The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria
Abstract
1. Introduction
2. Results
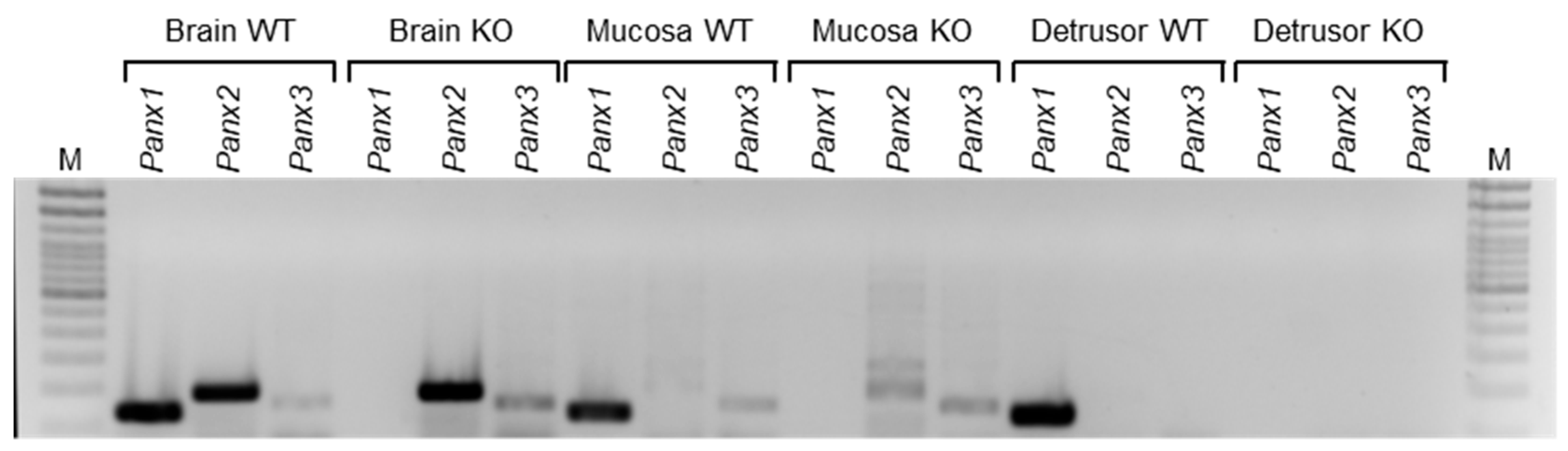
2.1. Effect of PANX1 on the Release of s-ENTDs in the Bladder LP
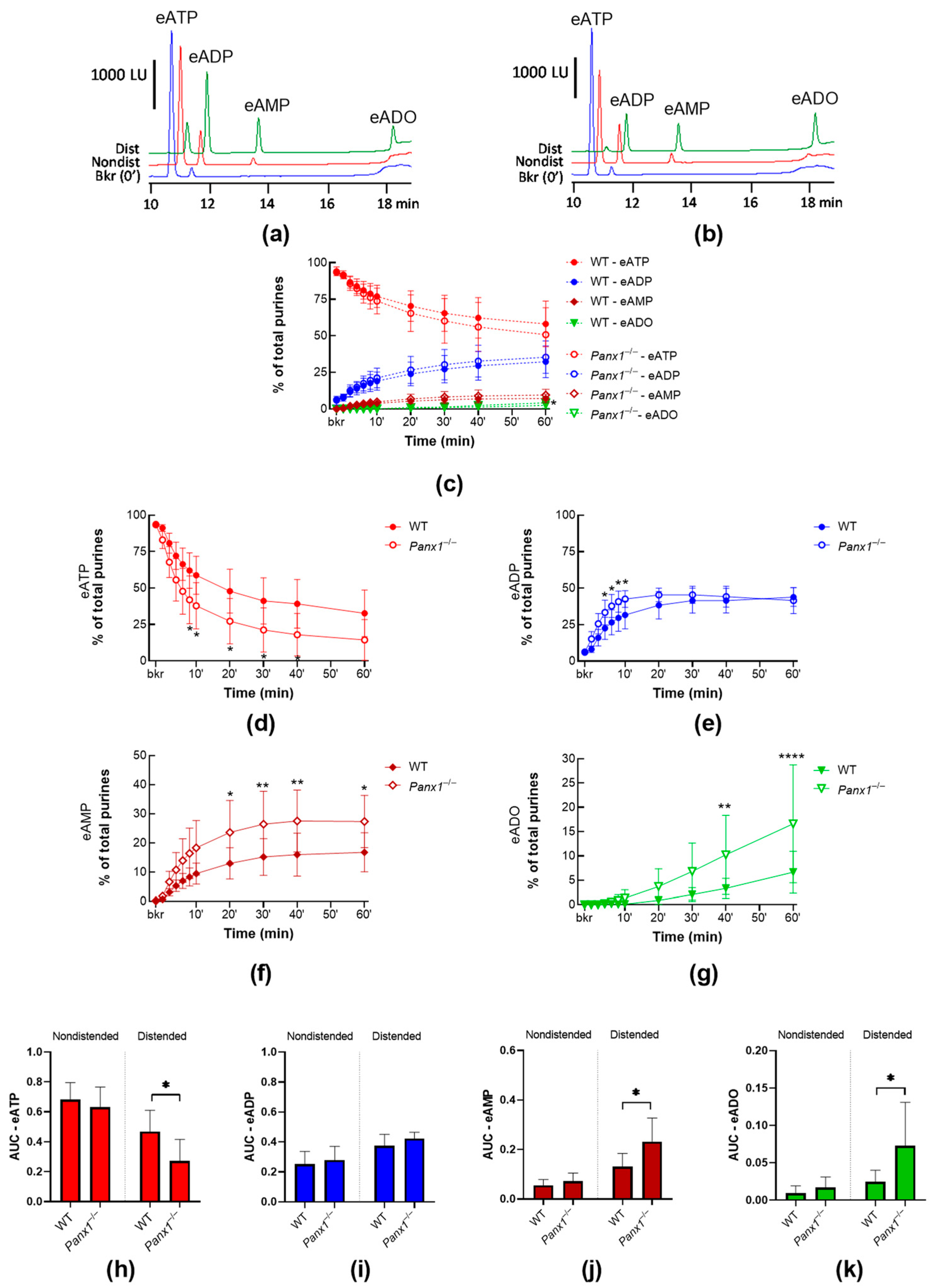
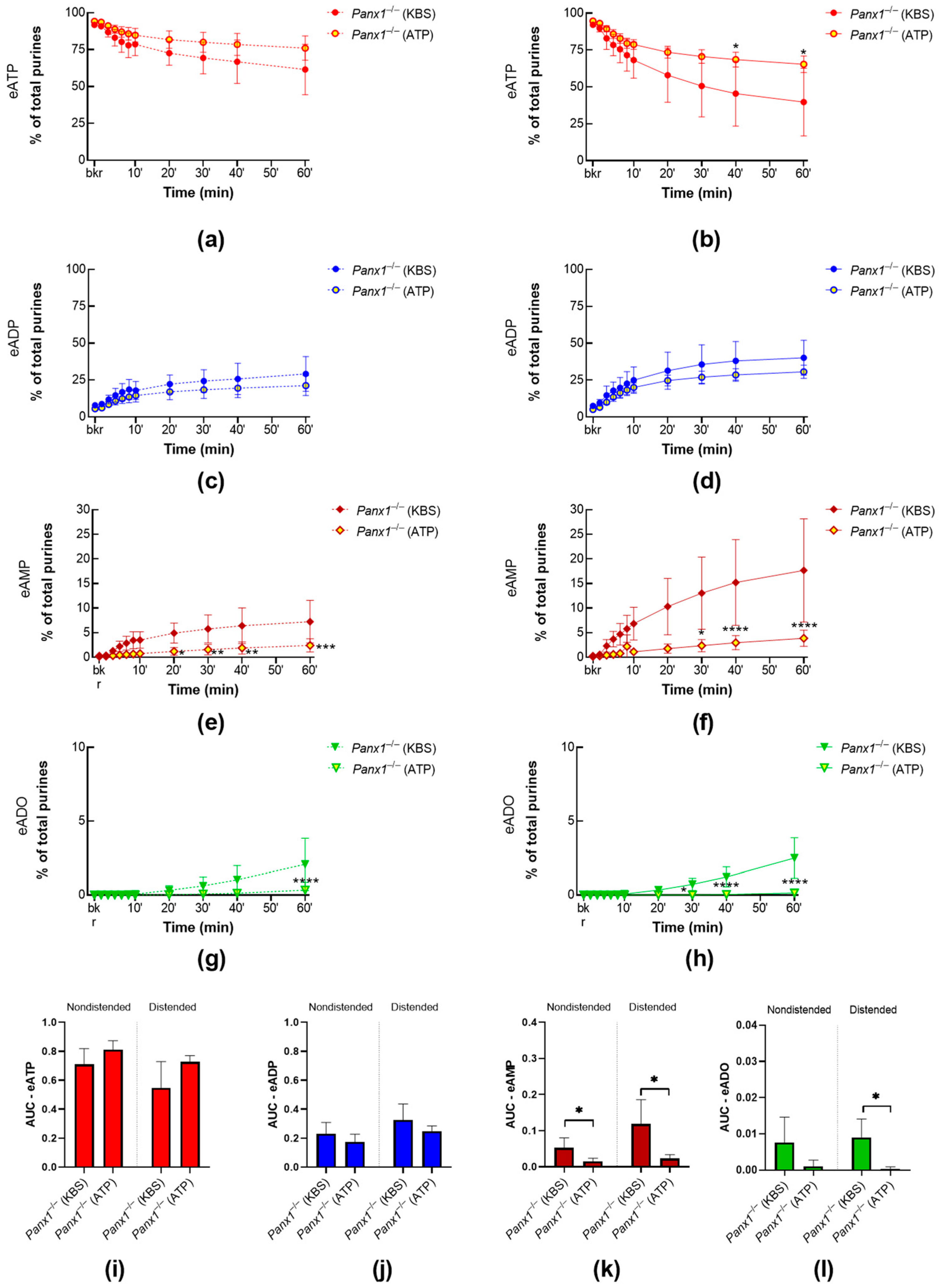
2.1.1. Panx1 Deletion Increases the Distension-Induced Release of s-ENTDs in the LP
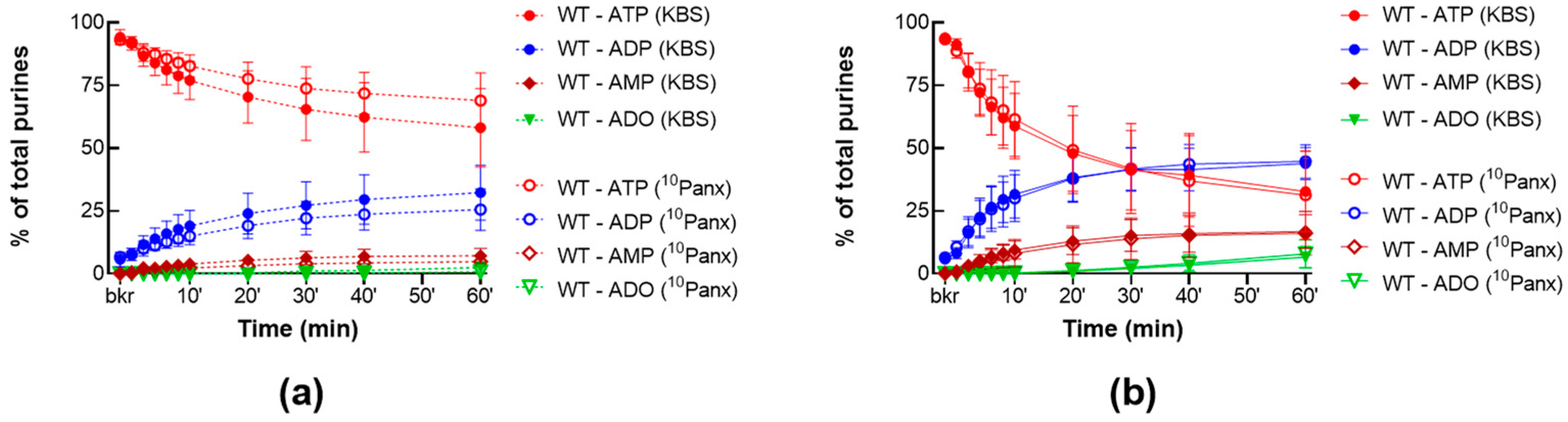
2.1.2. 10Panx Has no Effect on the Release of s-ENTDs in the LP
2.2. Role of P2X7R in the Release of s-ENTDs in LP
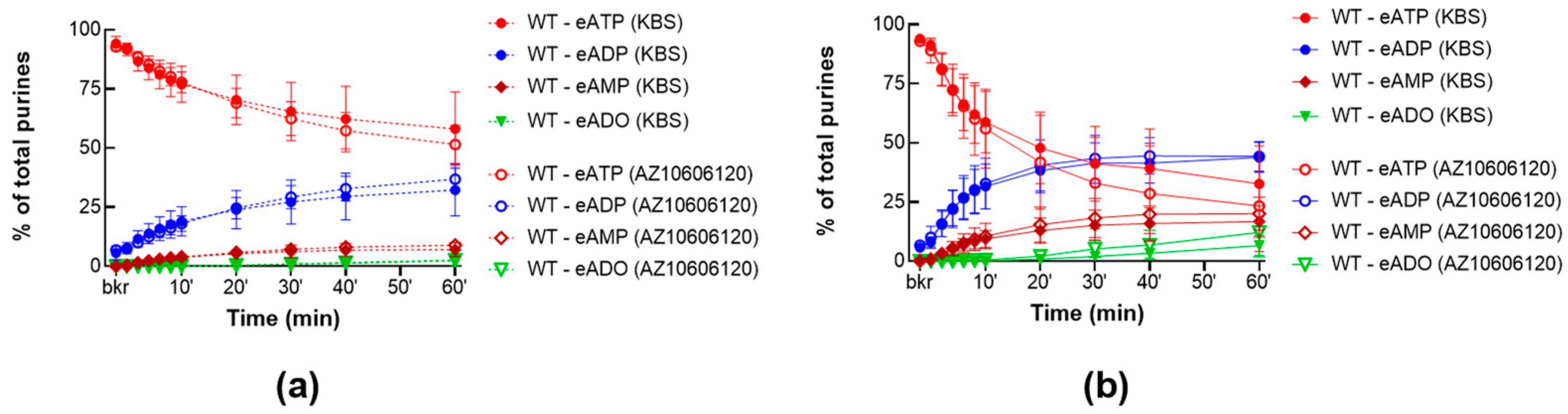
2.2.1. P2X7R Inhibition Does Not Alter the Constitutive nor the Distention-Induced Release of s-ENTDs in the LP of WT Denuded Bladders
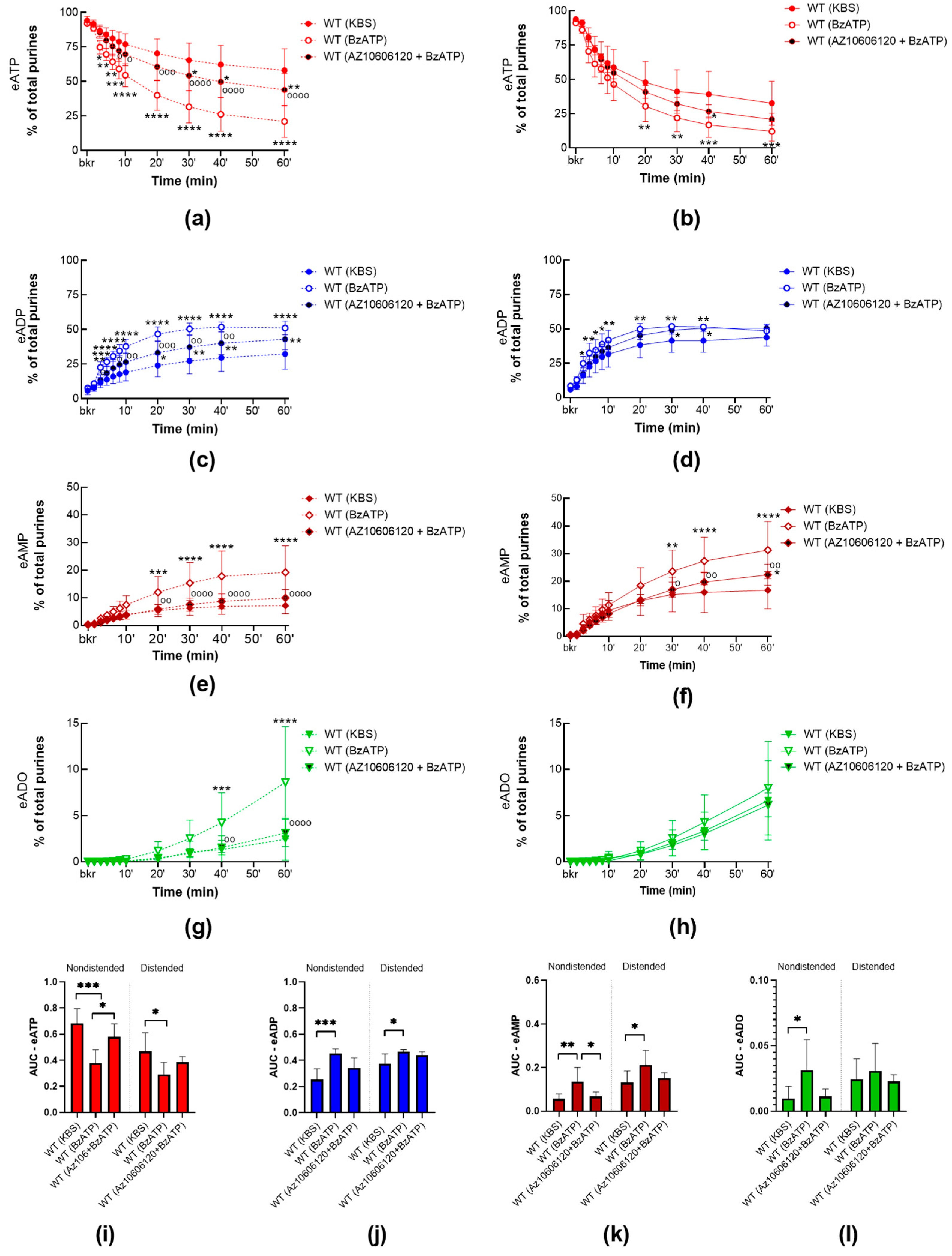
2.2.2. P2X7R Activation with BzATP Increases the Release of s-ENTDs in the LP of WT Bladders and Eliminates the Mechanosensitive Pattern of s-ENTDs Release
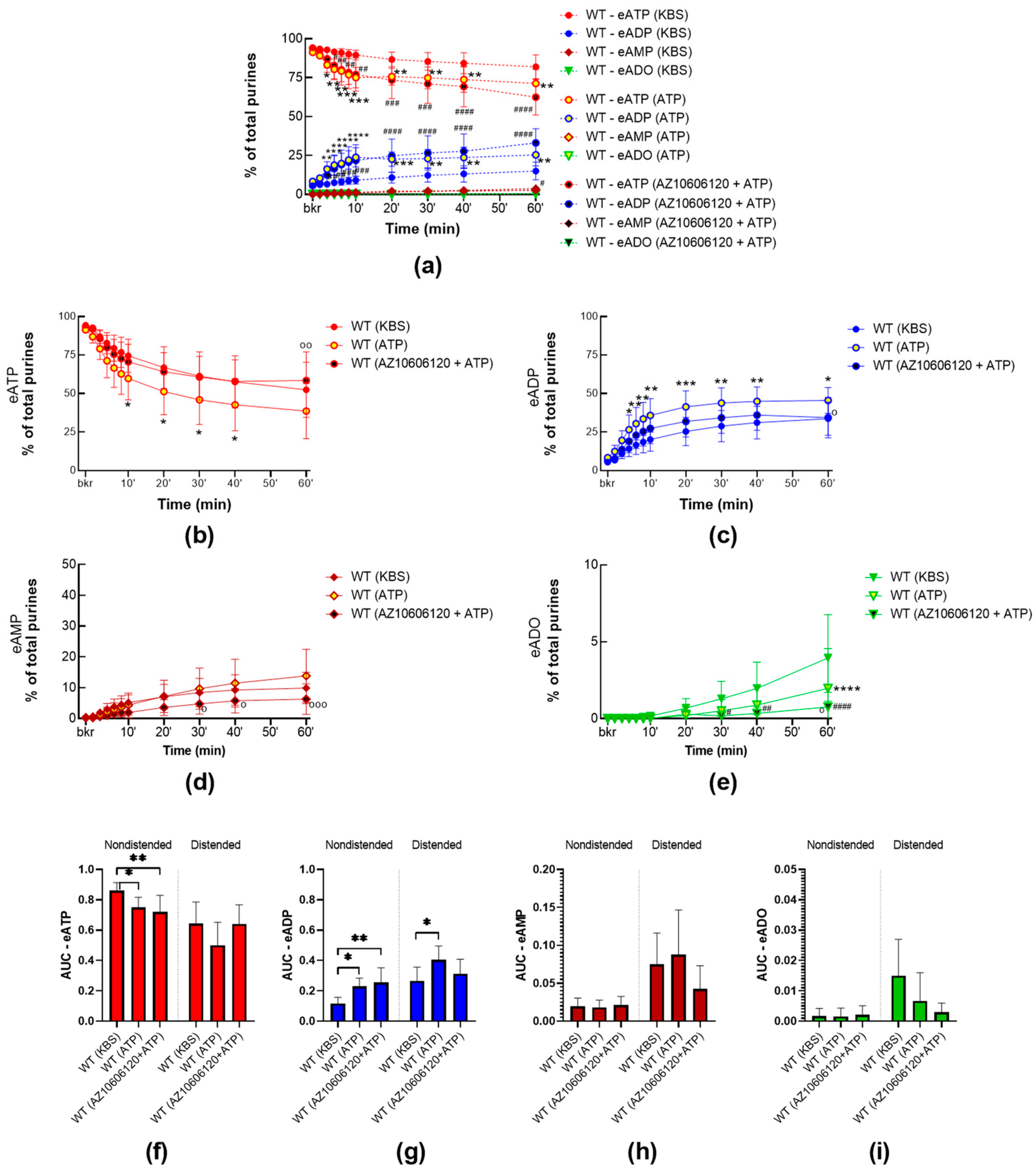
2.2.3. High Concentrations of ATP Facilitate the Release of s-ENTDs in the LP of WT Bladders
2.3. Interdependence of PANX1 and P2X7R in Mediating s-ENTDs Release in LP
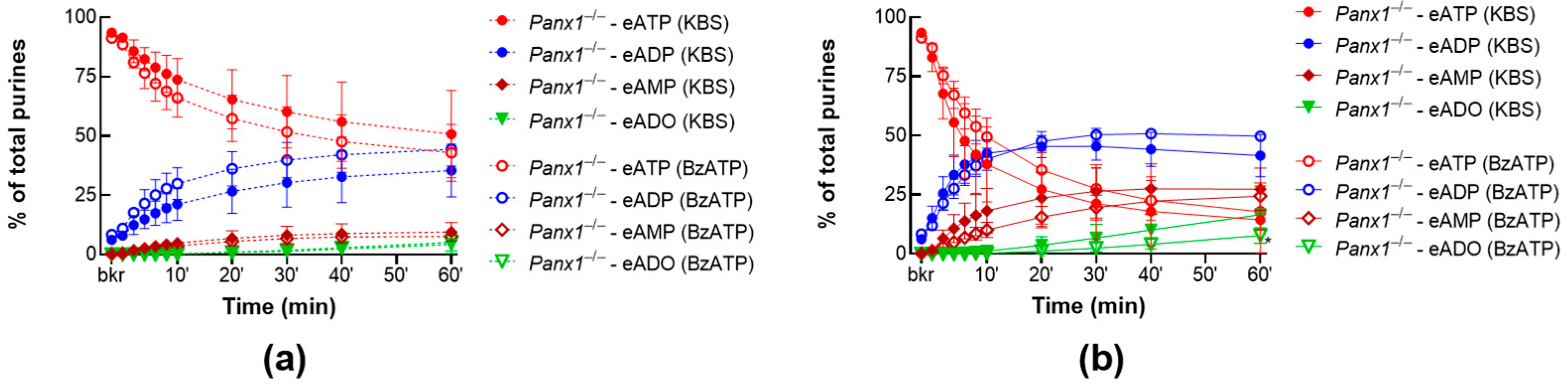
2.3.1. BzATP Has no Effect on the Release of s-ENTDs in the LP of Panx1−/− Detrusor-Free Bladders
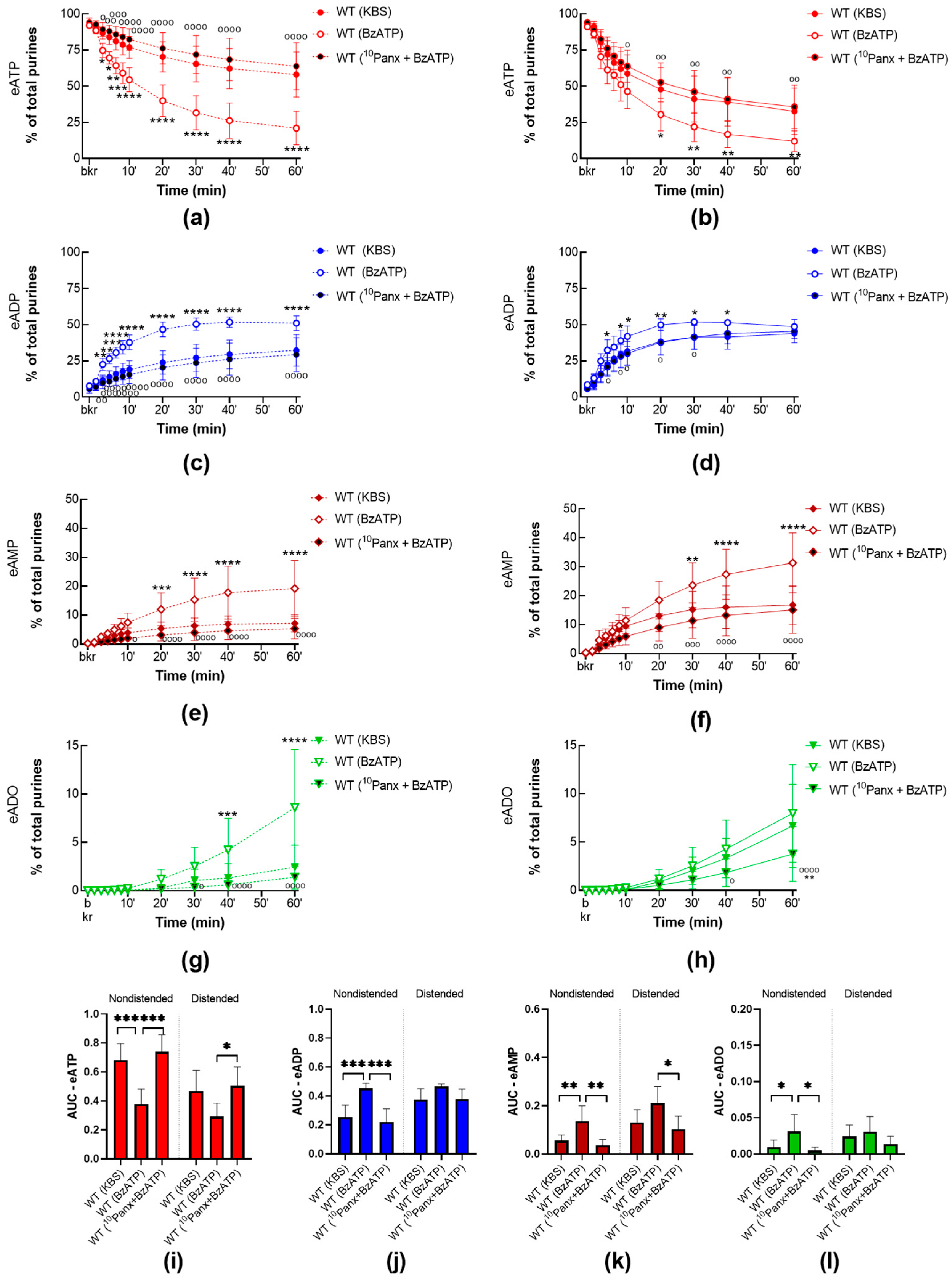
2.3.2. BzATP-Induced Release of s-ENTDS Is Blocked in the Presence of 10Panx
2.3.3. ATP-Induced Release of s-ENTDs during Distension Is Diminished in Panx1−/− Bladders
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethical Approval
4.3. RNA Isolation, Reverse Transcription, and RT-PCR
4.4. Detrusor-Free Bladder Preparation
4.5. Soluble/Releasable Nucleotidase Activity in the Lamina Propria of Detrusor-Free Bladder Preparations of WT and Panx1−/− Mice
4.6. Effect of Pharmacological Activation or Inhibition of P2X7R and/or PANX1 on the Release of Soluble Nucleotidases in the LP
4.7. Preparation of 1,N6-Etheno-Nucleotides
4.8. HPLC Analysis of 1,N6-Etheno-Nucleotides
4.9. Drugs and Reagents
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, S.A.; Lewis, J.R. Kinetics of Urothelial ATP Release. Am. J. Physiol.-Ren. Physiol. 2006, 291, F332–F340. [Google Scholar] [CrossRef] [PubMed]
- Durnin, L.; Kwok, B.; Kukadia, P.; McAvera, R.; Corrigan, R.D.; Ward, S.M.; Zhang, Y.; Chen, Q.; Koh, S.D.; Sanders, K.M.; et al. An Ex Vivo Bladder Model with Detrusor Smooth Muscle Removed to Analyse Biologically Active Mediators Released from the Suburothelium. J. Physiol. 2019, 597, 1467–1485. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.R.; Kennedy, I.; Burton, T.J. ATP Is Released from Rabbit Urinary Bladder Epithelial Cells by Hydrostatic Pressure Changes-Possible Sensory Mechanism? J. Physiol. 1997, 505, 503–511. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signalling in the Urinary Tract in Health and Disease. Purinergic Signal. 2014, 10, 103–155. [Google Scholar] [CrossRef] [PubMed]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Dayton, J.; Perrino, B.A.; Mutafova-Yambolieva, V.N. Mechanosensitive Hydrolysis of ATP and ADP in Lamina Propria of the Murine Bladder by Membrane-Bound and Soluble Nucleotidases. Front. Physiol. 2022, 13, 1185. [Google Scholar] [CrossRef]
- Todorov, L.D.; Mihaylova-Todorova, S.; Westfall, T.D.; Sneddon, P.; Kennedy, C.; Bjur, R.A.; Westfall, D.P. Neuronal Release of Soluble Nucleotidases and Their Role in Neurotransmitter Inactivation. Nature 1997, 387, 76–79. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular Function and Molecular Structure of Ecto-Nucleotidases. Purinergic Signal. 2012, 8, 437. [Google Scholar] [CrossRef]
- Negoro, H.; Urban-Maldonado, M.; Liou, L.S.; Spray, D.C.; Thi, M.M.; Suadicani, S.O. Pannexin 1 Channels Play Essential Roles in Urothelial Mechanotransduction and Intercellular Signaling. PLoS ONE 2014, 9, e106269. [Google Scholar] [CrossRef]
- Beckel, J.M.; Daugherty, S.L.; Tyagi, P.; Wolf-Johnston, A.S.; Birder, L.A.; Mitchell, C.H.; de Groat, W.C. Pannexin 1 Channels Mediate the Release of ATP into the Lumen of the Rat Urinary Bladder. J. Physiol. 2015, 593, 1857–1871. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 Mediates Large Pore Formation and Interleukin-1β Release by the ATP-Gated P2X7 Receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The Pannexin 1 Channel Activates the Inflammasome in Neurons and Astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef]
- Poornima, V.; Madhupriya, M.; Kootar, S.; Sujatha, G.; Kumar, A.; Bera, A.K. P2X7 Receptor–Pannexin 1 Hemichannel Association: Effect of Extracellular Calcium on Membrane Permeabilization. J. Mol. Neurosci. 2012, 46, 585–594. [Google Scholar] [CrossRef]
- Xu, X.J.; Boumechache, M.; Robinson, L.E.; Marschall, V.; Gorecki, D.C.; Masin, M.; Murrell-Lagnado, R. Splice-Variants of the P2X7 Receptor Reveal Differential Agonist-Dependence and Functional Coupling with Pannexin-1. J. Cell Sci. 2012, 125, 3776–3789. [Google Scholar] [CrossRef]
- Boyce, A.K.J.; Swayne, L.A. P2X7 Receptor Cross-Talk Regulates ATP-Induced Pannexin 1 Internalization. Biochem. J. 2017, 474, 2133–2144. [Google Scholar] [CrossRef]
- Rhodes, G.; Segars, K.L.; Lee, Y.K.; Hutcheon, A.E.K.; Rich, C.B.; Trinkaus-Randall, V. Pannexin1: Role as a Sensor to Injury Is Attenuated in Pretype 2 Corneal Diabetic Epithelium. Anal. Cell. Pathol. 2021, 2021, 4793338. [Google Scholar] [CrossRef]
- Bravo, D.; Zepeda-Morales, K.; Maturana, C.J.; Retamal, J.S.; Hernández, A.; Pelissier, T.; Barra, R.; Sáez-Briones, P.; Burgos, H.; Constandil, L. NMDA and P2X7 Receptors Require Pannexin 1 Activation to Initiate and Maintain Nociceptive Signaling in the Spinal Cord of Neuropathic Rats. Int. J. Mol. Sci. 2022, 23, 6705. [Google Scholar] [CrossRef]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin Channels Are Not Gap Junction Hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin Membrane Channels Are Mechanosensitive Conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- Iglesias, R.; Locovei, S.; Roque, A.; Alberto, A.P.; Dahl, G.; Spray, D.C.; Scemes, E. P2X 7 Receptor-Pannexin1 Complex: Pharmacology and Signaling. Am. J. Physiol.-Cell Physiol. 2008, 295, C752–C760. [Google Scholar] [CrossRef]
- Ma, W.; Compan, V.; Zheng, W.; Martin, E.; North, R.A.; Verkhratsky, A.; Surprenant, A. Pannexin 1 Forms an Anion-Selective Channel. Pflugers Arch. 2012, 463, 585–592. [Google Scholar] [CrossRef]
- Isakson, B.E.; Thompson, R.J. Pannexin-1 as a Potentiator of Ligand-Gated Receptor Signaling. Channels 2014, 8, 118–123. [Google Scholar] [CrossRef]
- Locovei, S.; Wang, J.; Dahl, G. Activation of Pannexin 1 Channels by ATP through P2Y Receptors and by Cytoplasmic Calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Zhang, M.; Nurse, C.A. Angiotensin II Mobilizes Intracellular Calcium and Activates Pannexin-1 Channels in Rat Carotid Body Type II Cells via AT1 Receptors. J. Physiol. 2014, 592, 4747–4762. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A. Connexin and Pannexin Hemichannels Are Regulated by Redox Potential. Front. Physiol. 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Zhou, N.; MacVicar, B.A. Ischemia Opens Neuronal Gap Junction Hemichannels. Science 2006, 312, 924–927. [Google Scholar] [CrossRef]
- Locovei, S.; Bao, L.; Dahl, G. Pannexin 1 in Erythrocytes: Function without a Gap. Proc. Natl. Acad. Sci. USA 2006, 103, 7655–7659. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Iglesias, R.; Wang, J.; Dahl, G.; Spray, D.C.; Scemes, E. ATP Signaling Is Deficient in Cultured Pannexin1-Null Mouse Astrocytes. Glia 2012, 60, 1106–1116. [Google Scholar] [CrossRef]
- DeLalio, L.J.; Billaud, M.; Ruddiman, C.A.; Johnstone, S.R.; Butcher, J.T.; Wolpe, A.G.; Jin, X.; Keller, T.C.S.; Keller, A.S.; Rivière, T.; et al. Constitutive SRC-Mediated Phosphorylation of Pannexin 1 at Tyrosine 198 Occurs at the Plasma Membrane. J. Biol. Chem. 2019, 294, 6940–6956. [Google Scholar] [CrossRef]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA Receptor Signaling Couples Src Family Kinases to Pannexin-1 during Excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Jin, X.; Medina, C.B.; Leonhardt, S.A.; Kiessling, V.; Bennett, B.C.; Shu, S.; Tamm, L.K.; Yeager, M.; Ravichandran, K.S.; et al. A Quantized Mechanism for Activation of Pannexin Channels. Nat. Commun. 2017, 8, 14324. [Google Scholar] [CrossRef]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 Channels Mediate ‘Find-Me’ Signal Release and Membrane Permeability during Apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dahl, G. Pannexin1: A Multifunction and Multiconductance and/or Permeability Membrane Channel. Am. J. Physiol.-Cell Physiol. 2018, 315, C290–C299. [Google Scholar] [CrossRef]
- Mim, C.; Perkins, G.; Dahl, G. Structure versus Function: Are New Conformations of Pannexin 1 yet to Be Resolved? J. Gen. Physiol. 2021, 153, e202012754. [Google Scholar] [CrossRef]
- Crespo Yanguas, S.; Willebrords, J.; Johnstone, S.R.; Maes, M.; Decrock, E.; De Bock, M.; Leybaert, L.; Cogliati, B.; Vinken, M. Pannexin1 as Mediator of Inflammation and Cell Death. Biochim. Biophys. Acta 2017, 1864, 51. [Google Scholar] [CrossRef]
- Willebrords, J.; Maes, M.; Pereira, I.V.A.; da Silva, T.C.; Govoni, V.M.; Lopes, V.V.; Crespo Yanguas, S.; Shestopalov, V.I.; Nogueira, M.S.; de Castro, I.A.; et al. Protective Effect of Genetic Deletion of Pannexin1 in Experimental Mouse Models of Acute and Chronic Liver Disease. Biochim. Biophys. Acta 2018, 1864, 819. [Google Scholar] [CrossRef]
- Leroy, K.; Vilas-Boas, V.; Gijbels, E.; Vanderborght, B.; Devisscher, L.; Cogliati, B.; Van Den Bossche, B.; Colle, I.; Vinken, M. Expression of Connexins and Pannexins in Diseased Human Liver. EXCLI J. 2022, 21, 1111. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. Cellular Distribution and Functions of P2 Receptor Subtypes in Different Systems. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 2004; Volume 240, pp. 31–304. [Google Scholar]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The Cytolytic P2Z Receptor for Extracellular ATP Identified as a P 2X Receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Klapperstück, M.; Büttner, C.; Schmalzing, G.; Markwardt, F. Functional Evidence of Distinct ATP Activation Sites at the Human P2X 7 Receptor. J. Physiol. 2001, 534, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 Receptor Pharmacology: Comparison of Recombinant Mouse, Rat and Human P2X7 Receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef]
- Gribble, F.M.; Loussouarn, G.; Tucker, S.J.; Zhao, C.; Nichols, C.G.; Ashcroft, F.M. A Novel Method for Measurement of Submembrane ATP Concentration. J. Biol. Chem. 2000, 275, 30046–30049. [Google Scholar] [CrossRef]
- Wang, E.C.Y.; Lee, J.-M.; Ruiz, W.G.; Balestreire, E.M.; von Bodungen, M.; Barrick, S.; Cockayne, D.A.; Birder, L.A.; Apodaca, G. ATP and Purinergic Receptor–Dependent Membrane Traffic in Bladder Umbrella Cells. J. Clin. Investig. 2005, 115, 2412–2422. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 Receptor: A Main Player in Inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 Purinergic Signalling in the Tumour Microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Silva, R.; Coutinho-Silva, R.; Takiya, C.; Battastini, A.; Morrone, F.; Campos, M. The Role of P2X7 Purinergic Receptors in Inflammatory and Nociceptive Changes Accompanying Cyclophosphamide-Induced Haemorrhagic Cystitis in Mice. Br. J. Pharmacol. 2012, 165, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Taidi, Z.; Zhou, T.; Moore, K.H.; Mansfield, K.J.; Liu, L. P2X7 Receptor Blockade Protects Against Acrolein-Induced Bladder Damage: A Potential New Therapeutic Approach for the Treatment of Bladder Inflammatory Diseases. Front. Pharmacol. 2021, 12, 682520. [Google Scholar] [CrossRef]
- Pellegatti, P.; Falzoni, S.; Pinton, P.; Rizzuto, R.; Di Virgilio, F. A Novel Recombinant Plasma Membrane-Targeted Luciferase Reveals a New Pathway for ATP Secretion. Mol. Biol. Cell 2005, 16, 3659–3665. [Google Scholar] [CrossRef]
- Peverini, L.; Beudez, J.; Dunning, K.; Chataigneau, T.; Grutter, T. New Insights Into Permeation of Large Cations Through ATP-Gated P2X Receptors. Front. Mol. Neurosci. 2018, 11, 265. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Ma, W.; Hui, H.; Pelegrin, P.; Surprenant, A. Pharmacological Characterization of Pannexin-1 Currents Expressed in Mammalian Cells. J. Pharmacol. Exp. Ther. 2009, 328, 409–418. [Google Scholar] [CrossRef]
- Qiu, F.; Dahl, G. A Permeant Regulating Its Permeation Pore: Inhibition of Pannexin 1 Channels by ATP. Am. J. Physiol.-Cell Physiol. 2009, 296, C250–C255. [Google Scholar] [CrossRef]
- Boyce, A.K.J.; Kim, M.S.; Wicki-Stordeur, L.E.; Swayne, L.A. ATP Stimulates Pannexin 1 Internalization to Endosomal Compartments. Biochem. J. 2015, 470, 319–330. [Google Scholar] [CrossRef]
- Purohit, R.; Bera, A.K. Pannexin 1 Plays a Pro-Survival Role by Attenuating P2X7 Receptor-Mediated Ca2+ Influx. Cell Calcium 2021, 99, 102458. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Dayl, S.; Schmid, R.; Evans, R.J. Unique Residues in the ATP Gated Human P2X7 Receptor Define a Novel Allosteric Binding Pocket for the Selective Antagonist AZ10606120. Sci. Rep. 2017, 7, 725. [Google Scholar] [CrossRef]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The Neural Control of Micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Borhani Peikani, M.; Mutafova-Yambolieva, V.N. Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 7322. [Google Scholar] [CrossRef]
- Gutierrez Cruz, A.; Aresta Branco, M.S.L.; Perrino, B.A.; Sanders, K.M.; Mutafova-Yambolieva, V.N. Urinary ATP Levels Are Controlled by Nucleotidases Released from the Urothelium in a Regulated Manner. Metabolites 2022, 13, 30. [Google Scholar] [CrossRef]
- Negoro, H.; Lutz, S.E.; Liou, L.S.; Kanematsu, A.; Ogawa, O.; Scemes, E.; Suadicani, S.O. Pannexin 1 Involvement in Bladder Dysfunction in a Multiple Sclerosis Model. Sci. Rep. 2013, 3, 2152. [Google Scholar] [CrossRef]
- Roberts, M.W.G.; Sui, G.; Wu, R.; Rong, W.; Wildman, S.; Montgomery, B.; Ali, A.; Langley, S.; Ruggieri, M.R.; Wu, C. TRPV4 Receptor as a Functional Sensory Molecule in Bladder Urothelium: Stretch-independent, Tissue-specific Actions and Pathological Implications. FASEB J. 2020, 34, 263–286. [Google Scholar] [CrossRef]
- Diem, K.; Fauler, M.; Fois, G.; Hellmann, A.; Winokurow, N.; Schumacher, S.; Kranz, C.; Frick, M. Mechanical Stretch Activates Piezo1 in Caveolae of Alveolar Type I Cells to Trigger ATP Release and Paracrine Stimulation of Surfactant Secretion from Alveolar Type II Cells. FASEB J. 2020, 34, 12785–12804. [Google Scholar] [CrossRef]
- Vial, C.; Evans, R.J. P2X Receptor Expression in Mouse Urinary Bladder and the Requirement of P2X 1 Receptors for Functional P2X Receptor Responses in the Mouse Urinary Bladder Smooth Muscle. Br. J. Pharmacol. 2000, 131, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Svennersten, K.; Hallén-Grufman, K.; de Verdier, P.J.; Wiklund, N.P.; Poljakovic, M. Localization of P2X Receptor Subtypes 2, 3 and 7 in Human Urinary Bladder. BMC Urol. 2015, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Lenertz, L.Y.; Gavala, M.L.; Zhu, Y.; Bertics, P.J. Transcriptional Control Mechanisms Associated with the Nucleotide Receptor P2X7, a Critical Regulator of Immunologic, Osteogenic, and Neurologic Functions. Immunol. Res. 2011, 50, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chai, T.C. Augmented Extracellular ATP Signaling in Bladder Urothelial Cells from Patients with Interstitial Cystitis. Am. J. Physiol.-Cell Physiol. 2006, 290, C27–C34. [Google Scholar] [CrossRef]
- Kumar, V.; Chapple, C.R.; Surprenant, A.M.; Chess-Williams, R. Enhanced Adenosine Triphosphate Release From the Urothelium of Patients With Painful Bladder Syndrome: A Possible Pathophysiological Explanation. J. Urol. 2007, 178, 1533–1536. [Google Scholar] [CrossRef]
- Kumar, V.; Chapple, C.R.; Rosario, D.; Tophill, P.R.; Chess-Williams, R. In Vitro Release of Adenosine Triphosphate from the Urothelium of Human Bladders with Detrusor Overactivity, Both Neurogenic and Idiopathic. Eur. Urol. 2010, 57, 1087–1092. [Google Scholar] [CrossRef]
- Sun, Y.; MaLossi, J.; Jacobs, S.C.; Chai, T.C. Effect of Doxazosin on Stretch-Activated Adenosine Triphosphate Release in Bladder Urothelial Cells from Patients with Benign Prostatic Hyperplasia. Urology 2002, 60, 351–356. [Google Scholar] [CrossRef]
- Birder, L.A.; Barrick, S.R.; Roppolo, J.R.; Kanai, A.J.; de Groat, W.C.; Kiss, S.; Buffington, C.A. Feline Interstitial Cystitis Results in Mechanical Hypersensitivity and Altered ATP Release from Bladder Urothelium. Am. J. Physiol.-Ren. Physiol. 2003, 285, F423–F429. [Google Scholar] [CrossRef]
- Munoz, A.; Smith, C.P.; Boone, T.B.; Somogyi, G.T. Overactive and Underactive Bladder Dysfunction Is Reflected by Alterations in Urothelial ATP and NO Release. Neurochem. Int. 2011, 58, 295–300. [Google Scholar] [CrossRef]
- Salas, N.A.; Somogyi, G.T.; Gangitano, D.A.; Boone, T.B.; Smith, C.P. Receptor Activated Bladder and Spinal ATP Release in Neurally Intact and Chronic Spinal Cord Injured Rats. Neurochem. Int. 2007, 50, 345–350. [Google Scholar] [CrossRef]
- Khera, M.; Somogyi, G.T.; Kiss, S.; Boone, T.B.; Smith, C.P. Botulinum Toxin A Inhibits ATP Release from Bladder Urothelium after Chronic Spinal Cord Injury. Neurochem. Int. 2004, 45, 987–993. [Google Scholar] [CrossRef]
- Smith, C.P.; Vemulakonda, V.M.; Kiss, S.; Boone, T.B.; Somogyi, G.T. Enhanced ATP Release from Rat Bladder Urothelium during Chronic Bladder Inflammation: Effect of Botulinum Toxin A. Neurochem. Int. 2005, 47, 291–297. [Google Scholar] [CrossRef]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid Secretion of Interleukin-1β by Microvesicle Shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Virginio, C.; MacKenzie, A.; North, R.A.; Surprenant, A. Kinetics of Cell Lysis, Dye Uptake and Permeability Changes in Cells Expressing the Rat P2X 7 Receptor. J. Physiol. 1999, 519, 335–346. [Google Scholar] [CrossRef]
- Hanley, P.J.; Kronlage, M.; Kirschning, C.; del Rey, A.; Di Virgilio, F.; Leipziger, J.; Chessell, I.P.; Sargin, S.; Filippov, M.A.; Lindemann, O.; et al. Transient P2X7 Receptor Activation Triggers Macrophage Death Independent of Toll-like Receptors 2 and 4, Caspase-1, and Pannexin-1 Proteins. J. Biol. Chem. 2012, 287, 10650–10663. [Google Scholar] [CrossRef] [PubMed]
- Banz, Y.; Beldi, G.; Wu, Y.; Atkinson, B.; Usheva, A.; Robson, S.C. CD39 Is Incorporated into Plasma Microparticles Where It Maintains Functional Properties and Impacts Endothelial Activation. Br. J. Haematol. 2008, 142, 627–637. [Google Scholar] [CrossRef]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. The P2X7 Receptor–Pannexin Connection to Dye Uptake and IL-1β Release. Purinergic Signal. 2009, 5, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.-J.; Illes, P. Involvement of P2X7 Receptors in Chronic Pain Disorders. Purinergic Signal. 2022, 18, 83–92. [Google Scholar] [CrossRef]
- Ren, W.; Rubini, P.; Tang, Y.; Engel, T.; Illes, P. Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. Int. J. Mol. Sci. 2021, 23, 232. [Google Scholar] [CrossRef]
- Atkinson, L.; Batten, T.F.C.; Moores, T.S.; Varoqui, H.; Erickson, J.D.; Deuchars, J. Differential Co-Localisation of the P2X7 Receptor Subunit with Vesicular Glutamate Transporters VGLUT1 and VGLUT2 in Rat CNS. Neuroscience 2004, 123, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Deuchars, S.A.; Atkinson, L.; Brooke, R.E.; Musa, H.; Milligan, C.J.; Batten, T.F.C.; Buckley, N.J.; Parson, S.H.; Deuchars, J. Neuronal P2X 7 Receptors Are Targeted to Presynaptic Terminals in the Central and Peripheral Nervous Systems. J. Neurosci. 2001, 21, 7143–7152. [Google Scholar] [CrossRef] [PubMed]
- Durnin, L.; Corrigan, R.D.; Sanders, K.M.; Mutafova-Yambolieva, V.N. A Decentralized (Ex Vivo) Murine Bladder Model with the Detrusor Muscle Removed for Direct Access to the Suburothelium during Bladder Filling. J. Vis. Exp. 2019, 2019, e60344. [Google Scholar] [CrossRef]
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 179, 659–670. [Google Scholar] [CrossRef]
- Levitt, B.; Head, R.J.; Westfall, D.P. High-Pressure Liquid Chromatographic-Fluorometric Detection of Adenosine and Adenine Nucleotides: Application to Endogenous Content and Electrically Induced Release of Adenyl Purines in Guinea Pig Vas Deferens. Anal. Biochem. 1984, 137, 93–100. [Google Scholar] [CrossRef]
- Bobalova, J.; Bobal, P.; Mutafova-Yambolieva, V.N. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Anal. Biochem. 2002, 305, 269–276. [Google Scholar] [CrossRef]
- Durnin, L.; Hayoz, S.; Corrigan, R.D.; Yanez, A.; Koh, S.D.; Mutafova-Yambolieva, V.N. Urothelial Purine Release during Filling of Murine and Primate Bladders. Am. J. Physiol.-Ren. Physiol. 2016, 311, F708–F716. [Google Scholar] [CrossRef]
- Vollert, J.; Macleod, M.; Dirnagl, U.; Kas, M.J.; Michel, M.C.; Potschka, H.; Riedel, G.; Wever, K.E.; Würbel, H.; Steckler, T.; et al. The EQIPD Framework for Rigor in the Design, Conduct, Analysis and Documentation of Animal Experiments. Nat. Methods 2022, 19, 1334–1337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aresta Branco, M.S.L.; Gutierrez Cruz, A.; Peri, L.E.; Mutafova-Yambolieva, V.N. The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria. Int. J. Mol. Sci. 2023, 24, 9964. https://doi.org/10.3390/ijms24129964
Aresta Branco MSL, Gutierrez Cruz A, Peri LE, Mutafova-Yambolieva VN. The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria. International Journal of Molecular Sciences. 2023; 24(12):9964. https://doi.org/10.3390/ijms24129964
Chicago/Turabian StyleAresta Branco, Mafalda S. L., Alejandro Gutierrez Cruz, Lauren E. Peri, and Violeta N. Mutafova-Yambolieva. 2023. "The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria" International Journal of Molecular Sciences 24, no. 12: 9964. https://doi.org/10.3390/ijms24129964
APA StyleAresta Branco, M. S. L., Gutierrez Cruz, A., Peri, L. E., & Mutafova-Yambolieva, V. N. (2023). The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria. International Journal of Molecular Sciences, 24(12), 9964. https://doi.org/10.3390/ijms24129964






