The Emerging Roles of the Cephalic Neural Crest in Brain Development and Developmental Encephalopathies
Abstract
1. Introduction
2. Vertebrate Neurulation: Ontogenesis of the Central Nervous System and the Neural Crest
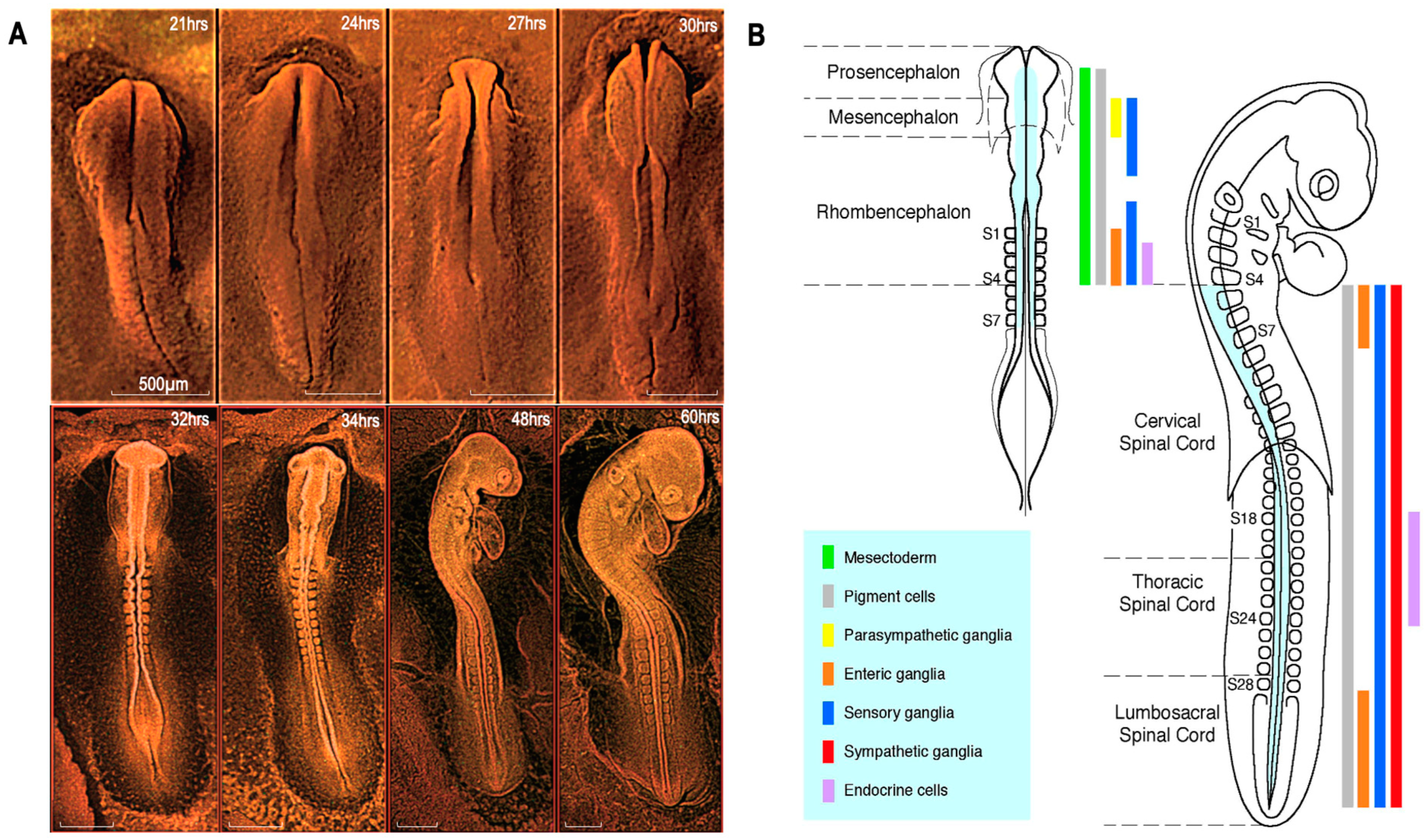
3. Neural Crest Discovery and Ontogenic Contribution
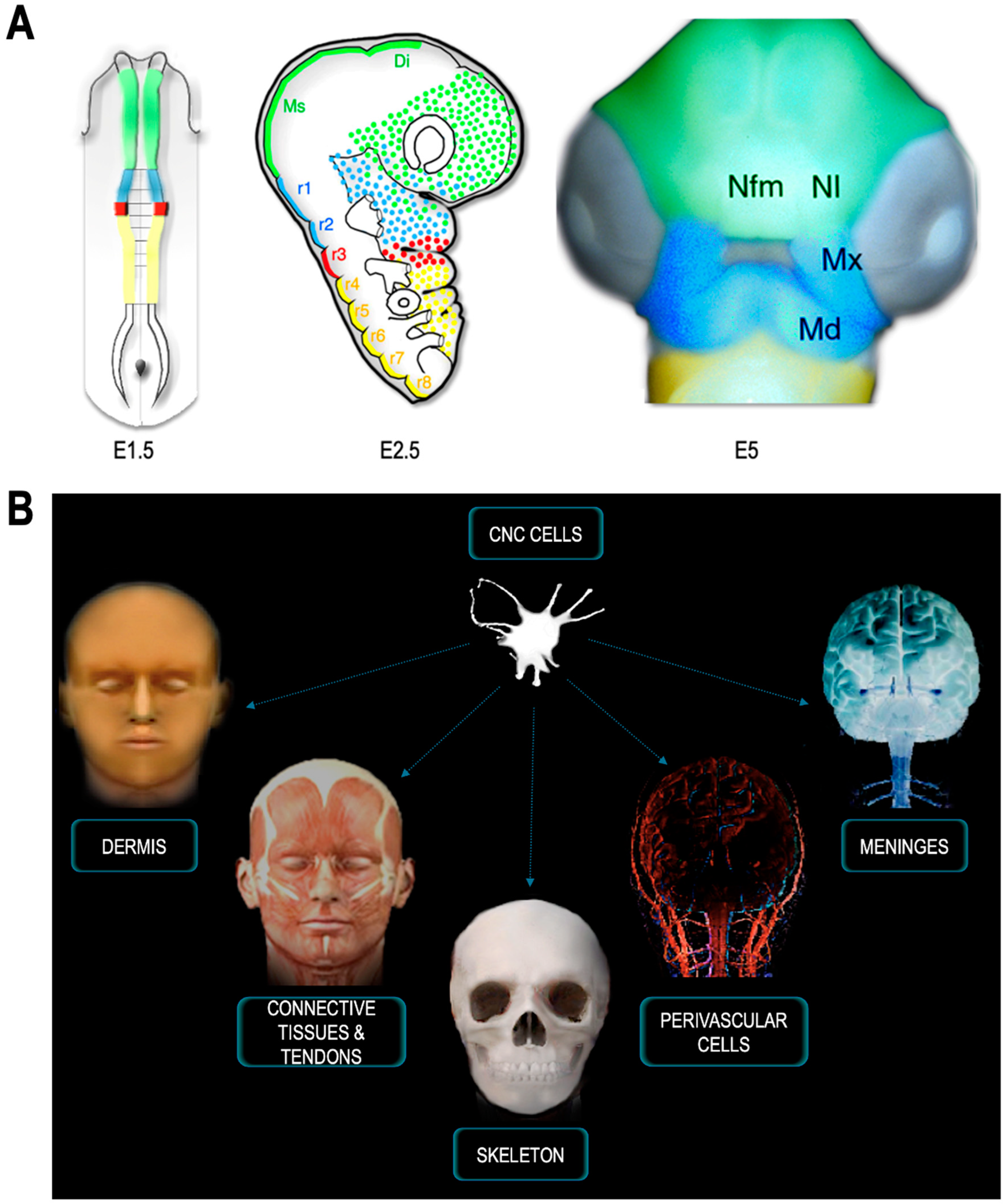
4. CNC: A Source of Mesenchyme for the Face and Pharynx
5. The CNC and Craniofacial Skeletogenesis: A Vertebrate Synapomorphy
6. Functional Partition in CNC Skeleton and Molecular Identities
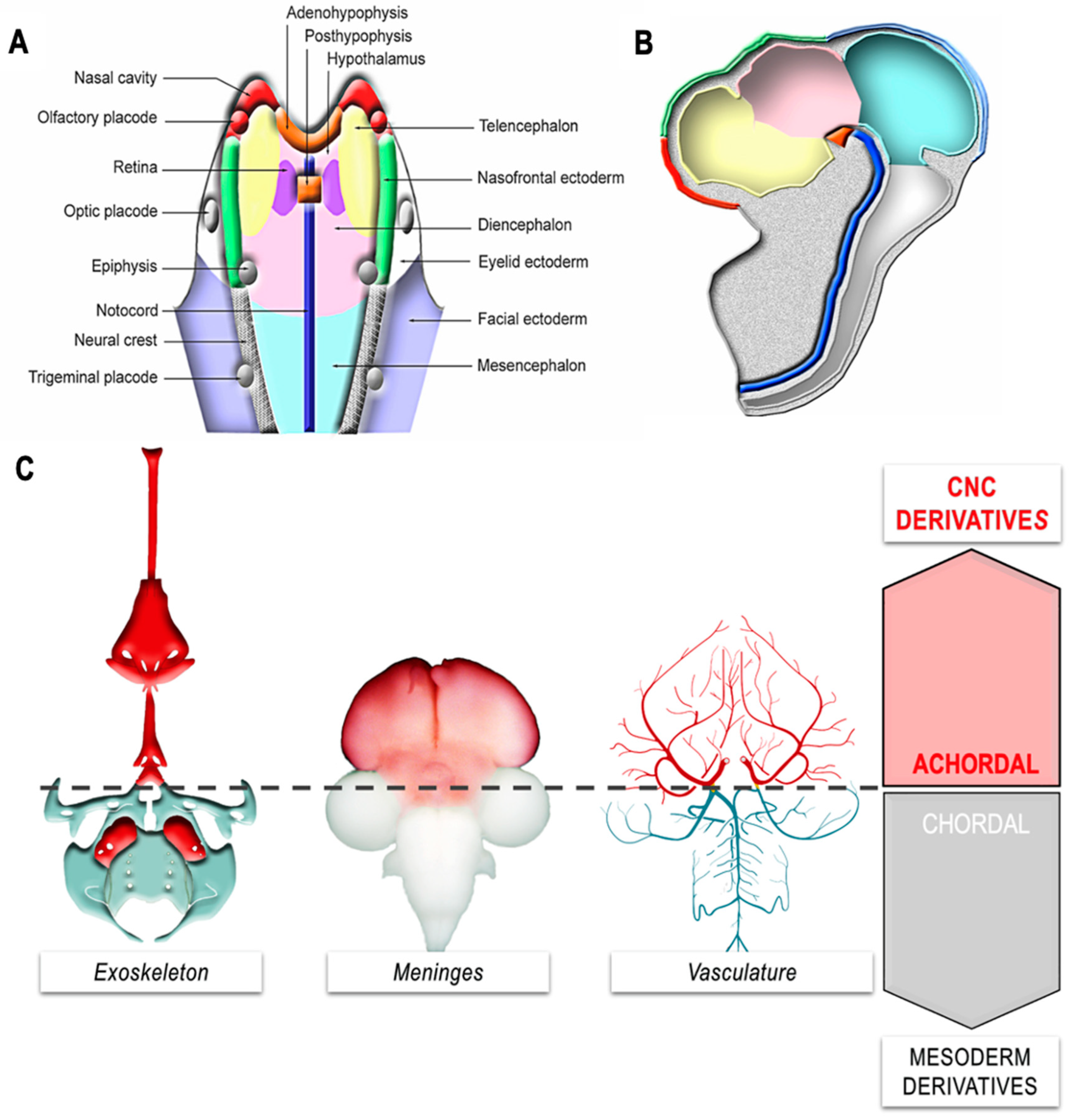
7. Contribution of CNC to Cardio-Vascular and Peri-Ocular Structures
8. CNC Contribution to the Emergence of an Achordal Segment: The “New Head”
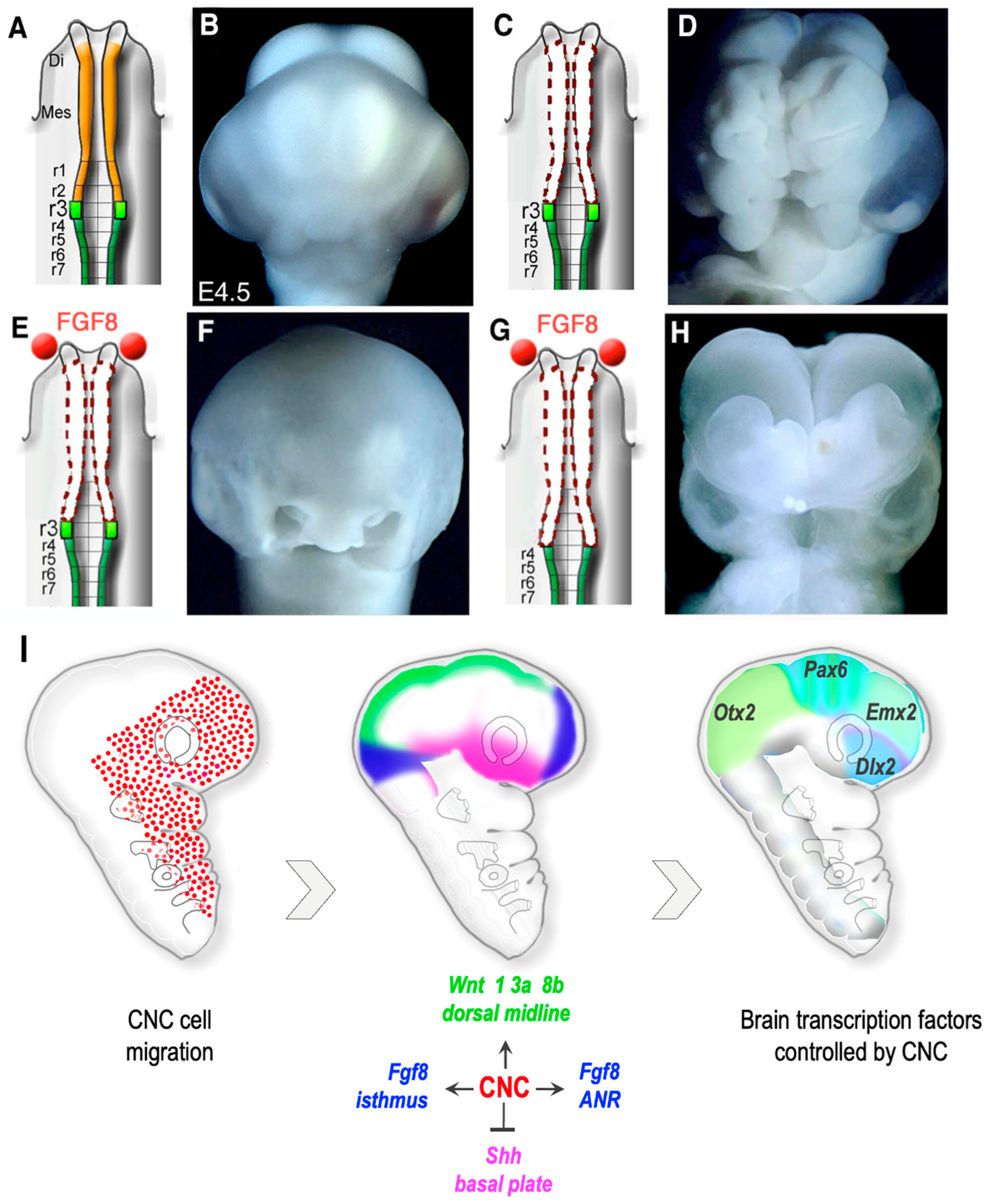
9. CNC Regulation of Pre-Otic Brain Morphogenesis
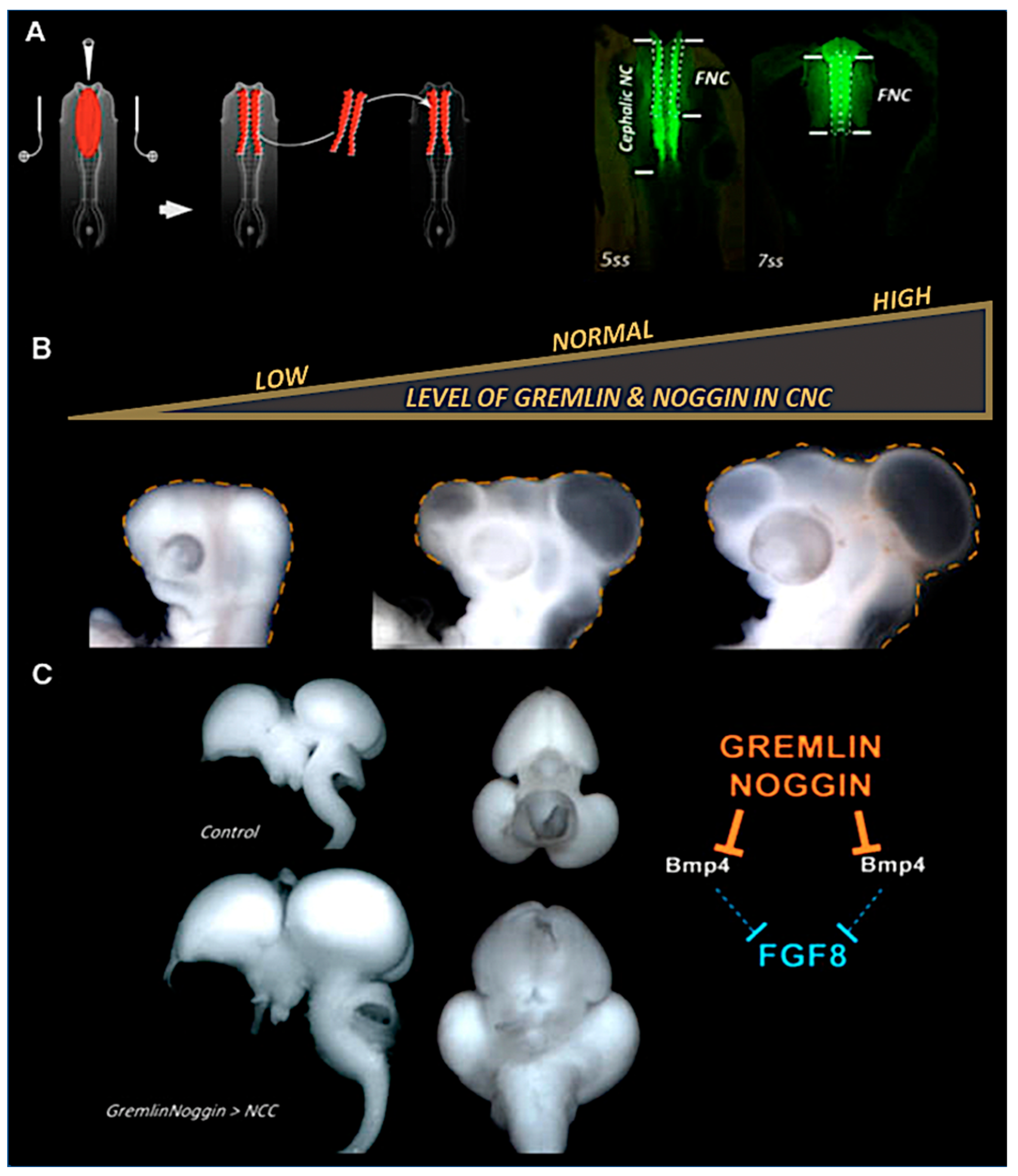
10. CNC Control of Brain Growth by Opposing Bmp Action
11. Role of Six Genes in CNC Cells for Forebrain Development
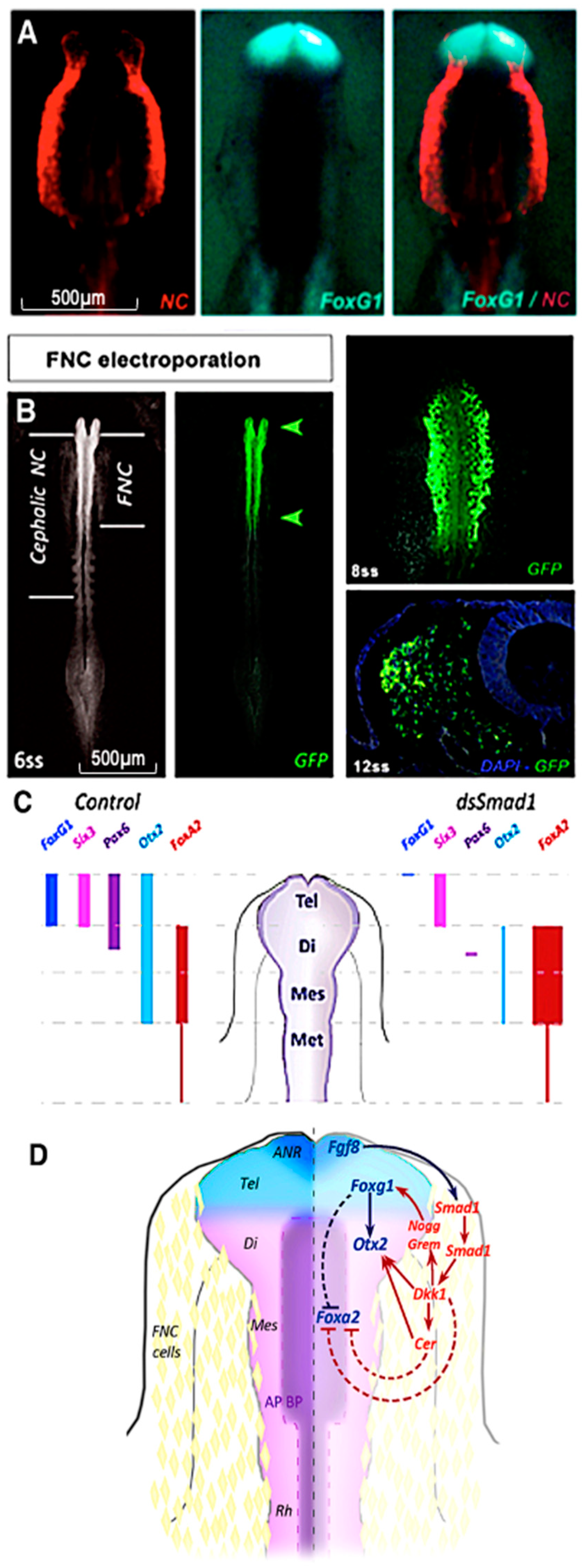
12. Integration of Fgf, Wnt and Bmp Signaling by the CNC for Forebrain Development
13. Concluding Remarks and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gans, C.; Northcutt, R.G. Neural crest and the origin of vertebrates: A new head. Science 1983, 220, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M. The Neural Crest; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Le Douarin, N.; Kalcheim, C. The Neural Crest; Cambridge University Press: Cambridge, UK, 1999; ISBN 9780521620109. [Google Scholar]
- Etchevers, H.; Couly, G.; Vincent, C.; Le Douarin, N. Anterior cephalic neural crest is required for forebrain viability. Development 1999, 126, 3533–3543. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, H.; Vincent, C.; Le Douarin, N.; Couly, G. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001, 128, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, H.C.; Amiel, J.; Lyonnet, S. Molecular bases of human neurocristopathies. Adv. Exp. Med. Biol. 2006, 589, 213–234. [Google Scholar] [CrossRef]
- Bolande, R.P. The neurocristopathies. A unifying concept of disease arising in neural crest maldevelopment. Hum. Pathol. 1974, 5, 409–429. [Google Scholar] [CrossRef]
- Langman, J.; Guerrant, R.L.; Freeman, B.G. Behavior of neuro-epithelial cells during closure of the neural tube. J. Comp. Neurol. 1966, 127, 399–411. [Google Scholar] [CrossRef]
- di Virgilio, G.; Lavenda, N.; Worden, J.L. Sequence of events in neural tube closure and the formation of neural crest in the chick embryo. Cells Tissues Organs 1967, 68, 127–146. [Google Scholar] [CrossRef]
- Smith, J.L.; Schoenwolf, G.C.; Quan, J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. J. Comp. Neurol. 1994, 342, 144–151. [Google Scholar] [CrossRef]
- Gont, L.K.; Steinbeisser, H.; Blumberg, B.; De Robertis, E.M. Tail formation as a continuation of gastrulation: The multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development 1993, 119, 991–1004. [Google Scholar] [CrossRef]
- Catala, M.; Teillet, M.; De Robertis, E.M.; Le Douarin, M.L. A spinal cord fate map in the avian embryo: While regressing, Hensen’s node lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development 1996, 122, 2599–2610. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Creuzet, S.E.; Couly, G.; Dupin, E. Neural Crest Cell Plasticity and its Limits. Development 2004, 131, 4637–4650. [Google Scholar] [CrossRef] [PubMed]
- His, W. Untersuchungen über die erste Anlage des Wirbelthierleibes: Die erste Entwickelung des Hühnchens im Ei; F.C.W. Vogel: Leipzig, Germany, 1868. [Google Scholar]
- Goronowitsch, N. Untersuchungen über die Entwicklung der sog.“Ganglienleisten” im Kopfe der Vögelembryonen. Morph. Jahrb. 1893, 20, 187–259. [Google Scholar]
- Kastschenko, N. Zur Entwicklungsgeschichte des Selachierembryos. Anat. Anz. 1888, 3, 445–467. [Google Scholar]
- Platt, J.B. Ectodermic Origin of the Cartilages of the Head. Anat. Anz. 1893, 8, 506–509. [Google Scholar]
- Platt, J.B. The Development of the Cartilaginous Skull and of the Branchial and Hypoglossal Musculature in Necturus. Morph. Jahrb. 1897, 25, 377–464. [Google Scholar]
- Detwiler, S.R. Observations upon the migration of neural crest cells, and upon the development of the spinal ganglia and vertebral arches in Amblystoma. Am. J. Anat. 1937, 61, 63–94. [Google Scholar] [CrossRef]
- Detwiler, S.R. Application of Vital Dyes to the Study of Sheath Cell Origin. Proc. Soc. Exp. Biol. Med. 1937, 37, 380–382. [Google Scholar] [CrossRef]
- Hörstadius, S.; Sellman, S. Experimental studies on the determination of the chondrocranium in Amblystoma mexicanum. Ark. Zool. A 1941, 33, 1–8. [Google Scholar]
- Hörstadius, S.; Sellman, S. Experimentelle Untersuchungen uber die Determination des Knorpeligen Kopfskelettes bei Urodellen; Almquist & Wiksell: Stockholm, Sweden, 1946. [Google Scholar]
- Raven, C.P. Zur Entwicklung der Ganglienleiste III. Die Induktionsfähigkeit des Kopfganglienleistenmaterials von Rana fusca. Wilhelm Roux’ Arch. Entwickl. Org. 1933, 130, 517–561. [Google Scholar] [CrossRef]
- Raven, C.P. Experiments on the origin of the sheath cells and sympathetic neuroblasts in amphibia. J. Comp. Neurol. 1937, 67, 221–240. [Google Scholar] [CrossRef]
- Stone, L.S. Further experiments on the extirpation and transplantation of mesectoderm in Amblystoma punctatum. J. Exp. Zool. 1926, 44, 95–131. [Google Scholar] [CrossRef]
- Stone, L.S. Experiments showing the role of migrating neural crest (mesectoderm) in the formation of head skeleton and loose connective tissue in Rana palustris. Wilhelm Roux’ Arch. Entwickl. Org. 1929, 118, 40–77. [Google Scholar] [CrossRef] [PubMed]
- Weston, J.A. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. 1963, 6, 279–310. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.C. A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat. Rec. 1966, 156, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M. Details of the interphase nucleus in Japanese quail (Cortunix cortunix japonica). Bull. Biol. Fr. Belg. 1969, 103, 435–452. [Google Scholar]
- Le Douarin, N. A biological cell labeling technique and its use in experimental embryology. Dev. Biol. 1973, 30, 217–222. [Google Scholar] [CrossRef]
- Alrajeh, M.; Vavrusova, Z.; Creuzet, S. Deciphering the Neural Crest Contribution to Cephalic Developlment with Avian Embryos. Methods Mol. Biol. 2019, 1976, 55–70. [Google Scholar] [CrossRef]
- Meulemans, D.; Bronner-Fraser, M. Gene-regulatory interactions in neural crest evolution and development. Dev. Cell 2004, 7, 291–299. [Google Scholar] [CrossRef]
- Nieto, M.A.; Sargent, M.G.; Wilkinson, D.G.; Cooke, J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 1994, 264, 835–839. [Google Scholar] [CrossRef]
- Epstein, J.A. Pax3, neural crest and cardiovascular development. Trends Cardiovasc. Med. 1996, 6, 255–260. [Google Scholar] [CrossRef]
- Kos, R.; Reedy, M.V.; Johnson, R.L.; Erickson, C.A. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 2001, 128, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Sasai, N.; Mizuseki, K.; Sasai, Y. Requirement of FoxD3 -class signaling for neural crest determination in Xenopus. Development 2001, 128, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Saint-Jeannet, J.P. Sox proteins and neural crest development. Semin. Cell Dev. Biol. 2005, 16, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.P.; Erickson, C.A. Morphology and behavior of quail neural crest cells in artificial three-dimensional extracellular matrices. Dev. Biol. 1984, 104, 390–405. [Google Scholar] [CrossRef]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P.; Han, J.; Rowitch, D.H.; Soriano, P.; Mcmahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 1679, 1671–1679. [Google Scholar] [CrossRef]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607–1616. [Google Scholar] [CrossRef]
- Jiang, X.; Iseki, S.; Maxson, R.E.; Sucov, H.M.; Morriss-Kay, G.M. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002, 241, 106–116. [Google Scholar] [CrossRef]
- Ito, Y.; Yeo, J.Y.; Chytil, A.; Han, J.; Bringas, P.; Nakajima, A.; Shuler, C.F.; Moses, H.L.; Chai, Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 2003, 130, 5269–5280. [Google Scholar] [CrossRef]
- Tallquist, M.D.; Soriano, P. Cell autonomous requirement for PDGFRα in populations of cranial and cardiac neural crest cells. Development 2003, 130, 507–518. [Google Scholar] [CrossRef]
- Brewer, S.; Feng, W.; Huang, J.; Sullivan, S.; Williams, T. Wnt1-Cre-mediated deletion of AP-2α causes multiple neural crest-related defects. Dev. Biol. 2004, 267, 135–152. [Google Scholar] [CrossRef]
- Matsuoka, T.; Ahlberg, P.E.; Kessaris, N.; Iannarelli, R.; Dennehy, U.; Richardson, W.D.; McMahon, A.P.; Koentges, G. Neural crest origins of the neck and shoulder. Nature 2005, 436, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.E.; Vasudevan, H.N.; O’Neill, A.K.; Soriano, P.; Bush, J.O. The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Dev. Biol. 2013, 379, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Pietri, T.; Eder, O.; Blanche, M.; Thiery, J.P.; Dufour, S. The human tissue plasminogen activator-Cre mouse: A new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev. Biol. 2003, 259, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M.; Brito, J.M.; Creuzet, S. The Role of the Neural Crest in Face and Brain Development. Brain Res. Rev. 2007, 55, 237–247. [Google Scholar] [CrossRef]
- Duband, J.L.; Thiery, J.P. Distribution of laminin and collagens during avian neural crest development. Development 1987, 101, 461–478. [Google Scholar] [CrossRef]
- Tucker, G.C.; Duband, J.L.; Dufour, S.; Thiery, J.P. Cell-adhesion and substrate-adhesion molecules: Their instructive roles in neural crest cell migration. Development 1988, 103, 81–94. [Google Scholar] [CrossRef]
- Perris, R.; Perissinotto, D. Role of the extracellular matrix during neural crest cell migration. Mech. Dev. 2000, 95, 3–21. [Google Scholar] [CrossRef]
- Osborne, N.J.; Begbie, J.; Chilton, J.K.; Schmidt, H.; Eickholt, B.J. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev. Dyn. 2005, 232, 939–949. [Google Scholar] [CrossRef]
- Yu, H.H.; Moens, C.B. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev. Biol. 2005, 280, 373–385. [Google Scholar] [CrossRef]
- Soo, K.; O’Rourke, M.P.; Khoo, P.L.; Steiner, K.A.; Wong, N.; Behringer, R.R.; Tam, P.P.L. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev. Biol. 2002, 247, 251–270. [Google Scholar] [CrossRef]
- Golding, J.P.; Trainor, P.; Krumlauf, R.; Gassmann, M. Defects in pathfinding by cranial neural crest cells in mice lacking the neuregulin receptor erbB4. Nat. Cell Biol. 2000, 2, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Golding, J.P.; Sobieszczuk, D.; Dixon, M.; Coles, E.; Christiansen, J.; Wilkinson, D.; Gassmann, M. Roles of erbB4, rhombomere-specific, and rhombomere-independent cues in maintaining neural crest-free zones in the embryonic head. Dev. Biol. 2004, 266, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Davy, A.; Aubin, J.; Soriano, P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004, 18, 572–583. [Google Scholar] [CrossRef]
- Lumsden, A.; Sprawson, N.; Graham, A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 1991, 113, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Heyman, I.; Lumsden, A. Even-numbered rhombomeres control the apoptotic elimination of neural crest cells from odd-numbered rhombomeres in the chick hindbrain. Development 1993, 119, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Francis-West, P.; Brickell, P.; Lumsden, A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature 1994, 372, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Theveneau, E.; Mayor, R. Collective cell migration of the cephalic neural crest: The art of integrating information. Genesis 2011, 49, 164–176. [Google Scholar] [CrossRef]
- Le Lievre, C.S.; Le Douarin, N.M. Mesenchymal derivatives of the neural crest: Analysis of chimaeric quail and chick embryos. J. Embryol. Exp. Morphol. 1975, 34, 125–154. [Google Scholar] [CrossRef]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development 1993, 117, 409–429. [Google Scholar] [CrossRef]
- Johnston, M.C.; Noden, D.M.; Hazelton, R.D.; Coulombre, J.L.; Coulombre, A.J. Origins of avian ocular and periocular tissues. Exp. Eye Res. 1979, 29, 27–43. [Google Scholar] [CrossRef]
- Le Lievre, C. Rôle des cellules mésectodermiques issues des crêtes neurales céphaliques dans la formation des arcs branchiaux et du squelette viscéral. Development 1974, 31, 453–477. [Google Scholar] [CrossRef]
- Le Lievre, C.S. Participation of neural crest-derived cells in the genesis of the skull in birds. J. Embryol. Exp. Morphol. 1978, 47, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Köntges, G.; Lumsden, A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 1996, 122, 3229–3242. [Google Scholar] [CrossRef]
- Couly, G.; Grapin-botton, A.; Coltey, P.; Douarin, N.M. Le Couly-1996 The regeneration of the cephalic neural crest a problem revisited. Development 1996, 3407, 3393–3407. [Google Scholar] [CrossRef] [PubMed]
- Creuzet, S.; Schuler, B.; Couly, G.; Le Douarin, N.M. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc. Natl. Acad. Sci. USA 2004, 101, 4843–4847. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.; Gulisano, M.; Cook, M.; Sham, M.-H.; Faiella, A.; Wilkinson, D.; Boncinelli, E.; Krumlauf, R. A Distinct Hox Code for the Branchial Region of the Vertebrate Head. Nature 1991, 353, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Krumlauf, R. Hox Genes in Vertebrate Development. Cell 1994, 78, 191–201. [Google Scholar] [CrossRef]
- Prince, V.; Lumsden, A. Hoxa-2 Expression in Normal and Transposed Rhombomeres: Independent Regulation in the Neural Tube and Neural Crest. Development 1994, 120, 911–923. [Google Scholar] [CrossRef]
- Couly, G.; Grapin-Botton, A.; Coltey, P.; Ruhin, B.; Le Douarin, N.M. Determination of the identity of the derivatives of the cephalic neural crest: Incompatibility between Hox gene expression and lower jaw development. Development 1998, 125, 3445–3459. [Google Scholar] [CrossRef]
- Creuzet, S.; Couly, G.; Vincent, C.; Le Douarin, N.M. Negative Effect of Hox Gene Expression on the Development of the Neural Crest-Derived Facial Skeleton. Development 2002, 129, 4301–4313. [Google Scholar] [CrossRef]
- Couly, G.; Creuzet, S.; Bennaceur, S.; Vincent, C.; Le Douarin, N.M. Interactions between Hox-Negative Cephalic Neural Crest Cells and the Foregut Endoderm in Patterning the Facial Skeleton in the Vertebrate Head. Development 2002, 129, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Gendron-Maguire, M.; Mallo, M.; Zhang, M.; Gridley, T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 1993, 75, 1317–1331. [Google Scholar] [CrossRef]
- Rijli, F.M.; Mark, M.; Lakkaraju, S.; Dierich, A.; Dollé, P.; Chambon, P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 1993, 75, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Solis, R.; Zheng, H.; Whiting, J.; Krumlauf, R.; Bradley, A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell 1993, 73, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Santagati, F.; Rijli, F.M. Cranial neural crest and the building of the vertebrate head. Nat. Rev. Neurosci. 2003, 4, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, G.A.; Bell, E.; Toole, L.; Lumsden, A.; Tucker, A.S. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development 2000, 127, 5355–5365. [Google Scholar] [CrossRef]
- Pasqualetti, M.; Ori, M.; Nardi, I.; Rijli, F.M. Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development 2000, 127, 5367–5378. [Google Scholar] [CrossRef]
- Kirby, M.L.; Stewart, D.E. Neural crest origin of cardiac ganglion cells in the chick embryo: Identification and extirpation. Dev. Biol. 1983, 97, 433–443. [Google Scholar] [CrossRef]
- Kirby, M.L.; Gale, T.F.; Stewart, D.E. Neural Crest Contribute to the APSpdf. Science 1983, 220, 1059–1061. [Google Scholar] [CrossRef]
- Creazzo, T.L.; Godt, R.E.; Leatherbury, L.; Conway, S.J.; Kirby, M.L. Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. 1998, 60, 267–286. [Google Scholar] [CrossRef]
- Kirby, M.L.; Turnage, K.L.; Hays, B.M. Characterization of conotruncal malformations following ablation of “cardiac” neural crest. Anat. Rec. 1985, 213, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Waldo, K.L.; Kumiski, D.H.; Kirby, M.L. Association of the cardiac neural crest with development of the coronary arteries in the chick embryo. Anat. Rec. 1994, 239, 315–331. [Google Scholar] [CrossRef]
- Escot, S.; Blavet, C.; Härtle, S.; Duband, J.L.; Fournier-Thibault, C. Misregulation of SDF1-CXCR4 signaling impairs early cardiac neural crest cell migration leading to conotruncal defects. Circ. Res. 2013, 113, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Waldo, K.; Zdanowicz, M.; Burch, J.; Kumiski, D.H.; Stadt, H.A.; Godt, R.E.; Creazzo, T.L.; Kirby, M.L. A novel role for cardiac neural crest in heart development. J. Clin. Investig. 1999, 103, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Creuzet, S.E. Neural Crest Contribution to Forebrain Development. Semin. Cell Dev. Biol. 2009, 20, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Hall, B. The neural crest as a fourth germ layer and vertebrate as a quadroblastic not triploblastic. Evol. Dev. 2000, 2, 3–5. [Google Scholar] [CrossRef]
- Baker, C.V. The Evolution and Elaboration of Vertebrate Neural Crest Cells. Curr. Opin. Genet. Dev. 2008, 18, 536–543. [Google Scholar] [CrossRef]
- Hall, B.K. Germ Layers and the Germ-Layer Theory Revisited. Evol. Biol. 1998, 30, 121–186. [Google Scholar] [CrossRef]
- Hanato, T.; Nakagawa, M.; Okamoto, N.; Nishijima, S.; Fujino, H.; Shimada, M.; Takeuchi, Y.; Imanaka-Yoshida, K. Developmental defects of coronary vasculature in rat embryos administered bis-diamine. Birth Defects Res. Part B-Dev. Reprod. Toxicol. 2011, 92, 10–16. [Google Scholar] [CrossRef]
- Creuzet, S.; Vincent, C.; Couly, G. Neural crest derivatives in ocular and periocular structures. Int. J. Dev. Biol. 2005, 49, 161–171. [Google Scholar] [CrossRef]
- Couly, G.F.; Le Douarin, N.M. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev. Biol. 1985, 110, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Couly, G.F.; Le Douarin, N.M. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: Implications for the genesis of cephalic human congenital abnormalities. Dev. Biol. 1987, 120, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Couly, G.; Le Douarin, N.M. The fate map of the cephalic neural primordium at the presomitic to the 3-somite stage in the avian embryo. Development 1988, 103, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, M.; Nieto, M.Á. A Celebration of the New Head and an Evaluation of the New Mouth. Neuron 2003, 37, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Creuzet, S.E.; Martinez, S.; Le Douarin, N.M. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc. Natl. Acad. Sci. USA 2006, 103, 14033–14038. [Google Scholar] [CrossRef]
- Simeone, A.; Acampora, D.; Gulisano, M.; Stornaiuolo, A.; Boncinelli, E. Nested expression domains of four homeobox genes in developing rostral brain. Nature 1992, 358, 687–690. [Google Scholar] [CrossRef]
- Rhinn, M.; Dierich, A.; Shawlot, W.; Behringer, R.R.; Le Meur, M.; Ang, S.L. Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development 1998, 125, 845–856. [Google Scholar] [CrossRef]
- Fernandez, A.S.; Pieau, C.; Repérant, J.; Boncinelli, E.; Wassef, M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: Implications for the evolution of telencephalic subdivisions in amniotes. Development 1998, 2111, 2099–2111. [Google Scholar] [CrossRef]
- Aoto, K.; Nishimura, T.; Eto, K.; Motoyama, J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev. Biol. 2002, 251, 320–332. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Chiang, C.; Rubenstein, J.L.R. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience 2002, 111, 1–17. [Google Scholar] [CrossRef]
- Crossley, P.H.; Martinez, S.; Ohkubo, Y.; Rubenstein, J.L.R. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience 2001, 108, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, D.; Klingensmith, J.; Kemp, C.; Belo, J.A.; Anderson, R.M.; May, S.R.; McMahon, J.A.; McMahon, A.P.; Harland, R.M.; Rossant, J.; et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 2000, 403, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Creuzet, S.E. Regulation of pre-otic brain development by the cephalic neural crest. Proc. Natl. Acad. Sci. USA 2009, 106, 15774–15779. [Google Scholar] [CrossRef] [PubMed]
- Garcez, R.C.; Le Douarin, N.M.; Creuzet, S.E. Combinatorial activity of Six1-2-4 genes in cephalic neural crest cells controls craniofacial and brain development. Cell. Mol. Life Sci. 2014, 71, 2149–2164. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Lawrence, A.R.; Stottmann, R.W.; Bachiller, D.; Klingensmith, J. Chd and Nog promote organising centres of forebrain development in the mouse. Development 2002, 4987, 4975–4987. [Google Scholar] [CrossRef] [PubMed]
- Bardot, B.; Lecoin, L.; Huillard, E.; Calothy, G.; Marx, M. Expression pattern of the drm/gremlin gene during chicken embryonic development. Mech. Dev. 2001, 101, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Tzahor, E.; Kempf, H.; Mootoosamy, R.C.; Poon, A.C.; Abzhanov, A.; Tabin, C.J.; Dietrich, S.; Lassar, A.B. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003, 17, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Kutejova, E.; Engist, B.; Mallo, M.; Kanzler, B.; Bobola, N. Hoxa2 downregulates Six2 in the neural crest-derived mesenchyme. Development 2005, 132, 469–478. [Google Scholar] [CrossRef]
- Cases, O.; Perea-Gomez, A.; Aguiar, D.P.; Nykjaer, A.; Amsellem, S.; Chandellier, J.; Umbhauer, M.; Cereghini, S.; Madsen, M.; Collignon, J.; et al. Cubilin, a high affinity receptor for fibroblast growth factor 8, is required for cell survival in the developing vertebrate head. J. Biol. Chem. 2013, 288, 16655–16670. [Google Scholar] [CrossRef]
- Xuan, S.; Baptista, C.A.; Balas, G.; Tao, W.; Soares, V.C.; Lai, E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 1995, 14, 1141–1152. [Google Scholar] [CrossRef]
- Tao, W.; Lai, E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron 1992, 8, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Papa, F.T.; Mencarelli, M.A.; Caselli, R.; Katzaki, E.; Sampieri, K.; Meloni, I.; Ariani, F.; Longo, I.; Maggio, A.; Balestri, P.; et al. Clinical Report A 3 Mb Deletion in 14q12 Causes Severe Mental Retardation, Mild Facial Dysmorphisms and Rett-like Features. Am. J. Med. Genet. Part A 2008, 1998, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Allou, L.; Lambert, L.; Amsallem, D.; Bieth, E.; Edery, P.; Destrée, A.; Rivier, F.; Amor, D.; Thompson, E.; Nicholl, J.; et al. 14q12 and severe Rett-like phenotypes: New clinical insights and physical mapping of FOXG1-regulatory elements. Eur. J. Hum. Genet. 2012, 20, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, K.; Rubenstein, J.L.R. Inductive interactions direct early regionalization of the mouse forebrain. Development 1997, 124, 2709–2718. [Google Scholar] [CrossRef]
- Houart, C.; Westerfield, M.; Wilson, S.W. A small population of anterior cells patterns the forebrain during zebrafish gastrulation. Nature 1998, 391, 788–792. [Google Scholar] [CrossRef]
- Aguiar, D.P.; Sghari, S.; Creuzet, S. The facial neural crest controls fore- and midbrain patterning by regulating Foxg1 expression through Smad1 activity. Development 2014, 141, 2494–2505. [Google Scholar] [CrossRef]
- Shanmugalingam, S.; Houart, C.; Picker, A.; Reifers, F.; Macdonald, R.; Barth, A.; Griffin, K.; Brand, M.; Wilson, S.W. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development 2000, 127, 2549–2561. [Google Scholar] [CrossRef]
- Storm, E.E.; Garel, S.; Borello, U.; Hebert, J.M.; Martinez, S.; McConnel, S.K.; Martin, G.R.; Rubenstein, J.L.R. Dose-dependent functions fo Fgf8 in regulating telencephalic patterning centers. Development 2006, 133, 1831–1844. [Google Scholar] [CrossRef]
- Fuentealba, L.C.; Eivers, E.; Ikeda, A.; Hurtado, C.; Kuroda, H.; Pera, E.M.; De Robertis, E.M. Integrating Patterning Signals: Wnt/GSK3 Regulates the Duration of the BMP/Smad1 Signal. Cell 2007, 131, 980–993. [Google Scholar] [CrossRef]
- De Morsier, G. Studies on malformation of cranio-encephalic sutures. III. Agenesis of the septum lucidum with malformation of the optic tract. Schweiz. Arch. Neurol. Psychiatr. 1956, 77, 267–292. [Google Scholar]
- Gregory, L.C.; Gergics, P.; Nakaguma, M.; Bando, H.; Patti, G.; Mccabe, M.J.; Fang, Q.; Ma, Q.; Ozel, A.B.; Li, J.Z.; et al. The phenotypic spectrum associated with OTX2 mutations in humans. Eur. J. Endocrinol. 2021, 185, 121–135. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruet, E.; Amarante-Silva, D.; Gorojankina, T.; Creuzet, S. The Emerging Roles of the Cephalic Neural Crest in Brain Development and Developmental Encephalopathies. Int. J. Mol. Sci. 2023, 24, 9844. https://doi.org/10.3390/ijms24129844
Bruet E, Amarante-Silva D, Gorojankina T, Creuzet S. The Emerging Roles of the Cephalic Neural Crest in Brain Development and Developmental Encephalopathies. International Journal of Molecular Sciences. 2023; 24(12):9844. https://doi.org/10.3390/ijms24129844
Chicago/Turabian StyleBruet, Emmanuel, Diego Amarante-Silva, Tatiana Gorojankina, and Sophie Creuzet. 2023. "The Emerging Roles of the Cephalic Neural Crest in Brain Development and Developmental Encephalopathies" International Journal of Molecular Sciences 24, no. 12: 9844. https://doi.org/10.3390/ijms24129844
APA StyleBruet, E., Amarante-Silva, D., Gorojankina, T., & Creuzet, S. (2023). The Emerging Roles of the Cephalic Neural Crest in Brain Development and Developmental Encephalopathies. International Journal of Molecular Sciences, 24(12), 9844. https://doi.org/10.3390/ijms24129844




