Roles of Fibroblast Growth Factors in the Axon Guidance
Abstract
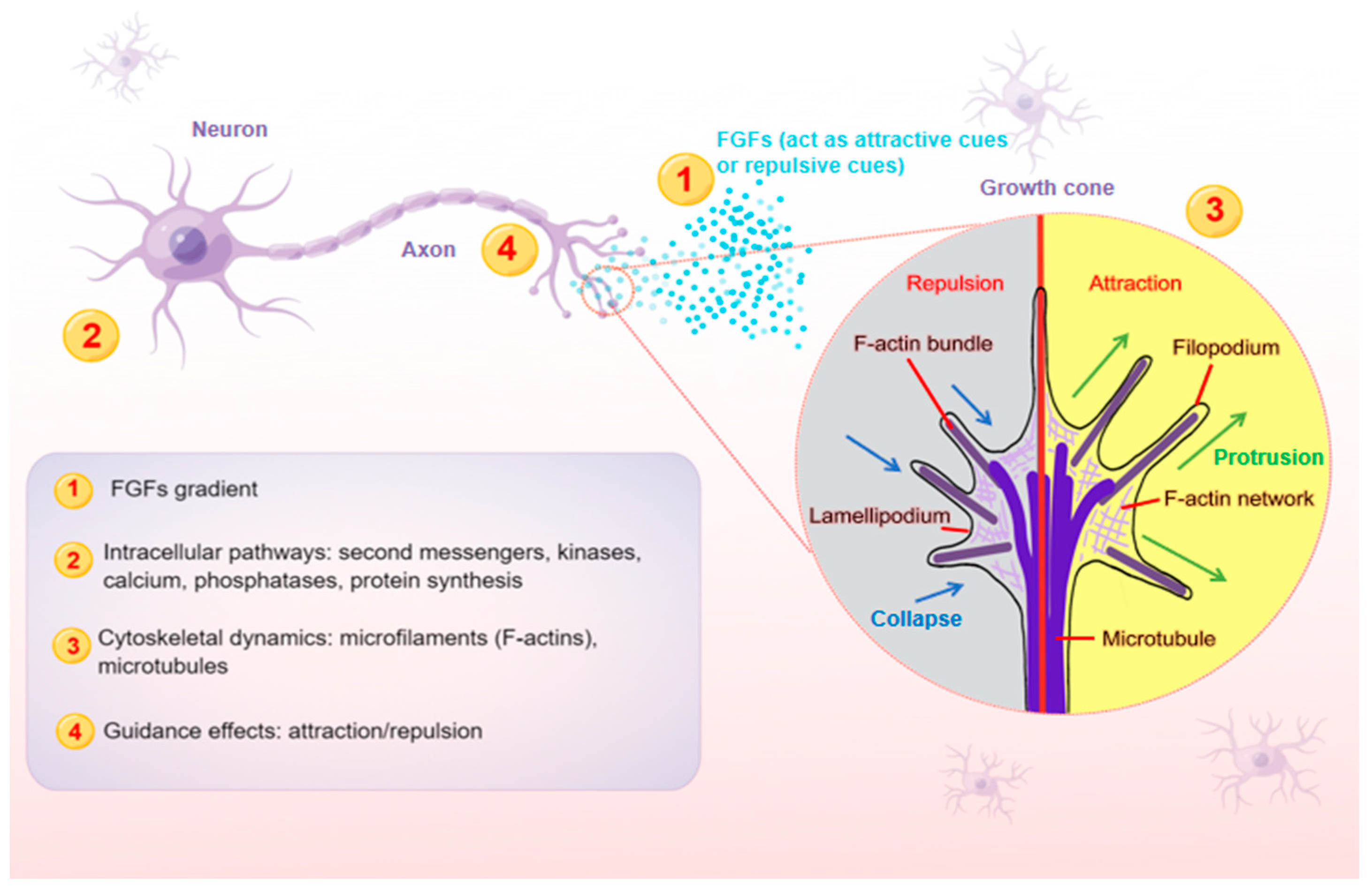
1. Introduction
2. Background of FGFs in the Development of Nervous System
3. Effects of FGFs on the Axon Guidance
3.1. Direct Axon Guidance Effects of FGFs in Vertebrates
3.2. Indirect Axon Guidance Effects of FGFs in Vertebrates
3.3. FGF’s Axon Guidance Functions in Invertebrates
4. Concentration-Dependent Responses of Growth Cones to FGFs
4.1. Continuous Changed Responses of the Same Axons over Time and Space
4.2. The Molecular Mechanism behind the Concentration-Dependent Responses
5. FGFs and FGFRs Interaction
5.1. Why FGFs Exert Distinct Effects on Different Axons
5.2. The Importance of the FGF-FGFR System in Axon Pathfinding
6. Common Downstream Signaling Mechanisms
6.1. Three Major FGF Signaling Pathways
6.2. Common Cytoskeletal Alterations
6.3. Recruitment and Convergence of FGF Signaling Pathways
7. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seiradake, E.; Jones, E.Y.; Klein, R. Structural Perspectives on Axon Guidance. Annu. Rev. Cell Dev. Biol. 2016, 32, 577–608. [Google Scholar] [CrossRef] [PubMed]
- Marin, O.; Valiente, M.; Ge, X.; Tsai, L.H. Guiding neuronal cell migrations. Cold Spring Harb. Perspect. Biol. 2010, 2, a001834. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Chance, R.K.; Bashaw, G.J. Axon growth and guidance: Receptor regulation and signal transduction. Annu. Rev. Neurosci. 2009, 32, 383–412. [Google Scholar] [CrossRef]
- Tessier-Lavigne, M.; Goodman, C.S. The molecular biology of axon guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, S.; Holt, C.E. Growth factors: A role in guiding axons? Trends Cell Biol. 1997, 7, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.T.; Charron, F. Signaling mechanisms of non-conventional axon guidance cues: The Shh, BMP and Wnt morphogens. Curr. Opin. Neurobiol. 2013, 23, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Kiecker, C.; Lumsden, A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat. Neurosci. 2004, 7, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Bluske, K.K.; Vue, T.Y.; Kawakami, Y.; Taketo, M.M.; Yoshikawa, K.; Johnson, J.E.; Nakagawa, Y. β-Catenin signaling specifies progenitor cell identity in parallel with Shh signaling in the developing mammalian thalamus. Development 2012, 139, 2692–2702. [Google Scholar] [CrossRef]
- Vue, T.Y.; Bluske, K.; Alishahi, A.; Yang, L.L.; Koyano-Nakagawa, N.; Novitch, B.; Nakagawa, Y. Sonic hedgehog signaling controls thalamic progenitor identity and nuclei specification in mice. J. Neurosci. 2009, 29, 4484–4497. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Itoh, N. The Fgf families in humans, mice, and zebrafish: Their evolutional processes and roles in development, metabolism, and disease. Biol. Pharm. Bull. 2007, 30, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Klimaschewski, L.; Claus, P. Fibroblast Growth Factor Signalling in the Diseased Nervous System. Mol. Neurobiol. 2021, 58, 3884–3902. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.A.; Eren-Kocak, E.; Inui, E.G.; Watson, S.J.; Akil, H. Dysregulated fibroblast growth factor (FGF) signaling in neurological and psychiatric disorders. Semin. Cell Dev. Biol. 2016, 53, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Gospodarowicz, D. Purification of a fibroblast growth factor from bovine pituitary. J. Biol. Chem. 1975, 250, 2515–2520. [Google Scholar] [CrossRef]
- Goetz, R.; Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013, 14, 166–180. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, reviews3005.1. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef]
- Nyhus, J.K.; Denburg, J.L. The in vivo regulation of pioneer axon growth by FGF-2 and heparan sulfate proteoglycans in cultured embryos of the cockroach. Mol. Cell. Neurosci. 1998, 11, 305–323. [Google Scholar] [CrossRef]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef]
- Mohammadi, M.; Olsen, S.K.; Ibrahimi, O.A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005, 16, 107–137. [Google Scholar] [CrossRef]
- Abraham, J.A.; Mergia, A.; Whang, J.L.; Tumolo, A.; Friedman, J.; Hjerrild, K.A.; Gospodarowicz, D.; Fiddes, J.C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science 1986, 233, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.A.; Whang, J.L.; Tumolo, A.; Mergia, A.; Friedman, J.; Gospodarowicz, D.; Fiddes, J.C. Human basic fibroblast growth factor: Nucleotide sequence and genomic organization. EMBO J. 1986, 5, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Baird, A. Fibroblast growth factors: Activities and significance of non-neurotrophin neurotrophic growth factors. Curr. Opin. Neurobiol. 1994, 4, 78–86. [Google Scholar] [CrossRef]
- Heikinheimo, M.; Lawshe, A.; Shackleford, G.M.; Wilson, D.B.; MacArthur, C.A. Fgf-8 expression in the post-gastrulation mouse suggests roles in the development of the face, limbs and central nervous system. Mech. Dev. 1994, 48, 129–138. [Google Scholar] [CrossRef]
- Crossley, P.H.; Martin, G.R. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 1995, 121, 439–451. [Google Scholar] [CrossRef]
- Crossley, P.H.; Martinez, S.; Martin, G.R. Midbrain development induced by FGF8 in the chick embryo. Nature 1996, 380, 66–68. [Google Scholar] [CrossRef]
- Chi, C.L.; Martinez, S.; Wurst, W.; Martin, G.R. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development 2003, 130, 2633–2644. [Google Scholar] [CrossRef]
- Basson, M.A.; Echevarria, D.; Ahn, C.P.; Sudarov, A.; Joyner, A.L.; Mason, I.J.; Martinez, S.; Martin, G.R. Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development 2008, 135, 889–898. [Google Scholar] [CrossRef]
- Chen, Y.; Mohammadi, M.; Flanagan, J.G. Graded levels of FGF protein span the midbrain and can instruct graded induction and repression of neural mapping labels. Neuron 2009, 62, 773–780. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Ornitz, D.M. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development 2000, 127, 1833–1843. [Google Scholar] [PubMed]
- Irving, C.; Malhas, A.; Guthrie, S.; Mason, I. Establishing the trochlear motor axon trajectory: Role of the isthmic organiser and Fgf8. Development 2002, 129, 5389–5398. [Google Scholar] [CrossRef]
- Nakamura, S.; Ito, Y.; Shirasaki, R.; Murakami, F. Local directional cues control growth polarity of dopaminergic axons along the rostrocaudal axis. J. Neurosci. 2000, 20, 4112–4119. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Sugiyama, S. Polarity and laminar formation of the optic tectum in relation to retinal projection. J. Neurobiol. 2004, 59, 48–56. [Google Scholar] [CrossRef]
- Volkmann, K.; Chen, Y.Y.; Harris, M.P.; Wullimann, M.F.; Koster, R.W. The zebrafish cerebellar upper rhombic lip generates tegmental hindbrain nuclei by long-distance migration in an evolutionary conserved manner. J. Comp. Neurol. 2010, 518, 2794–2817. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lyuksyutova, A.I. Morphogens as conserved axon guidance cues. Curr. Opin. Neurobiol. 2007, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M. FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. Bioessays 2000, 22, 108–112. [Google Scholar] [CrossRef]
- Dorey, K.; Amaya, E. FGF signalling: Diverse roles during early vertebrate embryogenesis. Development 2010, 137, 3731–3742. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J. Biochem. 2011, 149, 121–130. [Google Scholar] [CrossRef]
- Stifani, N. Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci. 2014, 8, 293. [Google Scholar] [CrossRef]
- Shirasaki, R.; Lewcock, J.W.; Lettieri, K.; Pfaff, S.L. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron 2006, 50, 841–853. [Google Scholar] [CrossRef]
- Fantetti, K.N.; Fekete, D.M. Members of the BMP, Shh, and FGF morphogen families promote chicken statoacoustic ganglion neurite outgrowth and neuron survival in vitro. Dev. Neurobiol. 2012, 72, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lv, Z.; Huang, H.; Yu, S.; Xiao, L.; Li, X.; Li, G.; Liu, F. FGF3 from the Hypothalamus Regulates the Guidance of Thalamocortical Axons. Dev. Neurosci. 2020, 42, 208–216. [Google Scholar] [CrossRef]
- Liu, K.; Lv, Z.; Huang, H.; Li, M.; Xiao, L.; Li, X.; Li, G.; Liu, F. FGF10 regulates thalamocortical axon guidance in the developing thalamus. Neurosci. Lett. 2020, 716, 134685. [Google Scholar] [CrossRef]
- Liu, F.; Pogoda, H.M.; Pearson, C.A.; Ohyama, K.; Lohr, H.; Hammerschmidt, M.; Placzek, M. Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis. Development 2013, 140, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mizushima, S.; Tamada, A.; Yamamoto, N.; Takashima, S.; Murakami, F. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J. Neurosci. 2009, 29, 4044–4055. [Google Scholar] [CrossRef]
- Webber, C.A.; Hyakutake, M.T.; McFarlane, S. Fibroblast growth factors redirect retinal axons In Vitro and In Vivo. Dev. Biol. 2003, 263, 24–34. [Google Scholar] [CrossRef]
- Webber, C.A.; Chen, Y.Y.; Hehr, C.L.; Johnston, J.; McFarlane, S. Multiple signaling pathways regulate FGF-2-induced retinal ganglion cell neurite extension and growth cone guidance. Mol. Cell. Neurosci. 2005, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Irving, C.; Mason, I. Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development 2000, 127, 177–186. [Google Scholar] [CrossRef]
- Partanen, J. FGF signalling pathways in development of the midbrain and anterior hindbrain. J. Neurochem. 2007, 101, 1185–1193. [Google Scholar] [CrossRef]
- Ebens, A.; Brose, K.; Leonardo, E.D.; Hanson, M.G., Jr.; Bladt, F.; Birchmeier, C.; Barres, B.A.; Tessier-Lavigne, M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron 1996, 17, 1157–1172. [Google Scholar] [CrossRef]
- O’Connor, R.; Tessier-Lavigne, M. Identification of maxillary factor, a maxillary process-derived chemoattractant for developing trigeminal sensory axons. Neuron 1999, 24, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Serafini, T.; Kennedy, T.E.; Galko, M.J.; Mirzayan, C.; Jessell, T.M.; Tessier-Lavigne, M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 1994, 78, 409–424. [Google Scholar] [CrossRef]
- Bovolenta, P. Morphogen signaling at the vertebrate growth cone: A few cases or a general strategy? J. Neurobiol. 2005, 64, 405–416. [Google Scholar] [CrossRef]
- Szebenyi, G.; Dent, E.W.; Callaway, J.L.; Seys, C.; Lueth, H.; Kalil, K. Fibroblast growth factor-2 promotes axon branching of cortical neurons by influencing morphology and behavior of the primary growth cone. J. Neurosci. 2001, 21, 3932–3941. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Bresnick, J.; Hornbruch, A.; Mahony, C.; Morton, N.; Colquhoun, K.; Martin, P.; Lumsden, A.; Dickson, C.; Mason, I. A role for FGF-8 in the initiation and maintenance of vertebrate limb bud outgrowth. Curr. Biol. 1995, 5, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Niswander, L.; Martin, G.R. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 1992, 114, 755–768. [Google Scholar] [CrossRef]
- Colvin, J.S.; Bohne, B.A.; Harding, G.W.; McEwen, D.G.; Ornitz, D.M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 1996, 12, 390–397. [Google Scholar] [CrossRef]
- Thisse, B.; Thisse, C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev. Biol. 2005, 287, 390–402. [Google Scholar] [CrossRef]
- Atkinson-Leadbeater, K.; Bertolesi, G.E.; Hehr, C.L.; Webber, C.A.; Cechmanek, P.B.; McFarlane, S. Dynamic expression of axon guidance cues required for optic tract development is controlled by fibroblast growth factor signaling. J. Neurosci. 2010, 30, 685–693. [Google Scholar] [CrossRef]
- Key, B.; Treloar, H.B.; Wangerek, L.; Ford, M.D.; Nurcombe, V. Expression and localization of FGF-1 in the developing rat olfactory system. J. Comp. Neurol. 1996, 366, 197–206. [Google Scholar] [CrossRef]
- Brittis, P.A.; Silver, J.; Walsh, F.S.; Doherty, P. Fibroblast growth factor receptor function is required for the orderly projection of ganglion cell axons in the developing mammalian retina. Mol. Cell. Neurosci. 1996, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, S.; McNeill, L.; Holt, C.E. FGF signaling and target recognition in the developing Xenopus visual system. Neuron 1995, 15, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, S.; Cornel, E.; Amaya, E.; Holt, C.E. Inhibition of FGF receptor activity in retinal ganglion cell axons causes errors in target recognition. Neuron 1996, 17, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, D.; Farrelly, O.; Miles, L.; Li, F.; Kim, S.E.; Lo, T.Y.; Wang, F.; Li, T.; Thompson-Peer, K.L.; et al. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 2019, 102, 373–389.e6. [Google Scholar] [CrossRef]
- Lee, S.M.; Danielian, P.S.; Fritzsch, B.; McMahon, A.P. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development 1997, 124, 959–969. [Google Scholar] [CrossRef]
- Logan, C.; Wizenmann, A.; Drescher, U.; Monschau, B.; Bonhoeffer, F.; Lumsden, A. Rostral optic tectum acquires caudal characteristics following ectopic engrailed expression. Curr. Biol. 1996, 6, 1006–1014. [Google Scholar] [CrossRef]
- Ciani, L.; Salinas, P.C. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005, 6, 351–362. [Google Scholar] [CrossRef]
- Miyake, A.; Itoh, N. Fgf22 regulated by Fgf3/Fgf8 signaling is required for zebrafish midbrain development. Biol. Open 2013, 2, 515–524. [Google Scholar] [CrossRef]
- Soundararajan, P.; Fawcett, J.P.; Rafuse, V.F. Guidance of postural motoneurons requires MAPK/ERK signaling downstream of fibroblast growth factor receptor 1. J. Neurosci. 2010, 30, 6595–6606. [Google Scholar] [CrossRef]
- Bulow, H.E.; Boulin, T.; Hobert, O. Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron 2004, 42, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Forni, J.J.; Romani, S.; Doherty, P.; Tear, G. Neuroglian and FasciclinII can promote neurite outgrowth via the FGF receptor Heartless. Mol. Cell. Neurosci. 2004, 26, 282–291. [Google Scholar] [CrossRef]
- Shimogori, T.; Grove, E.A. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J. Neurosci. 2005, 25, 6550–6560. [Google Scholar] [CrossRef] [PubMed]
- Braisted, J.E.; Ringstedt, T.; O’Leary, D.D. Slits are chemorepellents endogenous to hypothalamus and steer thalamocortical axons into ventral telencephalon. Cereb. Cortex 2009, 19 (Suppl. 1), i144–i151. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.A.; Ohyama, K.; Manning, L.; Aghamohammadzadeh, S.; Sang, H.; Placzek, M. FGF-dependent midline-derived progenitor cells in hypothalamic infundibular development. Development 2011, 138, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Hossain, W.A.; Zhou, X.; Rutledge, A.; Baier, C.; Morest, D.K. Basic fibroblast growth factor affects neuronal migration and differentiation in normotypic cell cultures from the cochleovestibular ganglion of the chick embryo. Exp. Neurol. 1996, 138, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Carnicero, E.; Garrido, J.J.; Alonso, M.T.; Schimmang, T. Roles of fibroblast growth factor 2 during innervation of the avian inner ear. J. Neurochem. 2001, 77, 786–795. [Google Scholar] [CrossRef]
- Hossain, W.A.; Brumwell, C.L.; Morest, D.K. Sequential interactions of fibroblast growth factor-2, brain-derived neurotrophic factor, neurotrophin-3, and their receptors define critical periods in the development of cochlear ganglion cells. Exp. Neurol. 2002, 175, 138–151. [Google Scholar] [CrossRef]
- Landmesser, L.T. Growth Cone Guidance in the Avian Limb: A Search for Cellular and Molecular Mechanisms; Raven Press: New York, NY, USA, 1992. [Google Scholar]
- Gomez, T.M.; Spitzer, N.C. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 1999, 397, 350–355. [Google Scholar] [CrossRef]
- Song, H.J.; Ming, G.L.; Poo, M.M. cAMP-induced switching in turning direction of nerve growth cones. Nature 1997, 388, 275–279. [Google Scholar] [CrossRef]
- Kater, S.B.; Davenport, R.W.; Guthrie, P.B. Filopodia as detectors of environmental cues: Signal integration through changes in growth cone calcium levels. Prog. Brain Res. 1994, 102, 49–60. [Google Scholar]
- Sutherland, D.J.; Goodhill, G.J. The interdependent roles of Ca2+ and cAMP in axon guidance. Dev. Neurobiol. 2015, 75, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Forbes, E.M.; Thompson, A.W.; Yuan, J.; Goodhill, G.J. Calcium and cAMP levels interact to determine attraction versus repulsion in axon guidance. Neuron 2012, 74, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Fukuda, T.; Tojima, T.; Nikolaev, V.O.; Kamiguchi, H. Cyclic Nucleotide Control of Microtubule Dynamics for Axon Guidance. J. Neurosci. 2016, 36, 5636–5649. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.J. Molecular mechanisms of axon guidance. Science 2002, 298, 1959–1964. [Google Scholar] [CrossRef]
- Johnson, D.E.; Williams, L.T. Structural and functional diversity in the FGF receptor multigene family. Adv. Cancer Res. 1993, 60, 1–41. [Google Scholar]
- Raffioni, S.; Thomas, D.; Foehr, E.D.; Thompson, L.M.; Bradshaw, R.A. Comparison of the intracellular signaling responses by three chimeric fibroblast growth factor receptors in PC12 cells. Proc. Natl. Acad. Sci. USA 1999, 96, 7178–7183. [Google Scholar] [CrossRef]
- Ota, S.; Tonou-Fujimori, N.; Yamasu, K. The roles of the FGF signal in zebrafish embryos analyzed using constitutive activation and dominant-negative suppression of different FGF receptors. Mech. Dev. 2009, 126, 1–17. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 2008, 237, 18–27. [Google Scholar] [CrossRef]
- Jaye, M.; Schlessinger, J.; Dionne, C.A. Fibroblast growth factor receptor tyrosine kinases: Molecular analysis and signal transduction. Biochim. Biophys. Acta 1992, 1135, 185–199. [Google Scholar] [CrossRef]
- Bottcher, R.T.; Niehrs, C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 2000, 6, 743–750. [Google Scholar] [CrossRef]
- Polanska, U.M.; Fernig, D.G.; Kinnunen, T. Extracellular interactome of the FGF receptor-ligand system: Complexities and the relative simplicity of the worm. Dev. Dyn. 2009, 238, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Berry, M.; Maher, P.A.; Logan, A.; Baird, A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995, 701, 201–226. [Google Scholar] [CrossRef]
- Tole, S.; Gutin, G.; Bhatnagar, L.; Remedios, R.; Hebert, J.M. Development of midline cell types and commissural axon tracts requires Fgfr1 in the cerebrum. Dev. Biol. 2006, 289, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, A.; Arruti, C.; Courtois, Y.; Jeanny, J.C. Localization of basic fibroblast growth factor binding sites in the chick embryonic neural retina. Differentiation 1990, 45, 161–167. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Xu, J.; Colvin, J.S.; McEwen, D.G.; MacArthur, C.A.; Coulier, F.; Gao, G.; Goldfarb, M. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996, 271, 15292–15297. [Google Scholar] [CrossRef]
- Philippe, J.M.; Garces, A.; deLapeyiere, O. Fgf-R3 is expressed in a subset of chicken spinal motorneurons. Mech. Dev. 1998, 78, 119–123. [Google Scholar] [CrossRef]
- Thomson, R.E.; Kind, P.C.; Graham, N.A.; Etherson, M.L.; Kennedy, J.; Fernandes, A.C.; Marques, C.S.; Hevner, R.F.; Iwata, T. Fgf receptor 3 activation promotes selective growth and expansion of occipitotemporal cortex. Neural Dev. 2009, 4, 4. [Google Scholar] [CrossRef]
- Asai, T.; Wanaka, A.; Kato, H.; Masana, Y.; Seo, M.; Tohyama, M. Differential expression of two members of FGF receptor gene family, FGFR-1 and FGFR-2 mRNA, in the adult rat central nervous system. Brain Res. Mol. Brain Res. 1993, 17, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Hattori, Y.; Ohta, M.; Itoh, N. Rat oligodendrocytes and astrocytes preferentially express fibroblast growth factor receptor-2 and -3 mRNAs. J. Neurosci. Res. 1996, 45, 534–541. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, V.; Kinkl, N.; Leveillard, T.; Sahel, J.; Hicks, D. Fibroblast growth factor receptor 4 (FGFR4) is expressed in adult rat and human retinal photoreceptors and neurons. J. Mol. Neurosci. 1999, 13, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.G.; Marie, J.; Wilson, E.; Ives, H.E.; Escobedo, J.; Del Rosario, M.; Mirda, D.; Williams, L.T. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature 1992, 358, 678–681. [Google Scholar] [CrossRef]
- Doherty, P.; Walsh, F.S. CAM-FGF receptor interactions: A model for axonal growth. Mol. Cell. Neurosci. 1996, 8, 99–111. [Google Scholar] [CrossRef]
- Jossin, Y.; Goffinet, A.M. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell. Biol. 2007, 27, 7113–7124. [Google Scholar] [CrossRef]
- Konno, D.; Yoshimura, S.; Hori, K.; Maruoka, H.; Sobue, K. Involvement of the phosphatidylinositol 3-kinase/rac1 and cdc42 pathways in radial migration of cortical neurons. J. Biol. Chem. 2005, 280, 5082–5088. [Google Scholar] [CrossRef]
- Poulopoulos, A.; Murphy, A.J.; Ozkan, A.; Davis, P.; Hatch, J.; Kirchner, R.; Macklis, J.D. Subcellular transcriptomes and proteomes of developing axon projections in the cerebral cortex. Nature 2019, 565, 356–360. [Google Scholar] [CrossRef]
- Yang, J.J.; Bertolesi, G.E.; Dueck, S.; Hehr, C.L.; McFarlane, S. The Expression of Key Guidance Genes at a Forebrain Axon Turning Point Is Maintained by Distinct Fgfr Isoforms but a Common Downstream Signal Transduction Mechanism. eNeuro 2019, 6, ENEURO.0086-19.2019. [Google Scholar] [CrossRef]
- Gallo, G.; Letourneau, P.C. Localized sources of neurotrophins initiate axon collateral sprouting. J. Neurosci. 1998, 18, 5403–5414. [Google Scholar] [CrossRef]
- Atwal, J.K.; Massie, B.; Miller, F.D.; Kaplan, D.R. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron 2000, 27, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Song, H.; Berninger, B.; Inagaki, N.; Tessier-Lavigne, M.; Poo, M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron 1999, 23, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.R.; Mazot, P.; Soriano, P. Genetic insights into the mechanisms of Fgf signaling. Genes Dev. 2016, 30, 751–771. [Google Scholar] [CrossRef]
- Reuss, B.; von Bohlen und Halbach, O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003, 313, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.W.; Gertler, F.B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 2003, 40, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.; Poo, M.M. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004, 14, 320–330. [Google Scholar] [CrossRef]
- Dent, E.W.; Kwiatkowski, A.V.; Mebane, L.M.; Philippar, U.; Barzik, M.; Rubinson, D.A.; Gupton, S.; Van Veen, J.E.; Furman, C.; Zhang, J.; et al. Filopodia are required for cortical neurite initiation. Nat. Cell Biol. 2007, 9, 1347–1359. [Google Scholar] [CrossRef]
- Tanaka, E.; Sabry, J. Making the connection: Cytoskeletal rearrangements during growth cone guidance. Cell 1995, 83, 171–176. [Google Scholar] [CrossRef]
- Schaefer, A.W.; Kabir, N.; Forscher, P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 2002, 158, 139–152. [Google Scholar] [CrossRef]

| Axons | FGFs | Effects | Note | References |
|---|---|---|---|---|
| Medial motor column (MMCm) axons | FGF2, 4, 8, 9 | Attractive | Directly | [39,40] |
| Statoacoustic ganglion (SAG) neurites | FGF8, 10, 19 | Promote asymmetric outgrowth | Directly | [41] |
| Thalamocortical axons (TCA) | FGF3, 10 | Low concentration: attractive | Directly | [42,43] |
| High concentration: repulsive | ||||
| Hypothalamic axons | FGF3, 10 | Low concentration: attractive | Directly | [44] |
| High concentration: repulsive | ||||
| Midbrain dopaminergic neuron (mDAN) axons | FGF8 | Repulsive | Indirectly | [45] |
| Trochlear motor axons | FGF8 | Attractive | Directly | [31] |
| Retinal ganglion cell (RGC) axons | FGF2 | Repulsive | Directly | [46,47] |
| RGC axons | FGF8 | Repulsive | Indirectly | [33] |
| FGFRs | Main Sites | Expression Phases |
|---|---|---|
| FGFR1 | Neurons [100,101] | In embryonic and adult development [97,102] |
| FGFR2 | Oligodendrocytes [100,101] | In embryonic and adult development [97,102] |
| FGFR3 | Astrocytes [101] | In embryonic and adult development [97,102] |
| FGFR4 | Neurons [101] | Only in embryonic development but not in the adult brain apart from the lateral habenular nucleus [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Luo, P.; Liu, X.; Cheng, R.; Zhang, S.; Qian, X.; Liu, F. Roles of Fibroblast Growth Factors in the Axon Guidance. Int. J. Mol. Sci. 2023, 24, 10292. https://doi.org/10.3390/ijms241210292
Zhang W, Luo P, Liu X, Cheng R, Zhang S, Qian X, Liu F. Roles of Fibroblast Growth Factors in the Axon Guidance. International Journal of Molecular Sciences. 2023; 24(12):10292. https://doi.org/10.3390/ijms241210292
Chicago/Turabian StyleZhang, Weiyun, Peiyi Luo, Xiaohan Liu, Ruoxi Cheng, Shuxian Zhang, Xiao Qian, and Fang Liu. 2023. "Roles of Fibroblast Growth Factors in the Axon Guidance" International Journal of Molecular Sciences 24, no. 12: 10292. https://doi.org/10.3390/ijms241210292
APA StyleZhang, W., Luo, P., Liu, X., Cheng, R., Zhang, S., Qian, X., & Liu, F. (2023). Roles of Fibroblast Growth Factors in the Axon Guidance. International Journal of Molecular Sciences, 24(12), 10292. https://doi.org/10.3390/ijms241210292






