Curcumin Attenuates Periodontal Injury via Inhibiting Ferroptosis of Ligature-Induced Periodontitis in Mice
Abstract
1. Introduction
2. Results
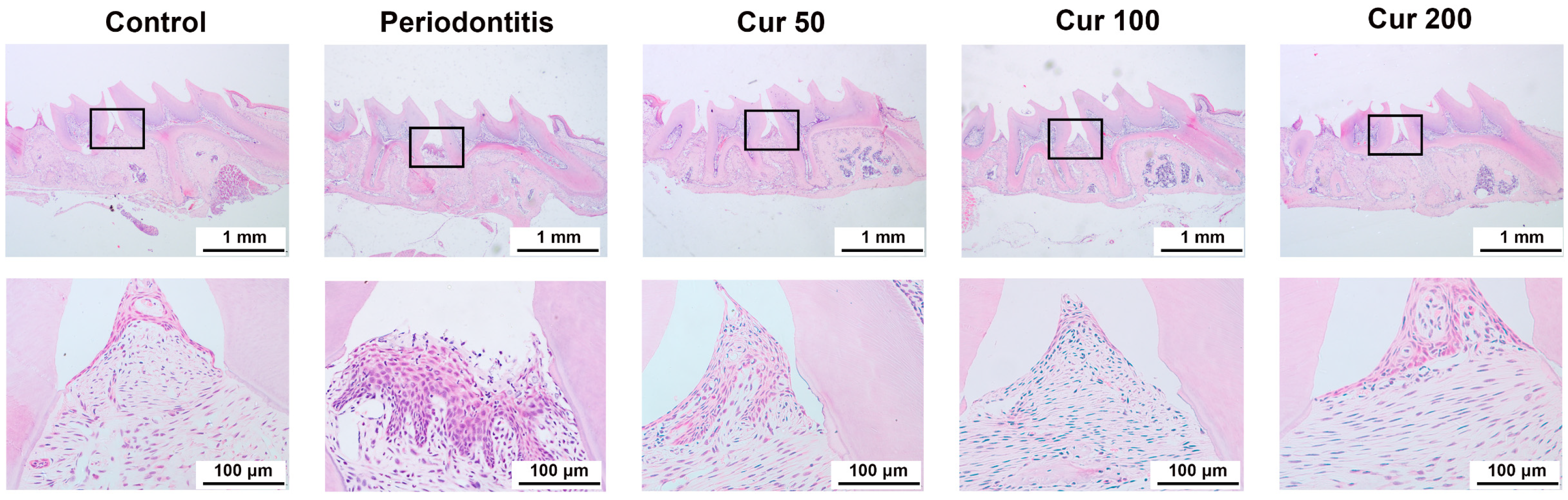
2.1. Curcumin Attenuates Periodontal Injury in Gingival Tissue of Ligature-Induced Periodontitis in Mice
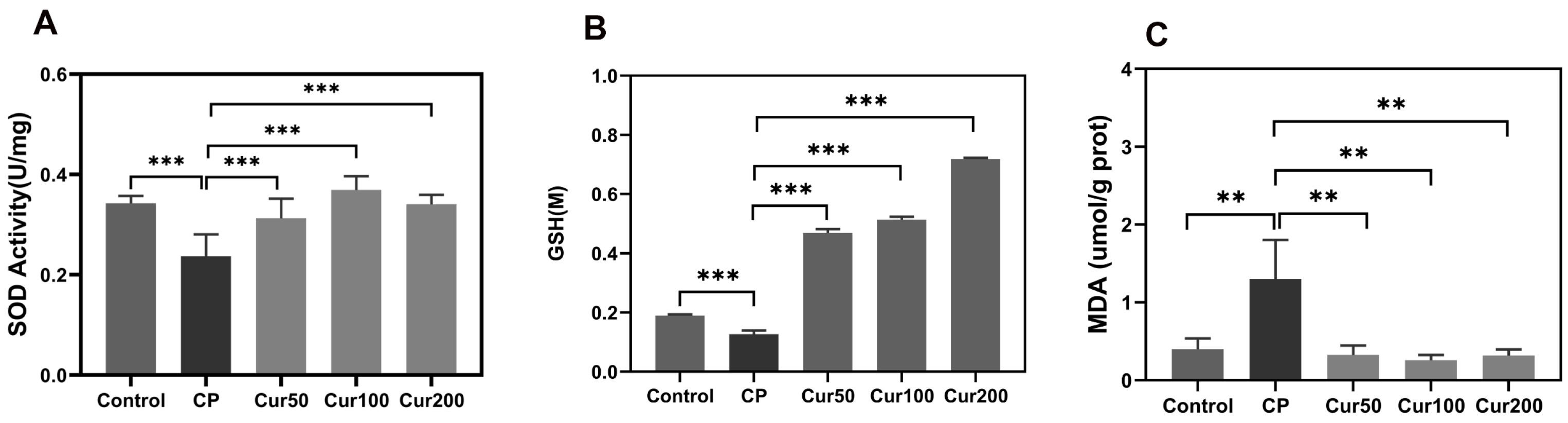
2.2. Curcumin Inhibits Ferroptosis-Related Marker in Gingival Tissue of Ligature-Induced Periodontitis in Mice
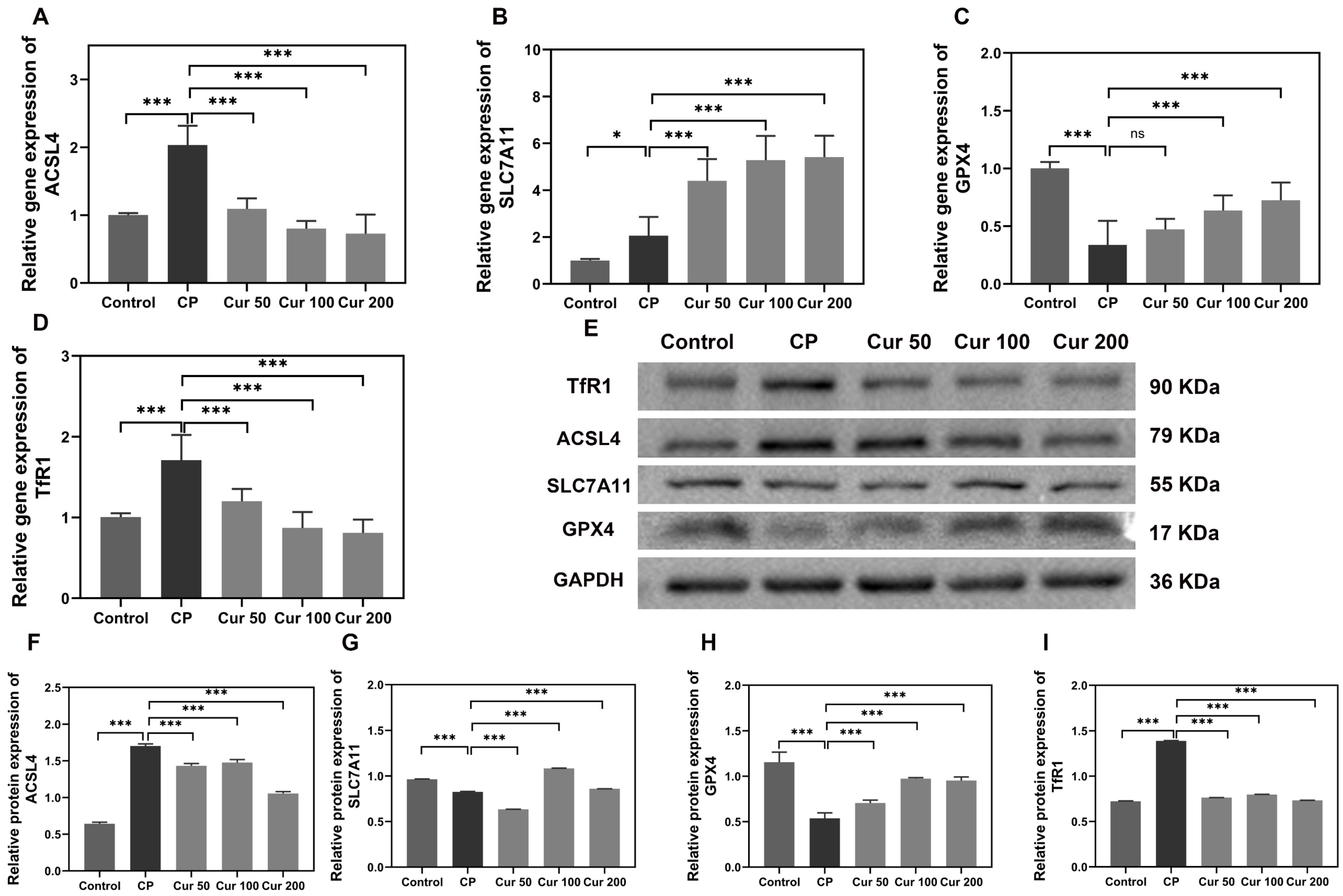
2.3. Curcumin Regulates Ferroptosis-Related Genes and Proteins in Gingival Tissue of Ligature-Induced Periodontitis Mice
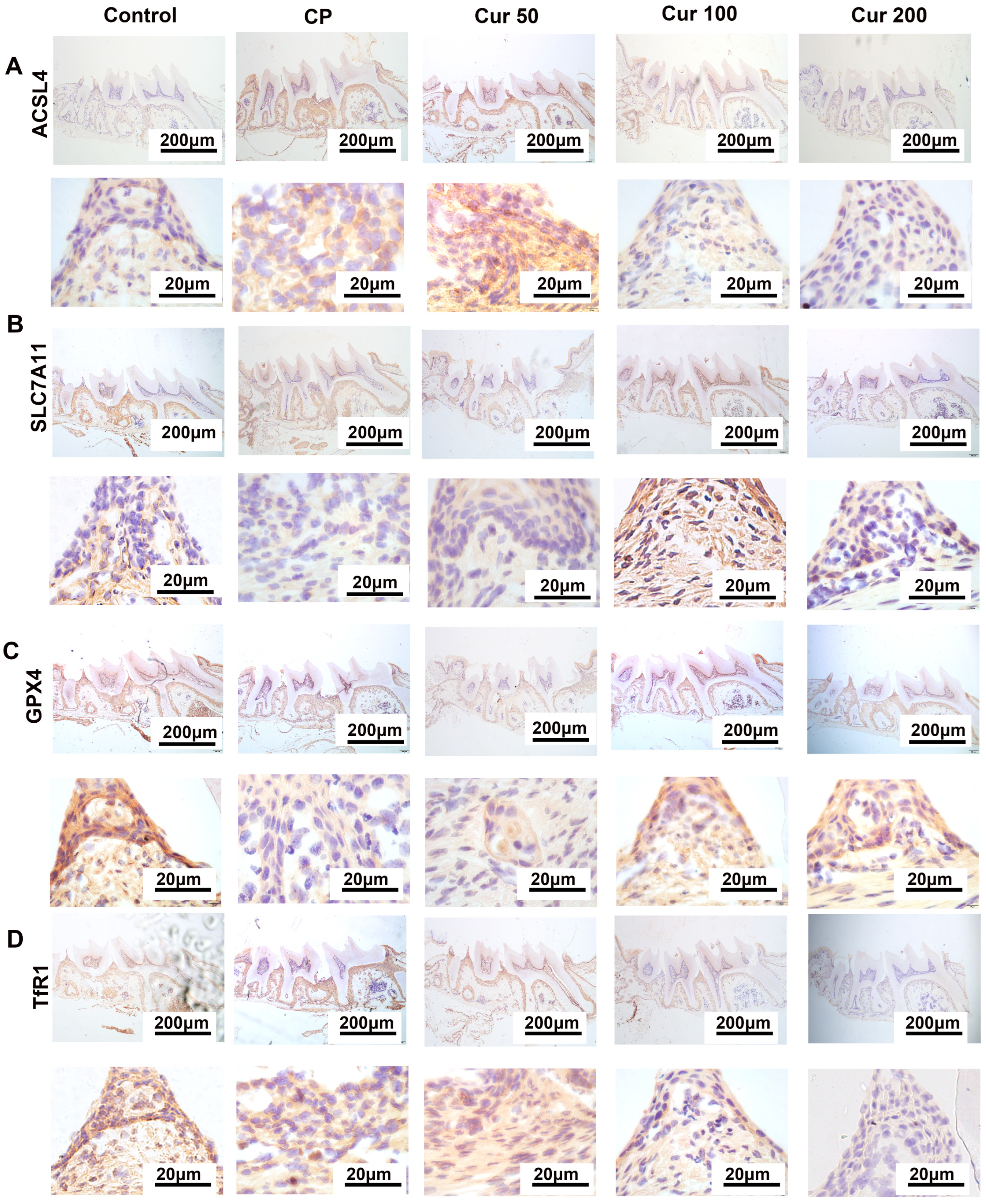
2.4. Curcumin Regulates Ferroptosis-Related Proteins in Gingival Tissue Detected by Immunohistochemistry (IHC)
2.5. Curcumin Inhibits Ferroptosis-Related Makers and Genes in Alveolar Bone of Ligature-Induced Periodontitis in Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals Experiment
4.3. Determination of SOD in Plasma
4.4. Detection of Lipid Peroxidation MDA in Maxillary Tissue
4.5. Detection of Total GSH in Maxillary Tissue
4.6. Reverse Transcription-Quantitative (qPCR)
4.7. Western Blot
4.8. H&E Staining
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Takedachi, M.; Shimabukuro, Y.; Sawada, K.; Koshimizu, M.; Shinada, K.; Asai, H.; Mizoguchi, A.; Hayashi, Y.; Tsukamoto, A.; Miyago, M.; et al. Evaluation of periodontitis-related tooth loss according to the new 2018 classification of periodontitis. Sci. Rep. 2022, 12, 11893. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, C.; Larsson, L.; Difloe-Geisert, J.C.; Zitzmann, N.U.; Berglundh, T. Cellular expression of epigenetic markers and oxidative stress in periodontitis lesions of smokers and non-smokers. J. Periodontal Res. 2022, 57, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.W.; Lin, S.L.; Hu, S.S.; Zhao, L. Structure and Function of Oral Microbial Community in Periodontitis Based on Integrated Data. Front. Cell. Infect. Microbiol. 2021, 11, 663756. [Google Scholar] [CrossRef]
- Wang, W.Z.; Zheng, C.X.; Yang, J.H.; Li, B. Intersection between macrophages and periodontal pathogens in periodontitis. J. Leukocyte Biol. 2021, 110, 577–583. [Google Scholar] [CrossRef]
- Liu, C.C.; Mo, L.Y.; Niu, Y.L.; Li, X.; Zhou, X.D.; Xu, X. The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox. Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Chen, E.; Wang, T.; Tu, Y.; Sun, Z.; Ding, Y.; Gu, Z.; Xiao, S. ROS-scavenging biomaterials for periodontitis. J. Mater. Chem. B 2022, 11, 482–499. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, J.; Wu, W.; Ma, P.; Ren, L.; Yi, Z.; Wu, J. Quercetin Prevents Oxidative Stress-Induced Injury of Periodontal Ligament Cells and Alveolar Bone Loss in Periodontitis. Drug Des. Devel. Ther. 2021, 15, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Veljovic, T.; Djuric, M.; Mirnic, J.; Gusic, I.; Maletin, A.; Ramic, B.; Neskovic, I.; Vukoje, K.; Brkic, S. Lipid Peroxidation Levels in Saliva and Plasma of Patients Suffering from Periodontitis. J. Clin. Med. 2022, 11, 3617. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.L.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Mao, H.M.; Zhao, Y.H.; Li, H.X.; Lei, L. Ferroptosis as an emerging target in inflammatory diseases. Prog. Biophys. Mol. Biol. 2020, 155, 20–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Guo, W.; Li, H.; Lei, L. Periodontitis-level butyrate-induced ferroptosis in periodontal ligament fibroblasts by activation of ferritinophagy. Cell Death Discov. 2020, 6, 119. [Google Scholar] [CrossRef]
- Qiao, S.; Li, B.; Cai, Q.; Li, Z.; Yin, Z.; He, J.; Li, Y.; Meng, W. Involvement of ferroptosis in Porphyromonas gingivalis lipopolysaccharide-stimulated periodontitis in vitro and in vivo. Oral. Dis. 2022, 14292. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Zhang, C.; Hou, J.; Qi, S.; LINC, L.N.-C.R.N. 00616 promotes ferroptosis of periodontal ligament stem cells via the microRNA-370/transferrin receptor axis. Bioengineered 2022, 13, 13070–13081. [Google Scholar] [CrossRef]
- Lee, S.; Cho, D.C.; Han, I.; Kim, K.T. Curcumin as a Promising Neuroprotective Agent for the Treatment of Spinal Cord Injury: A Review of the Literature. Neurospine 2022, 19, 249–261. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alotaibi, G.; Khan, F.; Alam, W.; Aschner, M.; Jeandet, P.; Saso, L. How Curcumin Targets Inflammatory Mediators in Diabetes: Therapeutic Insights and Possible Solutions. Molecules 2022, 27, 4058. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, H.; Bu, L.; Chen, C.; Ye, X. Review of the Effects and Mechanism of Curcumin in the Treatment of Inflammatory Bowel Disease. Front. Pharmacol. 2022, 13, 908077. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharm. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Okazaki, R.; Sato, M.; Oh-Hashi, K.; Takemori, H.; Furuta, K. Effect of ferroptosis inhibitors oxindole-curcumin hybrid compound and N,N-dimethylaniline derivatives on rotenone-induced oxidative stress. Eur. J. Pharmacol. 2022, 928, 175119. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Garcia-Caballero, C.; Palomino-Antolin, A.; Rubio-Navarro, A.; Vazquez-Carballo, C.; Herencia, C.; Martin-Sanchez, D.; Farre-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019, 33, 8961–8975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Z.; Zhang, M.; Wang, Y.; Hu, R.; Lin, W.; Huang, C.; Xu, C.; Wu, J.; Deng, H. Design, synthesis, and evaluation of mono-carbonyl analogues of curcumin (MCACs) as potential antioxidants against periodontitis. J. Periodontal. Res. 2021, 56, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pacheco, C.G.; Fernandes, N.A.R.; Primo, F.L.; Tedesco, A.C.; Bellile, E.; Retamal-Valdes, B.; Feres, M.; Guimaraes-Stabili, M.R.; Rossa, C., Jr. Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: Randomized, placebo-controlled, double-blind split-mouth clinical trial. Clin. Oral. Investig. 2021, 25, 3217–3227. [Google Scholar] [CrossRef]
- Iova, G.M.; Calniceanu, H.; Popa, A.; Szuhanek, C.A.; Marcu, O.; Ciavoi, G.; Scrobota, I. The Antioxidant Effect of Curcumin and Rutin on Oxidative Stress Biomarkers in Experimentally Induced Periodontitis in Hyperglycemic Wistar Rats. Molecules 2021, 26, 1332. [Google Scholar] [CrossRef]
- Sha, A.M.; Garib, B.T.; Azeez, S.H.; Gul, S.S. Effects of curcumin gel on osteoclastogenic bone markers in experimental periodontitis and alveolar bone loss in wistar rats. J. Dent. Sci. 2021, 16, 905–914. [Google Scholar] [CrossRef]
- Liu, M.F.; Sun, X.Y.; Chen, B.Y.; Dai, R.C.; Xi, Z.C.; Xu, H.X. Insights into Manganese Superoxide Dismutase and Human Diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xian, X.; Tan, Z.; Dong, F.; Xu, G.; Zhang, M.; Zhang, F. The Role of Iron Metabolism, Lipid Metabolism, and Redox Homeostasis in Alzheimer’s Disease: From the Perspective of Ferroptosis. Mol. Neurobiol. 2023, 60, 2832–2850. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xin, L.Y.; Xiang, M.; Shang, C.; Wang, Y.L.; Wang, Y.; Cui, X.N.; Lu, Y.D. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed. Pharmacother. 2022, 145, 112423. [Google Scholar] [CrossRef]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.M.; Zhang, X.Y.; Kang, R.; Tang, D.L. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Bioph. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.L.; Liu, Z.L.; Jiang, Z.W.; Peng, H. Dexamethasone induces ferroptosis via P53/SLC7A11/GPX4 pathway in glucocorticoid-induced osteonecrosis of the femoral head. Biochem. Bioph. Res. Commun. 2022, 602, 149–155. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Jung, J.I.; Kim, Y.; Kang, C.H.; Imm, J.Y. Effects of Lactobacillus reuteri MG5346 on Receptor Activator of Nuclear Factor-Kappa B Ligand (RANKL)-Induced Osteoclastogenesis and Ligature-Induced Experimental Periodontitis Rats. Food Sci. Anim. Resour. 2023, 43, 157–169. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Peng, H.; Gu, D.; Liu, C.; Ren, S.; Miao, L. Robust intervention for oxidative stress-induced injury in periodontitis via controllably released nanoparticles that regulate the ROS-PINK1-Parkin pathway. Front. Bioeng. Biotechnol. 2022, 10, 1081977. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases-Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharm. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pacheco, C.G.; Fernandes, N.A.R.; Camilli, A.C.; Ferrarezi, D.P.; Silva, A.F.; Zunareli, M.C.; Amantino, C.F.; Primo, F.L.; Guimaraes-Stabilli, M.R.; Junior, C.R. Local administration of curcumin-loaded nanoparticles enhances periodontal repair in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Justo, M.P.; Cardoso, C.D.M.; Cantiga-Silva, C.; de Oliveira, P.H.C.; Sivieri-Araujo, G.; Azuma, M.M.; Ervolino, E.; Cintra, L.T.A. Curcumin reduces inflammation in rat apical periodontitis. Int. Endod. J. 2022, 55, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Shi, L.; Ji, Y.; Zhao, S.; Li, H.; Jiang, Y.; Mao, J.; Chen, Y.; Zhang, X.; Mao, Y.; Sun, X.; et al. Crosstalk between reactive oxygen species and Dynamin-related protein 1 in periodontitis. Free Radic. Biol. Med. 2021, 172, 19–32. [Google Scholar] [CrossRef]
- Chen, K.; Ma, S.; Deng, J.; Jiang, X.; Ma, F.; Li, Z. Ferroptosis: A New Development Trend in Periodontitis. Cells 2022, 11, 3349. [Google Scholar] [CrossRef]
- Ma, F.; Luo, S.; Lu, C.; Jiang, X.; Chen, K.; Deng, J.; Ma, S.; Li, Z. The role of Nrf2 in periodontal disease by regulating lipid peroxidation, inflammation and apoptosis. Front. Endocrinol. 2022, 13, 963451. [Google Scholar] [CrossRef]
- Lin, W.; Wang, C.; Liu, G.; Bi, C.; Wang, X.; Zhou, Q.; Jin, H. SLC7A11/xCT in cancer: Biological functions and therapeutic implications. Am. J. Cancer Res. 2020, 10, 3106–3126. [Google Scholar]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Lang, X.T.; Green, M.D.; Wang, W.M.; Yu, J.L.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X. The Chemistry and Biology of Ferroptosis. Cell Chem. Biol. 2020, 27, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Y.; Zhao, X.L.; Shao, L.M.; Liu, G.P.; Sun, C.J.; Xu, R.; Zhang, Z.L. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav. Immun. 2021, 93, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.; Girnary, M.S.; Jing, L.; Miao, M.Z.; Zhang, S.; Sun, L.; Morelli, T.; Schoenfisch, M.H.; Inohara, N.; Offenbacher, S.; et al. An experimental murine model to study periodontitis. Nat. Protoc. 2018, 13, 2247–2267. [Google Scholar] [CrossRef] [PubMed]

| Primer | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| gapdh | GGAGATTGTTGCCATCAACGA | GAAGACACCATAGACTCCACG |
| acsl4 | CCTTTGGCTCATGTGCTGAACT | CAGCGGCCATAAGTGTGGGTTT |
| slc7a11 | TGGGTGGAACTGCTCGTAAT | AGGATGTAGCGTCCAAATGC |
| gpx4 | GCAACCAGTTTGGGAGGCAG | CCTCCATGGGACCATAGCGC |
| tfr1 | TCATGAGGGAAATCAATGATC | GCCCCAGAAGATATGTCGGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lin, H.; Huang, W.; Liu, Z.; Chen, Z.; Zhao, X.; Ding, T.; Qin, W.; Shen, Y. Curcumin Attenuates Periodontal Injury via Inhibiting Ferroptosis of Ligature-Induced Periodontitis in Mice. Int. J. Mol. Sci. 2023, 24, 9835. https://doi.org/10.3390/ijms24129835
Wang Y, Lin H, Huang W, Liu Z, Chen Z, Zhao X, Ding T, Qin W, Shen Y. Curcumin Attenuates Periodontal Injury via Inhibiting Ferroptosis of Ligature-Induced Periodontitis in Mice. International Journal of Molecular Sciences. 2023; 24(12):9835. https://doi.org/10.3390/ijms24129835
Chicago/Turabian StyleWang, Yawei, Hongbing Lin, Wenxin Huang, Zixian Liu, Zhen Chen, Xuetao Zhao, Tong Ding, Wenguang Qin, and Yuqin Shen. 2023. "Curcumin Attenuates Periodontal Injury via Inhibiting Ferroptosis of Ligature-Induced Periodontitis in Mice" International Journal of Molecular Sciences 24, no. 12: 9835. https://doi.org/10.3390/ijms24129835
APA StyleWang, Y., Lin, H., Huang, W., Liu, Z., Chen, Z., Zhao, X., Ding, T., Qin, W., & Shen, Y. (2023). Curcumin Attenuates Periodontal Injury via Inhibiting Ferroptosis of Ligature-Induced Periodontitis in Mice. International Journal of Molecular Sciences, 24(12), 9835. https://doi.org/10.3390/ijms24129835





