Analysis of MMP-2-735C/T (rs2285053) and MMP-9-1562C/T (rs3918242) Polymorphisms in the Risk Assessment of Developing Lung Cancer
Abstract
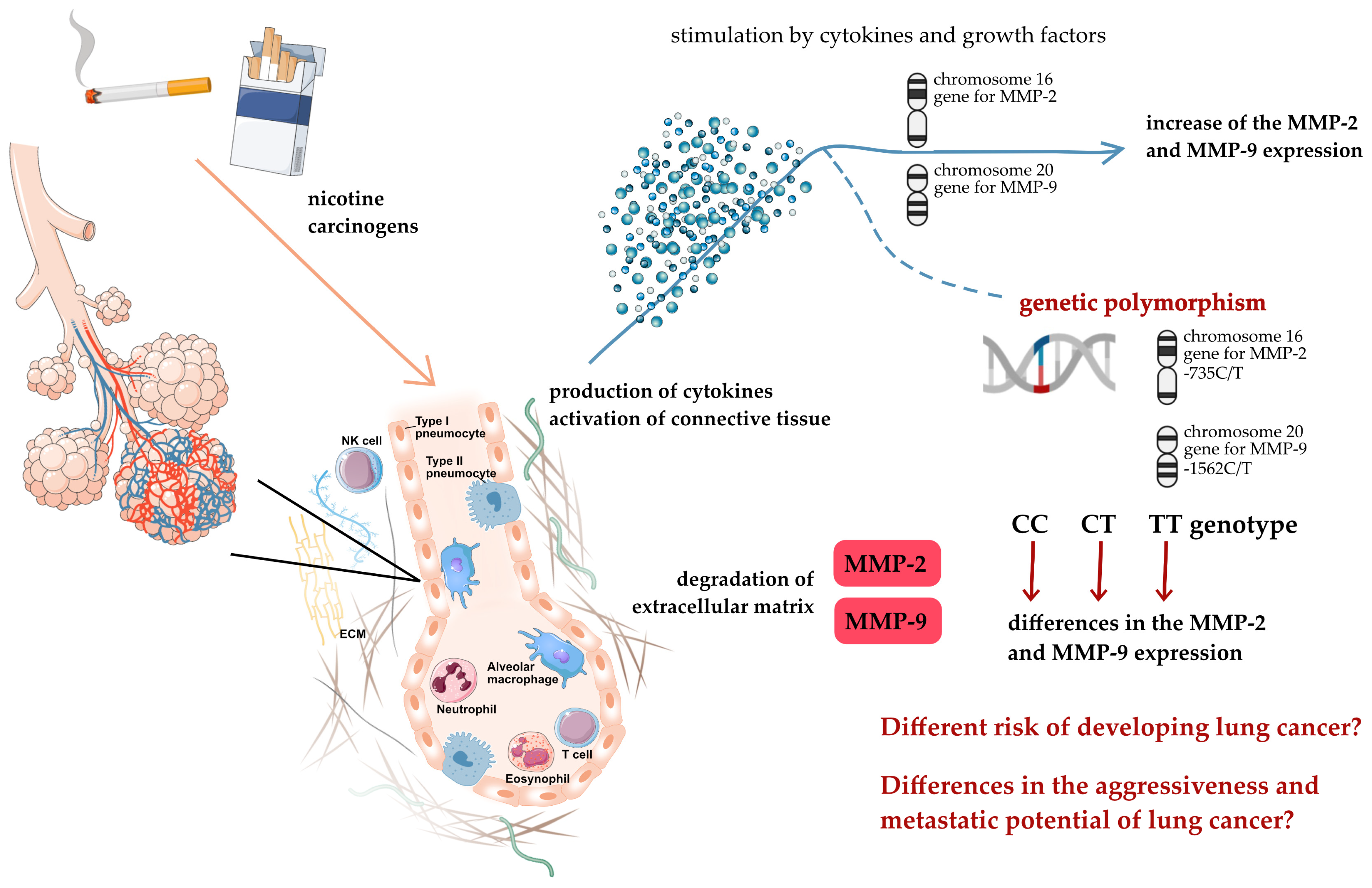
1. Introduction
2. Results
2.1. Characteristics of Cases and Controls
2.2. The Effect of Dependent Variables on the Risk of Developing Lung Cancer
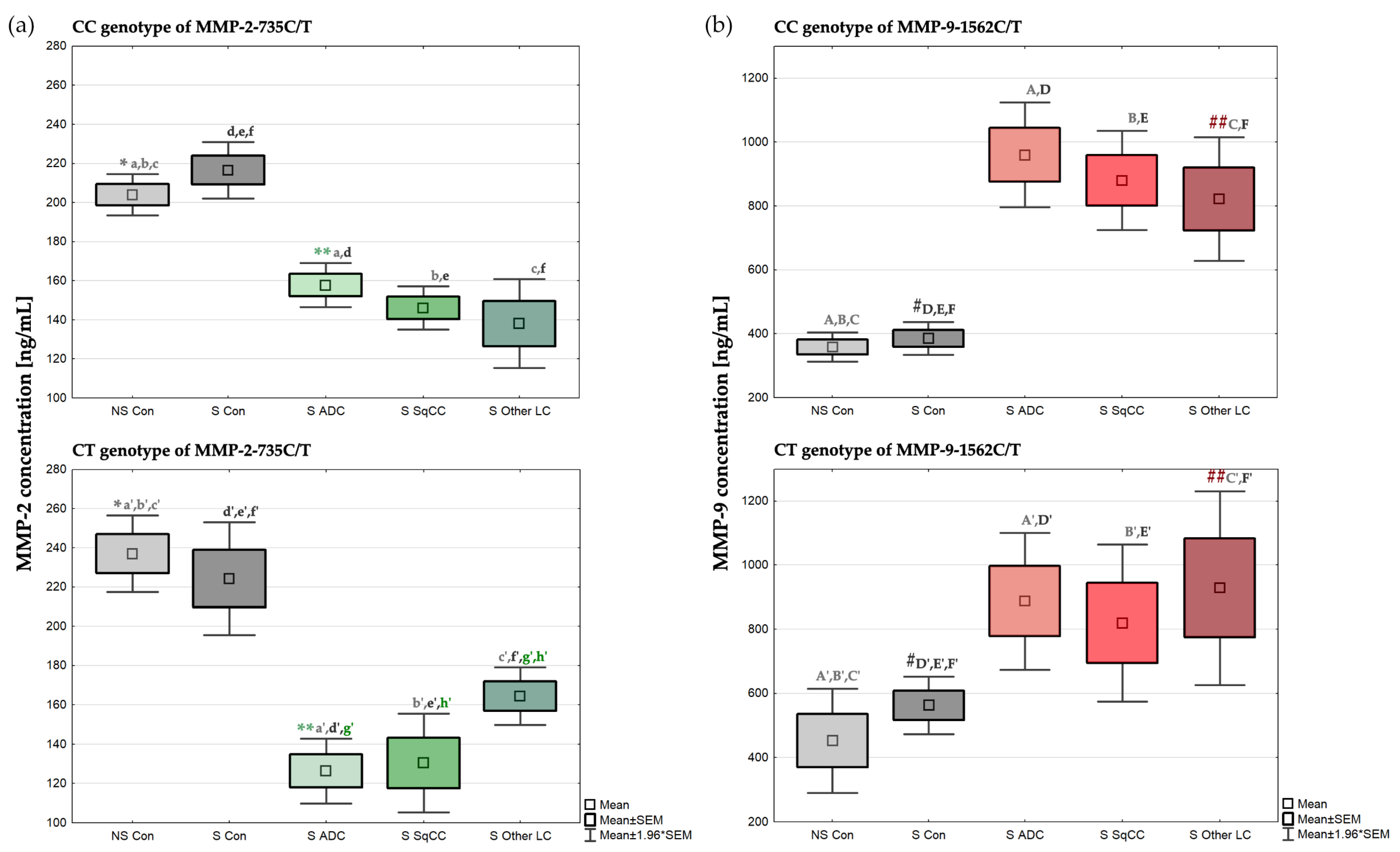
2.3. Concentration of MMP-2 Depending on the MMP-2-735C/T Genotypes
2.4. Concentration of MMP-9 Depending on the MMP-9-1562C/T Genotypes
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Liu, G.; Philp, A.M.; Corte, T.; Travis, M.A.; Schilter, H.; Hansbro, N.G.; Burns, C.J.; Eapen, M.S.; Sohal, S.S.; Burgess, J.K.; et al. Therapeutic Targets in Lung Tissue Remodelling and Fibrosis. Pharm. Ther. 2021, 225, 107839. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix Metalloproteinases and the Regulation of Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Murphy, G. Matrix Metalloproteinases. In Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 621–629. [Google Scholar]
- Lung Source: Globocan 2020 Number of New Cases in 2020, Both Sexes, All Ages. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 1 June 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Didkowska, J.; Wojciechowska, U.; Mańczuk, M.; Łobaszewski, J. Lung Cancer Epidemiology: Contemporary and Future Challenges Worldwide. Ann. Transl. Med. 2016, 4, 150. [Google Scholar] [CrossRef]
- Horn, L.; Lovly, C.M. Chapter 74: Neoplasms of the Lung. In Harrison’s Principles of Internal Medicine; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J.L., Eds.; McGraw Hill: New York, NY, USA, 2018. [Google Scholar]
- Hirsch, F.R.; Franklin, W.A.; Gazdar, A.F.; Bunn, P.A. Early Detection of Lung Cancer: Clinical Perspectives of Recent Advances in Biology and Radiology. Clin. Cancer Res. 2001, 7, 5–22. [Google Scholar]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Jakubek, Y.; Lang, W.; Vattathil, S.; Garcia, M.; Xu, L.; Huang, L.; Yoo, S.-Y.; Shen, L.; Lu, W.; Chow, C.-W.; et al. Genomic Landscape Established by Allelic Imbalance in the Cancerization Field of a Normal Appearing Airway. Cancer Res. 2016, 76, 3676–3683. [Google Scholar] [CrossRef]
- Kiyohara, C.; Otsu, A.; Shirakawa, T.; Fukuda, S.; Hopkin, J.M. Genetic Polymorphisms and Lung Cancer Susceptibility: A Review. Lung Cancer 2002, 37, 241–256. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Zou, X.; Jiang, Y.; Yao, J.; Liu, H.; Ni, B.; Ma, H. COX-2 Rs5275 and Rs689466 Polymorphism and Risk of Lung Cancer: A PRISMA-Compliant Meta-Analysis. Medicine 2018, 97, e11859. [Google Scholar] [CrossRef]
- Guo, X.T.; Wang, J.F.; Zhang, L.Y.; Xu, G.Q. Quantitative Assessment of the Effects of MMP-2 Polymorphisms on Lung Carcinoma Risk. Asian Pac. J. Cancer Prev. 2012, 13, 2853–2856. [Google Scholar] [CrossRef]
- Hecht, S.S. Cigarette Smoking and Lung Cancer: Chemical Mechanisms and Approaches to Prevention. Lancet Oncol. 2002, 3, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Ran, R.; Liu, G.; Yang, Y.; Zhao, W.; Xie, X.; Li, J. Matrix Metalloproteinase Family Gene Polymorphisms and Lung Cancer Susceptibility: An Updated Meta-Analysis. J. Thorac. Dis. 2020, 12, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, M.; Wang, C.; Liu, C.; Luo, K.; Yang, L. Study on the Relationship between MMP-2, MMP-9 Gene Polymorphisms, and the Risk of Colorectal Cancer. Evid.-Based Complement. Altern. Med. 2022, 2022, 7357160. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Prysak, G.M.; Bock, C.H.; Cote, M.L. The Molecular Epidemiology of Lung Cancer. Carcinogenesis 2006, 28, 507–518. [Google Scholar] [CrossRef]
- Shields, P.G.; Harris, C.C. Molecular Epidemiology and the Genetics of Environmental Cancer. JAMA 1991, 266, 681–687. [Google Scholar] [CrossRef]
- Blanco-Prieto, S.; Barcia-Castro, L.; Páez de la Cadena, M.; Rodríguez-Berrocal, F.J.; Vázquez-Iglesias, L.; Botana-Rial, M.I.; Fernández-Villar, A.; De Chiara, L. Relevance of Matrix Metalloproteases in Non-Small Cell Lung Cancer Diagnosis. BMC Cancer 2017, 17, 823. [Google Scholar] [CrossRef]
- Schveigert, D.; Cicenas, S.; Bruzas, S.; Samalavicius, N.; Gudleviciene, Z.; Didziapetriene, J. The Value of MMP-9 for Breast and Non-Small Cell Lung Cancer Patients’ Survival. Adv. Med. Sci. 2013, 58, 73–82. [Google Scholar] [CrossRef]
- Butkiewicz, D.; Krzesniak, M.; Drosik, A.; Giglok, M.; Gdowicz-Kłosok, A.; Kosarewicz, A.; Rusin, M.; Masłyk, B.; Gawkowska-Suwińska, M.; Suwiński, R. The VEGFR2, COX-2 and MMP-2 Polymorphisms Are Associated with Clinical Outcome of Patients with Inoperable Non-Small Cell Lung Cancer. Int. J. Cancer 2015, 137, 2332–2342. [Google Scholar] [CrossRef]
- Li, H.; Liang, X.; Qin, X.; Cai, S.; Yu, S. Association of Matrix Metalloproteinase Family Gene Polymorphisms with Lung Cancer Risk: Logistic Regression and Generalized Odds of Published Data. Sci. Rep. 2015, 5, 10056. [Google Scholar] [CrossRef]
- Dofara, S.G.; Chang, S.-L.; Diorio, C. Gene Polymorphisms and Circulating Levels of MMP-2 and MMP-9: A Review of Their Role in Breast Cancer Risk. Anticancer Res. 2020, 40, 3619–3631. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C. Matrix metalloproteinases. In Encyclopedia of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2006; pp. 18–25. [Google Scholar]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [PubMed]
- Iniesta, P.; Morán, A.; De Juan, C.; Gómez, A.; Hernando, F.; García-Aranda, C.; Frías, C.; Díaz-López, A.; Rodríguez-Jiménez, F.-J.; Balibrea, J.-L.; et al. Biological and Clinical Significance of MMP-2, MMP-9, TIMP-1 and TIMP-2 in Non-Small Cell Lung Cancer. Oncol. Rep. 2007, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Wang, S.-C.; Shen, T.-C.; Tsai, C.-W.; Chang, W.-S.; Li, H.-T.; Wu, C.-N.; Chao, C.-Y.; Hsia, T.-C.; Bau, D.-T. The Association of Matrix Metalloproteinas-2 Promoter Polymorphisms with Lung Cancer Susceptibility in Taiwan. Chin. J. Physiol. 2019, 62, 210. [Google Scholar] [CrossRef]
- Hsu, S.-W.; Gong, C.-L.; Hsu, H.-M.; Chao, C.-C.; Wang, Y.-C.; Chang, W.-S.; Tsai, Y.-T.; Shih, L.-C.; Tsai, C.-W.; Bau, D.-T. Contribution of Matrix Metalloproteinase-2 Promoter Genotypes to Nasopharyngeal Cancer Susceptibility and Metastasis in Taiwan. Cancer Genom.-Proteom. 2019, 16, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Yueh, T.-C.; Hung, Y.-C.; Lee, H.-T.; Yang, M.-D.; Wang, Z.-H.; Yang, Y.-C.; Ke, T.-W.; Pei, J.-S.; Tsai, C.-W.; Bau, D.-T.; et al. Role of Matrix Metallopeptidase-2 Genotypes in Taiwanese Patients With Colorectal Cancer. Anticancer Res. 2022, 42, 5335–5342. [Google Scholar] [CrossRef]
- Li, P.-H.; Liao, C.-H.; Huang, W.-C.; Chang, W.-S.; Wu, H.-C.; Hsu, S.-W.; Chen, K.-Y.; Wang, Z.-H.; Hsia, T.-C.; Bau, D.-T.; et al. Association of Matrix Metalloproteinase-2 Genotypes With Prostate Cancer Risk. Anticancer Res. 2023, 43, 343–349. [Google Scholar] [CrossRef]
- Liu, S.; Xu, C.; Wu, W.; Fu, Z.; He, S.; Qin, M.; Huang, J. Sphingosine Kinase 1 Promotes the Metastasis of Colorectal Cancer by Inducing the Epithelial-mesenchymal Transition Mediated by the FAK/AKT/MMPs Axis. Int. J. Oncol. 2018, 54, 41–52. [Google Scholar] [CrossRef]
- Kesanakurti, D.; Chetty, C.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Role of MMP-2 in the Regulation of IL-6/Stat3 Survival Signaling via Interaction with A5β1 Integrin in Glioma. Oncogene 2013, 32, 327–340. [Google Scholar] [CrossRef]
- Liu, C.J.; Hsia, T.C.; Wang, R.F.; Tsai, C.W.; Chu, C.C.; Hang, L.W.; Wang, C.H.; Lee, H.Z.; Tsai, R.Y.; Bau, D.T. Interaction of Cyclooxygenase 2 Genotype and Smoking Habit in Taiwanese Lung Cancer Patients. Anticancer Res. 2010, 30, 1195–1199. [Google Scholar]
- Wang, W.; Fan, X.; Zhang, Y.; Yang, Y.; Yang, S.; Li, G. Association between COX-2 Polymorphisms and Lung Cancer Risk. Med. Sci. Monit. 2015, 21, 3740–3747. [Google Scholar] [CrossRef] [PubMed]
- Campa, D.; Zienolddiny, S.; Maggini, V.; Skaug, V.; Haugen, A.; Canzian, F. Association of a Common Polymorphism in the Cyclooxygenase 2 Gene with Risk of Non-Small Cell Lung Cancer. Carcinogenesis 2004, 25, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wadowska, K.; Bil-Lula, I.; Trembecki, Ł.; Śliwińska-Mossoń, M. Genetic Markers in Lung Cancer Diagnosis: A Review. Int. J. Mol. Sci. 2020, 21, 4569. [Google Scholar] [CrossRef]
- Bayramoglu, A.; Gunes, H.V.; Metintas, M.; Deg, I.; Mutlu, F.; Alataş, F. The Association of MMP-9 Enzyme Activity, MMP-9 C1562T Polymorphism, and MMP-2 and-9 and TIMP-1,-2,-3, and-4 Gene Expression in Lung Cancer. Genet. Test. Mol. Biomark. 2009, 13, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Sanli, M.; Akar, E.; Pehlivan, S.; Bakir, K.; Tuncozgur, B.; Isik, A.F.; Pehlivan, M.; Elbeyli, L. The Relationship of Metalloproteinase Gene Polymorphisms and Lung Cancer. J. Surg. Res. 2013, 183, 517–523. [Google Scholar] [CrossRef]
- Hu, Z.; Huo, X.; Lu, D.; Qian, J.; Zhou, J.; Chen, Y.; Xu, L.; Ma, H.; Zhu, J.; Wei, Q.; et al. Functional Polymorphisms of Matrix Metalloproteinase-9 Are Associated with Risk of Occurrence and Metastasis of Lung Cancer. Clin. Cancer Res. 2005, 11, 5433–5439. [Google Scholar] [CrossRef]
- Li, W.; Jia, M.X.; Wang, J.H.; Lu, J.L.; Deng, J.; Tang, J.X.; Liu, C. Association of Mmp9-1562c/t and Mmp13-77a/g Polymorphisms with Non-Small Cell Lung Cancer in Southern Chinese Population. Biomolecules 2019, 9, 107. [Google Scholar] [CrossRef]
- Wang, J.; Cai, Y. Matrix Metalloproteinase 2 Polymorphisms and Expression in Lung Cancer: A Meta-Analysis. Tumour. Biol. 2012, 33, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- González-Arriaga, P.; Pascual, T.; García-Alvarez, A.; Fernández-Somoano, A.; López-Cima, M.F.; Tardón, A. Genetic Polymorphisms in MMP 2, 9 and 3 Genes Modify Lung Cancer Risk and Survival. BMC Cancer 2012, 12, 121. [Google Scholar] [CrossRef]
- Rollin, J.; Régina, S.; Vourc’h, P.; Iochmann, S.; Bléchet, C.; Reverdiau, P.; Gruel, Y. Influence of MMP-2 and MMP-9 Promoter Polymorphisms on Gene Expression and Clinical Outcome of Non-Small Cell Lung Cancer. Lung Cancer 2007, 56, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, C.; Miao, X.; Wang, Y.; Tan, W.; Sun, T.; Zhang, X.; Xiong, P.; Lin, D. Functional Haplotypes in the Promoter of Matrix Metalloproteinase-2 and Lung Cancer Susceptibility. Carcinogenesis 2005, 26, 1117–1121. [Google Scholar] [CrossRef]
- Nakamura, T.; Ebihara, I.; Shimada, N.; Koide, H. Effect of Cigarette Smoking on Plasma Metalloproteinase-9 Concentration. Clin. Chim. Acta 1998, 276, 173–177. [Google Scholar] [CrossRef]
- Cao, C.; Xu, N.; Zheng, X.; Zhang, W.; Lai, T.; Deng, Z.; Huang, X. Elevated Expression of MMP-2 and TIMP-2 Cooperatively Correlates with Risk of Lung Cancer. Oncotarget 2017, 8, 80560–80567. [Google Scholar] [CrossRef]
- Drzewiecka-Jędrzejczyk, M.; Wlazeł, R.; Terlecka, M.; Jabłoński, S. Serum Metalloproteinase-2 and Tissue Inhibitor of Metalloproteinase-2 in Lung Carcinoma Patients. J. Thorac. Dis. 2017, 9, 5306–5313. [Google Scholar] [CrossRef] [PubMed]
- Wadowska, K.; Błasiak, P.; Rzechonek, A.; Bil-Lula, I.; Śliwińska-Mossoń, M. New Insights on Old Biomarkers Involved in Tumor Microenvironment Changes and Their Diagnostic Relevance in Non-Small Cell Lung Carcinoma. Biomolecules 2021, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, L.; Shi, X.; Zuo, L. MMP2 Gene Polymorphism and Tumor Susceptibility Study. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
| Variable | Cases [n, (%)] n = 112 | Controls [n, (%)] n = 100 | p-Value (Pearson’s Chi-Square Test) |
|---|---|---|---|
| Age [years] | |||
| ≤60 | 22 (19.6%) | 85 (85.0%) | p < 0.00001 |
| >60 | 90 (80.4%) | 15 (15.0%) | |
| Gender | |||
| Male | 71 (63.4%) | 46 (46.0%) | p = 0.01102 |
| Female | 41 (36.6%) | 54 (54.0%) | |
| Smoking status | |||
| Never smoker | 3 (2.7%) | 47 (47.0%) | p < 0.00001 |
| Light smoker | 7 (6.3%) | 37 (37.0%) | |
| Moderate smoker | 33 (29.5%) | 11 (11.0%) | |
| Heavy smoker | 29 (25.9%) | 2 (2.0%) | |
| NA | 40 (35.7%) | 3 (3.0%) | |
| MMP-2-735 C/T | |||
| CC | 83 (74.1%) | 77 (77.0%) | p = 0.14379 |
| CT | 26 (23.2%) | 18 (18.0%) | |
| TT | 1 (0.9%) | 5 (5.0%) | |
| NA | 2 (1.8%) | 0 (0.0%) | |
| MMP-9-1562 C/T | |||
| CC | 76 (67.9%) | 70 (70.0%) | p = 0.83358 |
| CT | 30 (26.8%) | 25 (25.0%) | |
| TT | 3 (2.7%) | 4 (4.0%) | |
| NA | 3 (2.7%) | 1 (1.0%) |
| SNP | Gene | Band | Position | Alleles | Molecular Consequences | MAF—Cases | MAF—Controls | p-Value |
|---|---|---|---|---|---|---|---|---|
| rs2285053 | MMP-2 | 16q12.2 | 55478465 | C>T | 2KB upstream variant | 0.1273 | 0.1400 | 0.7376 |
| rs3918242 | MMP-9 | 20q13.2 | 46007337 | C>T | 2KB upstream variant | 0.1651 | 0.1667 | 0.9717 |
| Population | N | Mean ± SEM | Median | Min–Max |
|---|---|---|---|---|
| MMP-2-735C/T (rs2285053) | ||||
| European | 9 | 0.1101 ± 0.0087 a,b | 0.1133 | 0.0500–0.1346 |
| East Asian | 9 | 0.2591 ± 0.0047 a,c,d,e | 0.2604 | 0.2368–0.2784 |
| South Asian | 5 | 0.1454 ± 0.0280 c | 0.1186 | 0.1110–0.2570 |
| African | 5 | 0.1164 ± 0.0020 d,f | 0.1153 | 0.1105–0.1221 |
| American | 3 | 0.1761 ± 0.0048 b,e,f | 0.1801 | 0.1666–0.1816 |
| Semitic | 2 | 0.1411 ± 0.0024 | 0.1411 | 0.1387–0.1435 |
| Latin 1 | 2 | 0.1227 ± 0.0132 | 0.1227 | 0.1096–0.1358 |
| Latin 2 | 2 | 0.1872 ± 0.0068 | 0.1872 | 0.1803–0.1940 |
| Other | 2 | 0.1393 ± 0.0102 | 0.1393 | 0.1291–0.1494 |
| MMP-9-1562C/T (rs3918242) | ||||
| European | 8 | 0.1666 ± 0.0051 | 0.1671 | 0.1463–0.1873 |
| East Asian | 8 | 0.1583 ± 0.0110 | 0.1562 | 0.1294–0.2260 |
| South Asian | 4 | 0.2343 ± 0.0415 * | 0.2427 | 0.1250–0.3269 |
| African | 5 | 0.2199 ± 0.0788 ** | 0.1214 | 0.1103–0.5231 |
| American | 3 | 0.0809 ± 0.0039 *,** | 0.0796 | 0.0749–0.0883 |
| Semitic | 2 | 0.1610 ± 0.0057 | 0.1610 | 0.1553–0.1667 |
| Latin 1 | 1 | 0.1096 | 0.1096 | |
| Latin 2 | 1 | 0.0836 | 0.0836 | |
| Other | 2 | 0.1741 | 0.1741 | 0.1257–0.2225 |
| |||||||
| Variable | Cases | Controls | p-Value (Pearson’s Chi-Square Test) | ||||
| CC (n = 83) | CT (n = 26) | TT (n = 1) | CC (n = 77) | CT (n = 18) | TT (n = 5) | ||
| Age [years] | |||||||
| ≤60 | 18 (21.7%) | 3 (11.5%) | 1 (100.0%) | 65 (84.4%) | 16 (88.9%) | 4 (80.0%) | a p = 0.07026 |
| >60 | 65 (78.3%) | 23 (88.5%) | 0 (0.0%) | 12 (15.6%) | 2 (11.1%) | 1 (20.0%) | b p = 0.84697 |
| Gender | |||||||
| Male | 54 (65.1%) | 15 (57.7%) | 1 (100.0%) | 35 (45.5%) | 9 (50.0%) | 2 (40.0%) | a p = 0.59419 |
| Female | 29 (34.9%) | 11 (42.3%) | 0 (0.0%) | 42 (54.5%) | 9 (50.0%) | 3 (60.0%) | b p = 0.90591 |
| Smoking status | |||||||
| Never smoker | 2 (2.4%) | 1 (3.8%) | 0 (0.0%) | 39 (50.6%) | 5 (27.8%) | 3 (60.0%) | a p = 0.90223 |
| Light smoker | 7 (8.4%) | 0 (0.0%) | 0 (0.0%) | 27 (35.1%) | 8 (44.4%) | 2 (40.0%) | b p = 0.45752 |
| Moderate smoker | 25 (30.1%) | 7 (26.9%) | 0 (0.0%) | 7 (9.1%) | 4 (22.2%) | 0 (0.0%) | |
| Heavy smoker | 23 (27.7%) | 6 (23.1%) | 0 (0.0%) | 2 (2.6%) | 0 (0.0%) | 0 (0.0%) | |
| NA | 26 (31.3%) | 12 (46.2%) | 1 (100.0%) | 2 (2.6%) | 1 (5.6%) | 0 (0.0%) | |
| Lung cancer subtype | |||||||
| Adenocarcinoma | 39 (47.0%) | 10 (38.5%) | 0 (0.0%) | a p = 0.21120 | |||
| Squamous cell carcinoma | 28 (33.7%) | 7 (26.9%) | 0 (0.0%) | ||||
| Other lung neoplasms | 16 (19.3%) | 9 (34.6%) | 1 (100.0%) | ||||
| Metastases | |||||||
| No metastases | 40 (48.2%) | 11 (42.3%) | 1 (100.0%) | a p = 0.80839 | |||
| To lymph nodes | 32 (38.6%) | 12 (46.2%) | 0 (0.0%) | ||||
| Distant metastases | 11 (13.3%) | 3 (11.5%) | 0 (0.0%) | ||||
| |||||||
| Variable | Cases | Controls | p-Value (Pearson’s Chi-Square Test) | ||||
| CC (n = 76) | CT (n = 30) | TT (n = 3) | CC (n = 70) | CT (n = 25) | TT (n = 4) | ||
| Age [years] | |||||||
| ≤60 | 11 (14.5%) | 8 (26.7%) | 2 (66.7%) | 61 (87.1%) | 20 (80.0%) | 3 (75.0%) | a p = 0.03854 |
| >60 | 65 (85.5%) | 22 (73.3%) | 1 (33.3%) | 9 (12.9%) | 5 (20.0%) | 1 (25.0%) | b p = 0.59287 |
| Gender | |||||||
| Male | 47 (61.8%) | 20 (66.7%) | 3 (100.0%) | 30 (42.9%) | 13 (52.0%) | 3 (75.0%) | a p = 0.37972 |
| Female | 29 (38.2%) | 10 (33.3%) | 0 (0.0%) | 40 (57.1%) | 12 (48.0%) | 1 (25.0%) | b p = 0.37092 |
| Smoking status | |||||||
| Never smoker | 2 (2.6%) | 1 (3.3%) | 0 (0.0%) | 35 (50.0%) | 10 (40.0%) | 2 (50.0%) | a p = 0.84560 |
| Light smoker | 6 (7.9%) | 1 (3.3%) | 0 (0.0%) | 25 (35.7%) | 10 (40.0%) | 1 (25.0%) | b p = 0.91162 |
| Moderate smoker | 25 (32.9%) | 7 (23.3%) | 0 (0.0%) | 7 (10.0%) | 3 (12.0%) | 1 (25.0%) | |
| Heavy smoker | 18 (23.7%) | 11 (36.7%) | 0 (0.0%) | 1 (1.4%) | 1 (4.0%) | 0 (0.0%) | |
| NA | 25 (32.9%) | 10 (33.3%) | 3 (100.0%) | 2 (2.9%) | 1 (4.0%) | 0 (0.0%) | |
| Lung cancer subtype | |||||||
| Adenocarcinoma | 39 (51.3%) | 9 (30.0%) | 1 (33.3%) | a p = 0.11658 | |||
| Squamous cell carcinoma | 22 (28.9%) | 12 (40.0%) | 0 (0.0%) | ||||
| Other lung neoplasms | 15 (19.7%) | 9 (30.0%) | 2 (66.7%) | ||||
| Metastases | |||||||
| No metastases | 35 (46.1%) | 14 (46.7%) | 2 (66.7%) | a p = 0.94658 | |||
| To lymph nodes | 30 (39.5%) | 12 (40.0%) | 1 (33.3%) | ||||
| Distant metastases | 11 (14.5%) | 4 (13.3%) | 0 (0.0%) | ||||
| Variable | Adenocarcinoma [n, (%)] a,b n = 50 (44.6%) | Squamous Cell Carcinoma [n, (%)] a,c n = 35 (31.3%) | Other Lung Neoplasms [n, (%)] b,c n = 27 (24.1%) | p-Value (Pearson’s Chi-Square Test) |
|---|---|---|---|---|
| Age [years] | a p = 0.74016 | |||
| ≤60 | 9 (18.0%) | 6 (17.1%) | 5 (18.5%) | b p = 0.81861 |
| >60 | 41 (82.0%) | 29 (82.9%) | 22 (81.5%) | c p = 0.61571 |
| Gender | a p = 0.06694 | |||
| Male | 29 (58.0%) | 27 (77.1%) | 15 (56.6%) | b p = 0.83614 |
| Female | 21 (42.0%) | 8 (22.9%) | 12 (44.4%) | c p = 0.07140 |
| Smoking status | ||||
| Never smoker | 3 (6.0%) | 0 (0.0%) | 0 (0.0%) | |
| Light smoker | 4 (8.0%) | 3 (8.6%) | 0 (0.0%) | a p = 0.35977 |
| Moderate smoker | 17 (34.0%) | 11 (31.4%) | 5 (18.5%) | b p = 0.33032 |
| Heavy smoker | 11 (22.0%) | 12 (34.3%) | 6 (22.2%) | c p = 0.49688 |
| NA | 15 (30.0%) | 9 (25.7%) | 16 (59.3%) | |
| Metastases | ||||
| No metastases | 26 (52.0%) | 15 (42.9%) | 12 (44.4%) | a p = 0.57388 |
| To the lymph nodes | 20 (40.0%) | 18 (51.4%) | 6 (33.3%) | b p = 0.01420 |
| Distant | 4 (8.0%) | 2 (5.7%) | 9 (22.2%) | c p = 0.00701 |
| Variable | Lung Cancer Patient vs. Control | |
|---|---|---|
| OR (95% CI) | p-Value | |
| Gender–Male | 2.03 (1.17–3.52) | 0.011458 |
| Age | 1.23 (1.16–1.30) | <0.000001 |
| Pack-years | 1.15 (1.10–1.19) | <0.000001 |
| MMP-2 [ng/mL] | 0.96 (0.95–0.97) | <0.000001 |
| MMP-2-735 CC | 5.39 (0.62–47.17) | 0.238504 |
| MMP-2-735 CT | 7.22 (0.78–67.14) | 0.072836 |
| MMP-9 [ng/mL] | 1.01 (1.00–1.01) | <0.000001 |
| MMP-9-1562 CC | 1.45 (0.31–6.70) | 0.757914 |
| MMP-9-1562 CT | 1.60 (0.33–7.83) | 0.548801 |
| MMP-2 [ng/mL] | MMP-9 [ng/mL] | |||||
|---|---|---|---|---|---|---|
| MMP-2-735C/T Genotype | MMP-9-1562C/T Genotype | |||||
| CC | CT | TT | CC | CT | TT | |
| Non-smoking control (NSC) | ||||||
| (n = 39) | (n = 5) | (n = 3) | (n = 35) | (n = 10) | (n = 2) | |
| Mean ± SEM | 204.04 *,a,b,c ± 5.43 | 237.00 *,a’,b’,c’ ± 10.01 | 207.54 ± 3.97 | 358.74 ± 23.19 | 452.62 A’,B’,C’ ± 82.74 | 358.70 ± 32.02 |
| Median | 208.01 | 239.60 | 207.41 | 312.41 A,B,C | 396.93 | 358.70 |
| Min–Max | 145.21–275.71 | 207.97–269.46 | 200.74–214.47 | 192.94–697.64 | 152.14–941.59 | 326.68–390.72 |
| Smoking control (SC) | ||||||
| (n = 37) | (n = 12) | (n = 2) | (n = 34) | (n = 14) | (n = 2) | |
| Mean ± SEM | 216.56 d,e,f ± 7.37 | 224.34 d’,e’,f’ ± 14.69 | 190.92 ± 47.38 | 385.67 #,D,E,F ± 26.31 | 562.80 #,D’,E’,F’ ± 45.55 | 648.57 ± 139.44 |
| Median | 215.81 | 217.85 | 190.92 | 378.48 | 519.45 | 648.57 |
| Min–Max | 134.65–317.92 | 148.72–318.04 | 143.54–238.30 | 148.51–633.65 | 317.65–840.55 | 509.13–788.02 |
| Adenocarcinoma (ADC) | ||||||
| (n = 39) | (n = 10) | (n = 0) | (n = 39) | (n = 9) | (n = 1) | |
| Mean ± SEM | 157.69 **,a,d ± 5.75 | 126.37 **,a’,d’,g’ ± 8.41 | 959.95 D ± 83.76 | 887.55 A’,D’ ± 108.93 | 1307.07 | |
| Median | 154.72 | 128.25 | 936.72A | 779.53 | 1307.07 | |
| Min–Max | 94.93–237.57 | 90.70–175.39 | 73.03–2143.81 | 654.54–1310.73 | ||
| Squamous cell carcinoma (SqCC) | ||||||
| (n = 28) | (n = 7) | (n = 0) | (n = 22) | (n = 12) | (n = 0) | |
| Mean ± SEM | 146.11 b,e ± 5.69 | 130.43 b’,e’,h’ ± 12.81 | 880.26 E ± 79.06 | 819.39 B’,E’ ± 124.75 | ||
| Median | 142.00 | 137.85 | 778.66B | 817.61 | ||
| Min–Max | 90.70–209.82 | 81.92–180.09 | 403.44–1632.66 | 295.32–1514.18 | ||
| Other lung neoplasms (OLN) | ||||||
| (n = 16) | (n = 9) | (n = 1) | (n = 15) | (n = 9) | (n = 2) | |
| Mean ± SEM | 138.05 c,f ± 11.62 | 164.48 c’,f’,g’,h’ ± 7.51 | 231.50 | 821.64 ##,F ± 98.62 | 928.88 ##,C’,F’ ± 154.40 | 1527.66 ± 343.10 |
| Median | 134.93 | 164.78 | 231.50 | 932.81C | 802.18 | 1527.66 |
| Min–Max | 59.16–227.51 | 133.53–200.94 | 104.94–1322.75 | 413.85–1801.21 | 1184.56–1870.75 | |
| ELISA Kit | Standard Curve | Intra-Assay Precision | Inter-Assay Precision | Minimum Detectable Dose (MDD) |
|---|---|---|---|---|
| Cotinine | 5–100 ng/mL | 4.6–8.6% | 1 ng/mL | |
| MMP-2 | 0.5–32 ng/mL | 3.6–7.0% | 6.5–7.0% | 0.033 ng/mL |
| MMP-9 | 0.313–20 ng/mL | 1.9–2.9% | 6.9–7.9% | <0.156 ng/mL |
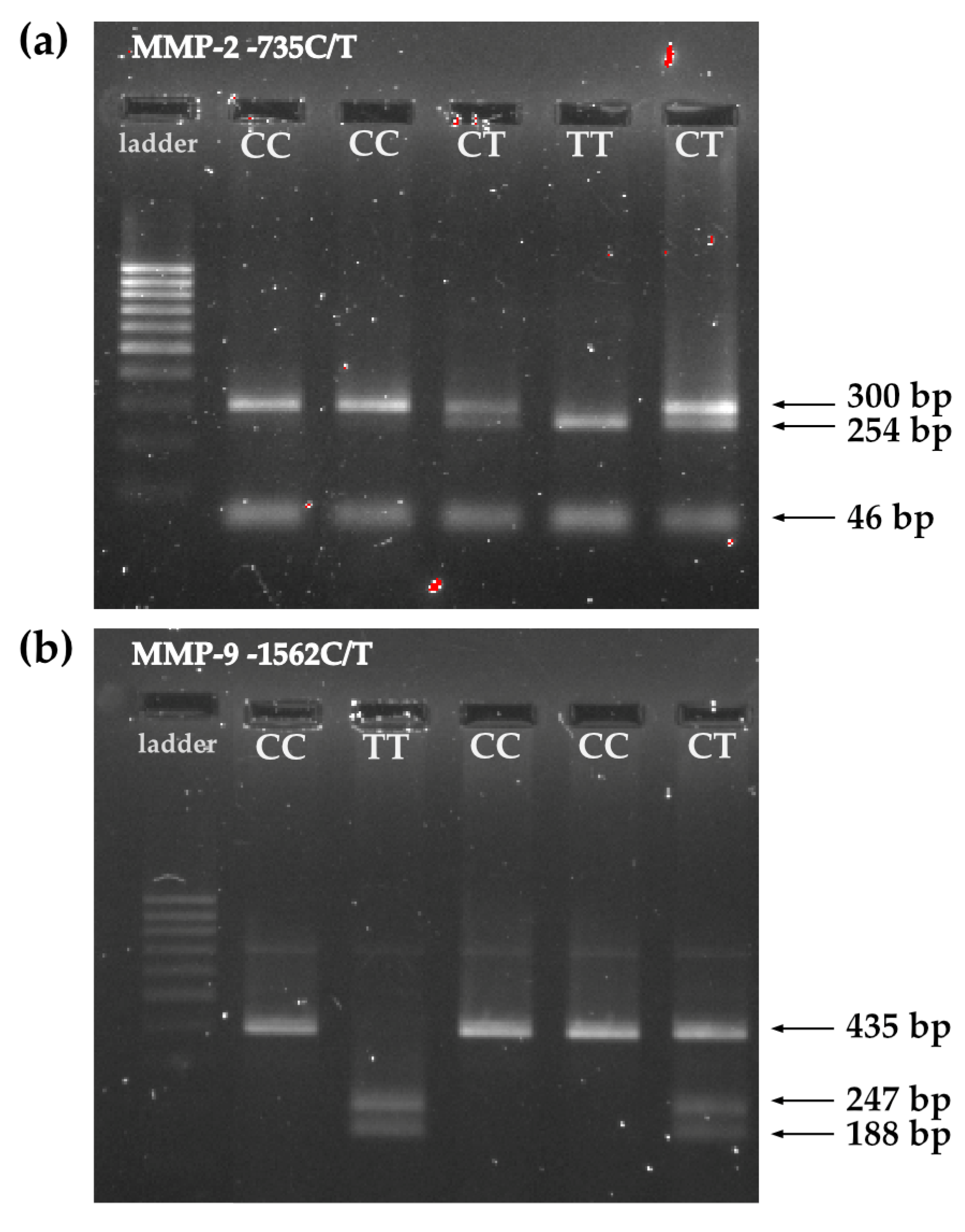
| MMP-2-735 C/T | MMP-9-1562 C/T | |
|---|---|---|
| ||
| PCR Mix: (given amounts are calculated for one reaction) | Forward primer: 0.6 μL Reverse primer: 0.6 μL Gold Taq polymerase (5 U/μL): 0.2 μL 10× Gold buffer: 2 μL 25 mM MgCl2: 1.6 μL 10 mM dNTP Mix: 0.4 μL DNA: 2 μL PCR water: 12.6 μL | Forward primer: 0.6 μL Reverse primer: 0.6 μL Gold Taq polymerase (5 U/μL): 0.2 μL 10× Gold buffer: 2 μL 25 mM MgCl2: 1.6 μL 10 mM dNTP Mix: 0.4 μL DNA: 2 μL PCR water: 12.6 μL |
| PCR conditions: | Activation: 15 min at 95 °C 35 cycles of:
Hold: ∞ at 4 °C | Activation: 15 min at 95 °C 35 cycles of:
Hold: ∞ at 4 °C |
| ||
| Reaction Mix: | PCR product: 10 μL Anza™ 10(×) Buffer: 2 µL HinfI enzyme: 1 µL PCR water: 7 µL | PCR product: 10 μL 10(×) Buffer B: 2 µL PaeI enzyme: 1 µL PCR water: 18 µL |
| Reaction conditions: | Incubation: 16 h at 37 °C Inactivation: 20 min at 65 °C | Incubation: 16 h at 37 °C Inactivation: 20 min at 65 °C |
| ||
| Agarose gel: | Agarose: 1.5 g | Agarose: 1.5 g |
| TBE buffer 1(×): 100 mL | TBE buffer 1(×): 100 mL | |
| Gold DNA gel stain: 5 µL | Gold DNA gel stain: 5 µL | |
| Electrophoresis conditions: | 50 V for 5 min | 50 V for 5 min |
| 120 V for 120 min | 120 V for 120 min | |
| Final products: | CC: 300 bp TT: 254 bp, 46 bp CT: 300 bp, 254 bp, 46 bp | CC: 435 bp TT: 247 bp, 188 bp CT: 435 bp, 247 bp, 188 bp |
| Genotype | Primer | Sequence |
|---|---|---|
| MMP-2-735 C/T | F primer: | 5′-ATA GGG TAA ACC TCC CCA CAT T-3′ |
| R primer: | 5′-GGT AAA ATG AGG CTG AGA CCT G-3′ | |
| MMP-9-1562 C/T | F primer: | 5′-GCC TGG CAC ATA GTA GGC CC-3′ |
| R primer: | 5′-TTC CTA GCC AGC CGG CAT C-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadowska, K.; Błasiak, P.; Rzechonek, A.; Śliwińska-Mossoń, M. Analysis of MMP-2-735C/T (rs2285053) and MMP-9-1562C/T (rs3918242) Polymorphisms in the Risk Assessment of Developing Lung Cancer. Int. J. Mol. Sci. 2023, 24, 10576. https://doi.org/10.3390/ijms241310576
Wadowska K, Błasiak P, Rzechonek A, Śliwińska-Mossoń M. Analysis of MMP-2-735C/T (rs2285053) and MMP-9-1562C/T (rs3918242) Polymorphisms in the Risk Assessment of Developing Lung Cancer. International Journal of Molecular Sciences. 2023; 24(13):10576. https://doi.org/10.3390/ijms241310576
Chicago/Turabian StyleWadowska, Katarzyna, Piotr Błasiak, Adam Rzechonek, and Mariola Śliwińska-Mossoń. 2023. "Analysis of MMP-2-735C/T (rs2285053) and MMP-9-1562C/T (rs3918242) Polymorphisms in the Risk Assessment of Developing Lung Cancer" International Journal of Molecular Sciences 24, no. 13: 10576. https://doi.org/10.3390/ijms241310576
APA StyleWadowska, K., Błasiak, P., Rzechonek, A., & Śliwińska-Mossoń, M. (2023). Analysis of MMP-2-735C/T (rs2285053) and MMP-9-1562C/T (rs3918242) Polymorphisms in the Risk Assessment of Developing Lung Cancer. International Journal of Molecular Sciences, 24(13), 10576. https://doi.org/10.3390/ijms241310576







