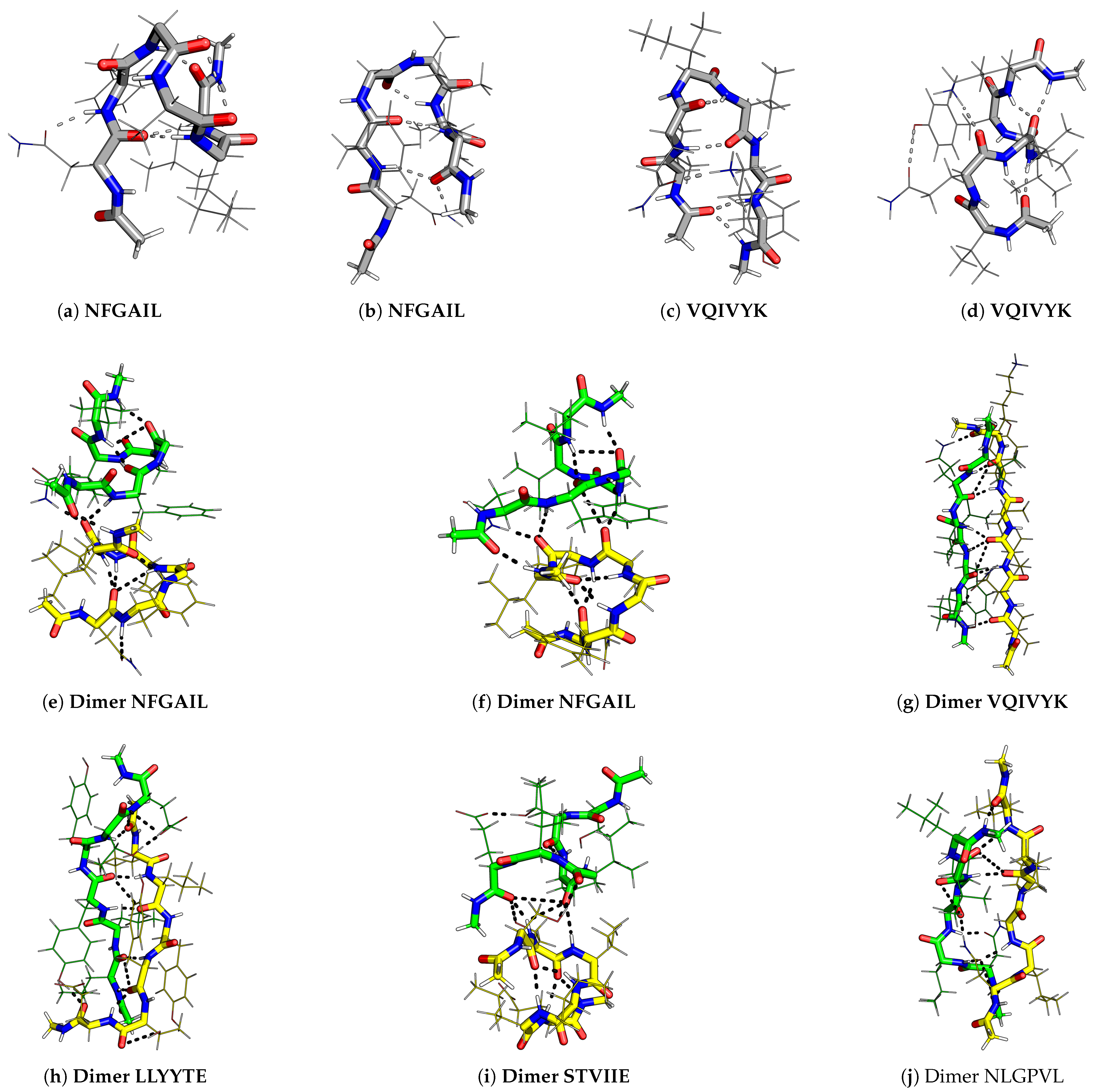
2.3. Correlation between Heat Capacity and Amyloid Formation Predictors

3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucciantini, M.; Giannoni, E.; Chiti, F.; Baroni, F.; Formigli, L.; Zurdo, J.; Taddei, N.; Ramponi, G.; Dobson, C.M.; Stefani, M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Blake, C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv. Protein Chem. 1997, 50, 123–159. [Google Scholar] [PubMed]
- Booth, D.R.; Sunde, M.; Bellotti, V.; Robinson, C.V.; Hutchinson, W.L.; Fraser, P.E.; Hawkins, P.N.; Dobson, C.M.; Radford, S.E.; Blake, C.C.; et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 1997, 385, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Fändrich, M.; Fletcher, M.A.; Dobson, C.M. Amyloid fibrils from muscle myoglobin. Nature 2001, 410, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.R.; Morozova-Roche, L.A.; Daniel, K.; Robinson, C.V.; Dobson, C.M. Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci. 2004, 13, 1933–1938. [Google Scholar] [CrossRef]
- Fernandez-Escamilla, A.M.; Rousseau, F.; Schymkowitz, J.; Serrano, L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 2004, 22, 1302–1306. [Google Scholar] [CrossRef]
- Balbirnie, M.; Grothe, R.; Eisenberg, D.S. An amyloid-forming peptide from the yeast prion Sup35 reveals a dehydrated β-sheet structure for amyloid. Proc. Natl. Acad. Sci. USA 2001, 98, 2375–2380. [Google Scholar] [CrossRef]
- Ventura, S.; Zurdo, J.; Narayanan, S.; Parreño, M.; Mangues, R.; Reif, B.; Chiti, F.; Giannoni, E.; Dobson, C.M.; Aviles, F.X.; et al. Short amino acid stretches can mediate amyloid formation in globular proteins: The Src homology 3 (SH3) case. Proc. Natl. Acad. Sci. USA 2004, 101, 7258–7263. [Google Scholar] [CrossRef]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.Ø.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.; McFarlane, H.T.; et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef]
- Tjernberg, L.O.; Callaway, D.J.; Tjernberg, A.; Hahne, S.; Lilliehöök, C.; Terenius, L.; Thyberg, J.; Nordstedt, C. A molecular model of Alzheimer amyloid β-peptide fibril formation. J. Biol. Chem. 1999, 274, 12619–12625. [Google Scholar] [CrossRef] [PubMed]
- Zanuy, D.; Haspel, N.; Tsai, H.H.G.; Ma, B.; Gunasekaran, K.; Wolfson, H.J.; Nussinov, R. Side chain interactions determine the amyloid organization: A single layer β-sheet molecular structure of the calcitonin peptide segment 15–19. Phys. Biol. 2004, 1, 89. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Fitzpatrick, A.W.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef]
- Wang, X.; Chapman, M.R. Sequence determinants of bacterial amyloid formation. J. Mol. Biol. 2008, 380, 570–580. [Google Scholar] [CrossRef]
- Gazit, E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tjernberg, L.; Hosia, W.; Bark, N.; Thyberg, J.; Johansson, J. Charge attraction and β propensity are necessary for amyloid fibril formation from tetrapeptides. J. Biol. Chem. 2002, 277, 43243–43246. [Google Scholar] [CrossRef]
- Marshall, K.E.; Morris, K.L.; Charlton, D.; O’Reilly, N.; Lewis, L.; Walden, H.; Serpell, L.C. Hydrophobic, aromatic, and electrostatic interactions play a central role in amyloid fibril formation and stability. Biochemistry 2011, 50, 2061–2071. [Google Scholar] [CrossRef]
- Berhanu, W.M.; Masunov, A.E. Can molecular dynamics simulations assist in design of specific inhibitors and imaging agents of amyloid aggregation? Structure, stability and free energy predictions for amyloid oligomers of VQIVYK, MVGGVV and LYQLEN. J. Mol. Model. 2011, 17, 2423–2442. [Google Scholar] [CrossRef]
- Chiti, F.; Stefani, M.; Taddei, N.; Ramponi, G.; Dobson, C.M. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 2003, 424, 805–808. [Google Scholar] [CrossRef]
- Tartaglia, G.G.; Cavalli, A.; Pellarin, R.; Caflisch, A. The role of aromaticity, exposed surface, and dipole moment in determining protein aggregation rates. Protein Sci. 2004, 13, 1939–1941. [Google Scholar] [CrossRef]
- Esler, W.P.; Stimson, E.R.; Fishman, J.B.; Ghilardi, J.R.; Vinters, H.V.; Mantyh, P.W.; Maggio, J.E. Stereochemical specificity of Alzheimer’s disease β-peptide assembly. Biopolymers 1999, 49, 505–514. [Google Scholar] [CrossRef]
- Pawar, A.P.; DuBay, K.F.; Zurdo, J.; Chiti, F.; Vendruscolo, M.; Dobson, C.M. Prediction of “aggregation-prone” and “aggregation-susceptible” regions in proteins associated with neurodegenerative diseases. J. Mol. Biol. 2005, 350, 379–392. [Google Scholar]
- Thompson, M.J.; Sievers, S.A.; Karanicolas, J.; Ivanova, M.I.; Baker, D.; Eisenberg, D. The 3D profile method for identifying fibril-forming segments of proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 4074–4078. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.G.; Vendruscolo, M. The Zyggregator method for predicting protein aggregation propensities. Chem. Soc. Rev. 2008, 37, 1395–1401. [Google Scholar]
- Goldschmidt, L.; Teng, P.K.; Riek, R.; Eisenberg, D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 2010, 107, 3487–3492. [Google Scholar] [CrossRef]
- Nekooki-Machida, Y.; Kurosawa, M.; Nukina, N.; Ito, K.; Oda, T.; Tanaka, M. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 9679–9684. [Google Scholar] [CrossRef] [PubMed]
- Eichner, T.; Radford, S.E. A diversity of assembly mechanisms of a generic amyloid fold. Mol. Cell 2011, 43, 8–18. [Google Scholar] [CrossRef]
- Marshall, K.E.; Hicks, M.R.; Williams, T.L.; Hoffmann, S.V.; Rodger, A.; Dafforn, T.R.; Serpell, L.C. Characterizing the assembly of the Sup35 yeast prion fragment, GNNQQNY: Structural changes accompany a fiber-to-crystal switch. Biophys. J. 2010, 98, 330–338. [Google Scholar] [CrossRef] [PubMed]
- DuBay, K.F.; Pawar, A.P.; Chiti, F.; Zurdo, J.; Dobson, C.M.; Vendruscolo, M. Prediction of the absolute aggregation rates of amyloidogenic polypeptide chains. J. Mol. Biol. 2004, 341, 1317–1326. [Google Scholar] [CrossRef]
- Buell, A.K. Stability matters, too–the thermodynamics of amyloid fibril formation. Chem. Sci. 2022, 13, 10177–10192. [Google Scholar] [CrossRef]
- Gómez, J.; Hilser, V.J.; Xie, D.; Freire, E. The heat capacity of proteins. Proteins Struct. Funct. Bioinform. 1995, 22, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Johnson, C.M.; Lakey, J.H.; Nöllmann, M. Heat does not come in different colours: Entropy–enthalpy compensation, free energy windows, quantum confinement, pressure perturbation calorimetry, solvation and the multiple causes of heat capacity effects in biomolecular interactions. Biophys. Chem. 2001, 93, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Loladze, V.V.; Ermolenko, D.N.; Makhatadze, G.I. Heat capacity changes upon burial of polar and nonpolar groups in proteins. Protein Sci. 2001, 10, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Yamamoto, K.; Hasegawa, K.; Naiki, H.; Goto, Y. Direct measurement of the thermodynamic parameters of amyloid formation by isothermal titration calorimetry. J. Biol. Chem. 2004, 279, 55308–55314. [Google Scholar] [CrossRef]
- Jeppesen, M.D.; Hein, K.; Nissen, P.; Westh, P.; Otzen, D.E. A thermodynamic analysis of fibrillar polymorphism. Biophys. Chem. 2010, 149, 40–46. [Google Scholar] [CrossRef]
- Rana, N.; Kodirov, R.; Shakya, A.; King, J.T. Protein unfolding thermodynamics predict multicomponent phase behavior. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nicy; Joseph, J.A.; Collepardo-Guevara, R.; Wales, D.J. Energy landscapes and heat capacity signatures for peptides correlate with phase separation propensity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kurnellas, M.P.; Adams, C.M.; Sobel, R.A.; Steinman, L.; Rothbard, J.B. Amyloid fibrils composed of hexameric peptides attenuate neuroinflammation. Sci. Transl. Med. 2013, 5, 179ra42. [Google Scholar] [CrossRef]
- Ivanova, M.I.; Thompson, M.J.; Eisenberg, D. A systematic screen of β2-microglobulin and insulin for amyloid-like segments. Proc. Natl. Acad. Sci. USA 2006, 103, 4079–4082. [Google Scholar] [CrossRef]
- Makin, O.S.; Atkins, E.; Sikorski, P.; Johansson, J.; Serpell, L.C. Molecular basis for amyloid fibril formation and stability. Proc. Natl. Acad. Sci. USA 2005, 102, 315–320. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The Universal Protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Tenidis, K.; Waldner, M.; Bernhagen, J.; Fischle, W.; Bergmann, M.; Weber, M.; Merkle, M.L.; Voelter, W.; Brunner, H.; Kapurniotu, A. Identification of a penta-and hexapeptide of islet amyloid polypeptide (IAPP) with amyloidogenic and cytotoxic properties. J. Mol. Biol. 2000, 295, 1055–1071. [Google Scholar] [CrossRef]
- Azriel, R.; Gazit, E. Analysis of the minimal amyloid-forming fragment of the islet amyloid polypeptide. J. Biol. Chem. 2001, 276, 34156–34161. [Google Scholar] [CrossRef] [PubMed]
- Von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.M.; Mandelkow, E. Assembly of τ protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129–5134. [Google Scholar] [CrossRef]
- López de la Paz, M.; Serrano, L. Sequence determinants of amyloid fibril formation. Proc. Natl. Acad. Sci. USA 2004, 101, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Louros, N.; Konstantoulea, K.; De Vleeschouwer, M.; Ramakers, M.; Schymkowitz, J.; Rousseau, F. WALTZ-DB 2.0: An updated database containing structural information of experimentally determined amyloid-forming peptides. Nucleic Acids Res. 2020, 48, D389–D393. [Google Scholar] [CrossRef]
- Conchillo-Solé, O.; de Groot, N.S.; Avilés, F.X.; Vendrell, J.; Daura, X.; Ventura, S. AGGRESCAN: A server for the prediction and evaluation of “hot spots" of aggregation in polypeptides. BMC Bioinform. 2007, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Ciryam, P.; Tartaglia, G.G.; Morimoto, R.I.; Dobson, C.M.; Vendruscolo, M. Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep. 2013, 5, 781–790. [Google Scholar] [CrossRef]
- Johnson, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef]
- Becker, O.M.; Karplus, M. The topology of multidimensional potential energy surfaces: Theory and application to peptide structure and kinetics. J. Chem. Phys. 1997, 106, 1495–1517. [Google Scholar] [CrossRef]
- Wales, D.J.; Miller, M.A.; Walsh, T.R. Archetypal energy landscapes. Nature 1998, 394, 758–760. [Google Scholar] [CrossRef]
- Chakraborty, D.; Straub, J.E.; Thirumalai, D. Energy landscapes of Aβ monomers are sculpted in accordance with Ostwald’s rule of stages. Sci. Adv. 2023, 9, eadd6921. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J.; Doye, J.P.K. Global optimization by basin-hopping and the lowest energy structures of Lennard-Jones clusters containing up to 110 atoms. J. Phys. Chem. A 1997, 101, 5111–5116. [Google Scholar] [CrossRef]
- Sormanni, P.; Aprile, F.A.; Vendruscolo, M. The CamSol method of rational design of protein mutants with enhanced solubility. J. Mol. Biol. 2015, 427, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ye, W.; Jiang, C.; Luo, R.; Chen, H.F. New force field on modeling intrinsically disordered proteins. Chem. Biol. Drug Des. 2014, 84, 253–269. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Case, D.A.; Duke, R.E.; Walker, R.C.; Skrynnikov, N.R.; Cheatham, T.E., III; Mikhailovskii, O.; Simmerling, C.; Xue, Y.; Roitberg, A.; Izmailov, S.A.; et al. AMBER 22 Reference Manual; University of California: Los Angeles, CA, USA, 2022. [Google Scholar]
- Malolepsza, E.; Strodel, B.; Khalili, M.; Trygubenko, S.; Fejer, S.N.; Wales, D.J. Symmetrization of the AMBER and CHARMM Force Fields. J. Comput. Chem. 2010, 31, 1402–1409. [Google Scholar] [CrossRef]
- Li, Z.; Scheraga, H.A. Monte Carlo-minimization approach to the multiple-minima problem in protein folding. Proc. Natl. Acad. Sci. USA 1987, 84, 6611–6615. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Scheraga, H.A. Structure and free energy of complex thermodynamic systems. J. Mol. Struct. THEOCHEM 1988, 179, 333–352. [Google Scholar] [CrossRef]
- Wales, D.J. GMIN: A Program for Finding Global Minima and Calculating Thermodynamic Properties from Basin-Sampling. 2023. Available online: http://www-wales.ch.cam.ac.uk/GMIN/ (accessed on 26 January 2023).
- Kusumaatmaja, H.; Whittleston, C.S.; Wales, D.J. A local rigid body framework for global optimization of biomolecules. J. Chem. Theory Comput. 2012, 8, 5159–5165. [Google Scholar] [CrossRef]
- Rühle, V.; Kusumaatmaja, H.; Chakrabarti, D.; Wales, D.J. Exploring energy landscapes: Metrics, pathways, and normal-mode analysis for rigid-body molecules. J. Chem. Theory Comput. 2013, 9, 4026–4034. [Google Scholar] [CrossRef]
- Mochizuki, K.; Whittleston, C.S.; Somani, S.; Kusumaatmaja, H.; Wales, D.J. A conformational factorisation approach for estimating the binding free energies of macromolecules. Phys. Chem. Chem. Phys. 2014, 16, 2842–2853. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, D.; Wales, D.J. Simulations of rigid bodies in an angle-axis framework. Phys. Chem. Chem. Phys. 2009, 11, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J. Discrete path sampling. Mol. Phys. 2002, 100, 3285–3305. [Google Scholar] [CrossRef]
- Murrell, J.N.; Laidler, K.J. Symmetries of activated complexes. Trans. Faraday Soc. 1968, 64, 371–377. [Google Scholar] [CrossRef]
- Wales, D.J. Energy Landscapes: Applications to Clusters, Biomolecules and Glasses; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Carr, J.M.; Trygubenko, S.A.; Wales, D.J. Finding pathways between distant local minima. J. Chem. Phys. 2005, 122, 234903. [Google Scholar] [CrossRef]
- Trygubenko, S.A.; Wales, D.J. A doubly nudged elastic band method for finding transition states. J. Chem. Phys. 2004, 120, 2082–2094. [Google Scholar] [CrossRef]
- Sheppard, D.; Terrell, R.; Henkelman, G. Optimization methods for finding minimum energy paths. J. Chem. Phys. 2008, 128, 134106. [Google Scholar] [CrossRef]
- Mills, G.; Jónsson, H.; Schenter, G.K. Reversible work transition state theory: Application to dissociative adsorption of hydrogen. Surf. Sci. 1995, 324, 305–337. [Google Scholar] [CrossRef]
- Jónsson, H.; Mills, G.; Jacobsen, K.W. Nudged elastic band method for fnding minimum energy paths of transitions. In Classical and Quantum Dynamics in Condensed Phase Simulations; World Scientific: Singapore, 1998; Chapter 16; pp. 385–404. [Google Scholar]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]
- Munro, L.J.; Wales, D.J. Defect migration in crystalline silicon. Phys. Rev. B 1999, 59, 3969–3980. [Google Scholar] [CrossRef]
- Kumeda, Y.; Wales, D.J.; Munro, L.J. Transition states and rearrangement mechanisms from hybrid eigenvector-following and density functional theory. Application to C10H10 and defect migration in crystalline silicon. Chem. Phys. Lett. 2001, 341, 185–194. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiao, P.; Henkelman, G. Unification of algorithms for minimum mode optimization. J. Chem. Phys. 2014, 140, 044115. [Google Scholar] [CrossRef] [PubMed]
- Nocedal, J. Updating quasi-Newton matrices with limited storage. Math. Comput. 1980, 35, 773–782. [Google Scholar] [CrossRef]
- Liu, D.C.; Nocedal, J. On the limited memory BFGS method for large scale optimization. Math. Program. 1989, 45, 503–528. [Google Scholar] [CrossRef]
- Dijkstra, E.W. A note on two problems in connexion with graphs. J. Numer. Math. 1959, 1, 269–271. [Google Scholar] [CrossRef]
- Wales, D.J. OPTIM: A Program for Optimising Geometries and Calculating Pathways. 2023. Available online: https://www-wales.ch.cam.ac.uk/OPTIM/ (accessed on 26 January 2023).
- Wales, D.J. PATHSAMPLE: A Program for Generating Connected Stationary Point Databases and Extracting Global Kinetics. 2023. Available online: https://www-wales.ch.cam.ac.uk/PATHSAMPLE/ (accessed on 26 January 2023).
- Strodel, B.; Whittleston, C.S.; Wales, D.J. Thermodynamics and kinetics of aggregation for the GNNQQNY peptide. J. Am. Chem. Soc. 2007, 129, 16005–16014. [Google Scholar] [CrossRef]
- McGinty, D.J. Vapor phase homogeneous nucleation and the thermodynamic properties of small clusters of argon atoms. J. Chem. Phys. 1971, 55, 580–588. [Google Scholar] [CrossRef]
- Burton, J. Vibrational frequencies and entropies of small clusters of atoms. J. Chem. Phys. 1972, 56, 3133–3138. [Google Scholar] [CrossRef]
- Hoare, M. Structure and dynamics of simple microclusters. Adv. Chem. Phys. 1979, 40, 49–135. [Google Scholar]
- Franke, G.; Hilf, E.; Borrmann, P. The structure of small clusters: Multiple normal-modes model. J. Chem. Phys. 1993, 98, 3496–3502. [Google Scholar] [CrossRef]
- Wales, D.J. Coexistence in small inert gas clusters. Mol. Phys. 1993, 78, 151–171. [Google Scholar] [CrossRef]
- Strodel, B.; Wales, D.J. Free energy surfaces from an extended harmonic superposition approach and kinetics for alanine dipeptide. Chem. Phys. Lett. 2008, 466, 105–115. [Google Scholar] [CrossRef]
- Wales, D.J. Decoding heat capacity features from the energy landscape. Phys. Rev. E 2017, 95, 030105. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham III, T.E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Nicy; Wales, D.J. Research Data Supporting—Energy Landscapes and Heat Capacity Signatures for Monomers and Dimers of Amyloid Forming Hexapeptides; University of Cambridge: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
| Disease | Protein | Amyloid | Control |
|---|---|---|---|
| Diabetes mellitus | Amylin (hIAPP) | NFGAIL [42] | NLGPVL, LIAGFN [42], NAGAIL [43] |
| Alzheimer’s | Tau | VQIVYK [44] | VQIVEK, NAEVYK [44] |
| - | De novo designed | STVIIE [45] | SPVIIE, STVIIP, STVVIE [23,45] |
| - | Insulin A chain | LYQLEN [10,39] | YQLENY [23,39] |
| Dialysis-related amyloidosis | -microglobulin | LLYYTE [23,39] | YYTEFT [23] |
| Protein | Amyloid |
|---|---|
| A-A4 | KLVFFA [11], GAIIGL, GGVVIA, MVGGVV [10] |
| Amylin | GAILSS, SSTNVG |
| Apolipoprotein E | SSQVTQ |
| Major prion protein | SNQNNF [10] |
| Ig chain | SVSSSY [38] |
| Serum amyloid P | GYVIIK |
| Protein | Control |
| -microglobulin | EVDLLK, LSFSKD, NGERIE |
| Waltz-DB [46] | KAFIIQ, KAILFL |
| Amyloid | Interactions | Control | Interactions |
|---|---|---|---|
| NFGAIL | N–F, F–I/L | LIAGFN | N–F |
| VQIVYK | I–Y, Q–K (U), Q–Y (X) | VQIVEK | K–Q/E, Q–E |
| STVIIE | S–E (U), T–E (X) | SPVIIE | S–E |
| LYQLEN | Y–Q, Q–E (U), N–E (X) | YQLENY | Y–Y, Q–E, Y–Q, Y–Q–Y |
| LLYYTE | Y–Y | YYTEFT | T–E, E–Y |
| KLVFFA | F–F | EVDLLK | K–D/E |
| SSQVTQ | S–T/Q, Q–Q | NAEVYK | E–Y/K |
| SNQNNF | S–N, N–N, Q–N, N–Q–N | STVVIE | S–E, T–E |
| SSTNVG | S–N/S | LSFSKD | K–D, L–F, S–D |
| SVSSSY | S–Y/S | NGERIE | E–R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicy; Wales, D.J. Energy Landscapes and Heat Capacity Signatures for Monomers and Dimers of Amyloid-Forming Hexapeptides. Int. J. Mol. Sci. 2023, 24, 10613. https://doi.org/10.3390/ijms241310613
Nicy, Wales DJ. Energy Landscapes and Heat Capacity Signatures for Monomers and Dimers of Amyloid-Forming Hexapeptides. International Journal of Molecular Sciences. 2023; 24(13):10613. https://doi.org/10.3390/ijms241310613
Chicago/Turabian StyleNicy, and David J. Wales. 2023. "Energy Landscapes and Heat Capacity Signatures for Monomers and Dimers of Amyloid-Forming Hexapeptides" International Journal of Molecular Sciences 24, no. 13: 10613. https://doi.org/10.3390/ijms241310613
APA StyleNicy, & Wales, D. J. (2023). Energy Landscapes and Heat Capacity Signatures for Monomers and Dimers of Amyloid-Forming Hexapeptides. International Journal of Molecular Sciences, 24(13), 10613. https://doi.org/10.3390/ijms241310613






