Molecular Mechanism and Clinical Effects of Probiotics in the Management of Cow’s Milk Protein Allergy
Abstract
1. Introduction
2. Probiotics
3. Cow’s Milk Protein Allergy (CMPA)
Pathogenesis of FA
4. Microbiota
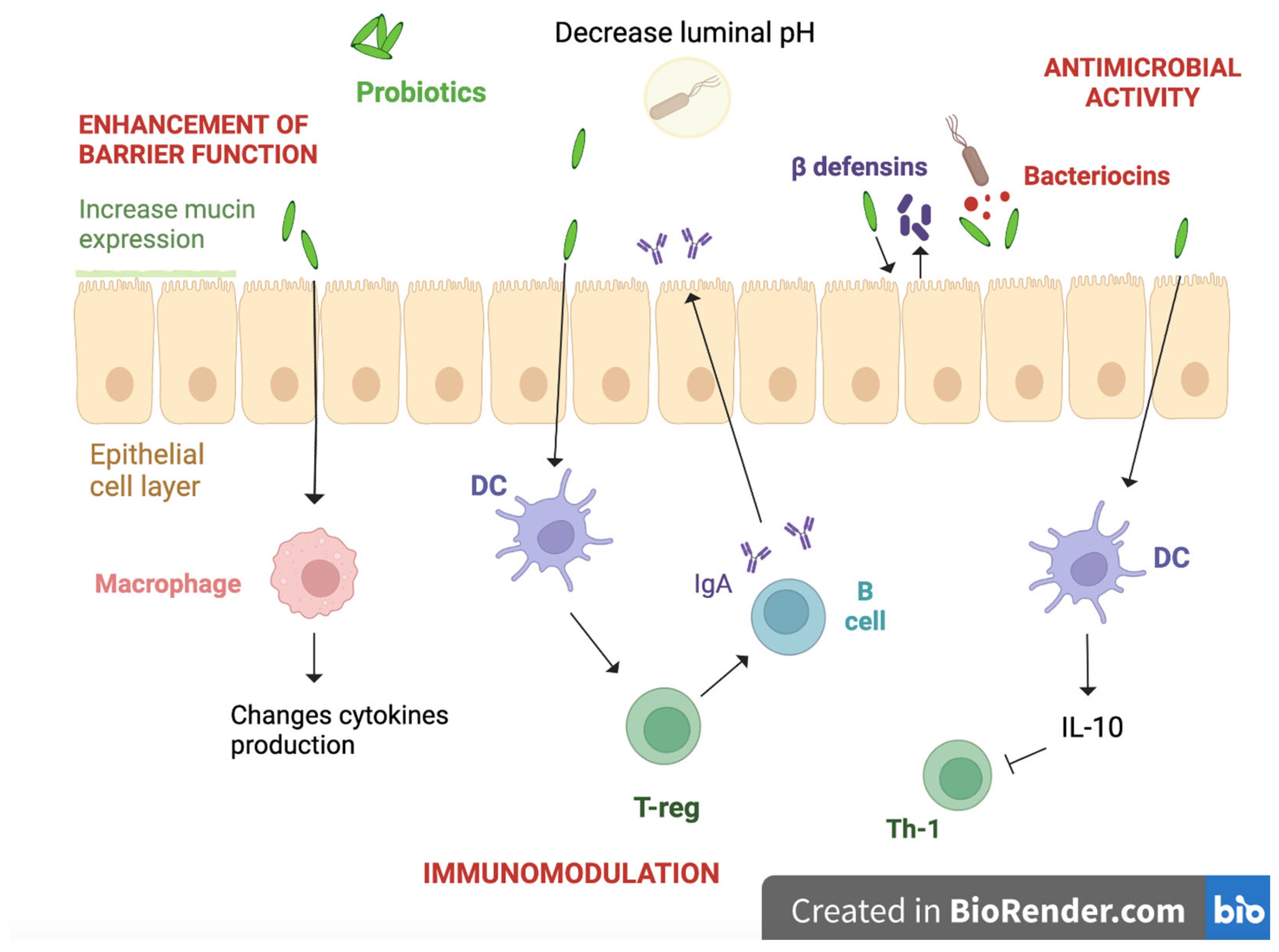
4.1. Role of Probiotics in Allergic Disorders
4.2. Microbiota Modulation
4.3. Enhancement of the Gut Barrier
4.4. Immunological Function
4.5. Reduction of IgE Levels
5. Discussion
5.1. Retrospective Studies
5.2. Non-Randomized Trials
5.3. Randomized Controlled Trials
6. Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shu, S.-A.; Yuen, A.W.T.; Woo, E.; Chu, K.-H.; Kwan, H.-S.; Yang, G.-X.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Moriki, D.; Francino, M.P.; Koumpagioti, D.; Boutopoulou, B.; Rufián-Henares, J.Á.; Priftis, K.N.; Douros, K. The Role of the Gut Microbiome in Cow’s Milk Allergy: A Clinical Approach. Nutrients 2022, 14, 4537. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Brough, H.A.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current Guidelines and Future Strategies for the Management of Cow’s Milk Allergy. J. Asthma Allergy 2021, 14, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Brindisi, G.; Martinelli, I.; Bonucci, E.; D’Orsi, M.; Ialongo, S.; Nyffenegger, A.; Raso, T.; Spatuzzo, M.; De Castro, G.; et al. Probiotics Function in Preventing Atopic Dermatitis in Children. Int. J. Mol. Sci. 2022, 23, 5409. [Google Scholar] [CrossRef]
- Anania, C.; Di Marino, V.P.; Olivero, F.; De Canditiis, D.; Brindisi, G.; Iannilli, F.; De Castro, G.; Zicari, A.M.; Duse, M. Treatment with a Probiotic Mixture Containing Bifidobacterium Animalis Subsp. Lactis BB12 and Enterococcus Faecium L3 for the Prevention of Allergic Rhinitis Symptoms in Children: A Randomized Controlled Trial. Nutrients 2021, 13, 1315. [Google Scholar] [CrossRef]
- Havenaar, R.; Huis In’t Veld, J.H.J. Probiotics: A General View. In The Lactic Acid Bacteria Volume 1: The Lactic Acid Bacteria in Health and Disease; Wood, B.J.B., Ed.; Springer US: Boston, MA, USA, 1992; pp. 151–170. ISBN 978-1-4615-3522-5. [Google Scholar]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M.M. Gut Microbiota and Gastrointestinal Health: Current Concepts and Future Directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Scott, K.P.; Antoine, J.-M.; Midtvedt, T.; van Hemert, S. Manipulating the Gut Microbiota to Maintain Health and Treat Disease. Microb. Ecol. Health Dis. 2015, 26, 25877. [Google Scholar] [CrossRef]
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-Analysis. Pediatrics 2016, 137, e20153684. [Google Scholar] [CrossRef]
- Bakirtzi, K.; Law, I.K.M.; Xue, X.; Iliopoulos, D.; Shah, Y.M.; Pothoulakis, C. Neurotensin Promotes the Development of Colitis and Intestinal Angiogenesis via Hif-1α-MiR-210 Signaling. J. Immunol. 2016, 196, 4311–4321. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Song, J.-H.; Xu, H.; Li, D.; Goldsmith, J.; Zeng, H.; Parsons-Wingerter, P.A.; Reinecker, H.-C.; Kelly, C.P. Probiotic Yeast Inhibits VEGFR Signaling and Angiogenesis in Intestinal Inflammation. PLoS ONE 2013, 8, e64227. [Google Scholar] [CrossRef] [PubMed]
- Mousan, G.; Kamat, D. Cow’s Milk Protein Allergy. Clin. Pediatr. 2016, 55, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and Natural History of Challenge-Proven Cow’s Milk Allergy in European Children--EuroPrevall Birth Cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G.; Elsayed, S.; Hosking, C.S.; Hill, D.J. Cow’s Milk Allergy in Infancy. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 217–225. [Google Scholar] [CrossRef]
- Tsabouri, S.; Douros, K.; Priftis, K.N. Cow’s Milk Allergenicity. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 16–26. [Google Scholar] [CrossRef]
- de Jong, N.W.; van Splunter, M.E.; Emons, J.A.M.; Hettinga, K.A.; Gerth van Wijk, R.; Wichers, H.J.; Savelkoul, H.F.J.; Sprikkelman, A.B.; van Neerven, R.J.J.; Liu, L.; et al. Introduction of Heated Cow’s Milk Protein in Challenge-Proven Cow’s Milk Allergic Children: The IAGE Study. Nutrients 2022, 14, 629. [Google Scholar] [CrossRef]
- Tosca, M.A.; Schiavetti, I.; Olcese, R.; Trincianti, C.; Ciprandi, G. Molecular Allergy Diagnostics in Children with Cow’s Milk Allergy: Prediction of Oral Food Challenge Response in Clinical Practice. J. Immunol. Res. 2023, 2023, 1129449. [Google Scholar] [CrossRef]
- Wal, J.-M. Bovine Milk Allergenicity. Ann. Allergy Asthma Immunol. 2004, 93, S2–S11. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to Extensively Heated Milk in Children with Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347. [Google Scholar] [CrossRef]
- Ehn, B.-M.; Ekstrand, B.; Bengtsson, U.; Ahlstedt, S. Modification of IgE Binding during Heat Processing of the Cow’s Milk Allergen Beta-Lactoglobulin. J. Agric. Food Chem. 2004, 52, 1398–1403. [Google Scholar] [CrossRef]
- Høst, A. Cow’s Milk Protein Allergy and Intolerance in Infancy. Some Clinical, Epidemiological and Immunological Aspects. Pediatr. Allergy Immunol. 1994, 5, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.C.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.U.A.; Santoro, A.; et al. Non-IgE- or Mixed IgE/Non-IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen-Seppo, K.M. Milk Allergy: Clinical Features and Diagnosis—UpToDate. Available online: https://www.uptodate.com/contents/milk-allergy-clinical-features-and-diagnosis?search=Milk%20allergy:%20Clinical%20features%20and%20diagnosis.%20UptoDate&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#topicContent (accessed on 23 April 2023).
- Sampson, H.A. Food Allergy. Part 1: Immunopathogenesis and Clinical Disorders. J. Allergy Clin. Immunol. 1999, 103, 717–728. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non-IgE-Mediated Gastrointestinal Food Allergy. J. Allergy Clin. Immunol. 2015, 135, 1114–1124. [Google Scholar] [CrossRef]
- Cianferoni, A. Non-IgE Mediated Food Allergy. Curr. Pediatr. Rev. 2020, 16, 95–105. [Google Scholar] [CrossRef]
- Matthai, J.; Sathiasekharan, M.; Poddar, U.; Sibal, A.; Srivastava, A.; Waikar, Y.; Malik, R.; Ray, G.; Geetha, S.; Yachha, S.K.; et al. Guidelines on Diagnosis and Management of Cow’s Milk Protein Allergy. Indian Pediatr. 2020, 57, 723–729. [Google Scholar] [CrossRef]
- Venter, C.; Brown, T.; Meyer, R.; Walsh, J.; Shah, N.; Nowak-Węgrzyn, A.; Chen, T.-X.; Fleischer, D.M.; Heine, R.G.; Levin, M.; et al. Better Recognition, Diagnosis and Management of Non-IgE-Mediated Cow’s Milk Allergy in Infancy: IMAP-an International Interpretation of the MAP (Milk Allergy in Primary Care) Guideline. Clin. Transl. Allergy 2017, 7, 26. [Google Scholar] [CrossRef]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr. Allergy Immunol. 2010, 21 (Suppl. 21), 1–125. [Google Scholar] [CrossRef]
- Dupont, C.; Chouraqui, J.-P.; Linglart, A.; Bocquet, A.; Darmaun, D.; Feillet, F.; Frelut, M.-L.; Girardet, J.-P.; Hankard, R.; Rozé, J.-C.; et al. Nutritional Management of Cow’s Milk Allergy in Children: An Update. Arch. Pediatr. 2018, 25, 236–243. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T.; Standards of Care Committee (SOCC) of the British Society for Allergy and Clinical Immunology (BSACI). BSACI Guideline for the Diagnosis and Management of Cow’s Milk Allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Espín Jaime, B.; Díaz Martín, J.J.; Blesa Baviera, L.C.; Claver Monzón, Á.; Hernández Hernández, A.; García Burriel, J.I.; Mérida, M.J.G.; Pinto Fernández, C.; Coronel Rodríguez, C.; Román Riechmann, E.; et al. Non-IgE-mediated cow’s milk allergy: Consensus document of the Spanish Society of Paediatric Gastroenterology, Hepatology, and Nutrition (SEGHNP), the Spanish Association of Paediatric Primary Care (AEPAP), the Spanish Society of Extra-hospital Paediatrics and Primary Health Care (SEPEAP), and the Spanish Society of Paediatric ClinicaL Immunology, Allergy, and Asthma (SEICAP). An. Pediatría 2019, 90, 193.e1. [Google Scholar] [CrossRef]
- de Boissieu, D.; Dupont, C. Allergy to Extensively Hydrolyzed Cow’s Milk Proteins in Infants: Safety and Duration of Amino Acid-Based Formula. J. Pediatr. 2002, 141, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A Workshop Report on the Development of the Cow’s Milk-Related Symptom Score Awareness Tool for Young Children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Bajerova, K.; Dupont, C.; Eigenmann, P.; Kuitunen, M.; Meyer, R.; Ribes-Koninckx, C.; Salvatore, S.; Shamir, R.; Szajewska, H. The Cow’s Milk Related Symptom Score: The 2022 Update. Nutrients 2022, 14, 2682. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Koletzko, S.; Isolauri, E.; Hill, D.; Oranje, A.P.; Brueton, M.; Staiano, A.; Dupont, C. Guidelines for the Diagnosis and Management of Cow’s Milk Protein Allergy in Infants. Arch. Dis. Child. 2007, 92, 902–908. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and Management of Food Allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Agostoni, C.; Terracciano, L.; Varin, E.; Fiocchi, A. The Nutritional Value of Protein-Hydrolyzed Formulae. Crit. Rev. Food Sci. Nutr. 2016, 56, 65–69. [Google Scholar] [CrossRef]
- Borschel, M.W.; Ziegler, E.E.; Wedig, R.T.; Oliver, J.S. Growth of Healthy Term Infants Fed an Extensively Hydrolyzed Casein-Based or Free Amino Acid-Based Infant Formula: A Randomized, Double-Blind, Controlled Trial. Clin. Pediatr. 2013, 52, 910–917. [Google Scholar] [CrossRef]
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Pract. 2018, 6, 383–399. [Google Scholar] [CrossRef]
- Tosca, M.A.; Olcese, R.; Marinelli, G.; Schiavetti, I.; Ciprandi, G. Oral Immunotherapy for Children with Cow’s Milk Allergy: A Practical Approach. Children 2022, 9, 1872. [Google Scholar] [CrossRef] [PubMed]
- Cronin, C.; Ramesh, Y.; De Pieri, C.; Velasco, R.; Trujillo, J. “Early Introduction” of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management. Nutrients 2023, 15, 1397. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, Y.; Pu, X.; Chen, J. Oral Immunotherapy for Immunoglobulin E-Mediated Cow’s Milk Allergy in Children: A Systematic Review and Meta Analysis. Immun. Inflamm. Dis. 2022, 10, e704. [Google Scholar] [CrossRef]
- Akarsu, A.; Brindisi, G.; Fiocchi, A.; Zicari, A.M.; Arasi, S. Oral Immunotherapy in Food Allergy: A Critical Pediatric Perspective. Front. Pediatr. 2022, 10, 842196. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the Epithelial Barrier Hypothesis Explain the Increase in Allergy, Autoimmunity and Other Chronic Conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial Barrier Hypothesis: Effect of the External Exposome on the Microbiome and Epithelial Barriers in Allergic Disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Yazici, D.; Ogulur, I.; Kucukkase, O.; Li, M.; Rinaldi, A.O.; Pat, Y.; Wallimann, A.; Wawrocki, S.; Celebi Sozener, Z.; Buyuktiryaki, B.; et al. Epithelial Barrier Hypothesis and the Development of Allergic and Autoimmune Diseases. Allergo J. Int. 2022, 31, 91–102. [Google Scholar] [CrossRef]
- Akdis, C.A. The Epithelial Barrier Hypothesis Proposes a Comprehensive Understanding of the Origins of Allergic and Other Chronic Noncommunicable Diseases. J. Allergy Clin. Immunol. 2022, 149, 41–44. [Google Scholar] [CrossRef]
- Vickery, B.P.; Scurlock, A.M.; Jones, S.M.; Burks, A.W. Mechanisms of Immune Tolerance Relevant to Food Allergy. J. Allergy Clin. Immunol. 2011, 127, 576–584, quiz 585–586. [Google Scholar] [CrossRef]
- Iweala, O.I.; Nagler, C.R. The Microbiome and Food Allergy. Annu. Rev. Immunol. 2019, 37, 377–403. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed. Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, U.; Sikder, S.; Lee, D.; Fisher, C. T Follicular Helper Cells in IgE-Mediated Pathologies. Curr. Opin. Immunol. 2022, 74, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; MacLennan, I.C.M. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef]
- Qi, H. T Follicular Helper Cells in Space-Time. Nat. Rev. Immunol. 2016, 16, 612–625. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, C.-L.; Yu, D.; Liu, Z. Roles of Follicular Helper and Regulatory T Cells in Allergic Diseases and Allergen Immunotherapy. Allergy 2021, 76, 456–470. [Google Scholar] [CrossRef]
- Grydziuszko, E.; Phelps, A.; Bruton, K.; Jordana, M.; Koenig, J.F.E. Heterogeneity, Subsets, and Plasticity of T Follicular Helper Cells in Allergy. J. Allergy Clin. Immunol. 2022, 150, 990–998. [Google Scholar] [CrossRef]
- Dolence, J.J.; Kobayashi, T.; Iijima, K.; Krempski, J.; Drake, L.Y.; Dent, A.L.; Kita, H. Airway Exposure Initiates Peanut Allergy by Involving the IL-1 Pathway and T Follicular Helper Cells in Mice. J. Allergy Clin. Immunol. 2018, 142, 1144–1158.e8. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Baumjohann, D.; Craft, J.; Fazilleau, N.; Ma, C.S.; Tangye, S.G.; Vinuesa, C.G.; Linterman, M.A. CD4+ T Cells That Help B Cells—A Proposal for Uniform Nomenclature. Trends Immunol. 2021, 42, 658–669. [Google Scholar] [CrossRef]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T Follicular Helper Cell Subset That Drives Anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.M.; Chen, Q.; Liu, H.; Yang, K.; Koh, B.; Wu, H.; Maleki, S.J.; Hurlburt, B.K.; Cook-Mills, J.; Kaplan, M.H.; et al. T Follicular Regulatory Cells and IL-10 Promote Food Antigen-Specific IgE. J. Clin. Invest. 2020, 130, 3820–3832. [Google Scholar] [CrossRef] [PubMed]
- Suto, A.; Nakajima, H.; Hirose, K.; Suzuki, K.; Kagami, S.; Seto, Y.; Hoshimoto, A.; Saito, Y.; Foster, D.C.; Iwamoto, I. Interleukin 21 Prevents Antigen-Induced IgE Production by Inhibiting Germ Line C(Epsilon) Transcription of IL-4-Stimulated B Cells. Blood 2002, 100, 4565–4573. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, C.-A.M.; Targ, S.; Allen, C.D.C. IL-21 Is a Broad Negative Regulator of IgE Class Switch Recombination in Mouse and Human B Cells. J. Exp. Med. 2020, 217, e20190472. [Google Scholar] [CrossRef]
- Sage, P.T.; Sharpe, A.H. The Multifaceted Functions of Follicular Regulatory T Cells. Curr. Opin. Immunol. 2020, 67, 68–74. [Google Scholar] [CrossRef]
- Lu, Y.; Craft, J. T Follicular Regulatory Cells: Choreographers of Productive Germinal Center Responses. Front. Immunol. 2021, 12, 679909. [Google Scholar] [CrossRef]
- Wing, J.B.; Lim, E.L.; Sakaguchi, S. Control of Foreign Ag-Specific Ab Responses by Treg and Tfr. Immunol. Rev. 2020, 296, 104–119. [Google Scholar] [CrossRef]
- Gonzalez-Figueroa, P.; Roco, J.A.; Papa, I.; Núñez Villacís, L.; Stanley, M.; Linterman, M.A.; Dent, A.; Canete, P.F.; Vinuesa, C.G. Follicular Regulatory T Cells Produce Neuritin to Regulate B Cells. Cell 2021, 184, 1775–1789.e19. [Google Scholar] [CrossRef]
- Sampath, V.; Sindher, S.B.; Alvarez Pinzon, A.M.; Nadeau, K.C. Can Food Allergy Be Cured? What Are the Future Prospects? Allergy 2020, 75, 1316–1326. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.-Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Ravel, J. The Vocabulary of Microbiome Research: A Proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; McCray, A.T. ‘Ome Sweet ‘Omics—A Genealogical Treasury of Words. Scientist 2001, 15, 8. [Google Scholar]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and Cellular Mechanisms of Food Allergy and Food Tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of Short-Chain Fatty Acids and Their Receptors in Inflammation and Carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D.; Cait, J.; Sbihi, H.; Stiemsma, L.; Subbarao, P.; Mandhane, P.J.; et al. Reduced Genetic Potential for Butyrate Fermentation in the Gut Microbiome of Infants Who Develop Allergic Sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647.e3. [Google Scholar] [CrossRef] [PubMed]
- Herbst, T.; Sichelstiel, A.; Schär, C.; Yadava, K.; Bürki, K.; Cahenzli, J.; McCoy, K.; Marsland, B.J.; Harris, N.L. Dysregulation of Allergic Airway Inflammation in the Absence of Microbial Colonization. Am. J. Respir. Crit. Care Med. 2011, 184, 198–205. [Google Scholar] [CrossRef]
- Cahenzli, J.; Köller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal Microbial Diversity during Early-Life Colonization Shapes Long-Term IgE Levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef]
- Smolinska, S.; Jutel, M.; Crameri, R.; O’Mahony, L. Histamine and Gut Mucosal Immune Regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef]
- Jutel, M.; Watanabe, T.; Klunker, S.; Akdis, M.; Thomet, O.A.; Malolepszy, J.; Zak-Nejmark, T.; Koga, R.; Kobayashi, T.; Blaser, K.; et al. Histamine Regulates T-Cell and Antibody Responses by Differential Expression of H1 and H2 Receptors. Nature 2001, 413, 420–425. [Google Scholar] [CrossRef]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L.; et al. Healthy Infants Harbor Intestinal Bacteria That Protect against Food Allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Yang, Y.; Shoaie, S.; Zhang, C.; Ji, B.; Wei, Y. Advances in the Relationships Between Cow’s Milk Protein Allergy and Gut Microbiota in Infants. Front. Microbiol. 2021, 12, 716667. [Google Scholar] [CrossRef]
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut Microbiota, Probiotics, and Their Interactions in Prevention and Treatment of Atopic Dermatitis: A Review. Front. Immunol. 2021, 12, 720393. [Google Scholar] [CrossRef] [PubMed]
- Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Di Costanzo, M.; Buono, A.; Bruno, C.; Berni Canani, R. Targeting Food Allergy with Probiotics. Adv. Exp. Med. Biol. 2019, 1125, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Tosca, M.A. Probiotics in Children with Asthma. Children 2022, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ni, B.; Liu, Z.; Liu, X.; Xie, W.; Wu, I.X.Y.; Li, X. The Role of Probiotics in the Prevention and Treatment of Atopic Dermatitis in Children: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Paediatr. Drugs 2020, 22, 535–549. [Google Scholar] [CrossRef]
- Zhao, M.; Shen, C.; Ma, L. Treatment Efficacy of Probiotics on Atopic Dermatitis, Zooming in on Infants: A Systematic Review and Meta-Analysis. Int. J. Dermatol. 2018, 57, 635–641. [Google Scholar] [CrossRef]
- Elazab, N.; Mendy, A.; Gasana, J.; Vieira, E.R.; Quizon, A.; Forno, E. Probiotic Administration in Early Life, Atopy, and Asthma: A Meta-Analysis of Clinical Trials. Pediatrics 2013, 132, e666–e676. [Google Scholar] [CrossRef]
- Berni Canani, R.; Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Maddalena, Y.; Buono, A.; Bruno, C.; Voto, L.; Ercolini, D. Gut Microbiome as Target for Innovative Strategies Against Food Allergy. Front. Immunol. 2019, 10, 191. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Todorov, S.D.; Furtado, D.N.; Saad, S.M.; Gombossy de Melo Franco, B.D. Bacteriocin Production and Resistance to Drugs Are Advantageous Features for Lactobacillus Acidophilus La-14, a Potential Probiotic Strain. New Microbiol. 2011, 34, 357–370. [Google Scholar]
- Butel, M.-J. Probiotics, Gut Microbiota and Health. Médecine Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- de Moreno de LeBlanc, A.; Dogi, C.A.; Galdeano, C.M.; Carmuega, E.; Weill, R.; Perdigón, G. Effect of the Administration of a Fermented Milk Containing Lactobacillus Casei DN-114001 on Intestinal Microbiota and Gut Associated Immune Cells of Nursing Mice and after Weaning until Immune Maturity. BMC Immunol. 2008, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 Mucin Secretion Follows Adherence of Lactobacillus Strains to Intestinal Epithelial Cells in Vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef]
- Mattar, A.F.; Teitelbaum, D.H.; Drongowski, R.A.; Yongyi, F.; Harmon, C.M.; Coran, A.G. Probiotics Up-Regulate MUC-2 Mucin Gene Expression in a Caco-2 Cell-Culture Model. Pediatr. Surg. Int. 2002, 18, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.-M.; Podolsky, D.K. Functional Modulation of Enterocytes by Gram-Positive and Gram-Negative Microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G613–G626. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Live Probiotics Protect Intestinal Epithelial Cells from the Effects of Infection with Enteroinvasive Escherichia Coli (EIEC). Gut 2003, 52, 988–997. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Eslami, M.; Bahar, A.; Keikha, M.; Karbalaei, M.; Kobyliak, N.M.; Yousefi, B. Probiotics Function and Modulation of the Immune System in Allergic Diseases. Allergol. Immunopathol. 2020, 48, 771–788. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigón, G. Role of Viability of Probiotic Strains in Their Persistence in the Gut and in Mucosal Immune Stimulation. J. Appl. Microbiol. 2004, 97, 673–681. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Xiong, N.; Hu, S. Regulation of Intestinal IgA Responses. Cell. Mol. Life. Sci. 2015, 72, 2645–2655. [Google Scholar] [CrossRef]
- Phalipon, A.; Cardona, A.; Kraehenbuhl, J.P.; Edelman, L.; Sansonetti, P.J.; Corthésy, B. Secretory Component: A New Role in Secretory IgA-Mediated Immune Exclusion in Vivo. Immunity 2002, 17, 107–115. [Google Scholar] [CrossRef]
- Kadaoui, K.A.; Corthésy, B. Secretory IgA Mediates Bacterial Translocation to Dendritic Cells in Mouse Peyer’s Patches with Restriction to Mucosal Compartment. J. Immunol. 2007, 179, 7751–7757. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Macpherson, A.J. Immune Adaptations That Maintain Homeostasis with the Intestinal Microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Perdigón, G.; Maldonado Galdeano, C.; Valdez, J.C.; Medici, M. Interaction of Lactic Acid Bacteria with the Gut Immune System. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 4), S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Cao, S.; Cui, J.Z.; Matsubara, J.A. Immuno-Modulatory Effect of IFN-Gamma in AMD and Its Role as a Possible Target for Therapy. J. Clin. Exp. Ophthalmol. 2013, S2, 71–76. [Google Scholar] [CrossRef]
- Pochard, P.; Gosset, P.; Grangette, C.; Andre, C.; Tonnel, A.-B.; Pestel, J.; Mercenier, A. Lactic Acid Bacteria Inhibit TH2 Cytokine Production by Mononuclear Cells from Allergic Patients. J. Allergy Clin. Immunol. 2002, 110, 617–623. [Google Scholar] [CrossRef]
- Niers, L.E.M.; Timmerman, H.M.; Rijkers, G.T.; van Bleek, G.M.; van Uden, N.O.P.; Knol, E.F.; Kapsenberg, M.L.; Kimpen, J.L.L.; Hoekstra, M.O. Identification of Strong Interleukin-10 Inducing Lactic Acid Bacteria Which down-Regulate T Helper Type 2 Cytokines. Clin. Exp. Allergy 2005, 35, 1481–1489. [Google Scholar] [CrossRef]
- Nonaka, Y.; Izumo, T.; Izumi, F.; Maekawa, T.; Shibata, H.; Nakano, A.; Kishi, A.; Akatani, K.; Kiso, Y. Antiallergic Effects of Lactobacillus Pentosus Strain S-PT84 Mediated by Modulation of Th1/Th2 Immunobalance and Induction of IL-10 Production. Int. Arch. Allergy Immunol. 2008, 145, 249–257. [Google Scholar] [CrossRef]
- Hol, J.; van Leer, E.H.G.; Elink Schuurman, B.E.E.; de Ruiter, L.F.; Samsom, J.N.; Hop, W.; Neijens, H.J.; de Jongste, J.C.; Nieuwenhuis, E.E.S. Cow’s Milk Allergy Modified by Elimination and Lactobacilli study group The Acquisition of Tolerance toward Cow’s Milk through Probiotic Supplementation: A Randomized, Controlled Trial. J. Allergy Clin. Immunol. 2008, 121, 1448–1454. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of Lactobacillus GG on Tolerance Acquisition in Infants with Cow’s Milk Allergy: A Randomized Trial. J. Allergy Clin. Immunol. 2012, 129, 580–582. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Steenhout, P.; Planoudis, Y.; Grathwohl, D.; Althera Study Group. Treating Cow’s Milk Protein Allergy: A Double-Blind Randomized Trial Comparing Two Extensively Hydrolysed Formulas with Probiotics. Acta Paediatr. 2013, 102, 990–998. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula Selection for Management of Children with Cow’s Milk Allergy Influences the Rate of Acquisition of Tolerance: A Prospective Multicenter Study. J. Pediatr. 2013, 163, 771–777.e1. [Google Scholar] [CrossRef] [PubMed]
- Ahanchian, H.; Nouri, Z.; Jafari, S.-A.; Moghiman, T.; Amirian, M.-H.; Ezzati, A.; Kianifar, H.-R. Synbiotics in Children with Cow’s Milk Allergy: A Randomized Controlled Trial. Iran J. Pediatr. 2014, 24, 29–34. [Google Scholar]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively Hydrolyzed Casein Formula Containing Lactobacillus Rhamnosus GG Reduces the Occurrence of Other Allergic Manifestations in Children with Cow’s Milk Allergy: 3-Year Randomized Controlled Trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef] [PubMed]
- Candy, D.C.A.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Wopereis, H.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E.; et al. A Synbiotic-Containing Amino-Acid-Based Formula Improves Gut Microbiota in Non-IgE-Mediated Allergic Infants. Pediatr. Res. 2018, 83, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, R.; Di Costanzo, M.; Bedogni, G.; Cosenza, L.; Maddalena, Y.; Di Scala, C.; Della Gatta, G.; Carucci, L.; Voto, L.; Coppola, S.; et al. Dietary Treatment with Extensively Hydrolyzed Casein Formula Containing the Probiotic Lactobacillus Rhamnosus GG Prevents the Occurrence of Functional Gastrointestinal Disorders in Children with Cow’s Milk Allergy. J. Pediatr. 2019, 213, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Fuller, G.W. Effectiveness of Using an Extensively Hydrolyzed Casein Formula Supplemented with Lactobacillus Rhamnosus GG Compared with an Extensively Hydrolysed Whey Formula in Managing Cow’s Milk Protein Allergic Infants. J. Comp. Eff. Res. 2019, 8, 1317–1326. [Google Scholar] [CrossRef]
- Jing, W.; Liu, Q.; Wang, W. Bifidobacterium Bifidum TMC3115 Ameliorates Milk Protein Allergy in by Affecting Gut Microbiota: A Randomized Double-Blind Control Trial. J. Food Biochem. 2020, 44, e13489. [Google Scholar] [CrossRef]
- Basturk, A.; Isik, İ.; Atalay, A.; Yılmaz, A. Investigation of the Efficacy of Lactobacillus Rhamnosus GG in Infants With Cow’s Milk Protein Allergy: A Randomised Double-Blind Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2020, 12, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, K.; Cawood, A.L.; Cooke, L.H.; Acosta-Mena, D.; Stratton, R.J. The Use of an Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy-Effect on Clinical Outcomes. Nutrients 2021, 13, 2205. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Atwal, K.; Graham, L.; Narayanan, S.; Cooke, L.; Casewell, C.; Denton, S.-A.; Gavin, J.; Browne, R.M.; Kinnear, F.J.; et al. Synbiotic Containing Extensively Hydrolyzed Formula Improves Gastrointestinal and Atopic Symptom Severity, Growth, Caregiver Quality of Life, and Hospital-Related Healthcare Use in Infants with Cow’s Milk Allergy. Immun. Inflamm. Dis. 2022, 10, e636. [Google Scholar] [CrossRef] [PubMed]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Harthoorn, L.F.; Langford, J.E.; et al. Tolerance Development in Cow’s Milk-Allergic Infants Receiving Amino Acid-Based Formula: A Randomized Controlled Trial. J. Allergy Clin. Immunol. 2022, 149, 650–658.e5. [Google Scholar] [CrossRef]
- Nocerino, R.; Coppola, S.; Carucci, L.; de Giovanni di Santa Severina, A.F.; Oglio, F.; De Michele, R.; Di Sessa, I.; Masino, A.; Bedogni, G.; Berni Canani, R. The Step-down Approach in Children with Cow’s Milk Allergy: Results of a Randomized Controlled Trial. Allergy, 2023; in print. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Sato, M.; Toyokuni, K.; Irahara, M.; Hiraide-Kotaki, E.; Harima-Mizusawa, N.; Morita, H.; Matsumoto, K.; Ohya, Y. Combination of Heat-Killed Lactiplantibacillus Plantarum YIT 0132 (LP0132) and Oral Immunotherapy in Cow’s Milk Allergy: A Randomised Controlled Trial. Benef. Microbes 2023, 14, 17–29. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Vitale, A.; Perna, F.; Garziano, F.; Dolce, P.; Vitale, S.; Micillo, T.; Oglio, F.; Del Giudice, M.M.; Matarese, G.; et al. Bifidobacteria Modulate Immune Response in Pediatric Patients with Cow’s Milk Protein Allergy. Pediatr. Res. 2023. in print. [Google Scholar] [CrossRef] [PubMed]


| Author, Year, Nationality | Study Design | Sample Size at Baseline | Sample Size at Follow-Up | Probiotics | Period of Administration | Follow-Up | Results |
|---|---|---|---|---|---|---|---|
| Hol et al., 2008, Netherlands [113] | Randomized double-blind placebo-controlled study | 119 (1.4–6.0 months) SG = 59 CG = 60 | At 6 months: 111 infants At 12 months: 48 infants SG = 23 CG = 25 | Lactobacillus casei CRL431 Bifidobacterium lactis Bb-12 | 4 weeks | 6–12 months | At 6 and 12 months, no difference in obtaining CT (p = 0.92, p = 0.58). |
| Berni Canani et al., 2012, Italy [114] | Randomized trial | 80 infants (1–12 months) CG = 40 SG = 40 | Initial groups: CG = 36 SG = 37 At 1 month: CG = 28 SG = 27 At 6 months: CG = 22 SG = 11 At 12 months: CG = 13 SG = 5 | Lactobacillus rhamnosus GG | 12 months | 1–6–12 months | Supplementation of eHCF with LGG accelerated CT to CMP. |
| Vandenplas et al., 2013, Belgium [115] | Double-blind randomized trial | 116 infants | 85 infants eHWF = 41 eHCF = 44 | Bifidobacterium lactis, Lactobacillus rhamnosus GG | 1 month | Until 1 year | eHWF leads to CT faster than eHCF (p = 0.037). The SBS decreased significantly (p < 0.001) in both groups. |
| Berni Canani et al., 2013, Italy [116] | Open prospective non-randomized trial | 260 infants (0–12 months) eHCF = 55 eHCF + LGG = 71 HRF = 46 SF = 55 AAF = 33 | 260 infants | Lactobacillus rhamnosus GG | 12 months | 12 months | Achieving CT at 12 months more frequently in eHCF and eHCF + LGG groups (p < 0.05). |
| Ahanchian et al., 2014, Iran [117] | Double-blind randomized trial | 45 infants: (1–12 months) SG = 21 CG = 24 | 32 infants: SG = 16 CG = 16 | Symbiotic: Lactobacillus casei, Lactobacillus rhamnosus, Streptooccus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus bulgaricus and FOS | At least 1 week | 72 h, 1 week, 2 weeks, 3 months | No significant differences in daily vomiting or diarrhea in SG and CG (p > 0.005). Improvement in rectal bleeding and intestinal colic in both groups (p > 0.005). |
| Berni Canani et al., 2017, Italy [118] | Parallel arm randomized controlled trial | 220 infants (1–12 months) SG = 110 CG = 110 | At 12 months: SG = 108 CG = 107 At 24 months: SG = 103 CG = 101 At 36 months: 193 infants: SG = 98 CG = 95 | Lactobacillus rhamnosus GG | 36 months | 12, 24, 36 months | At 36 months lower risk (p < 0.001) in developing another allergy and significantly higher probability of acquiring CT at 36 months (p < 0.001). |
| Candy et al., 2018, UK, Italy, Belgium, Sweden [119] | Multicenter double-blind, randomized controlled trial | 122 subjects under 13 months CG = 36 SG = 35 HS = 51 | CG = 28 SG = 32 | Prebiotic blend of chicory-derived neutral oligofructose and long-chain inulin and a probiotic strain Bifidobacterium breve M-16 V | 8 weeks | 0, 8, 12, and 26 weeks | The stool frequency score was lower in the SG than in the CG (p = 0.015). |
| Nocerino et al., 2019, Italy [120] | Non-randomized trial | 330 subjects, (4–6 years) CG = 110 SG = 110 HS = 110 | = | Lactobacillus rhamnosus GG | 12 months | 5 years | eHCF + LGG could lower the occurrence of FGIDs in patients with history of CMPA (p < 0.001). |
| Guest et al., 2019, UK [121] | Retrospective cohort analysis | 940 infants under 1 year CG = 470 SG = 470 | = | Lactobacillus rhamnosus GG | Mean duration: 8.3 ± 5.30 months | 24 months after starting the formula | eHCF + LGG accelerated CT (p = 0.001). Clinical wellbeing in SG infants compared to CG (p < 0.02). |
| Jing et al., 2020, China [122] | Double-blind, randomized controlled trial | 256 infants (0.5 to 12 months) SG = 128 CG = 128 | At 6 months: 244 infants: SG = 123 CG = 121 | Bifidobacterium bifidum TMC3115 | 6 months | 6 months | Allergic symptoms improvement in SG (p < 0.05). |
| Basturk et al., 2020, Turkey [123] | Randomized multicenter double-blind placebo-controlled study | 106 infants (0–12 months) SG = 51 CG = 55 | At 4 weeks: 100 infants: SG = 48 CG = 52 | Lactobacillus rhamnosus GG | 4 weeks | 4 weeks | Clinical improvements in both groups |
| Sorensen et al., 2021, UK [124] | Retrospective matched cohort study | 148 infants SG = 74 CG = 74 (0–12 months) in the analyzed database | / | Symbiotic: Bifidobacterium breve M16-V and prebiotics (including chicory-derived oligo-fructose and long-chain inulin) | Mean duration: SG: 6.65 ± 5.30 months CG: 8.44 ± 5.62 months | Mean observation period: 1.19 years | In SG, improvement in clinical symptoms and better prognosis (p < 0.001). |
| Hubbard et al., 2022, UK [125] | Prospective single-arm longitudinal interventional multicenter study | 33 infants (<13 months) | 29 infants | SeHF with galacto-oligosaccharides, fructo-oligosaccharides, and Bifidobacterium Breve M-16V | 28 days | 6 months before and 6 months after SeHF initiation | Improvements in GI symptoms (p ≤ 0.005). |
| Chatchatee et al., 2022, Thailand [126] | Multicenter prospective randomized double-blind controlled clinical study | 169 infants (<13 months) SG = 80 CG = 89 | At 12 months: 63 infants: SG = 71 CG = 81 At 24 months: 55 infants: SG = 64 CG = 71 | Prebiotic oligosaccharides (oligofructose, inulin) and probiotic Bifidobacterium breve M-16V | 12 months | 12–24 months | At 12 and 24 months, no difference in obtaining CT (p = 0.401, p = 0.53). |
| Nocerino et al., 2023, Italy [127] | Randomized controlled trial | 59 infants (<6 months) AAF = 30 EHCF + LGG = 29 | = | Lacticaseibacillus rhamnosus GG | Until 12 months of age | 12 months | Step-down from AAF to EHCF + LGG could facilitate the CT. |
| Yamamoto-Hanada et al., 2023, Japan [128] | Double-blind randomized two-arm parallel group placebo-controlled phase 2 trial | 61 children (1–18 years) SG = 31 CG = 30 | 59 children: SG = 30 CG = 29 | Lactiplantibacillus plantarum YIT 0132 (LP0132) | 24 weeks | 24 weeks | No significant differences between two groups in CT (p = 1.00). |
| Strisciuglio et al., 2023, Italy [129] | Prospective non-randomized pilot trial | 8 infants (6–12 months) | = | Bifidobacterium Longum BB536, Bifidobacterium Infantis M-63, Bifidobacterium breve M-16V | 45 days | After 45 days and 45 days after the probiotic wash-out | Bifidobacteria could have a role in the acquisition of CT to CMPs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cela, L.; Brindisi, G.; Gravina, A.; Pastore, F.; Semeraro, A.; Bringheli, I.; Marchetti, L.; Morelli, R.; Cinicola, B.; Capponi, M.; et al. Molecular Mechanism and Clinical Effects of Probiotics in the Management of Cow’s Milk Protein Allergy. Int. J. Mol. Sci. 2023, 24, 9781. https://doi.org/10.3390/ijms24129781
Cela L, Brindisi G, Gravina A, Pastore F, Semeraro A, Bringheli I, Marchetti L, Morelli R, Cinicola B, Capponi M, et al. Molecular Mechanism and Clinical Effects of Probiotics in the Management of Cow’s Milk Protein Allergy. International Journal of Molecular Sciences. 2023; 24(12):9781. https://doi.org/10.3390/ijms24129781
Chicago/Turabian StyleCela, Ludovica, Giulia Brindisi, Alessandro Gravina, Francesca Pastore, Antonio Semeraro, Ivana Bringheli, Lavinia Marchetti, Rebecca Morelli, Bianca Cinicola, Martina Capponi, and et al. 2023. "Molecular Mechanism and Clinical Effects of Probiotics in the Management of Cow’s Milk Protein Allergy" International Journal of Molecular Sciences 24, no. 12: 9781. https://doi.org/10.3390/ijms24129781
APA StyleCela, L., Brindisi, G., Gravina, A., Pastore, F., Semeraro, A., Bringheli, I., Marchetti, L., Morelli, R., Cinicola, B., Capponi, M., Gori, A., Pignataro, E., Piccioni, M. G., Zicari, A. M., & Anania, C. (2023). Molecular Mechanism and Clinical Effects of Probiotics in the Management of Cow’s Milk Protein Allergy. International Journal of Molecular Sciences, 24(12), 9781. https://doi.org/10.3390/ijms24129781







