β-Lapachone Exerts Anticancer Effects by Downregulating p53, Lys-Acetylated Proteins, TrkA, p38 MAPK, SOD1, Caspase-2, CD44 and NPM in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
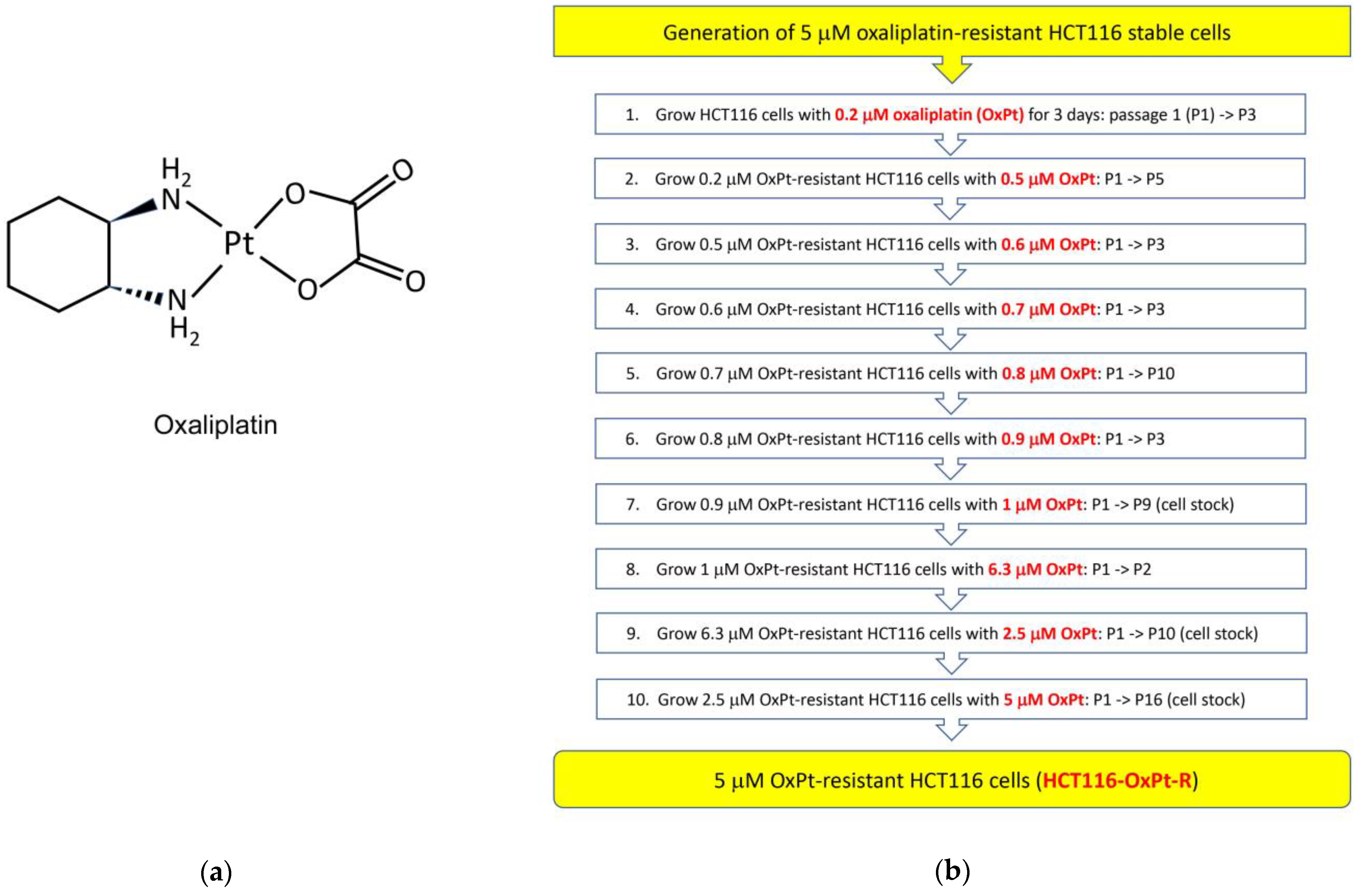
2.1. Generation of 5 μM OxPt-Resistant HCT116 Cells
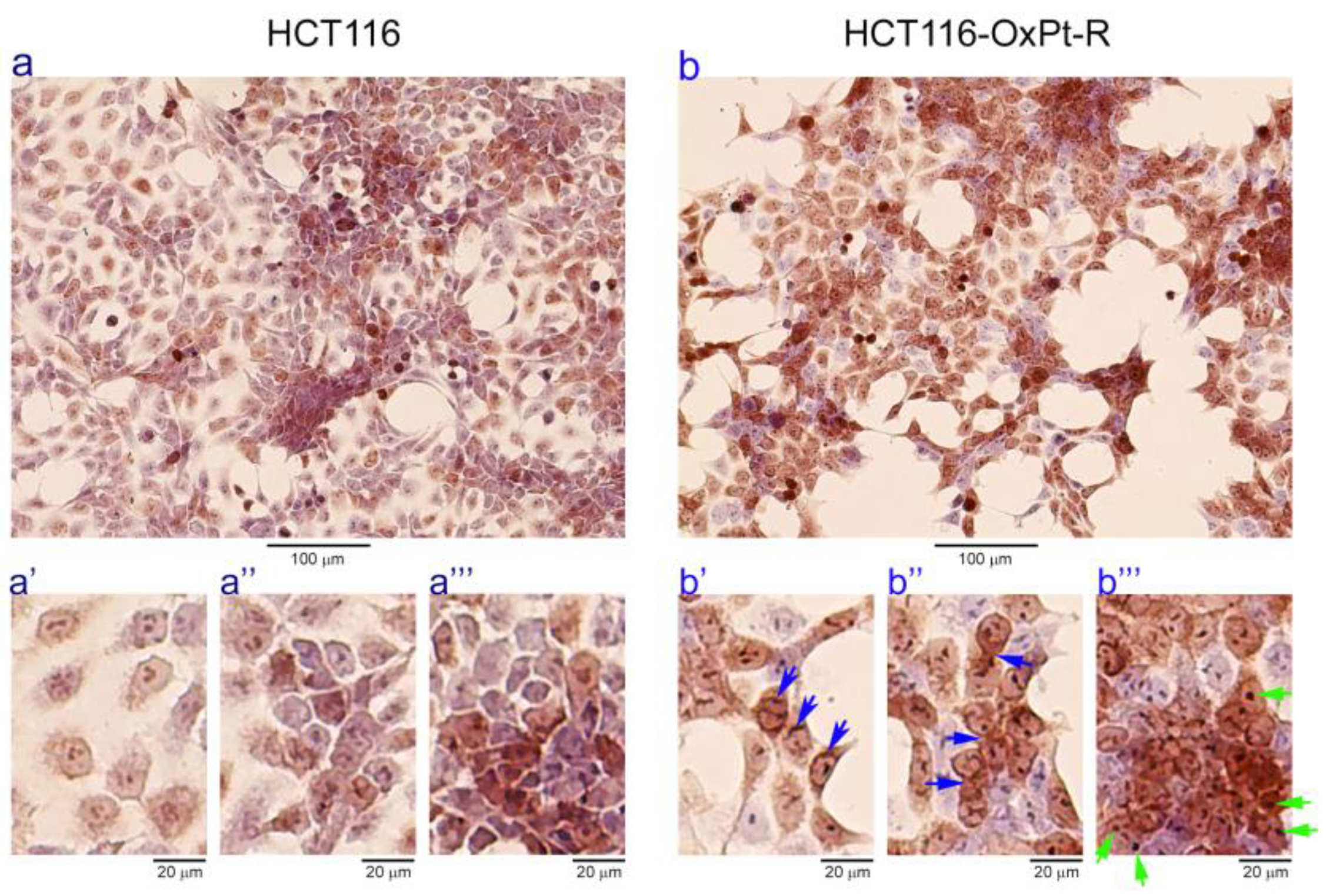
2.2. Characterization of HCT116-OxPt-R Cells
2.2.1. Morphological Phenotype
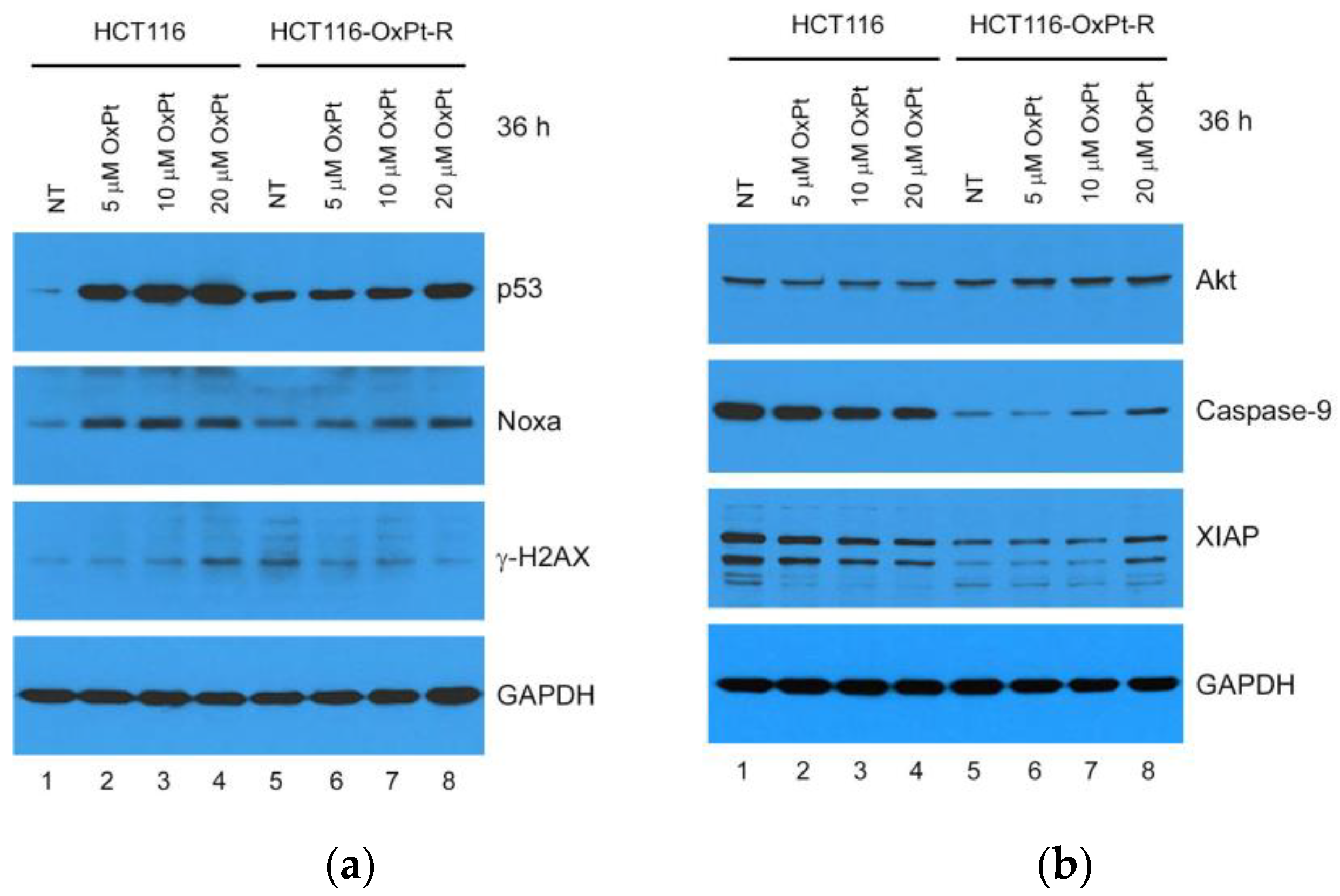
2.2.2. OxPt-Specific Resistance
2.2.3. Molecular Mechanisms
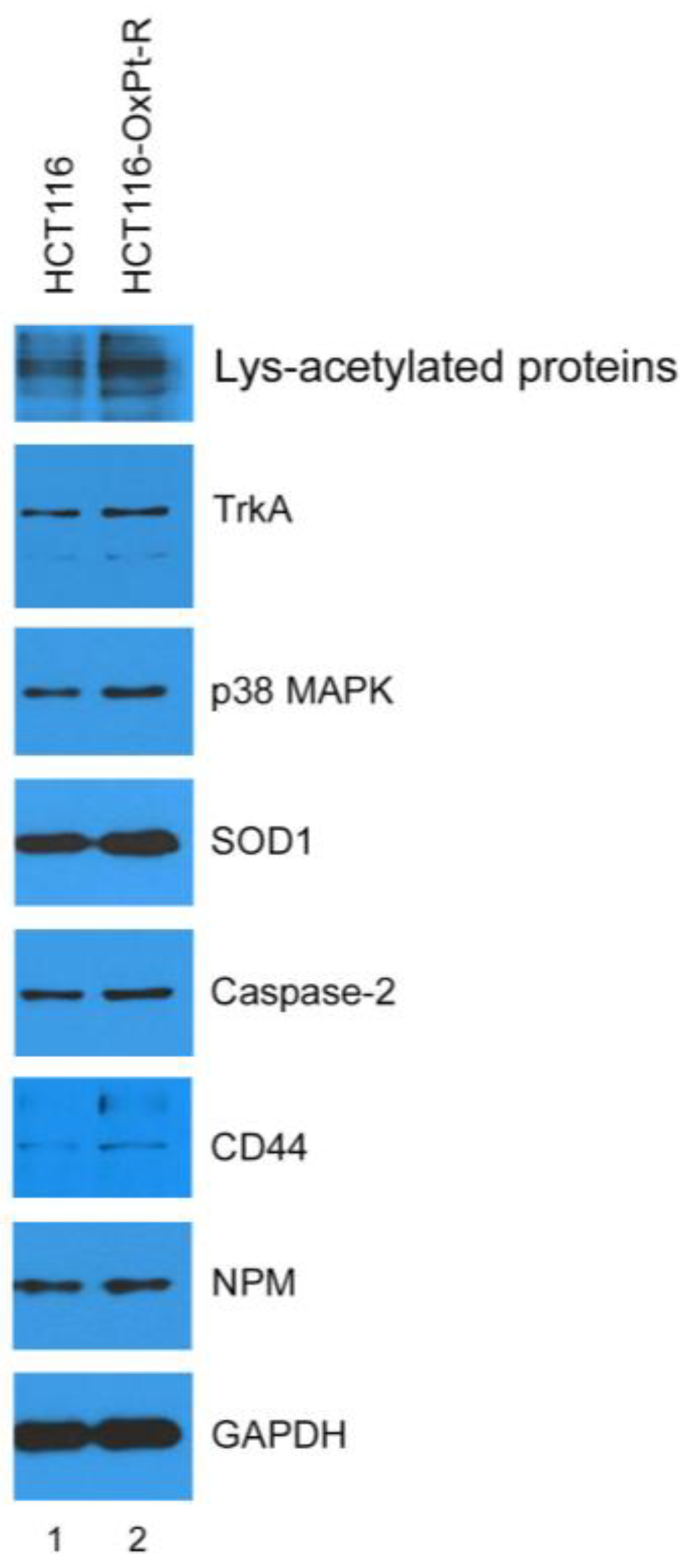
2.3. Identification of New OxPt-R-Related Proteins in HCT116-OxPt-R Cells
2.4. Verification of OxPt-R-Related Proteins Identified via Antibody Array
2.5. Bioinformatics Analysis for OxPt-R-Related Proteins
2.5.1. Gene Ontology Analysis
2.5.2. Protein–Protein Interaction Network Analysis
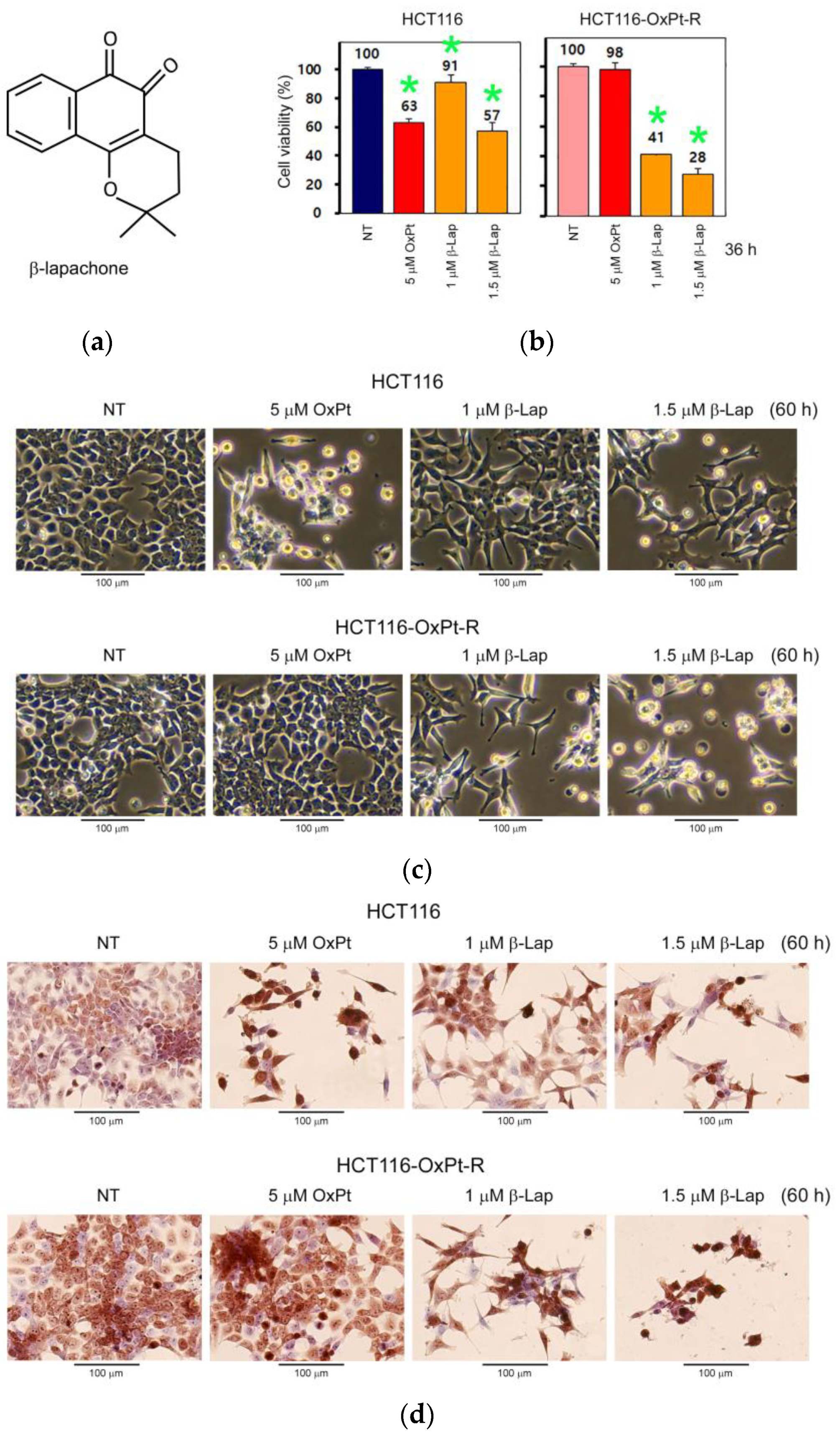
2.6. Anticancer Effect by β-Lap in HCT116-OxPt-R Cells
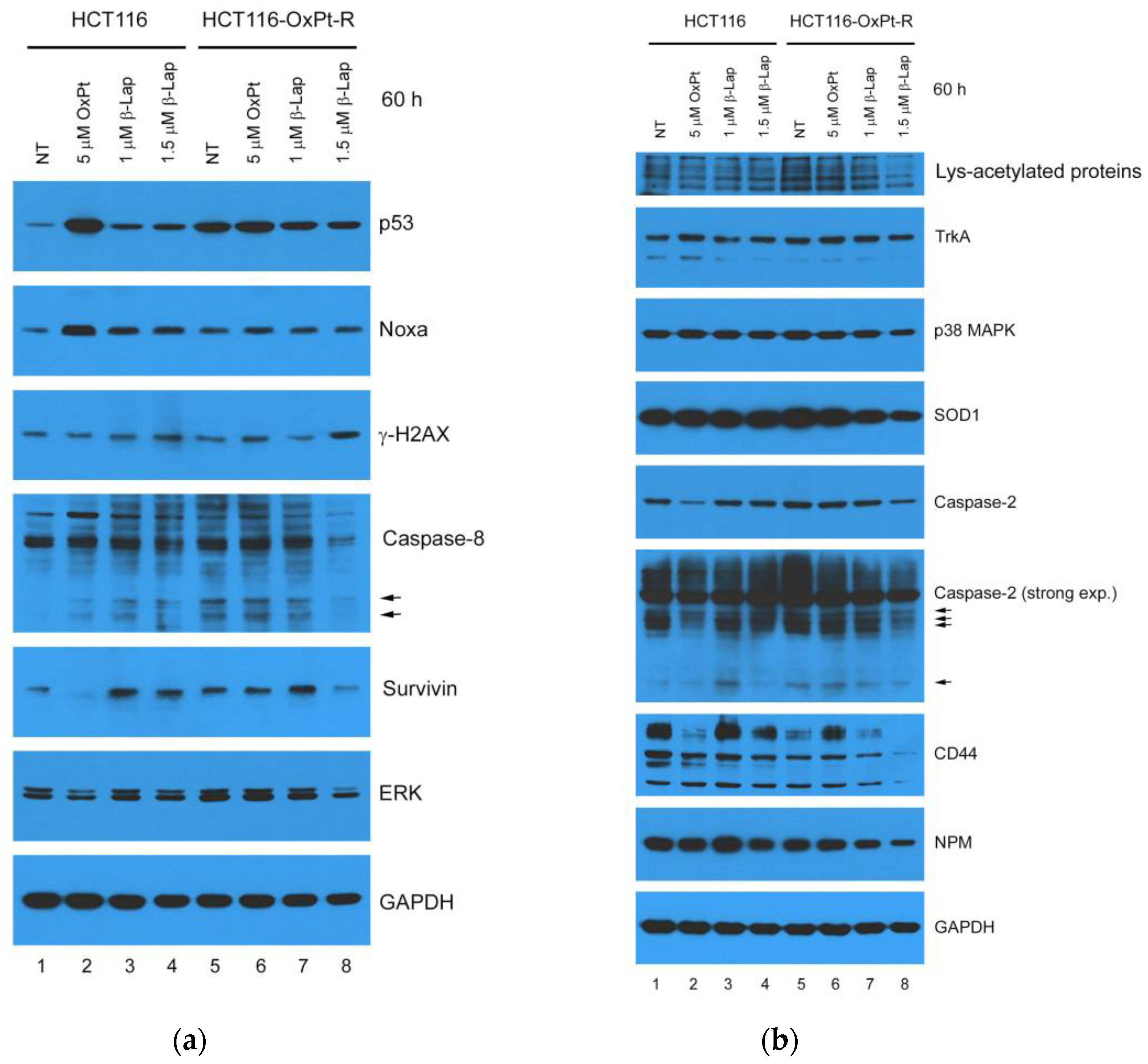
2.7. Anticancer Mechanisms of β-Lap in HCT116-OxPt-R Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Generation of 5 μM OxPt-Resistant HCT116 Cells
4.3. Phase-Contrast Microscopy
4.4. Phase-Contrast Microscopy of Hematoxylin-Stained Cells
4.5. Cell Viability Analysis
4.6. Western Blot Analysis
4.7. Sample Preparation for Antibody Array
4.8. Signaling Explorer Antibody Array Analysis
4.9. Bioinformatics Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, C.L.; de Albuquerque Wanderley Sales, V.; Gomes de Melo, C.; Ferreira da Silva, R.M.; Vicente Nishimura, R.H.; Rolim, L.A.; Rolim Neto, P.J. Beta-lapachone: Natural occurrence, physicochemical properties, biological activities, toxicity and synthesis. Phytochemistry 2021, 186, 112713. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Hu, J.; Wang, P.; Li, X.; Zhang, X. A comprehensive review on beta-lapachone: Mechanisms, structural modifications, and therapeutic potentials. Eur. J. Med. Chem. 2021, 210, 112962. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, D.W.; Jo, E.J.; Kim, Y.K.; Jo, Y.S.; Park, J.H.; Yoo, S.K.; Park, M.K.; Kwak, T.H.; Kho, Y.L.; et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes 2009, 58, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pardee, A.B. beta-lapachone induces cell cycle arrest and apoptosis in human colon cancer cells. Mol. Med. 1999, 5, 711–720. [Google Scholar] [CrossRef]
- Planchon, S.M.; Wuerzberger, S.; Frydman, B.; Witiak, D.T.; Hutson, P.; Church, D.R.; Wilding, G.; Boothman, D.A. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: A p53-independent response. Cancer Res. 1995, 55, 3706–3711. [Google Scholar]
- Choi, B.T.; Cheong, J.; Choi, Y.H. beta-Lapachone-induced apoptosis is associated with activation of caspase-3 and inactivation of NF-kappaB in human colon cancer HCT-116 cells. Anticancer Drugs 2003, 14, 845–850. [Google Scholar] [CrossRef]
- D'Anneo, A.; Augello, G.; Santulli, A.; Giuliano, M.; di Fiore, R.; Messina, C.; Tesoriere, G.; Vento, R. Paclitaxel and beta-lapachone synergistically induce apoptosis in human retinoblastoma Y79 cells by downregulating the levels of phospho-Akt. J. Cell Physiol. 2010, 222, 433–443. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Yang, H.Z.; Ma, Y.; Zhou, Y.; Xu, L.M.; Chen, X.J.; Ding, W.B.; Zou, H.B. Autophagy contributes to the enrichment and survival of colorectal cancer stem cells under oxaliplatin treatment. Cancer Lett. 2015, 361, 128–136. [Google Scholar] [CrossRef]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L. Colon cancer: Cancer stem cells markers, drug resistance and treatment. Biomed. Pharmacother. 2014, 68, 911–916. [Google Scholar] [CrossRef]
- Huang, R.; Wang, G.; Song, Y.; Tang, Q.; You, Q.; Liu, Z.; Chen, Y.; Zhang, Q.; Li, J.; Muhammand, S.; et al. Colorectal cancer stem cell and chemoresistant colorectal cancer cell phenotypes and increased sensitivity to Notch pathway inhibitor. Mol. Med. Rep. 2015, 12, 2417–2424. [Google Scholar] [CrossRef]
- Faivre, S.; Chan, D.; Salinas, R.; Woynarowska, B.; Woynarowski, J.M. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem. Pharmacol. 2003, 66, 225–237. [Google Scholar] [CrossRef]
- Ahmad, S. Platinum-DNA interactions and subsequent cellular processes controlling sensitivity to anticancer platinum complexes. Chem. Biodivers. 2010, 7, 543–566. [Google Scholar] [CrossRef]
- Chiu, S.J.; Chao, J.I.; Lee, Y.J.; Hsu, T.S. Regulation of gamma-H2AX and securin contribute to apoptosis by oxaliplatin via a p38 mitogen-activated protein kinase-dependent pathway in human colorectal cancer cells. Toxicol. Lett. 2008, 179, 63–70. [Google Scholar] [CrossRef]
- Chiu, S.J.; Lee, Y.J.; Hsu, T.S.; Chen, W.S. Oxaliplatin-induced gamma-H2AX activation via both p53-dependent and -independent pathways but is not associated with cell cycle arrest in human colorectal cancer cells. Chem. Biol. Interact. 2009, 182, 173–182. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Hsu, S.H.; Lin, C.Y.; Liaw, H.J.; Li, T.W.; Jiang, K.Y.; Chiang, N.J.; Chen, S.H.; Lin, B.W.; Chen, P.C.; et al. CHK2 activation contributes to the development of oxaliplatin resistance in colorectal cancer. Br. J. Cancer 2022, 127, 1615–1628. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chen, M.C.; Baskaran, R.; Lin, Y.M.; Day, C.H.; Lin, Y.J.; Tu, C.C.; Vijaya Padma, V.; Kuo, W.W.; Huang, C.Y. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell Physiol. 2018, 233, 5458–5467. [Google Scholar] [CrossRef]
- Liao, X.; Bu, Y.; Chang, F.; Jia, F.; Song, G.; Xiao, X.; Zhang, M.; Ning, P.; Jia, Q. Remodeling of hepatic stellate cells orchestrated the stroma-derived oxaliplatin-resistance through CCN3 paracrine in hepatocellular carcinoma. BMC Cancer 2019, 19, 1192. [Google Scholar] [CrossRef]
- Lin, L.; Li, X.; Pan, C.; Lin, W.; Shao, R.; Liu, Y.; Zhang, J.; Luo, Y.; Qian, K.; Shi, M.; et al. ATXN2L upregulated by epidermal growth factor promotes gastric cancer cell invasiveness and oxaliplatin resistance. Cell Death Dis. 2019, 10, 173. [Google Scholar] [CrossRef]
- Tang, X.; Liang, Y.; Sun, G.; He, Q.; Hou, Z.; Jiang, X.; Gao, P.; Qu, H. Upregulation of CRABP2 by TET1-mediated DNA hydroxymethylation attenuates mitochondrial apoptosis and promotes oxaliplatin resistance in gastric cancer. Cell Death Dis. 2022, 13, 848. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Wang, Y.; Shen, H.; Wang, X.; Wu, L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-beta/Smad2/3 signaling pathway. OncoTargets Ther. 2019, 12, 3893–3903. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Balibrea, E.; Martinez-Cardus, A.; Gines, A.; Ruiz de Porras, V.; Moutinho, C.; Layos, L.; Manzano, J.L.; Buges, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Podar, K.; Tai, Y.T.; Lin, B.; Hideshima, T.; Akiyama, M.; LeBlanc, R.; Catley, L.; Mitsiades, N.; Mitsiades, C.; et al. beta-lapachone, a novel plant product, overcomes drug resistance in human multiple myeloma cells. Exp. Hematol. 2002, 30, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, X.; Niu, H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018, 13, 4107–4119. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Koh, A.E.; Kothandan, S.; Alsrhani, A.; Mok, P.L.; Subbiah, S.K. Treatment of HT29 Human Colorectal Cancer Cell Line with Nanocarrier-Encapsulated Camptothecin Reveals Histone Modifier Genes in the Wnt Signaling Pathway as Important Molecular Cues for Colon Cancer Targeting. Int. J. Mol. Sci. 2021, 22, 2286. [Google Scholar] [CrossRef]
- Hevener, K.; Verstak, T.A.; Lutat, K.E.; Riggsbee, D.L.; Mooney, J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B 2018, 8, 844–861. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Martins-Dinis, M.; Ferretti, G.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, beta-Lapachone and Their Analogues. Molecules 2020, 25, 893. [Google Scholar] [CrossRef]
- Ye, M.; Han, Y.; Tang, J.; Piao, Y.; Liu, X.; Zhou, Z.; Gao, J.; Rao, J.; Shen, Y. A Tumor-Specific Cascade Amplification Drug Release Nanoparticle for Overcoming Multidrug Resistance in Cancers. Adv. Mater. 2017, 29, 1702342. [Google Scholar] [CrossRef]
- Ghanbarian, M.; Afgar, A.; Yadegarazari, R.; Najafi, R.; Teimoori-Toolabi, L. Through oxaliplatin resistance induction in colorectal cancer cells, increasing ABCB1 level accompanies decreasing level of miR-302c-5p, miR-3664-5p and miR-129-5p. Biomed. Pharmacother. 2018, 108, 1070–1080. [Google Scholar] [CrossRef]
- Yang, A.D.; Fan, F.; Camp, E.R.; van Buren, G.; Liu, W.; Somcio, R.; Gray, M.J.; Cheng, H.; Hoff, P.M.; Ellis, L.M. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin. Cancer Res. 2006, 12, 4147–4153. [Google Scholar] [CrossRef]
- Liao, X.; Song, G.; Xu, Z.; Bu, Y.; Chang, F.; Jia, F.; Xiao, X.; Ren, X.; Zhang, M.; Jia, Q. Oxaliplatin resistance is enhanced by saracatinib via upregulation Wnt-ABCG1 signaling in hepatocellular carcinoma. BMC Cancer 2020, 20, 31. [Google Scholar] [CrossRef]
- Noordhuis, P.; Laan, A.C.; van de Born, K.; Honeywell, R.J.; Peters, G.J. Coexisting Molecular Determinants of Acquired Oxaliplatin Resistance in Human Colorectal and Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2019, 20, 3619. [Google Scholar] [CrossRef]
- Sun, W.; Ge, Y.; Cui, J.; Yu, Y.; Liu, B. Scutellarin resensitizes oxaliplatin-resistant colorectal cancer cells to oxaliplatin treatment through inhibition of PKM2. Mol. Ther. Oncolytics 2021, 21, 87–97. [Google Scholar] [CrossRef]
- Chian, S.; Li, Y.Y.; Wang, X.J.; Tang, X.W. Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac. J. Cancer Prev. 2014, 15, 2911–2916. [Google Scholar] [CrossRef]
- Ho, C.J.; Lin, R.W.; Zhu, W.H.; Wen, T.K.; Hu, C.J.; Lee, Y.L.; Hung, T.I.; Wang, C. Transcription-independent and -dependent p53-mediated apoptosis in response to genotoxic and non-genotoxic stress. Cell Death Discov. 2019, 5, 131. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, J.; Chen, Z.R.; Chng, W.J. P53 mutations in colorectal cancer—Molecular pathogenesis and pharmacological reactivation. World J. Gastroenterol. 2015, 21, 84–93. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. The Role of p53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, Q.; Li, M.; Tong, X.; Sun, J.; Qing, Y.; Sun, L.; Yang, X.; Hu, X.; Jiang, J.; et al. Upregulation of CYP2S1 by oxaliplatin is associated with p53 status in colorectal cancer cell lines. Sci. Rep. 2016, 6, 33078. [Google Scholar] [CrossRef]
- Boyer, J.; McLean, E.G.; Aroori, S.; Wilson, P.; McCulla, A.; Carey, P.D.; Longley, D.B.; Johnston, P.G. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin. Cancer Res. 2004, 10, 2158–2167. [Google Scholar] [CrossRef]
- Komlodi-Pasztor, E.; Trostel, S.; Sackett, D.; Poruchynsky, M.; Fojo, T. Impaired p53 binding to importin: A novel mechanism of cytoplasmic sequestration identified in oxaliplatin-resistant cells. Oncogene 2009, 28, 3111–3120. [Google Scholar] [CrossRef]
- Li, C.J.; Averboukh, L.; Pardee, A.B. beta-Lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J. Biol. Chem. 1993, 268, 22463–22468. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wu, X.W.; Agama, K.; Pommier, Y.; Du, J.; Li, D.; Gu, L.Q.; Huang, Z.S.; An, L.K. A novel DNA topoisomerase I inhibitor with different mechanism from camptothecin induces G2/M phase cell cycle arrest to K562 cells. Biochemistry 2010, 49, 10131–10136. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, M.J.; Lee, S.Y.; Lee, Y.N.; Chi, G.Y.; Eom, H.S.; Kim, N.D.; Choi, B.T. Phosphorylation of p53, induction of Bax and activation of caspases during beta-lapachone-mediated apoptosis in human prostate epithelial cells. Int. J. Oncol. 2002, 21, 1293–1299. [Google Scholar] [PubMed]
- Toth, M.; Boros, I.M.; Balint, E. Elevated level of lysine 9-acetylated histone H3 at the MDR1 promoter in multidrug-resistant cells. Cancer Sci. 2012, 103, 659–669. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Tran, E.L.; Tokars, V.; Dean, A.E.; Quan, S.; Gius, D. MnSOD Lysine 68 acetylation leads to cisplatin and doxorubicin resistance due to aberrant mitochondrial metabolism. Int. J. Biol. Sci. 2021, 17, 1203–1216. [Google Scholar] [CrossRef]
- Aubert, L.; Guilbert, M.; Corbet, C.; Genot, E.; Adriaenssens, E.; Chassat, T.; Bertucci, F.; Daubon, T.; Magne, N.; Le Bourhis, X.; et al. NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib. Oncotarget 2015, 6, 9807–9819. [Google Scholar] [CrossRef]
- Chocry, M.; Leloup, L.; Kovacic, H. Reversion of resistance to oxaliplatin by inhibition of p38 MAPK in colorectal cancer cell lines: Involvement of the calpain / Nox1 pathway. Oncotarget 2017, 8, 103710–103730. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Lakshman, M.; Subramaniam, V.; Rubenthiran, U.; Jothy, S. CD44 promotes resistance to apoptosis in human colon cancer cells. Exp. Mol. Pathol. 2004, 77, 18–25. [Google Scholar] [CrossRef]
- Sun, Z.; Yue, L.; Shen, Z.; Li, Y.; Sui, A.; Li, T.; Tang, Q.; Yao, R.; Sun, Y. Downregulation of NPM expression by Her-2 reduces resistance of gastric cancer to oxaliplatin. Oncol. Lett. 2017, 13, 2377–2384. [Google Scholar] [CrossRef]
- Atale, N.; Gupta, S.; Yadav, U.C.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef]



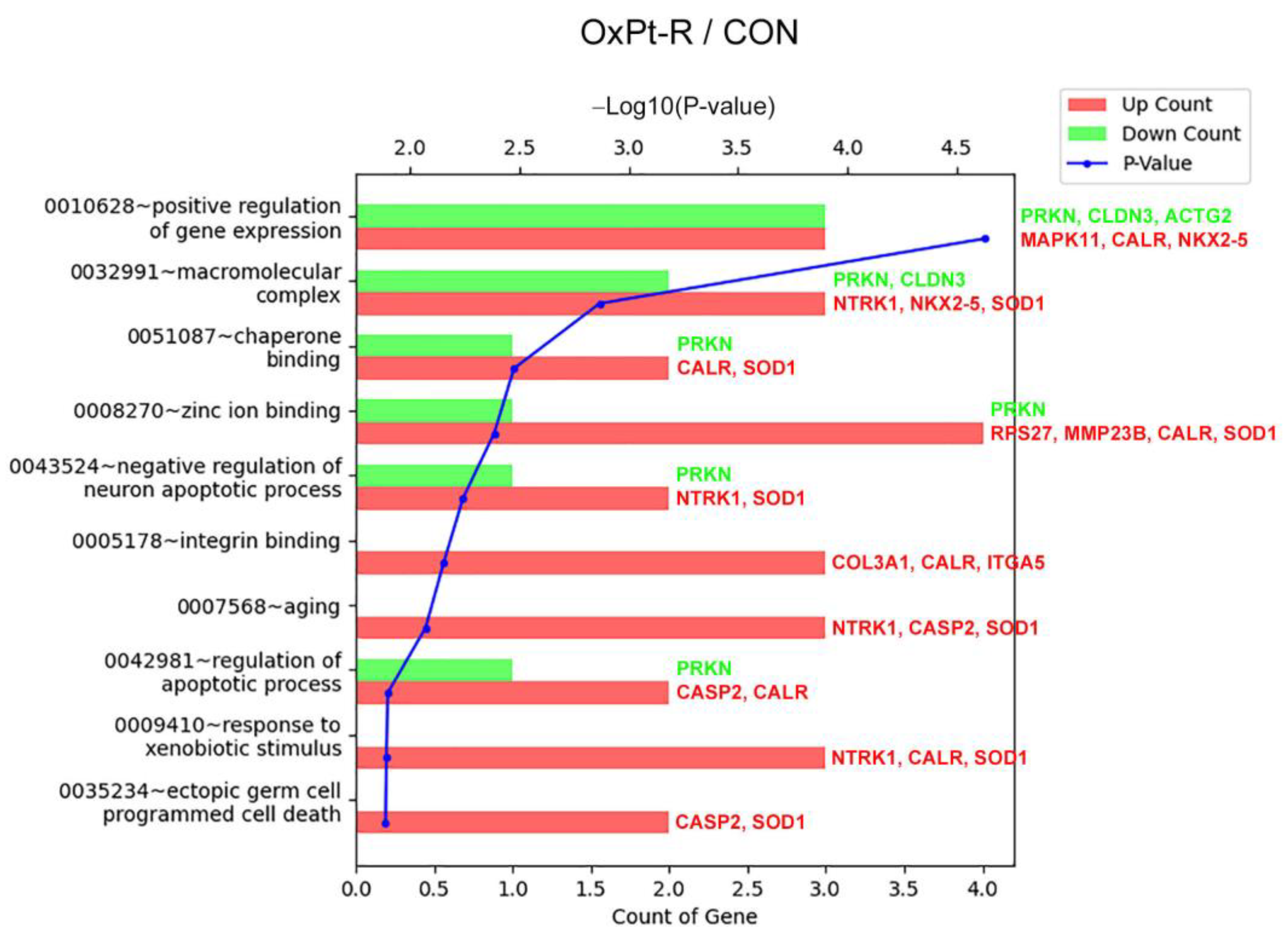
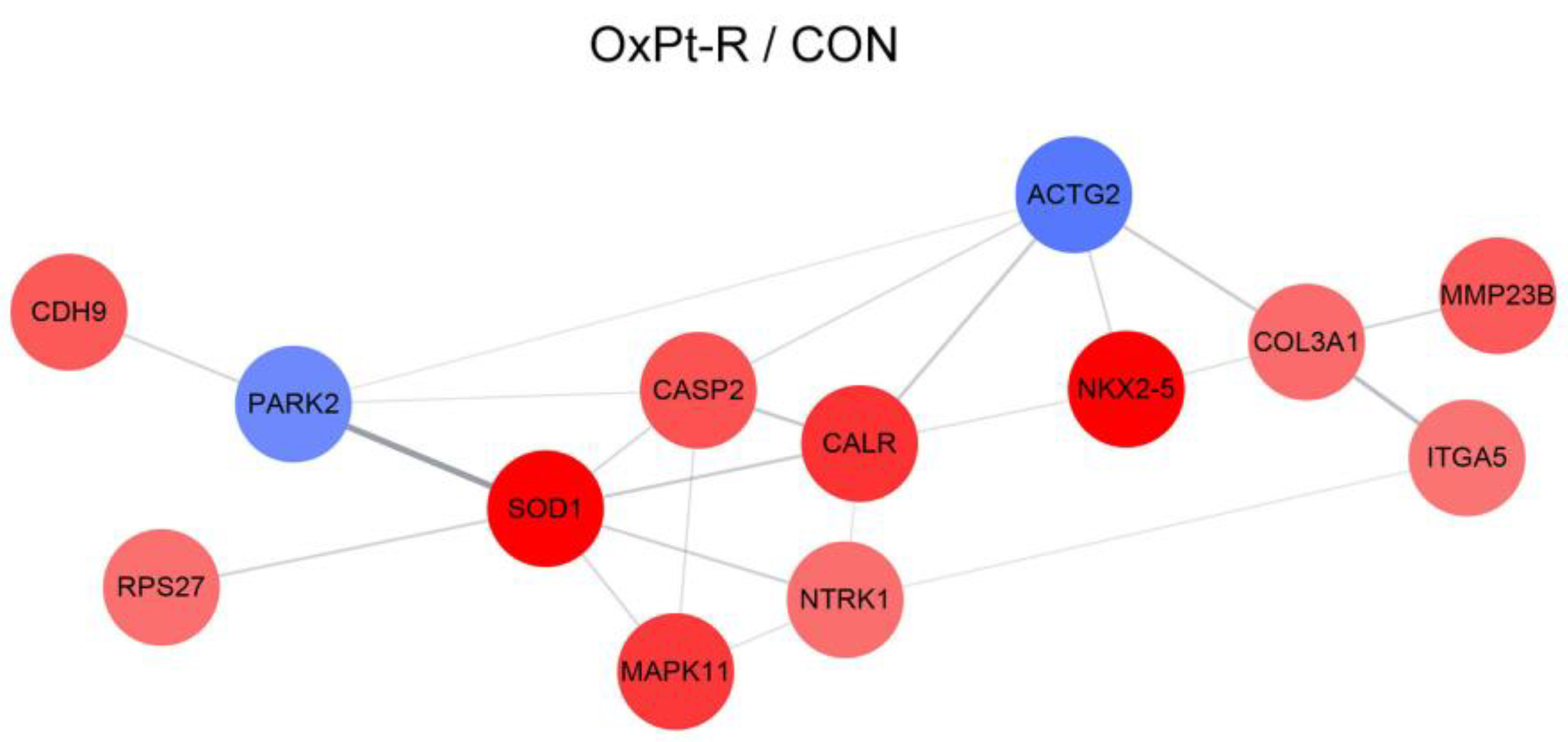
| Gene Symbol | Antibody Name | Protein Name | Fold Change OxPt-R/CON | UniProt ID |
|---|---|---|---|---|
| NPM1 | Nucleophosmin (NPM) | Nucleophosmin | 6.433 | P06748 |
| CD37 | CD37 | Leukocyte antigen CD37 | 5.477 | P11049 |
| NKX2-5 | NKX2.5 | Homeobox protein Nkx-2.5 | 4.541 | P52952 |
| SOD1 | SOD1 | Superoxide dismutase [Cu-Zn] | 4.460 | P00441 |
| H2BS1 | Histone H2B (Acetyl-Lys15) | Histone H2B type F-S | 3.147 | P57053 |
| CALR | Calreticulin | Calreticulin | 3.017 | P27797 |
| MAPK11 | MAPK 11 | Mitogen-activated protein kinase 11 | 2.925 | Q15759 |
| CASP2 | CASP2 (p18, Cleaved-Thr325) | Caspase-2 | 2.518 | P42575 |
| CDH9 | CDH9 | Cadherin-9 | 2.434 | Q9ULB4 |
| MMP23B | MMP23 (Cleaved-Tyr79) | Matrix metalloproteinase-23 | 2.413 | O75900 |
| ACOT2 | ACOT2 | Acyl-coenzyme A thioesterase 2, mitochondrial | 2.256 | P49753 |
| N/A | Lys-acetylated proteins | Lys-acetylated proteins | 2.241 | N/A |
| COL3A1 | Collagen III alpha1 (Cleaved-Gly1221) | Collagen alpha-1(III) chain | 2.188 | P02461 |
| NTRK1 | TrkA | High-affinity nerve growth factor receptor | 2.162 | P04629 |
| RPS27 | MPS-1 | 40S ribosomal protein S27 | 2.144 | P42677 |
| CD44 | CD44 | CD44 antigen | 2.090 | P16070 |
| ITGA5 | Integrin alpha-5 (ITGA5) | Integrin alpha-5 | 2.084 | P08648 |
| CLDN3 | Claudin 3 | Claudin-3 | 0.480 | O15551 |
| PRKN | Parkin | E3 ubiquitin-protein ligase parkin | 0.465 | O60260 |
| ACTG2 | Actin-gamma2 | Actin, gamma-enteric smooth muscle | 0.411 | P63267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, E.J.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.-M.; Hong, S.C.; Kim, C.W.; Lee, W.S. β-Lapachone Exerts Anticancer Effects by Downregulating p53, Lys-Acetylated Proteins, TrkA, p38 MAPK, SOD1, Caspase-2, CD44 and NPM in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 9867. https://doi.org/10.3390/ijms24129867
Jung EJ, Kim HJ, Shin SC, Kim GS, Jung J-M, Hong SC, Kim CW, Lee WS. β-Lapachone Exerts Anticancer Effects by Downregulating p53, Lys-Acetylated Proteins, TrkA, p38 MAPK, SOD1, Caspase-2, CD44 and NPM in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells. International Journal of Molecular Sciences. 2023; 24(12):9867. https://doi.org/10.3390/ijms24129867
Chicago/Turabian StyleJung, Eun Joo, Hye Jung Kim, Sung Chul Shin, Gon Sup Kim, Jin-Myung Jung, Soon Chan Hong, Choong Won Kim, and Won Sup Lee. 2023. "β-Lapachone Exerts Anticancer Effects by Downregulating p53, Lys-Acetylated Proteins, TrkA, p38 MAPK, SOD1, Caspase-2, CD44 and NPM in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells" International Journal of Molecular Sciences 24, no. 12: 9867. https://doi.org/10.3390/ijms24129867
APA StyleJung, E. J., Kim, H. J., Shin, S. C., Kim, G. S., Jung, J.-M., Hong, S. C., Kim, C. W., & Lee, W. S. (2023). β-Lapachone Exerts Anticancer Effects by Downregulating p53, Lys-Acetylated Proteins, TrkA, p38 MAPK, SOD1, Caspase-2, CD44 and NPM in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells. International Journal of Molecular Sciences, 24(12), 9867. https://doi.org/10.3390/ijms24129867








