Molecular-Biology-Driven Frontline Treatment for Chronic Lymphocytic Leukemia: A Network Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Results
2.1. Trials Characteristics
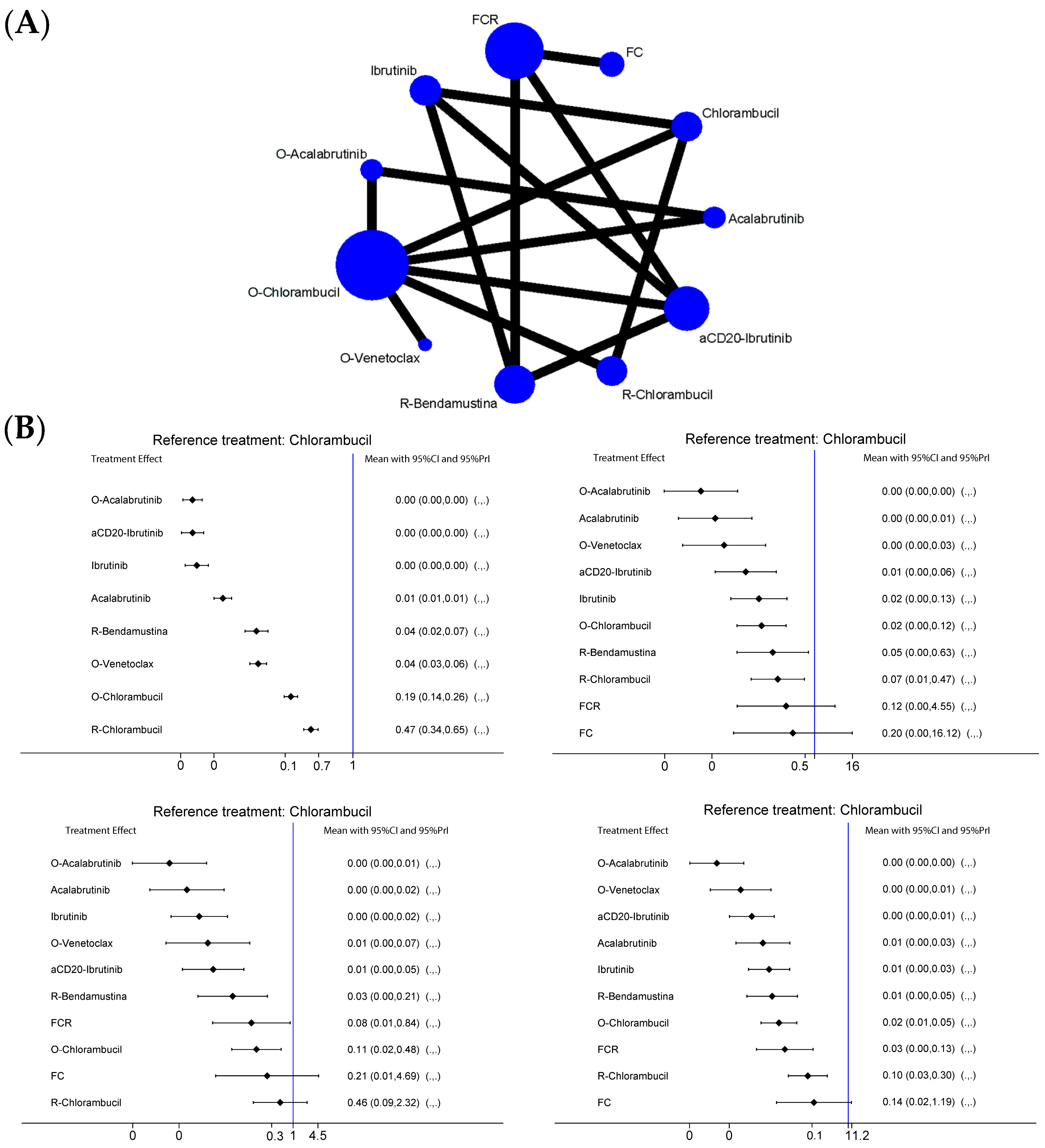
2.2. PFS Analysis
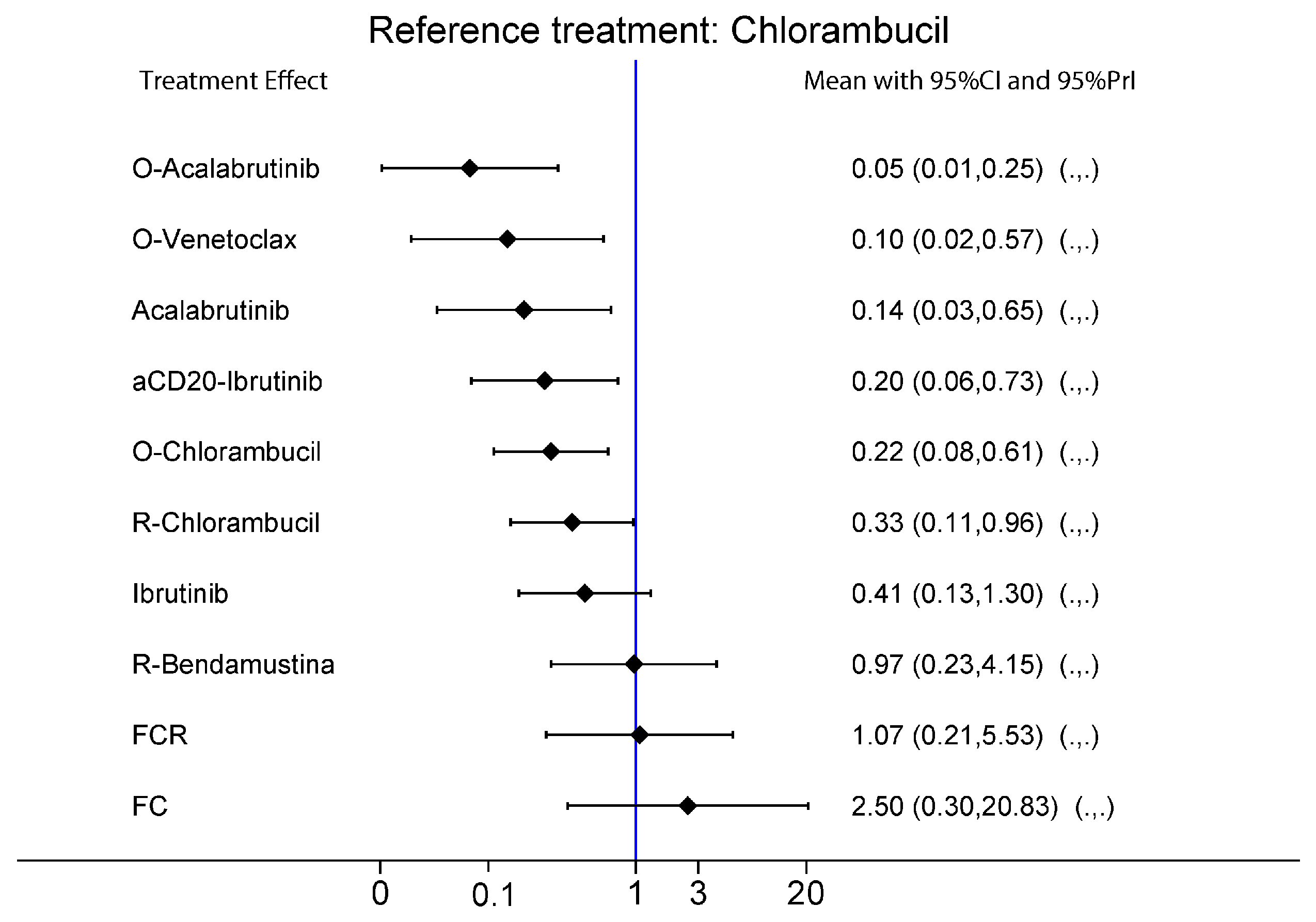
2.2.1. Del17/p53mut Population
2.2.2. No Del17/p53wt Population
2.2.3. IgHV-Hypermutated Population
2.2.4. IgHV-Germline Population
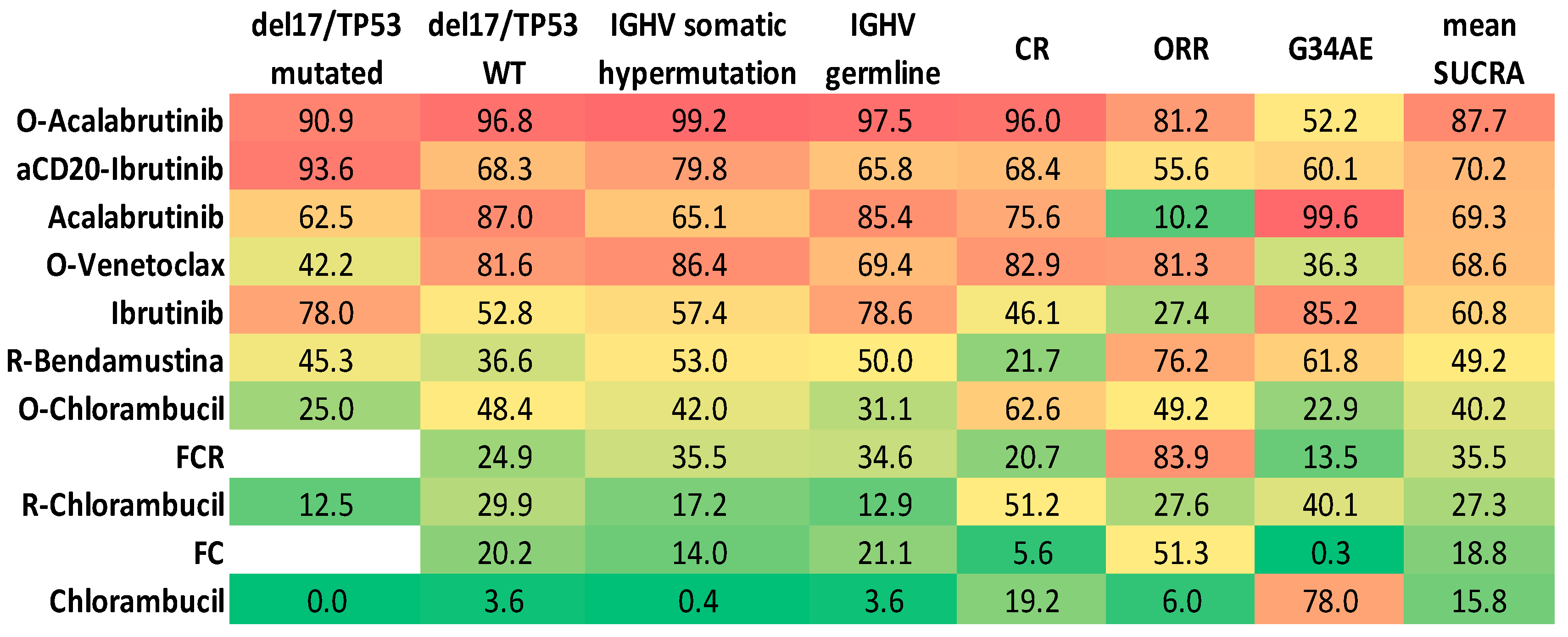
2.3. ORR and CRR
2.4. Safety
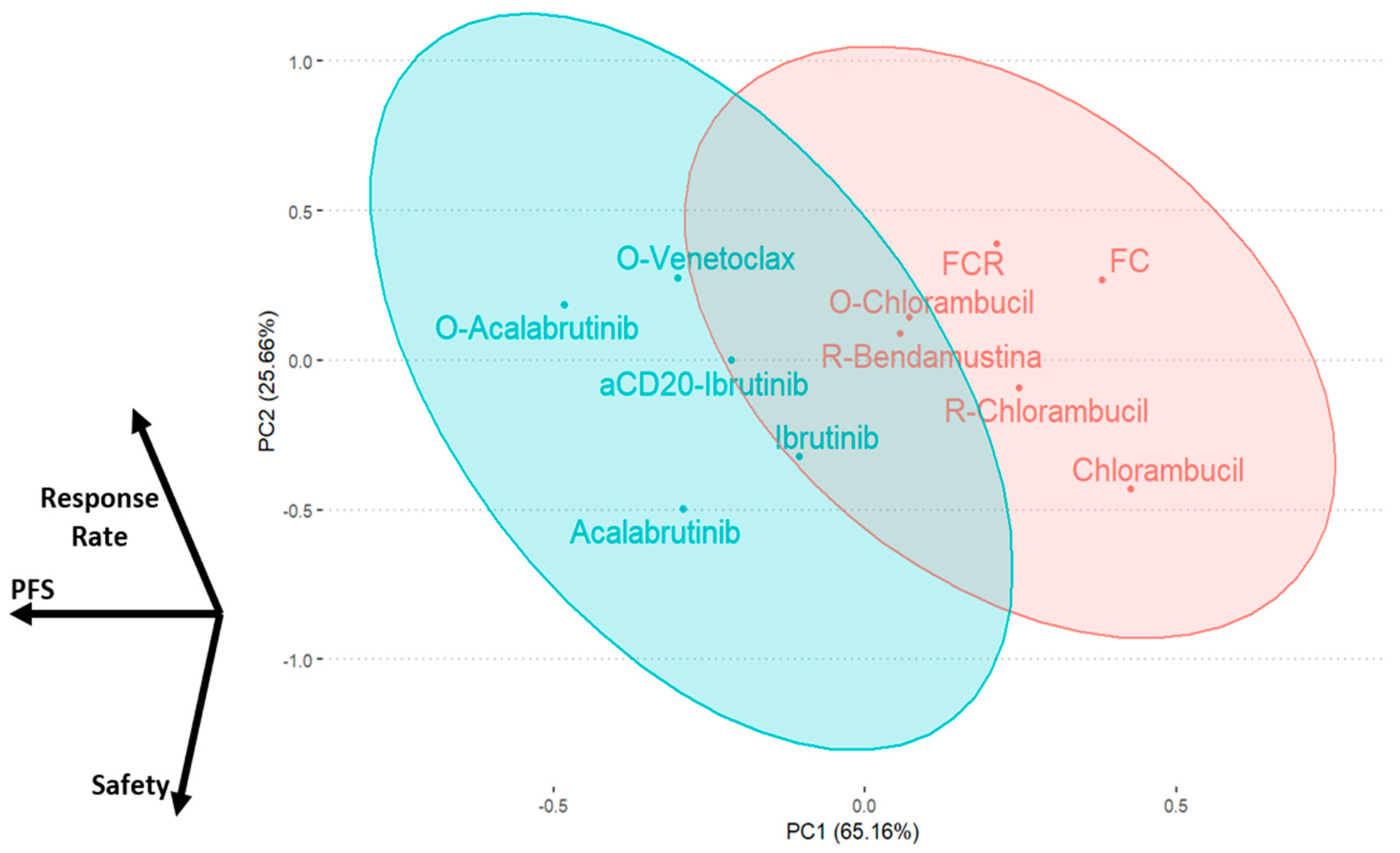
2.5. Results Integration by Principal Component Analysis
3. Discussion
4. Materials and Methods
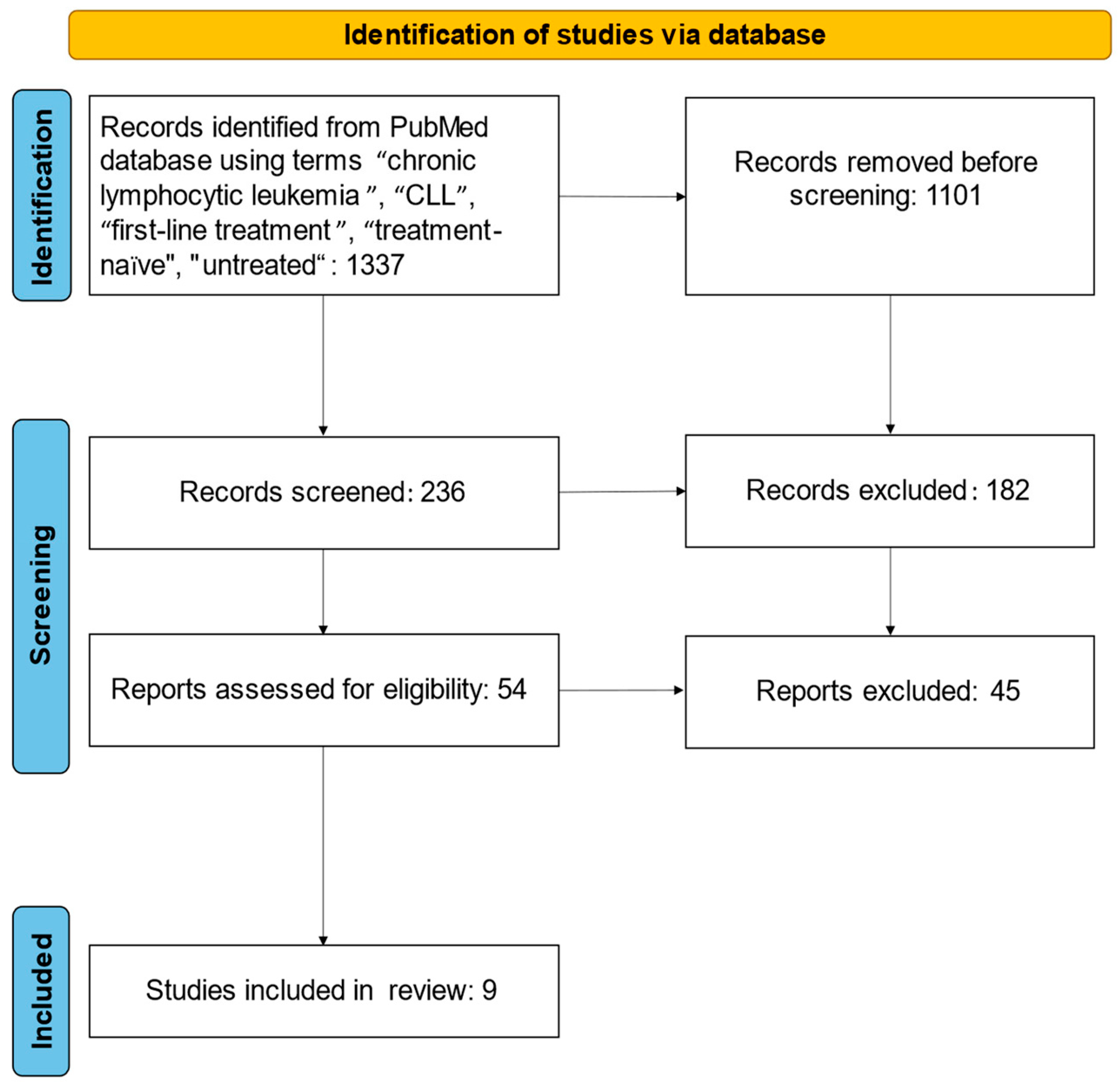
4.1. Search Strategy
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction
4.4. Network Meta-Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dighiero, G.; Binet, J.L. Chronic lymphocytic leukemia. Hematol. Cell Ther. 1996, 38 (Suppl. S2), S41–S61. [Google Scholar]
- Chronic Lymphocytic Leukemia—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/clyl.html (accessed on 7 July 2022).
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef]
- Döhner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Kröber, A.; Bullinger, L.; Döhner, K.; Bentz, M.; Lichter, P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef]
- Buccheri, V.; Barreto, W.G.; Fogliatto, L.M.; Capra, M.; Marchiani, M.; Rocha, V. Prognostic and therapeutic stratification in CLL: Focus on 17p deletion and p53 mutation. Ann. Hematol. 2018, 97, 2269–2278. [Google Scholar] [CrossRef]
- Zenz, T.; Eichhorst, B.; Busch, R.; Denzel, T.; Häbe, S.; Winkler, D.; Bühler, A.; Edelmann, J.; Bergmann, M.; Hopfinger, G.; et al. TP53 mutation and survival in chronic lymphocytic leukemia. J. Clin. Oncol. 2010, 28, 4473–4479. [Google Scholar] [CrossRef]
- Kröber, A.; Seiler, T.; Benner, A.; Bullinger, L.; Brückle, E.; Lichter, P.; Döhner, H.; Stilgenbauer, S. VH mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood 2002, 100, 1410–1416. [Google Scholar] [CrossRef]
- Parikh, S.A.; Strati, P.; Tsang, M.; West, C.P.; Shanafelt, T.D. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? A systematic review and meta-analysis. Blood 2016, 127, 1752–1760. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Mato, A.R.; Hill, B.T.; Lamanna, N.; Barr, P.M.; Ujjani, C.S.; Brander, D.M.; Howlett, C.; Skarbnik, A.P.; Cheson, B.D.; Zent, C.S.; et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: Results from a multicenter study of 683 patients. Ann. Oncol. 2017, 28, 1050–1056. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib–Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): A randomised, controlled, phase 3 trial. Lancet Lond. Engl. 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.-M.; Robrecht, S.; Samoylova, O.; Liberati, A.M.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef]
- Eichhorst, B.; Fink, A.-M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Engelke, A.; Schlag, R.; Lepretre, S.; Montero, L.F.C.; Montillo, M.; Fegan, C.; Asikanius, E.; Humphrey, K.; et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: Updated results of the CLL11 study. Leukemia 2015, 29, 1602–1604. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grünhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet Lond. Engl. 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.-S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef]
- Molica, S.; Giannarelli, D.; Montserrat, E. Comparison Between Venetoclax-based and Bruton Tyrosine Kinase Inhibitor-based Therapy as Upfront Treatment of Chronic Lymphocytic Leukemia (CLL): A Systematic Review and Network Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2021, 21, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Alrawashdh, N.; Persky, D.O.; McBride, A.; Sweasy, J.; Erstad, B.; Abraham, I. Comparative Efficacy of First-Line Treatments of Chronic Lymphocytic Leukemia: Network Meta-Analyses of Survival Curves. Clin. Lymphoma Myeloma Leuk. 2021, 21, e820–e831. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Duvvuri, S.; Ruvolo, V.; Samaniego, F.; Younes, A.; Andreeff, M. Decreased sensitivity of 17p-deleted chronic lymphocytic leukemia cells to a small molecule BCL-2 antagonist ABT-737. Cancer 2012, 118, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib–rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Botta, C.; Ciliberto, D.; Rossi, M.; Staropoli, N.; Cucè, M.; Galeano, T.; Tagliaferri, P.; Tassone, P. Network meta-analysis of randomized trials in multiple myeloma: Efficacy and safety in relapsed/refractory patients. Blood Adv. 2017, 1, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Martino, E.A.; Conticello, C.; Mendicino, F.; Vigna, E.; Romano, A.; Palumbo, G.A.; Cerchione, C.; Martinelli, G.; Morabito, F.; et al. Treatment of Lenalidomide Exposed or Refractory Multiple Myeloma: Network Meta-Analysis of Lenalidomide-Sparing Regimens. Front. Oncol. 2021, 11, 643490. [Google Scholar] [CrossRef]
- Botta, C.; Gigliotta, E.; Paiva, B.; Anselmo, R.; Santoro, M.; Otero, P.R.; Carlisi, M.; Conticello, C.; Romano, A.; Solimando, A.G.; et al. Network meta-analysis of randomized trials in multiple myeloma: Efficacy and safety in frontline therapy for patients not eligible for transplant. Hematol. Oncol. 2022, 40, 987–998. [Google Scholar] [CrossRef]
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P.T. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, F. Robust clustering using exponential power mixtures. Biometrics 2010, 66, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.4. 2022. Available online: https://CRAN.R-project.org/package=cluster (accessed on 22 February 2023).

| Trials | Authors | Treatments |
|---|---|---|
| Resonate II | Burger et al. [13] | Ibrutinib vs. Chlorambucil |
| A041202 | Woyach et al. [11] | Rituximab-Ibrutinib vs. Ibrutinib vs. Rituximab-Bendamustine |
| E1912 | Shanafelt et al. [12] | Rituximab-Ibrutinib vs. Fludarabine-Cyclophosphamide-Rituximab |
| iLLUMINATE | Moreno et al. [18] | Obinutuzumab-Ibrutinib vs. Obinutuzumab-Chlorambucil |
| ELEVATE-TN | Sharman et al. [14] | Obinutuzumab-Acalabrutinib vs. Acalabrutinib vs. Obinutuzumab-Chlorambucil |
| CLL-14 | Al-Sawaf et al. [15] | Obinutuzumab-Venetoclax vs. Obinutuzumab-Chlorambucil |
| CLL-10 | Eichorst et al. [16] | Fludarabine-Cyclophosphamide-Rituximab vs. Rituximab-Bendamustine |
| CLL-11 | Goede et al. [21] | Obinutuzumab-Chlorambucil vs. Rituximab-Chlorambucil vs. Chlorambucil |
| CLL-8 | Hallek et al. [19] | Fludarabine-Cyclophosphamide-Rituximab vs. Cyclophosphamide-Rituximab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzuto, A.; Pirrera, A.; Gigliotta, E.; Mancuso, S.; Vullo, C.; Camarda, G.M.; Rotolo, C.; Roppolo, A.; Spoto, C.; Gentile, M.; et al. Molecular-Biology-Driven Frontline Treatment for Chronic Lymphocytic Leukemia: A Network Meta-Analysis of Randomized Clinical Trials. Int. J. Mol. Sci. 2023, 24, 9930. https://doi.org/10.3390/ijms24129930
Rizzuto A, Pirrera A, Gigliotta E, Mancuso S, Vullo C, Camarda GM, Rotolo C, Roppolo A, Spoto C, Gentile M, et al. Molecular-Biology-Driven Frontline Treatment for Chronic Lymphocytic Leukemia: A Network Meta-Analysis of Randomized Clinical Trials. International Journal of Molecular Sciences. 2023; 24(12):9930. https://doi.org/10.3390/ijms24129930
Chicago/Turabian StyleRizzuto, Andrea, Angelo Pirrera, Emilia Gigliotta, Salvatrice Mancuso, Candida Vullo, Giulia Maria Camarda, Cristina Rotolo, Arianna Roppolo, Corinne Spoto, Massimo Gentile, and et al. 2023. "Molecular-Biology-Driven Frontline Treatment for Chronic Lymphocytic Leukemia: A Network Meta-Analysis of Randomized Clinical Trials" International Journal of Molecular Sciences 24, no. 12: 9930. https://doi.org/10.3390/ijms24129930
APA StyleRizzuto, A., Pirrera, A., Gigliotta, E., Mancuso, S., Vullo, C., Camarda, G. M., Rotolo, C., Roppolo, A., Spoto, C., Gentile, M., Botta, C., & Siragusa, S. (2023). Molecular-Biology-Driven Frontline Treatment for Chronic Lymphocytic Leukemia: A Network Meta-Analysis of Randomized Clinical Trials. International Journal of Molecular Sciences, 24(12), 9930. https://doi.org/10.3390/ijms24129930






