Recent Advances of 68Ga-Labeled PET Radiotracers with Nitroimidazole in the Diagnosis of Hypoxia Tumors
Abstract
1. Introduction
2. Results
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Gambhir, S.S. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, M.; Darijani, M.; Momeni, F.; Moradi, P.; Ebrahimnejad, H.; Masoudifar, A.; Mirzaei, H. Molecular Imaging and Oral Cancer Diagnosis and Therapy. J. Cell. Biochem. 2017, 118, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, F.A.; Weissleder, R. Molecular Imaging in the Clinical Arena. JAMA 2005, 293, 855–862. [Google Scholar] [CrossRef]
- Guo, J.; Pan, X.; Wang, C.; Liu, H. Molecular Imaging-Guided Sonodynamic Therapy. Bioconjug. Chem. 2021, 33, 993–1010. [Google Scholar] [CrossRef]
- Kim, N.H.; Huh, Y.; Kim, D. Benzo[g]coumarin-benzothiazole hybrid: A fluorescent probe for the detection of amyloid-beta aggregates. Bull. Korean Chem. Soc. 2022, 43, 764–768. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Zhang, X. Radiotracers for Nuclear Imaging of Reactive Oxygen Species: Advances Made So Far. Bioconjug. Chem. 2022, 33, 749–766. [Google Scholar] [CrossRef]
- Cerami, C.; Iaccarino, L.; Perani, D. Molecular imaging of neuroinflammation in neurodegenerative dementias: The role of in vivo PET imaging. Int. J. Mol. Sci. 2017, 18, 993. [Google Scholar] [CrossRef]
- Ariztia, J.; Solmont, K.; Moise, N.P.; Specklin, S.; Heck, M.P.; Lamande-Langle, S.; Kuhnast, B. PET/fluorescence imaging: An overview of the chemical strategies to build dual imaging tools. Bioconjug. Chem. 2022, 33, 24–52. [Google Scholar] [CrossRef]
- Kim, H.; Shin, M.; Kim, E. Fluorescent fluoride sensor based on indolizine core skeleton for bioimaging. Bull. Korean Chem. Soc. 2021, 42, 95–98. [Google Scholar] [CrossRef]
- Han, Z.; Ke, M.; Liu, X.; Wang, J.; Guan, Z.; Qiao, L.; Wu, Z.; Sun, Y.; Sun, X. Molecular imaging, how close to clinical precision medicine in lung, brain, prostate and breast cancers. Mol. Imaging Biol. 2022, 24, 8–22. [Google Scholar] [CrossRef]
- Grus, T.; Lahnif, H.; Klasen, B.; Moon, E.S.; Greifenstein, L.; Roesch, F. Squaric acid-based radiopharmaceuticals for tumor imaging and therapy. Bioconjug. Chem. 2021, 32, 1223–1231. [Google Scholar] [CrossRef]
- Lim, C.S.; Kim, Y.C.; Kim, H.M. Analyzing Nonmelanoma Skin Cancer Using Enzyme-Activatable Two-Photon Probes. Bull. Korean Chem. Soc. 2021, 42, 103–106. [Google Scholar] [CrossRef]
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Tian, J.; Chen, X. Intraoperative imaging-guided cancer surgery: From current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 2014, 4, 1072. [Google Scholar] [CrossRef]
- Palmer, G.M.; Fontanella, A.N.; Zhang, G.; Hanna, G.; Fraser, C.L.; Dewhirst, M.W. Optical imaging of tumor hypoxia dynamics. J. Biomed. Opt. 2010, 15, 066021. [Google Scholar] [CrossRef]
- Sun, X.; Niu, G.; Chan, N.; Shen, B.; Chen, X. Tumor Hypoxia Imaging. Mol. Imaging Biol. 2010, 13, 399–410. [Google Scholar] [CrossRef]
- Histed, S.N.; Lindenberg, M.L.; Mena, E.; Turkbey, B.; Choyke, P.L.; Kurdziel, K.A. Review of Functional/ Anatomic Imaging in Oncology. Nucl. Med. Commun. 2012, 33, 349. [Google Scholar] [CrossRef]
- Balyasnikova, S.; Löfgren, J.; de Nijs, R.; Zamogilnaya, Y.; Højgaard, L.; Fischer, B.M. PET/MR in Oncology: An Introduction with Focus on MR and Future Perspectives for Hybrid Imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 458. [Google Scholar]
- Even-Sapir, E.; Keidar, Z.; Bar-Shalom, R. Hybrid Imaging (SPECT/CT and PET/CT)—Improving the Diagnostic Accuracy of Functional/Metabolic and Anatomic Imaging. Semin. Nucl. Med. 2009, 39, 264–275. [Google Scholar] [CrossRef]
- Van Dort, M.; Rehemtulla, A.; Ross, B. PET and SPECT Imaging of Tumor Biology: New Approaches towards Oncology Drug Discovery and Development. Curr. Comput. Aided. Drug Des. 2008, 4, 46–53. [Google Scholar] [CrossRef]
- Kuhl, D.E.; Edwards, R.Q. Image Separation Radioisotope Scanning. Radiology 1963, 80, 653–662. [Google Scholar] [CrossRef]
- Al Badarin, F.J.; Malhotra, S. Diagnosis and Prognosis of Coronary Artery Disease with SPECT and PET. Curr. Cardiol. Rep. 2019, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Raijmakers, P.G.; Driessen, R.S.; Leipsic, J.; Raju, R.; Naoum, C.; Knuuti, J.; Mäki, M.; Underwood, R.S.; Min, J.K.; et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017, 2, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Klunk, W.E.; Debnath, M.L.; Huang, G.F.; Holt, D.P.; Shao, L.; Mathis, C.A. Development of a PET/SPECT Agent for Amyloid Imaging in Alzheimer’s Disease. J. Mol. Neurosci. 2004, 24, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Svedberg, M.M.; Rahman, O.; Hall, H. Preclinical Studies of Potential Amyloid Binding PET/SPECT Ligands in Alzheimer’s Disease. Nucl. Med. Biol. 2012, 39, 484–501. [Google Scholar] [CrossRef]
- Lu, F.-M.; Yuan, Z. PET/SPECT Molecular Imaging in Clinical Neuroscience: Recent Advances in the Investigation of CNS Diseases. Quant. Imaging Med. Surg. 2015, 5, 433. [Google Scholar]
- Lee, H.J.; Ehlerding, E.B.; Cai, W. Antibody-Based Tracers for PET/SPECT Imaging of Chronic Inflammatory Diseases. ChemBioChem 2019, 20, 422–436. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Miao, Z.; Deng, Z.; Shen, B.; Hong, X.; Cheng, Z. Development of 18F-Labeled Picolinamide Probes for PET Imaging of Malignant Melanoma. J. Med. Chem. 2013, 56, 895–901. [Google Scholar] [CrossRef]
- Dannoon, S.; Ganguly, T.; Cahaya, H.; Geruntho, J.J.; Galliher, M.S.; Beyer, S.K.; Choy, C.J.; Hopkins, M.R.; Regan, M.; Blecha, J.E.; et al. Structure-Activity Relationship of 18F-Labeled Phosphoramidate Peptidomimetic Prostate-Specific Membrane Antigen (PSMA)-Targeted Inhibitor Analogues for PET Imaging of Prostate Cancer. J. Med. Chem. 2016, 59, 5684–5694. [Google Scholar] [CrossRef]
- Janssen, J.C.; Meißner, S.; Woythal, N.; Prasad, V.; Brenner, W.; Diederichs, G.; Hamm, B.; Makowski, M.R. Comparison of Hybrid 68Ga-PSMA-PET/CT and 99mTc-DPD-SPECT/CT for the Detection of Bone Metastases in Prostate Cancer Patients: Additional Value of Morphologic Information from Low Dose CT. Eur. Radiol. 2018, 28, 610–619. [Google Scholar] [CrossRef]
- Luu, T.G.; Kim, H.K. 18F-Radiolabeled translocator protein (TSPO) PET tracers: Recent development of TSPO radioligands and their application to PET study. Pharmaceutics. 2022, 14, 2545. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Byun, Y.; Kim, H.K. 18F-labelled BODIPY dye as a dual imaging agent: Radiofluorination and applications in PET and optical imaging. Nucl. Med. Biol. 2021, 93, 22–36. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Lee, J.Y.; La, M.T.; Lee, S.J.; Lee, S.H.; Park, J.H.; Kim, H.K. Novel multifunctional 18F-labelled PET tracer with prostate-specific membrane antigen-targeting and hypoxia-sensitive moieties. Eur. J. Med. Chem. 2020, 189, 112099. [Google Scholar] [CrossRef]
- Tran, V.H.; Park, H.; Park, J.; Kwon, Y.D.; Kang, S.; Jung, J.H.; Chang, K.A.; Lee, B.C.; Lee, S.Y.; Kang, S.; et al. Synthesis and evaluation of novel potent TSPO PET ligands with 2-phenylpyrazolo[1,5-a]pyrimidin-3-yl acetamide. Bioorg. Med. Chem. 2019, 27, 4069–4080. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Kang, S.; Park, H.; Cheong, I.K.; Chang, K.A.; Lee, S.Y.; Jung, J.H.; Lee, B.C.; Lim, S.T.; Kim, H.K. Novel potential pyrazolopyrimidine based translocator protein ligands for the evaluation of neuroinflammation with PET. Eur. J. Med. Chem. 2018, 159, 292–306. [Google Scholar] [CrossRef]
- Kim, M.H.; Jung, W.J.; Jeong, H.J.; Lee, K.; Kil, H.S.; Chung, W.S.; Nam, K.R.; Lee, Y.J.; Lee, K.C.; Lim, S.M.; et al. Off-target screening of amyloid-beta plaque targeting [18F]florapronol ([18F]FC119S) in postmortem Alzheimer’s disease tissues. Bull. Korean Chem. Soc. 2022, 43, 859–867. [Google Scholar] [CrossRef]
- Oh, K.; Chi, D.Y. Direct fluorination strategy for the synthesis of fluorine-18 labeled oligopeptide–[18F]ApoPep-7. Bull. Korean Chem. Soc. 2021, 42, 1161–1166. [Google Scholar] [CrossRef]
- van der Vaart, M.G.; Meerwaldt, R.; Slart, R.H.J.A.; van Dam, G.M.; Tio, R.A.; Zeebregts, C.J. Application of PET/SPECT imaging in vascular disease. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 507–513. [Google Scholar] [CrossRef]
- Dobrucki, L.W.; Sinusas, A.J. PET and SPECT in cardiovascular molecular imaging. Nat. Rev. Cardiol. 2010, 7, 38–47. [Google Scholar] [CrossRef]
- Abdelhafez, Y.; Raychaudhuri, S.P.; Mazza, D.; Sarkar, S.; Hunt, H.L.; McBride, K.; Nguyen, M.; Caudle, D.T.; Spencer, B.A.; Omidvari, N.; et al. Total-Body 18F-FDG PET/CT in Autoimmune Inflammatory Arthritis at Ultra-Low Dose: Initial Observations. J. Nucl. Med. 2022, 63, 1579–1585. [Google Scholar] [CrossRef]
- Grimm, J.; Kirsch, D.G.; Windsor, S.D.; Kim, C.F.B.; Santiago, P.M.; Ntziachristos, V.; Jacks, T.; Weissleder, R. Use of Gene Expression Profiling to Direct in Vivo Molecular Imaging of Lung Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 14404–14409. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. Molecular Imaging in Cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, D.; Doddamane, I.; Cheng, D. Targeted Radionuclide Therapy. Cancers 2011, 3, 3838–3855. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.J. Targeted Radionuclide Therapy: A Historical and Personal Review. Semin. Nucl. Med. 2020, 50, 87–97. [Google Scholar] [CrossRef]
- Li, Z.; Conti, P.S. Radiopharmaceutical Chemistry for Positron Emission Tomography. Adv. Drug Deliv. Rev. 2010, 62, 1031–1051. [Google Scholar] [CrossRef]
- Ido, T.; Wan, C.-N.; Casella, V.; Fowler, J.S.; Wolf, A.P.; Reivich, M.; Kuhl, D.E. Labeled 2-Deoxy-D-Glucose Analogs. 18F-Labeled 2-Deoxy-2-Fluoro-D-Glucose, 2-Deoxy-2-Fluoro-D-Mannose and 14C-2-Deoxy-2-Fluoro-D-Glucose. J. Label. Compd. Radiopharm. 1978, 14, 175–183. [Google Scholar] [CrossRef]
- Phelps, M.E.; Huang, S.C.; Hoffman, E.J.; Selin, C.; Sokoloff, L.; Kuhl, D.E. Tomographic Measurement of Local Cerebral Glucose Metabolic Rate in Humans with (F-18)2-Fluoro-2-Deoxy-D-Glucose: Validation of Method. Ann. Neurol. 1979, 6, 371–388. [Google Scholar] [CrossRef]
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the Use of 18F-FDG PET in Oncology. J. Nucl. Med. 2008, 49, 480–508. [Google Scholar] [CrossRef]
- Höckel, M.; Vaupel, P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular Imaging of Hypoxia. J. Nucl. Med. 2008, 49, 129S–148S. [Google Scholar] [CrossRef]
- Dunn, T. Oxygen and Cancer. N. C. Med. J. 1997, 58, 140–143. [Google Scholar]
- Lewis, J.; Welch, M. PET Imaging of Hypoxia. Q. J. Nucl. Med. Mol. Imaging 2001, 45, 183. [Google Scholar]
- Höckel, M.; Schienger, K.; Aral, B.; Milze, M.; Schäffer, U.; Vaupel, P. Association between Tumor Hypoxia and Malignant Progression in Advanced Cancer of the Uterine Cervix. Cancer Res. 1996, 56, 4509–4515. [Google Scholar]
- Ammirante, M.; Shalapour, S.; Kang, Y.; Jamieson, C.A.M.; Karin, M. Tissue Injury and Hypoxia Promote Malignant Progression of Prostate Cancer by Inducing CXCL13 Expression in Tumor Myofibroblasts. Proc. Natl. Acad. Sci. USA 2014, 111, 14776–14781. [Google Scholar] [CrossRef]
- Abu el Maaty, M.A.; Terzic, J.; Keime, C.; Rovito, D.; Lutzing, R.; Yanushko, D.; Parisotto, M.; Grelet, E.; Namer, I.J.; Lindner, V.; et al. Hypoxia-Mediated Stabilization of HIF1A in Prostatic Intraepithelial Neoplasia Promotes Cell Plasticity and Malignant Progression. Sci. Adv. 2022, 8, 2295. [Google Scholar] [CrossRef]
- Liao, S.; Apaijai, N.; Luo, Y.; Wu, J.; Chunchai, T.; Singhanat, K.; Arunsak, B.; Benjanuwattra, J.; Chattipakorn, N.; Chattipakorn, S.C. Cell Death Inhibitors Protect against Brain Damage Caused by Cardiac Ischemia/Reperfusion Injury. Cell Death Discov. 2021, 7, 312. [Google Scholar] [CrossRef]
- Yashiro, M.; Kinoshita, H.; Tsujio, G.; Fukuoka, T.; Yamamoto, Y.; Sera, T.; Sugimoto, A.; Nishimura, S.; Kushiyama, S.; Togano, S.; et al. SDF1α/CXCR4 Axis May Be Associated with the Malignant Progression of Gastric Cancer in the Hypoxic Tumor Microenvironment. Oncol. Lett. 2020, 21, 38. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Ma, X.; Liang, X.; Liu, X.; Wang, Y. Clinicopathological Characteristics of Gynecological Cancer Associated with Hypoxia-Inducible Factor 1α Expression: A Meta-Analysis Including 6,612 Subjects. PLoS ONE 2015, 10, e0127229. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ding, Q.; Desaki, R.; Maemura, K.; Mataki, Y.; Shinchi, H.; Natsugoe, S.; Takao, S. Hypoxia Inducible Factor-1 Alpha Plays a Pivotal Role in Hepatic Metastasis of Pancreatic Cancer: An Immunohistochemical Study. J. Hepatobiliary Pancreat. Sci. 2014, 21, 105–112. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anticancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef]
- Ullah, A.; Leong, S.W.; Wang, J.; Wu, Q.; Ghauri, M.A.; Sarwar, A.; Su, Q.; Zhang, Y. Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1α interaction in lung cancer. Cell Death Dis. 2021, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Briest, S.; Höckel, M. Hypoxia in Breast Cancer: Pathogenesis, Characterization and Biological/Therapeutic Implications. Wien. Med. Wochenschr. 2002, 152, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.; Blackwell, K. Hypoxia and Anemia: Factors in Decreased Sensitivity to Radiation Therapy and Chemotherapy? Oncologist 2004, 9, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.Y.; Yan, M.; Campo, L.; Han, C.; Takano, E.; Turley, H.; Candiloro, I.; Pezzella, F.; Gatter, K.C.; Millar, E.K.A.; et al. The Key Hypoxia Regulated Gene CAIX Is Upregulated in Basal-like Breast Tumours and Is Associated with Resistance to Chemotherapy. Br. J. Cancer 2009, 100, 405–411. [Google Scholar] [CrossRef]
- Brizel, D.M.; Sibley, G.S.; Prosnitz, L.R.; Scher, R.L.; Dewhirst, M.W. Tumor Hypoxia Adversely Affects the Prognosis of Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 285–289. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ibusuki, M.; Okumura, Y.; Kawasoe, T.; Kai, K.; Iyama, K.; Iwase, H. Hypoxia-Inducible Factor 1α Is Closely Linked to an Aggressive Phenotype in Breast Cancer. Breast Cancer Res. Treat. 2008, 110, 465–475. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, H.; Li, Y.; Liu, Y.; Zou, J.; Zhu, Y. Clinicopathological and Prognostic Value of Hypoxia-Inducible Factor-1α in Breast Cancer: A Meta-Analysis Including 5177 Patients. Clin. Transl. Oncol. 2020, 22, 1892–1906. [Google Scholar] [CrossRef]
- Höckel, M.; Knoop, C.; Schlenger, K.; Vorndran, B.; Baußnann, E.; Mitze, M.; Knapstein, P.G.; Vaupel, P. Intratumoral PO2 Predicts Survival in Advanced Cancer of the Uterine Cervix. Radiother. Oncol. 1993, 26, 45–50. [Google Scholar] [CrossRef]
- Ballinger, J.R. Imaging Hypoxia in Tumors. Semin. Nucl. Med. 2001, 31, 321–329. [Google Scholar] [CrossRef]
- Bussink, J.; Kaanders, J.H.A.M.; Van Der Kogel, A.J. Tumor Hypoxia at the Micro-Regional Level: Clinical Relevance and Predictive Value of Exogenous and Endogenous Hypoxic Cell Markers. Radiother. Oncol. 2003, 67, 3–15. [Google Scholar] [CrossRef]
- Lu, X.G.; Xing, C.G.; Feng, Y.Z.; Chen, J.; Deng, C. Clinical Significance of Immunohistochemical Expression of Hypoxia-Inducible Factor–1α as a Prognostic Marker in Rectal Adenocarcinoma. Clin. Colorectal Cancer 2006, 5, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Durand, R.E. Detection of Hypoxic Cells in a Murine Tumor with the Use of the Comet Assay. J. Natl. Cancer Inst. 1992, 84, 707–711. [Google Scholar] [CrossRef]
- Olive, P.L. The Comet Assay in Clinical Practice. Acta Oncol. 2009, 38, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Banáth, J.P. The Comet Assay: A Method to Measure DNA Damage in Individual Cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Mees, G.; Dierckx, R.; Vangestel, C.; Van De Wiele, C. Molecular Imaging of Hypoxia with Radiolabelled Agents. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1674–1686. [Google Scholar] [CrossRef]
- Nunn, A.; Linder, K.; Strauss, H.W. Nitroimidazoles and Imaging Hypoxia. Eur. J. Nucl. Med. 1995, 22, 265–280. [Google Scholar] [CrossRef]
- Kizaka-Kondoh, S.; Konse-Nagasawa, H. Significance of Nitroimidazole Compounds and Hypoxia-Inducible Factor-1 for Imaging Tumor Hypoxia. Cancer Sci. 2009, 100, 1366–1373. [Google Scholar] [CrossRef]
- Liu, J.N.; Bu, W.; Shi, J. Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chem. Rev. 2017, 117, 6160–6224. [Google Scholar] [CrossRef]
- Kubota, K.; Tada, M.; Yamada, S.; Hori, K.; Saito, S.; Iwata, R.; Sato, K.; Fukuda, H.; Ido, T. Comparison of the Distribution of Fluorine-18 Fluoromisonidazole, Deoxyglucose and Methionine in Tumour Tissue. Eur. J. Nucl. Med. 1999, 26, 750–757. [Google Scholar] [CrossRef]
- Rasey, J.S.; Casciari, J.J.; Hofstrand, P.D.; Muzi, M.; Graham, M.M.; Chin, L.K. Determining Hypoxic Fraction in a Rat Glioma by Uptake of Radiolabeled Fluoromisonidazole. Radiat. Res. 2000, 153, 84–92. [Google Scholar] [CrossRef]
- Zimny, M.; Gagel, B.; DiMartino, E.; Hamacher, K.; Coenen, H.H.; Westhofen, M.; Eble, M.; Buell, U.; Reinartz, P. FDG—A Marker of Tumour Hypoxia? A Comparison with [18F] Fluoromisonidazole and PO2-Polarography in Metastatic Head and Neck Cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Rischin, D.; Fisher, R.; Binns, D.; Scott, A.M.; Peters, L.J. Utility of FMISO PET in advanced head and neck cancer treated with chemoradiation incorporating a hypoxia-targeting chemotherapy agent. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, K.; Phillips, M.; Smith, W.; Peterson, L.; Krohn, K.; Rajendran, J. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: Potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother. Oncol. 2011, 101, 369–375. [Google Scholar] [CrossRef]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.-M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1383–1392. [Google Scholar] [CrossRef]
- Chang, C.W.; Chou, T.K.; Liu, R.S.; Wang, S.J.; Lin, W.J.; Chen, C.H.; Wang, H.E. A robotic synthesis of [18F]fluoromisonidazole ([18F]FMISO). Appl. Radiat. Isot. 2007, 65, 682–686. [Google Scholar] [CrossRef]
- Wanek, T.; Kreis, K.; Križková, P.; Schweifer, A.; Denk, C.; Stanek, J.; Mairinger, S.; Filip, T.; Sauberer, M.; Edelhofer, P.; et al. Synthesis and preclinical characterization of 1-(6′-deoxy-6′-[18F] fluoro-b-D-allofuranosyl)-2-nitroimidazole (β-6′ -[18F]FAZAL) as a positron emission tomography radiotracer to assess tumor hypoxia. Bioorg. Med. Chem. 2016, 24, 5326–5339. [Google Scholar] [CrossRef]
- Maier, F.C.; Schweifer, A.; Damaraju, V.L.; Cass, C.E.; Bowden, G.D.; Ehrlichmann, W.; Kneilling, M.; Pichler, B.J.; Hammerschmidt, F.; Reischl, G. 2-Nitroimidazole-Furanoside Derivatives for Hypoxia Imaging—Investigation of Nucleoside Transporter Interaction, 18F-Labeling and Preclinical PET Imaging. Pharmaceuticals 2019, 12, 31. [Google Scholar] [CrossRef]
- Koh, W.J.; Rasey, J.S.; Evans, M.L.; Grierson, J.R.; Lewellen, T.K.; Graham, M.M.; Krohn, K.A.; Griffin, T.W. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 199–212. [Google Scholar] [CrossRef]
- Grunbaum, Z.; Freauff, S.J.; Krohn, K.A.; Wilbur, D.S.; Magee, S.; Rasey, J.S. Synthesis and characterization of congeners of misonidazole for imaging hypoxia. J. Nucl. Med. 1987, 28, 68–75. [Google Scholar]
- Yang, D.J.; Wallace, S.; Cherif, A.; Li, C.; Gretzer, M.B.; Kim, E.E.; Podoloff, D.A. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology 1995, 194, 795–800. [Google Scholar] [CrossRef]
- Piert, M.; Machulla, H.-J.; Picchio, M.; Reischl, G.; Ziegler, S.; Kumar, P.; Wester, H.-J.; Beck, R.; McEwan, A.J.B.; Wiebe, L.I.; et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J. Nucl. Med. 2005, 46, 106–113. [Google Scholar]
- Postema, E.J.; McEwan, A.J.; Riauka, T.A.; Kumar, P.; Richmond, D.A.; Abrams, D.N.; Wiebe, L.I. Initial results of hypoxia imaging using 1-α-D-(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole (18F-FAZA). Eur. J. Nucl. Med. Mol. Imaging. 2009, 36, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Mannan, R.H.; Somayaji, V.V.; Lee, J.; Mercer, J.R.; Chapman, J.D.; Wiebe, L.I. Radioiodinated 1-(5-Iodo-5-Deoxy--Darabinofuranosyl)-2-Nitroimidazole (Iodoazomycin Arabinoside: IAZA): A Novel Marker of Tissue Hypoxia. J. Nucl. Med. 1991, 32, 1764–1770. [Google Scholar]
- Reischl, G.; Dorow, D.S.; Cullinane, C.; Katsifis, A.; Roselt, P.; Binns, D.; Hicks, R.J. Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA: First small animal PET results. J. Pharm. Pharmaceut. Sci. 2007, 10, 203–211. [Google Scholar]
- Carlin, S.; Humm, J.L. PET of Hypoxia: Current and Future Perspectives. J. Nucl. Med. 2012, 53, 1171–1174. [Google Scholar] [CrossRef]
- Souvatzoglou, M.; Grosu, A.L.; Röper, B.; Krause, B.J.; Beck, R.; Reischl, G.; Picchio, M.; Machulla, H.-J.; Wester, H.-J.; Piert, M. Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: A pilot study. Eur. J. Nucl. Med. Mol. Imaging. 2007, 34, 1566–1575. [Google Scholar] [CrossRef]
- Yue, J.; Yang, Y.; Cabrera, A.R.; Sun, X.; Zhao, S.; Xie, P.; Zheng, J.; Ma, L.; Fu, Z.; Yu, J. Measuring tumor hypoxia with 18F-FETNIM PET in esophageal squamous cell carcinoma: A pilot clinical study. Dis. Esophagus 2012, 25, 54–61. [Google Scholar] [CrossRef]
- Hu, M.; Xie, P.; Lee, N.Y.; Li, M.; Ho, F.; Lian, M.; Zhao, S.; Yang, G.; Fu, Z.; Zheng, J.; et al. Hypoxia with 18F-fluoroerythronitroimidazole integrated positron emission tomography and computed tomography (18F-FETNIM PET/CT) in locoregionally advanced head and neck cancer: Hypoxia changes during chemoradiotherapy and impact on clinical outcome. Medicine 2019, 98, e17067. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, W.; Huang, Y.; Yu, Q.; Zhu, S.; Wang, S.; Zhao, S.; Hu, X.; Yu, J.; Yuan, S. A Comparative Study of Noninvasive Hypoxia Imaging with 18F-Fluoroerythronitroimidazole and 18F-Fluoromisonidazole PET/CT in Patients with Lung Cancer. PLoS ONE 2016, 11, e0157606. [Google Scholar] [CrossRef]
- Komar, G.; Seppänen, M.; Eskola, O.; Lindholm, P.; Grönroos, T.J.; Forsback, S.; Sipilä, H.; Evans, S.M.; Solin, O.; Minn, H. 18F-EF5: A New PET Tracer for Imaging Hypoxia in Head and Neck Cancer. J. Nucl. Med. 2008, 49, 1944–1951. [Google Scholar] [CrossRef]
- Mahy, P.; De Bast, M.; de Groot, T.; Cheguillaume, A.; Gillart, J.; Haustermans, K.; Labar, D.; Grégoire, V. Comparative pharmacokinetics, biodistribution, metabolism and hypoxia-dependent uptake of [18F]-EF3 and [18F]-MISO in rodent tumor models. Radiother. Oncol. 2009, 89, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Christian, N.; Bol, A.; De Bast, M.; Labar, D.; Lee, J.; Mahy, P.; Grégoire, V. Determination of tumour hypoxia with the PET tracer [18F]EF3: Improvement of the tumour-to-background ratio in a mouse tumour model. Eur. J. Nucl. Med. Mol. Imaging. 2007, 34, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.; Landuyt, W.; Cloetens, L.; Bol, A.; Bormans, G.; Haustermans, K.; Labar, D.; Nuyts, J.; Grégoire, V.; Mortelmans, L. [18F]EF3 is not superior to [18F] FMISO for PET-based hypoxia evaluation as measured in a rat rhabdomyosarcoma tumour model. Eur. J. Nucl. Med. Mol. Imaging 2008, 36, 209–218. [Google Scholar] [CrossRef]
- Doss, M.; Zhang, J.J.; Bélanger, M.-J.; Stubbs, J.B.; Hostetler, E.D.; Alpaugh, K.; Kolb, H.C.; Yu, J.Q.; Alpaugh, R.K. Biodistribution and radiation dosimetry of the hypoxia marker 18F-HX4 in monkeys and humans determined by using whole-body PET/CT. Nucl. Med. Commun. 2010, 31, 1016–1024. [Google Scholar] [CrossRef]
- Sanduleanu, S.; Wiel, A.M.A.v.d.; Lieverse, R.I.Y.; Marcus, D.; Ibrahim, A.; Primakov, S.; Wu, G.; Theys, J.; Yaromina, A.; Dubois, L.J.; et al. Hypoxia PET Imaging with [18F]-HX4—A Promising Next-Generation Tracer. Cancers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Kaneta, T.; Takai, Y.; Iwata, R.; Hakamatsuka, T.; Yasuda, H.; Nakayama, K.; Ishikawa, Y.; Watanuki, S.; Furumoto, S.; Funaki, Y.; et al. Initial evaluation of dynamic human imaging using 18F-FRP170 as a new PET tracer for imaging hypoxia. Ann. Nucl. Med. 2007, 21, 101–107. [Google Scholar] [CrossRef]
- Li, Z.; Chu, T. Recent Advances on Radionuclide Labeled Hypoxia-Imaging Agents. Curr. Pharm. Des. 2012, 18, 1084–1097. [Google Scholar] [CrossRef]
- Lewis, J.S.; Laforest, R.; Dehdashti, F.; Grigsby, P.W.; Welch, M.J.; Siegel, B.A. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J. Nucl. Med. 2008, 49, 1177–1182. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Jacobson, O.; Chen, X. PET designated flouride-18 production and chemistry. Curr. Top. Med. Chem. 2010, 10, 1048–1105. [Google Scholar] [CrossRef]
- Rösch, F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013, 76, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68Ga-based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Suwada, J.; Sano, K.; Okada, M.; Yamamoto, F.; Maeda, M. Design of Ga–DOTA-based bifunctional radiopharmaceuticals: Two functional moieties can be conjugated to radiogallium–DOTA without reducing the complex stability. Bioorg. Med. Chem. 2009, 17, 4285–4289. [Google Scholar] [CrossRef] [PubMed]
- Hoigebazar, L.; Jeong, J.M.; Choi, S.Y.; Choi, J.Y.; Shetty, D.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Lee, M.C.; Chung, Y.K. Synthesis and characterization of nitroimidazole derivatives for 68Ga-labeling and testing in tumor xenografted mice. J. Med. Chem. 2010, 53, 6378–6385. [Google Scholar] [CrossRef]
- Hoigebazar, L.; Jeong, J.M.; Hong, M.K.; Kim, Y.J.; Lee, J.Y.; Shetty, D.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Lee, M.C. Synthesis of 68Ga-labeled DOTA-nitroimidazole derivatives and their feasibilities as hypoxia imaging PET tracers. Bioorg. Med. Chem. 2011, 19, 2176–2181. [Google Scholar] [CrossRef]
- Fernández, S.; Dematteis, S.; Giglio, J.; Cerecetto, H.; Rey, A. Synthesis, in vitro and in vivo characterization of two novel 68Ga-labelled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl. Med. Biol. 2013, 40, 273–279. [Google Scholar] [CrossRef]
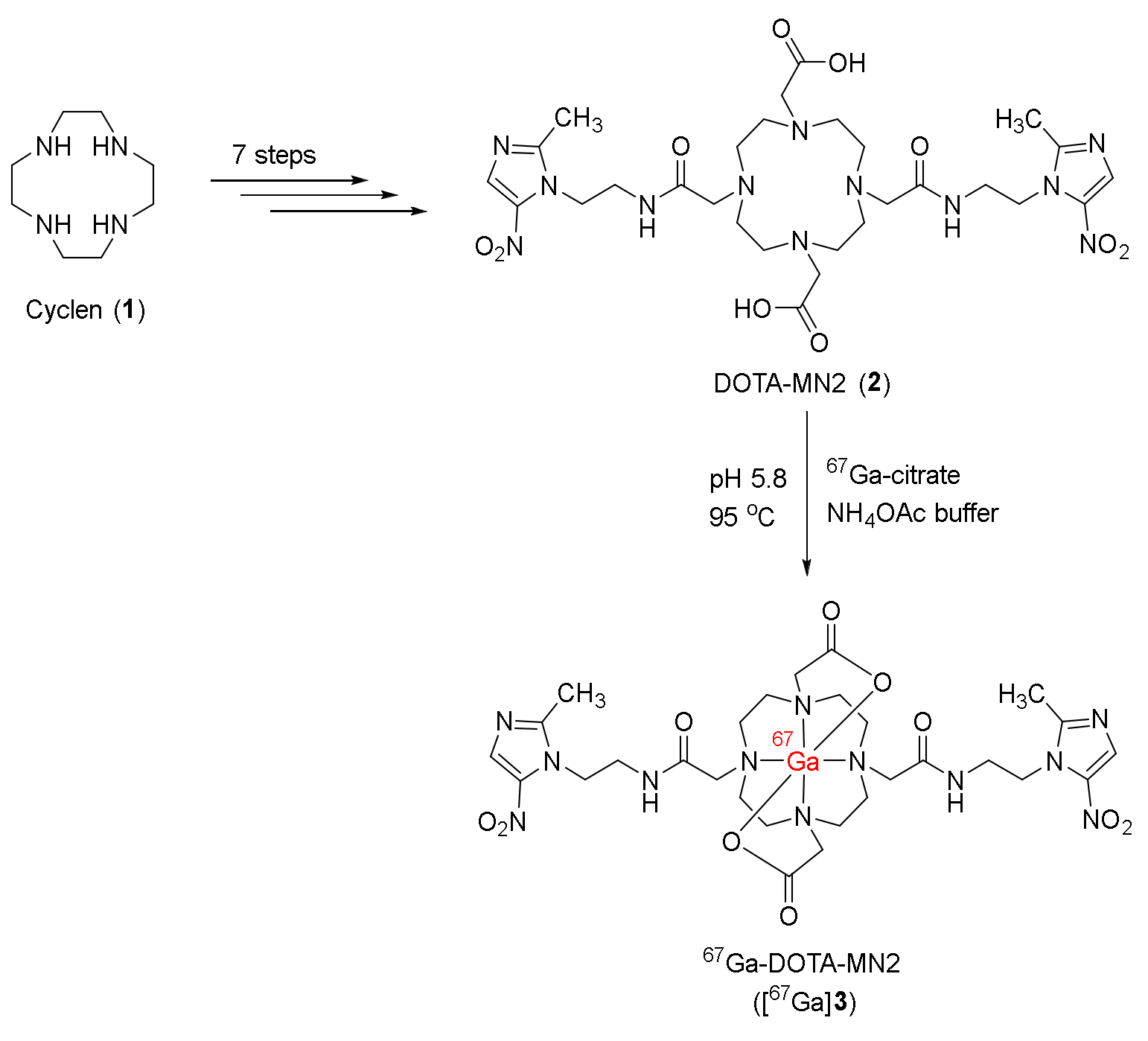
- Sano, K.; Okada, M.; Hisada, H.; Shimokawa, K.; Saji, H.; Maeda, M.; Mukai, T. In vivo evaluation of a radiogallium-labeled bifunctional radiopharmaceutical, Ga-DOTA-MN2, for hypoxic tumor imaging. Biol. Pharm. Bull. 2013, 36, 602–608. [Google Scholar] [CrossRef]
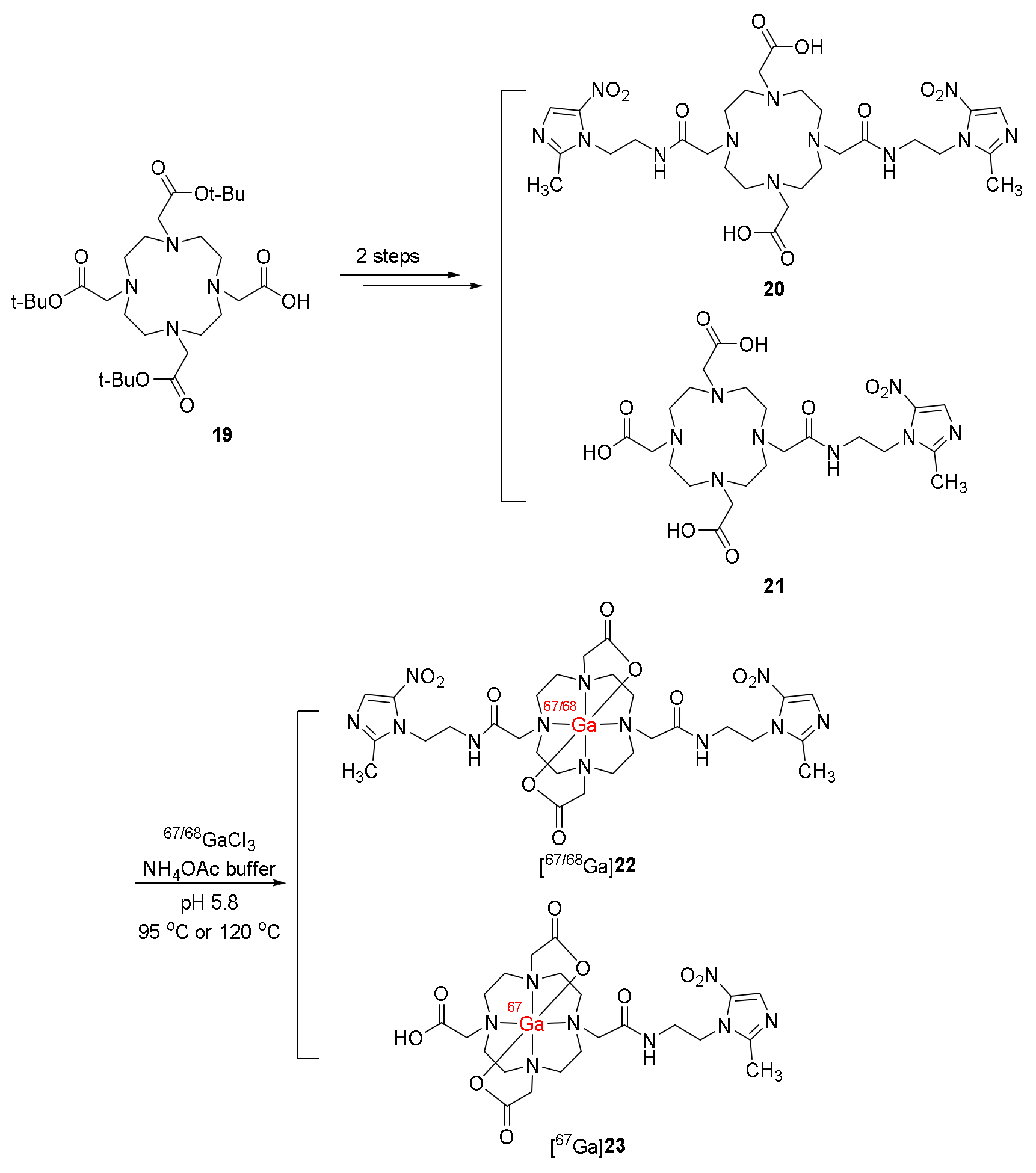
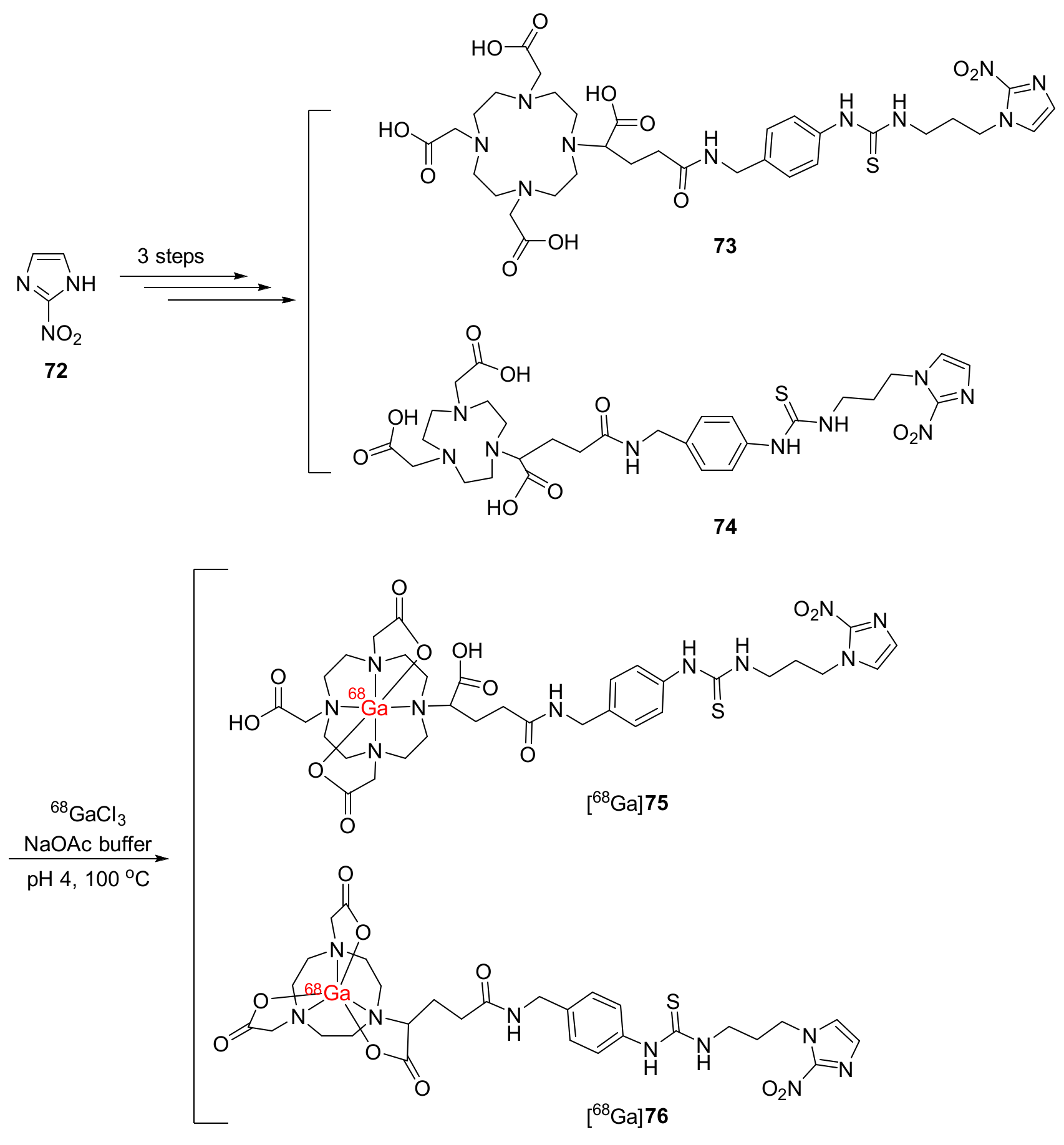
- Seelam, S.R.; Lee, J.Y.; Lee, Y.S.; Hong, M.K.; Kim, Y.J.; Banka, V.K.; Lee, D.S.; Chung, J.K.; Jeong, J.M. Development of 68Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Bioorg. Med. Chem. 2015, 23, 7743–7750. [Google Scholar] [CrossRef]
- Singh, A.N.; Liu, W.; Hao, G.; Kumar, A.; Gupta, A.; Öz, O.K.; Hsieh, J.T.; Sun, X. Multivalent bifunctional chelator scaffolds for gallium-68 based positron emission tomography imaging probe design: Signal amplification via multivalency. Bioconjug. Chem. 2011, 22, 1650–1662. [Google Scholar] [CrossRef]
- Mokoala, K.M.; Lawal, I.O.; Maserumule, L.C.; Hlongwa, K.N.; Ndlovu, H.; Reed, J.; Bida, M.; Maes, A.; Van de Wiele, C.; Mahapane, J.; et al. A Prospective Investigation of Tumor Hypoxia Imaging with 68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry. J. Clin. Med. 2022, 11, 962. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Pan, J.; Ferreira, C.L.; Patrick, B.O.; Rebullar, K.; Yapp, D.T.; Lin, K.S.; Adam, M.J.; Orvig, C. Nitroimidazole-containing H2dedpa and H2-CHX-dedpa derivatives as potential PET imaging agents of hypoxia with 68Ga. Inorg. Chem. 2015, 54, 4953–4965. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, G.; Ramezani, S.; Saha, D.; Zhao, D.; Sun, X.; Sherry, A.D. [68Ga]-HP-DO3A-nitroimidazole: A promising agent for PET detection of tumor hypoxia. Contrast Media Mol. Imaging 2015, 10, 465–472. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakai, Y.; Iikuni, S.; Watanabe, H.; Nakamoto, Y.; Ono, M. Synthesis and Evaluation of Gallium-68-Labeled Nitroimidazole-Based Imaging Probes for PET Diagnosis of Tumor Hypoxia. Ann. Nucl. Med. 2021, 35, 360–369. [Google Scholar] [CrossRef]
- Mittal, S.; Sharma, R.; Mallia, M.B.; Sarma, H.D. 68Ga-labeled PET tracers for targeting tumor hypoxia: Role of bifunctional chelators on pharmacokinetics. Nucl. Med. Biol. 2021, 96, 61–67. [Google Scholar] [CrossRef]
- Lau, J.; Rousseau, E.; Kwon, D.; Lin, K.S.; Bénard, F.; Chen, X. Insight into the Development of PET Radiopharmaceuticals for Oncology. Cancers 2020, 12, 1312. [Google Scholar] [CrossRef]
- Jeong, J.M.; Hong, M.K.; Chang, Y.S.; Lee, Y.S.; Kim, Y.J.; Cheon, G.J.; Lee, D.S.; Chung, J.K.; Lee, M.C. Preparation of a promising angiogenesis PET imaging agent: 68Ga-labeled c(RGDyK)–isothiocyanatobenzyl-1,4,7-triazacyclononane-1,4,7 -triacetic acid and feasibility studies in mice. J. Nucl. Med. 2008, 49, 830–836. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Jiang, T.; Xu, H.; Ran, Q.; Shu, Z.; Wu, J.; Li, Y.; Zhou, S.; Zhang, B. Comparison of gallium-68 somatostatin receptor and 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of neuroendocrine tumours: A systematic review and meta-analysis. Hell. J. Nucl. Med. 2020, 23, 188–200. [Google Scholar]
- De Man, K.; Van Laeken, N.; Schelfhout, V.; Fendler, W.P.; Lambert, B.; Kersemans, K.; Piron, S.; Lumen, N.; Decaestecker, K.; Fonteyne, V.; et al. 18F-PSMA-11 Versus 68Ga-PSMA-11 Positron Emission Tomography/Computed Tomography for Staging and Biochemical Recurrence of Prostate Cancer: A Prospective Double-blind Randomised Cross-over Trial. Eur. Urol. 2022, 82, 501–509. [Google Scholar] [CrossRef]
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 2013, 76, 55–62. [Google Scholar] [CrossRef]


| Radiotracers for Tumor Hypoxia with PET | |
|---|---|
| 18F-labeled hypoxia tracers | 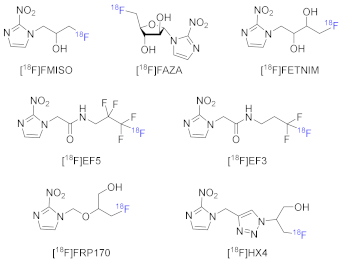 |
| I-labeled hypoxia tracer | 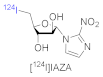 |
| 64Cu-labeled hypoxia tracer |  |
| 68Ga-Labeled Radiopharmaceuticals | Logp/Stability/Protein Binding | In Vitro Study (Cell Lines, Findings) | In Vivo Study (Animal Models, Findings) | Comments (Clinical Study, Comparison with RP) |
|---|---|---|---|---|
| 67Ga-DOTA-MN2 ([67Ga]3) [113] | N.R. | - | C3H/He mice bearing NFSa tumors | No clinical study |
| Stable in saline and mouse plasma for 24 h | - | Lower nonspecific retention in normal mice than 67Ga-citrate Higher tumor uptake at 1 h p.i. (0.49 ± 0.12 %ID/g) than 67Ga-DOTA Good T/B and T/M ratios | ||
| 68Ga-NOTA-NI ([68Ga]7) 68Ga-SCN-NOTA-NI ([68Ga]8) [114] | Logp([68Ga]7) = −2.71 Logp([68Ga]8) = −2.27 | Ovarian cancer cell line CHO Colon cancer cell line CT-26 | Mice bearing CT-26 xenografts | No clinical study |
| Stable in prepared solutions and in human serum Low protein binding | Higher uptake values under hypoxic than normoxic environment | [68Ga]7 showed better SUV than [68Ga]8 Tumor uptakes of [68Ga]7 and [68Ga]8 were 0.73 ± 0.18 and 0.61 ± 0.06 %ID/g, respectively, at 1 h p.i. | Both showed lower T/B yet similar T/M ratios compared to [18F]FAZA and [18F]FMISO Both showed higher SUV than [18F]FAZA and [18F]FMISO | |
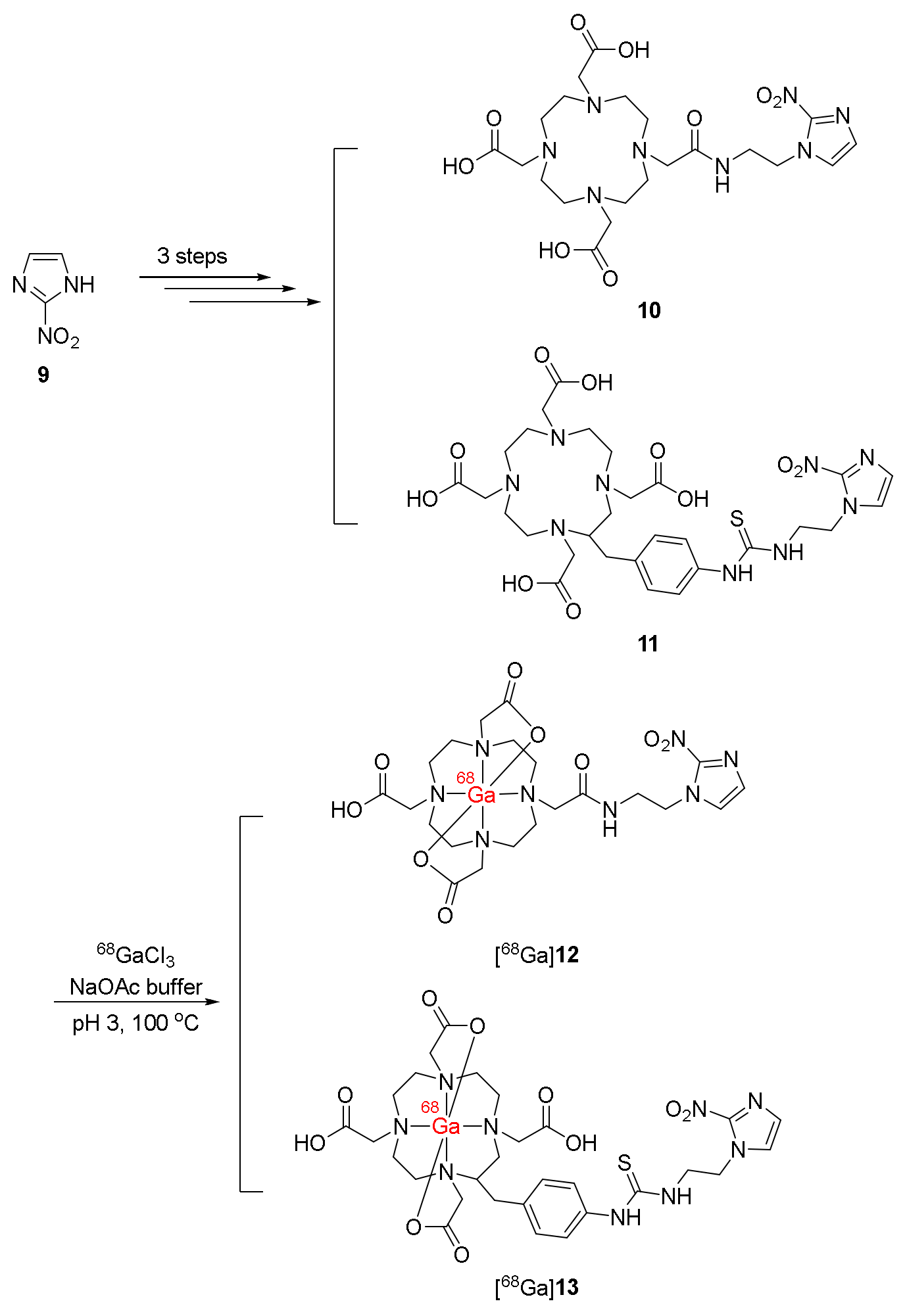
| 68Ga-labeled derivatives of 2-nitroimidazole with DOTA chelate [68Ga]12 and [68Ga]13 [115] | Logp([68Ga]12) = −4.6 Logp([68Ga]13) = −4.5 | Hela, CHO, and CT-26 cancer cell lines | Mice bearing CT-26 xenografts | No clinical study |
| Stable in prepared solutions and human serum for 2 h Desirable protein binding | Both complexes showed higher uptake values under hypoxic than normoxic environments. | T/B and T/M ratios of [68Ga]12 and [68Ga]13 were relatively high at 2 h p.i. due to rapid clearance from blood and muscle Significantly higher tumor uptake of [68Ga]12 compared to [68Ga]13 in PET imaging | ||
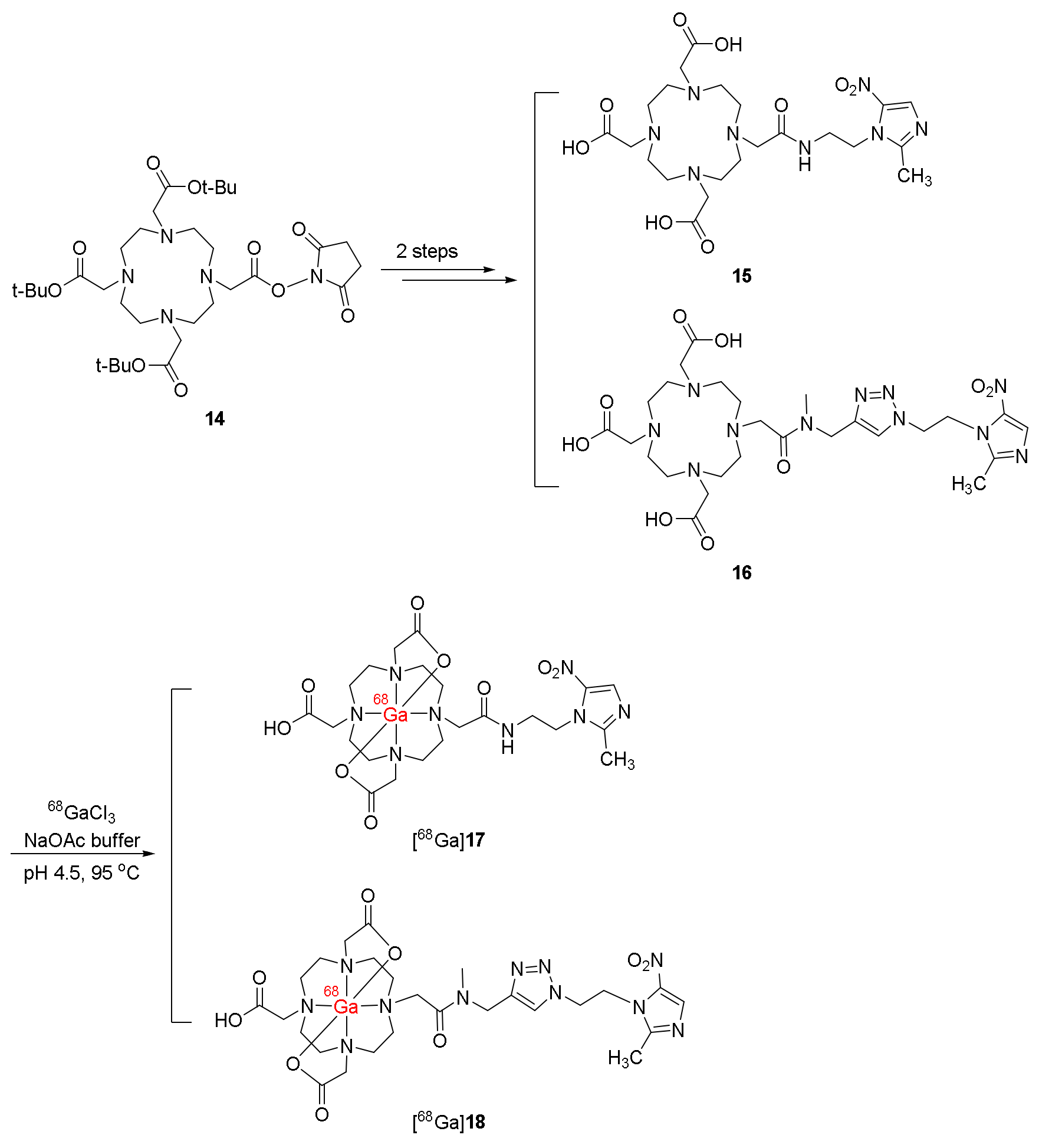
| 68Ga-labeled derivatives of 5-nitroimidazole with DOTA chelate [68Ga]17 and [68Ga]18 [116] | Logp([68Ga]17) = −1.65± 0.05 Logp([68Ga]18) = −3.30± 0.10 | HCT-15 cell lines | C57 mice bearing Lewis carcinoma | No clinical study |
| Stable in labeling milieu and human plasma Low protein binding | [68Ga]17 exhibited a higher hypoxic/normoxic ratio than both [18F]FMISO and [68Ga]18 | Rapid blood and liver clearance [68Ga]18 exhibited good retention in tumor | Both exhibited higher hydrophilicity than [18F]FMISO and significantly higher T/M ratios than [18F]FMISO | |
| 67/68Ga-DOTA-MN2 ([67/68Ga]22) 67Ga-DOTA-MN1 ([67Ga]23) [117] | - | - | C3H/He mice bearing FM3A tumors | No clinical study |
| [67Ga]22 exhibited higher T/B and T/M ratios than [67Ga]23 due to the fast blood clearance [67Ga]22 accumulated in hypoxic regions Clearly observed tumors in the mice injected with [68Ga]22 | ||||
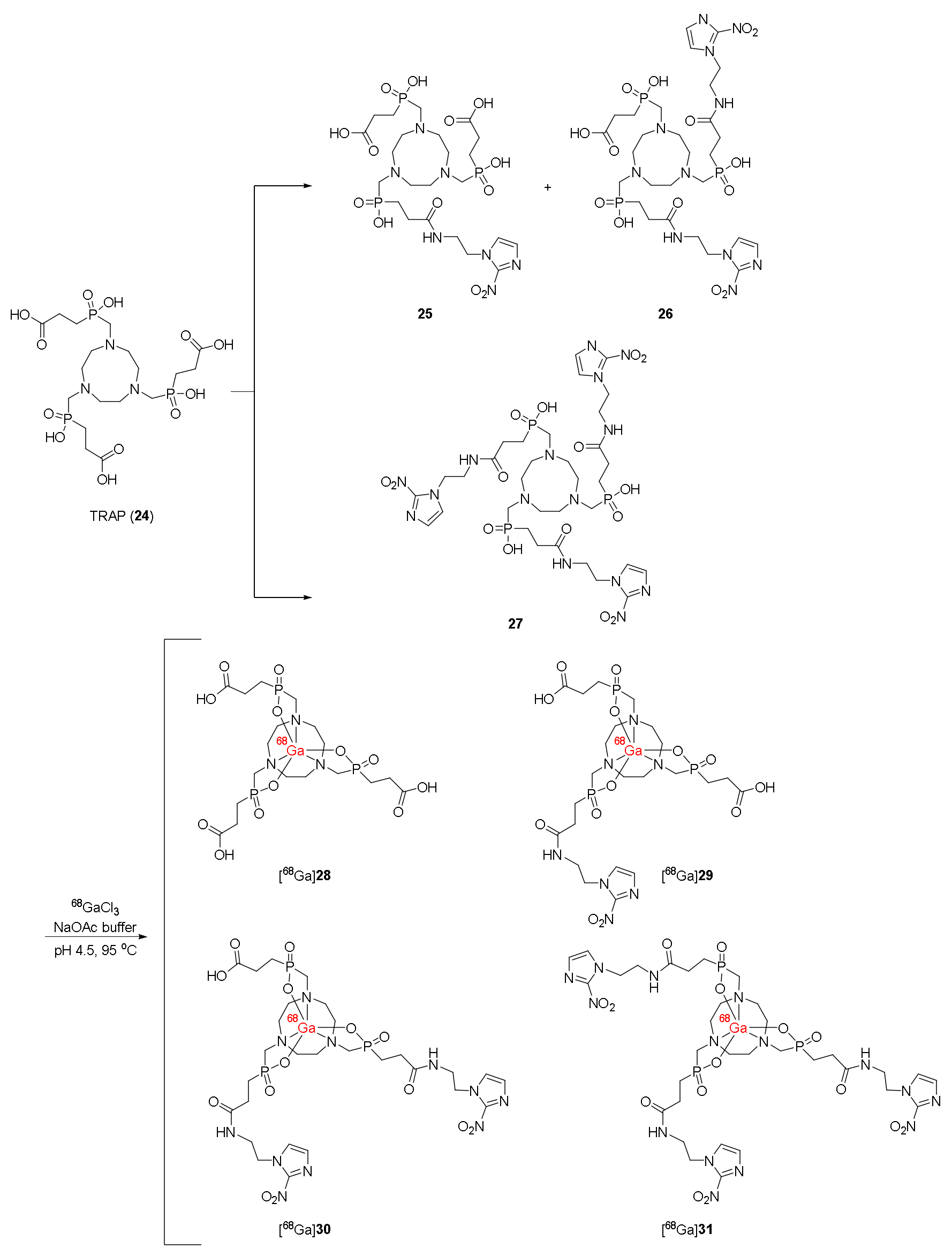
| 68Ga-labeled trivalent complexes with TRAP chelator ([68Ga]28–31) [118,120] | Logp([68Ga]30) = −3.64 Logp([68Ga]31) = −3.28 | U87MG and CT-26 cell lines | BALB/c mice bearing CT-26 xenograft | Clinical application: Patients with cervical cancer No significant correlation of PET imaging results with the immunohistochemical HIF-1α positive regions [120] |
| Lower protein bindings than 68Ga-NOTA-SCN-NI, 68Ga-DOTA-NI, and 68Ga-DOTA-SCN-NI | [68Ga]31 showed the highest hypoxic/normoxic ratios | [68Ga]29–31 were selectively uptaken in tumors [68Ga]31 indicated the best contrast No significant correlation between PET imaging results and immunohistochemical HIF-1α positive regions | ||
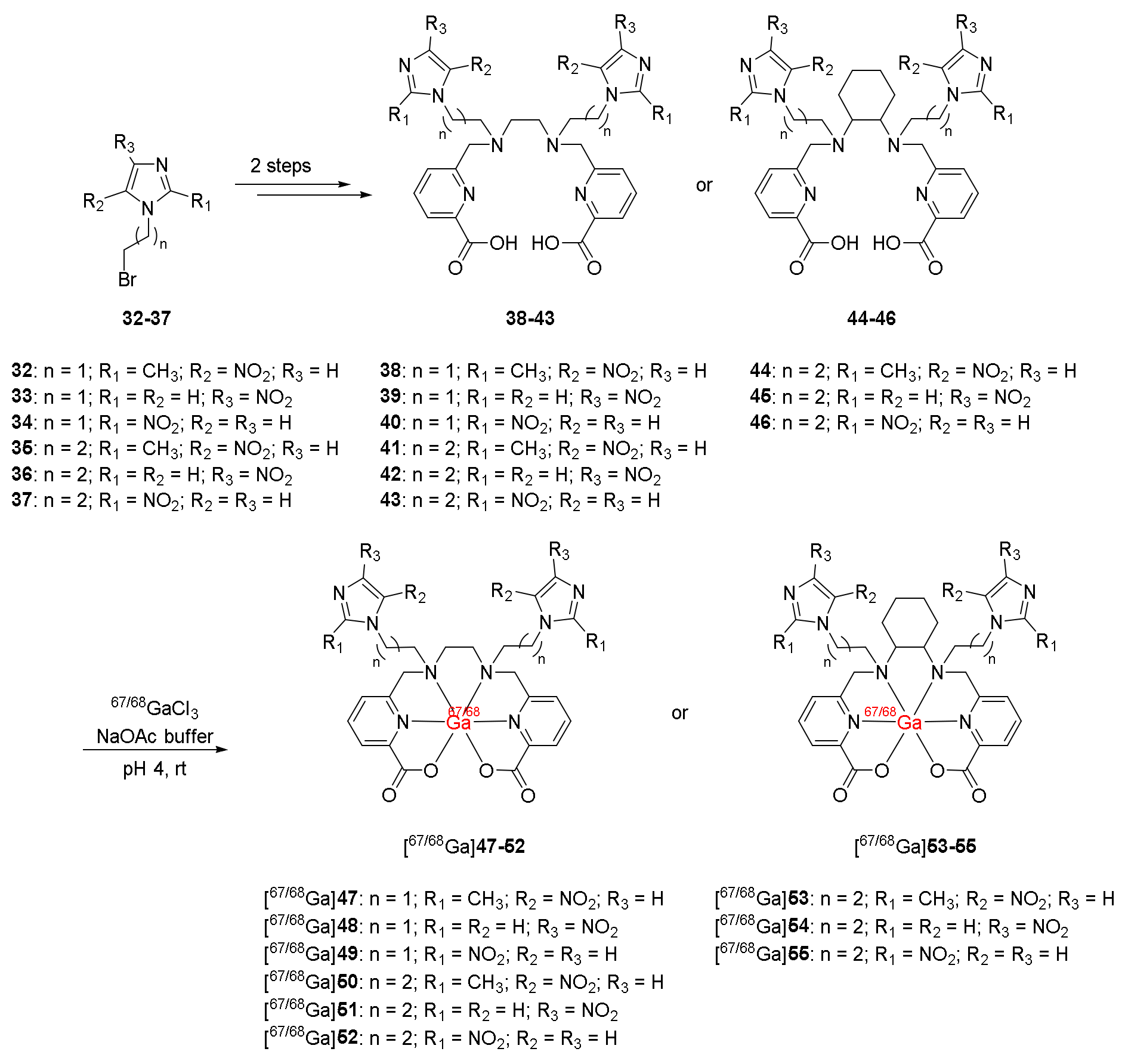
| 68Ga-labeled complexes containing nitroimidazole moieties and ligand H2dedpa/ H2CHXdedpa ([68Ga]47–55) [121] | - | HT-29, LCC6HER-2, and CHO cancer cell lines | - | No clinical study |
| Good stability Low protein binding No trans-chelation | 68Ga complexes with CHXdedpa showed higher hypoxic/normoxic ratios than 68Ga-DOTA and 68Ga-DOTA-NI Position of the nitro group of nitroimidazole did not affect the cellular uptake and retention of 68Ga-(CHX)dedpa-NI | |||
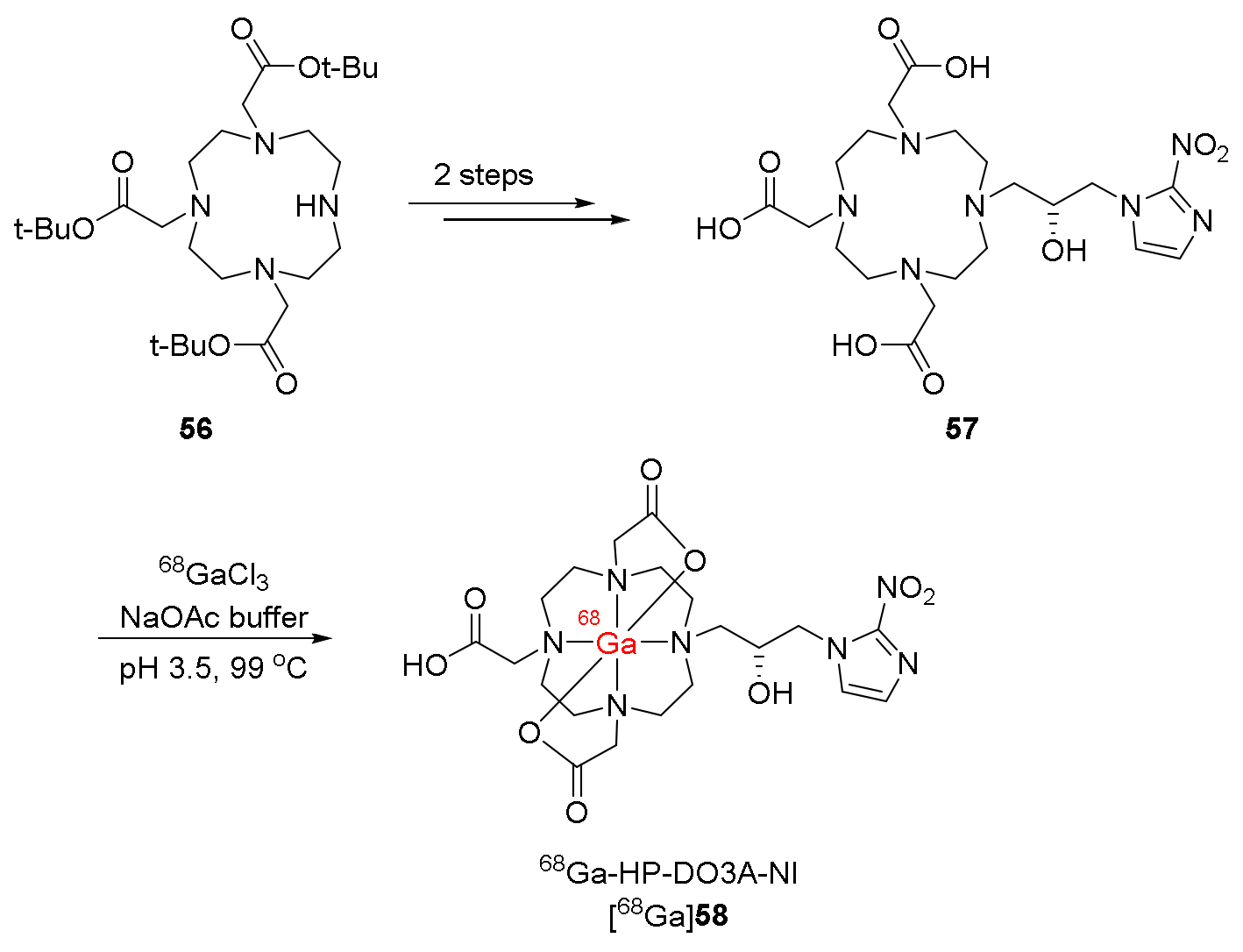
| 68Ga-HP-DO3A-NI, ([68Ga]58) [122] | Logp = −4.6 ± 0.1 | A549 cancer cell lines | SCID mice bearing A549 tumors | No clinical study |
| Higher accumulation under hypoxia than under air Hypoxia/normoxia ratios comparable to those of [68Ga]-DOTA-NI in CT-26 cancer cell lines | Selectively uptaken at the maximum in the tumor at 10 min p.i. and showed good retention until 2 h p.i. High T/M ratio due to rapid muscle clearance | |||
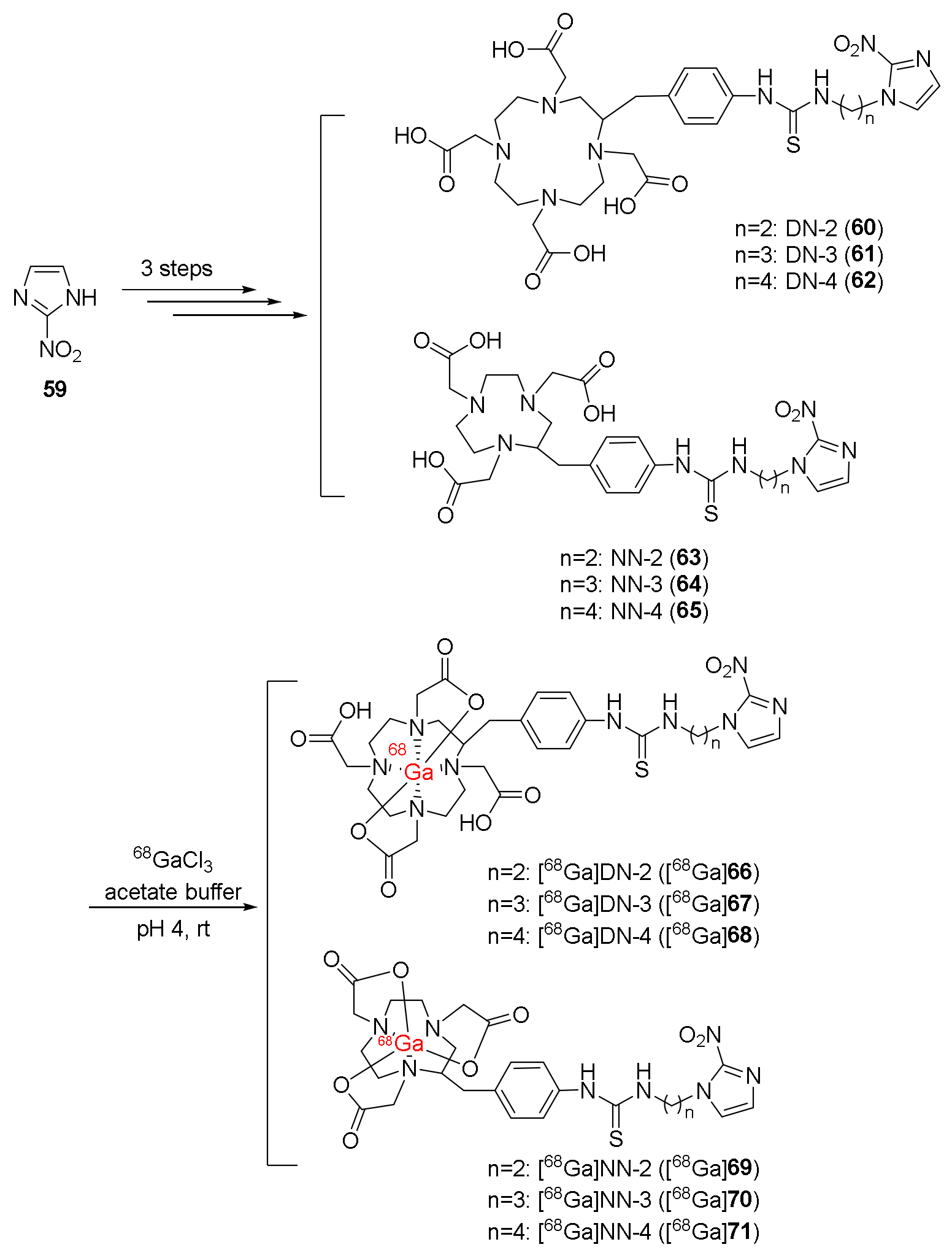
| [68Ga]DN-3 ([68Ga]67), [68Ga]DN-4 ([68Ga]68), [68Ga]NN-3 ([68Ga]70), [68Ga]NN-4 ([68Ga]71) [123] | - | FaDu cancer cell lines | Balb/c mice bearing FaDu xenograft | No clinical study |
| Cellular uptakes were significantly improved compared to [68Ga]DN-2 and [68Ga]NN-2 | T/B ratios over one and T/M ratios over three Tumors were observed in PET/CT imaging. | |||
| 68Ga-DOTAGA-2-NIM ([68Ga]75) 68Ga-NODAGA-2-NIM ([68Ga]76) [124] | Logp([68Ga]75) = −2.42± 0.19 Logp([68Ga]76) = −2.62± 0.14 | CHO cell lines | Swiss mice bearing Fibrosarcoma tumors | No clinical study |
| Hypoxia/normoxia ratios over one | [68Ga]76 exhibited both T/B and T/M ratios over one due to low binding to serum proteins | Compared to [18F]FMISO, [68Ga]76 exhibited a higher T/B ratio but a lower T/M ratio. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.T.; Kim, H.-K. Recent Advances of 68Ga-Labeled PET Radiotracers with Nitroimidazole in the Diagnosis of Hypoxia Tumors. Int. J. Mol. Sci. 2023, 24, 10552. https://doi.org/10.3390/ijms241310552
Nguyen AT, Kim H-K. Recent Advances of 68Ga-Labeled PET Radiotracers with Nitroimidazole in the Diagnosis of Hypoxia Tumors. International Journal of Molecular Sciences. 2023; 24(13):10552. https://doi.org/10.3390/ijms241310552
Chicago/Turabian StyleNguyen, Anh Thu, and Hee-Kwon Kim. 2023. "Recent Advances of 68Ga-Labeled PET Radiotracers with Nitroimidazole in the Diagnosis of Hypoxia Tumors" International Journal of Molecular Sciences 24, no. 13: 10552. https://doi.org/10.3390/ijms241310552
APA StyleNguyen, A. T., & Kim, H.-K. (2023). Recent Advances of 68Ga-Labeled PET Radiotracers with Nitroimidazole in the Diagnosis of Hypoxia Tumors. International Journal of Molecular Sciences, 24(13), 10552. https://doi.org/10.3390/ijms241310552






