Anti-Inflammatory and Protective Effects of Water Extract and Bioferment from Sambucus nigra Fruit in LPS-Induced Human Skin Fibroblasts
Abstract
1. Introduction
2. Results
2.1. Phytochemical Investigation and Quantitative Analysis of Main Components
2.2. Antioxidant Assay
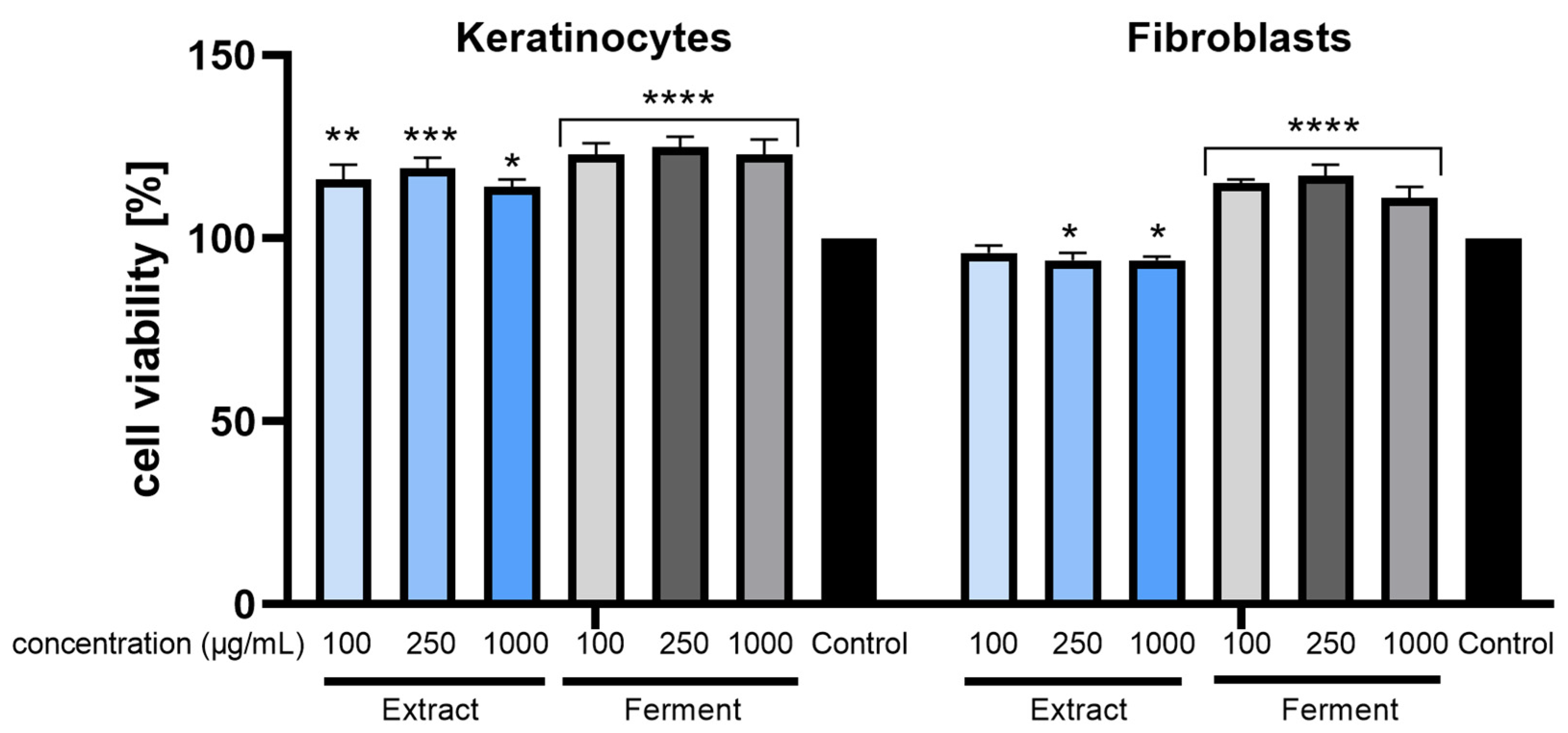
2.3. Cytotoxicity Assessment
2.4. Assessment of Matrix Metallopeptidase Inhibition
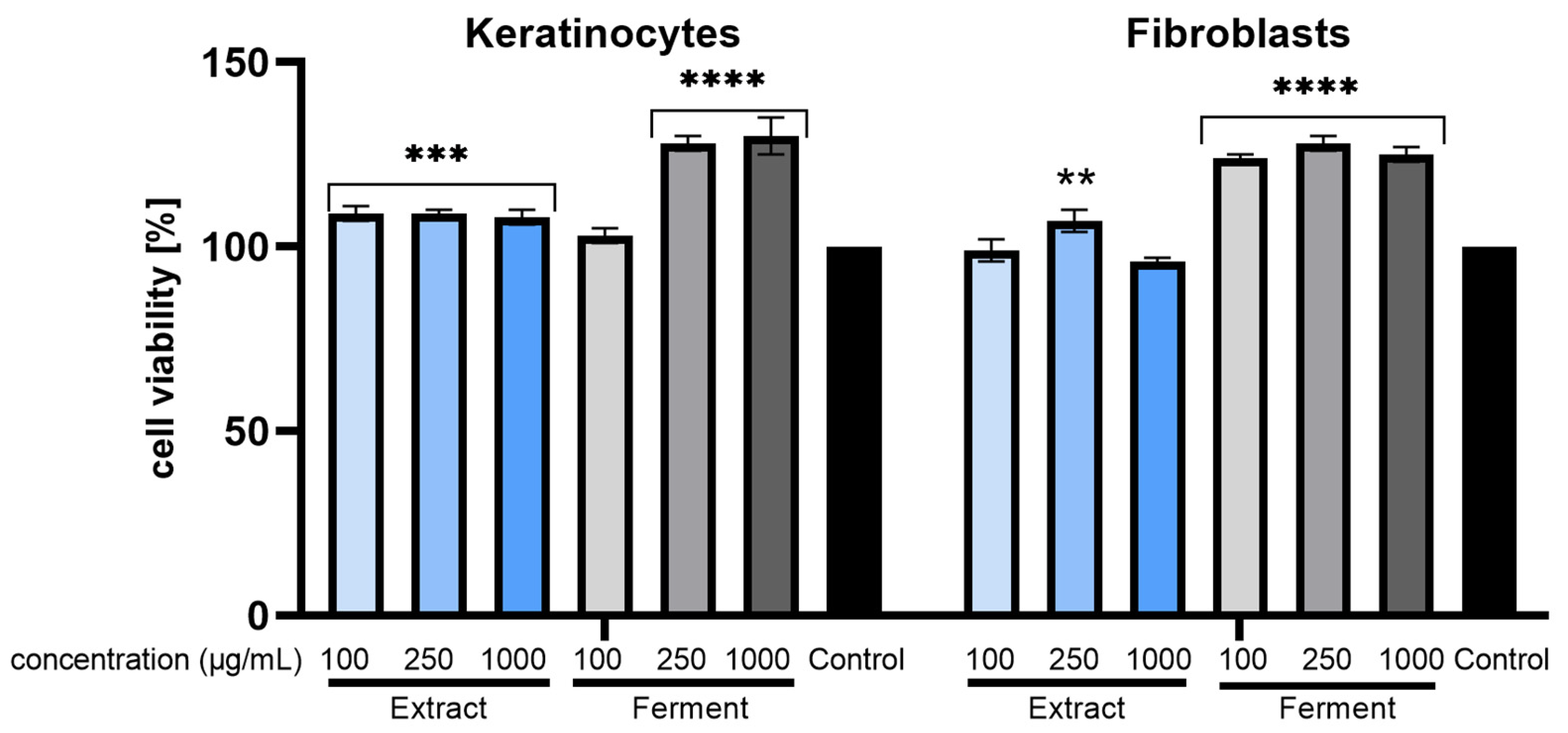
2.5. Protecive Effect of Extracts on LPS-Induced Cytotoxicity
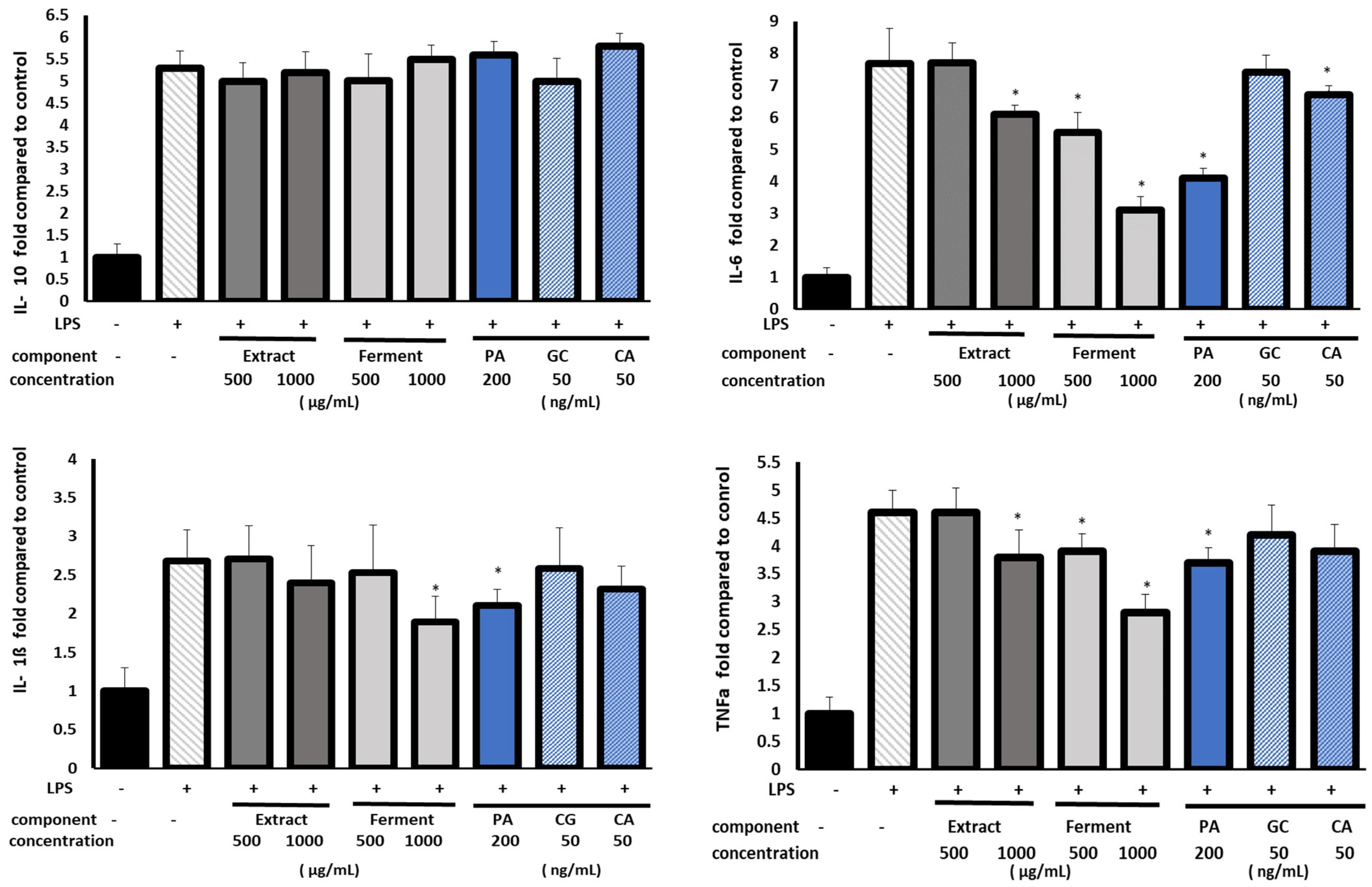
2.6. Anti-Inflammatory Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Preparation
4.2. UHPLC-MS Analysis
4.3. Antioxidative Activity
4.3.1. DPPH Radical Scavenging Assay
4.3.2. ABTS+ Scavenging Assay
4.4. Cell Culture
4.4.1. Cell Viability Test
Alamar Blue Assay (AB)
Neutral Red Assay (NR)
4.5. Assessment of Matrix Metallopeptidase Inhibition
4.5.1. Determination of Anti-Collagenase Activity
4.5.2. Determination of Anti-Elastase Activity
4.6. Anti-Inflammatory Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction Methods, LC-ESI-MS/MS Analysis of Phenolic Compounds and Antiradical Properties of Functional Food Enriched with Elderberry Flowers or Fruits. Arab. J. Chem. 2019, 12, 4719–4730. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive Properties of Sambucus nigra L. as a Functional Ingredient for Food and Pharmaceutical Industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Mahboubi, M. Sambucus nigra (Black Elder) as Alternative Treatment for Cold and Flu. Adv. Tradit. Med. 2021, 21, 405–414. [Google Scholar] [CrossRef]
- Waswa, E.N.; Li, J.; Mkala, E.M.; Wanga, V.O.; Mutinda, E.S.; Nanjala, C.; Odago, W.O.; Katumo, D.M.; Gichua, M.K.; Gituru, R.W.; et al. Ethnobotany, Phytochemistry, Pharmacology, and Toxicology of the Genus Sambucus L. (Viburnaceae). J. Ethnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The Antioxidant Properties of Alcoholic Extracts from Sambucus nigra L. (Antioxidant Properties of Extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial Activity of Elder (Sambucus nigra L.) Flower or Berry against Hospital Pathogens. J. Med. Plants Res. 2020, 4, 1805–1809. [Google Scholar]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as Potential Source of Antioxidants. Characterization, Optimization of Extraction Parameters and Bioactive Properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Michalak, M.; Paradowska, K.; Zielińska, A. Phenolic Content, Antioxidant Activity and Pharmaceutical Availability of Hydrogels with Extracts of Rosmarinus officinalis L. and Sambucus nigra L. Acta Pol. Pharm. Drug. Res. 2021, 78, 219–226. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Yang, D.; Moh, S.; Son, D.; You, S.; Kinyua, A.; Ko, C.; Song, M.; Yeo, J.; Choi, Y.-H.; Kim, K. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Jullian, C.; Miranda, S.; Zapata-Torres, G.; Mendizábal, F.; Olea-Azar, C. Studies of Inclusion Complexes of Natural and Modified Cyclodextrin with (+)Catechin by NMR and Molecular Modeling. Bioorg. Med. Chem. 2007, 15, 3217–3224. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Rodrigues, R.; Oliveira, M.B.P.P.; Alves, R.C. Chlorogenic Acids and Caffeine from Coffee By-Products: A Review on Skincare Applications. Cosmetics 2023, 10, 12. [Google Scholar] [CrossRef]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New Insights into Quercetin Nanoformulations for Topical Delivery. Phytomedicine Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Moriguchi, C.; Shigematsu, Y.; Tanabe, K.; Haraguchi, N.; Iwashita, S.; Tokudome, Y.; Kitagaki, H. Fermented Cosmetics and Metabolites of Skin Microbiota—A New Approach to Skin Health. Fermentation 2022, 8, 703. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The Importance of Both Fibroblasts and Keratinocytes in a Bilayered Living Cellular Construct Used in Wound Healing. Wound Repair. Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef]
- Russo, B.; Brembilla, N.C.; Chizzolini, C. Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders. Front. Immunol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In Vitro Screening of Elastase, Collagenase, Hyaluronidase, and Tyrosinase Inhibitory and Antioxidant Activities of 22 Halophyte Plant Extracts for Novel Cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- González-Minero, F.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Kielar, P.; Mołoń, M.; Szczepanek, D.; Sowa, I.; Wójciak, M. In Vitro Evaluation of Antioxidant and Protective Potential of Kombucha-Fermented Black Berry Extracts against H2O2-Induced Oxidative Stress in Human Skin Cells and Yeast Model. Int. J. Mol. Sci. 2023, 24, 4388. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Wójciak, M.; Szczepanek, D.; Sowa, I. Assessment of Cosmetic and Dermatological Properties and Safety of Use of Model Skin Tonics with Kombucha-Fermented Red Berry Extracts. Int. J. Mol. Sci. 2022, 23, 14675. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Bujak, T.; Wójciak, M.; Sowa, I. Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products. Molecules 2022, 27, 2345. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of Fermentation Time on the Content of Bioactive Compounds with Cosmetic and Dermatological Properties in Kombucha Yerba Mate Extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) Extracts Promote Anti-Inflammatory and Cellular Antioxidant Activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Samoticha, J.; Eler, K.; Stampar, F.; Veberic, R. Traditional Elderflower Beverages: A Rich Source of Phenolic Compounds with High Antioxidant Activity. J. Agric. Food Chem. 2015, 63, 1477–1487. [Google Scholar] [CrossRef]
- Mota, A.H.; Duarte, N.; Serra, A.T.; Ferreira, A.; Bronze, M.R.; Custódio, L.; Gaspar, M.M.; Simões, S.; Rijo, P.; Ascensão, L.; et al. Further Evidence of Possible Therapeutic Uses of Sambucus nigra L. Extracts by the Assessment of the In Vitro and In Vivo Anti-Inflammatory Properties of Its PLGA and PCL-Based Nanoformulations. Pharmaceutics 2020, 12, 1181. [Google Scholar] [CrossRef]
- Hung, S.-J.; Tang, S.-C.; Liao, P.-Y.; Ge, J.-S.; Hsiao, Y.-P.; Yang, J.-H. Photoprotective Potential of Glycolic Acid by Reducing NLRC4 and AIM2 Inflammasome Complex Proteins in UVB Radiation-Induced Normal Human Epidermal Keratinocytes and Mice. DNA Cell. Biol. 2017, 36, 177–187. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.; Yi, T.H. Gallic Acid Regulates Skin Photoaging in UVB-Exposed Fibroblast and Hairless Mice: Protective Effects of Ga Against Uvb-Mediated Skin Aging. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Son, J.H.; Kim, S.-Y.; Jang, H.H.; Lee, S.N.; Ahn, K.J. Protective Effect of Protocatechuic Acid against Inflammatory Stress Induced in Human Dermal Fibroblasts. Biomed. Dermatol. 2018, 2, 9. [Google Scholar] [CrossRef]
- Daré, R.G.; Oliveira, M.M.; Truiti, M.C.T.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Abilities of Protocatechuic Acid and Its Alkyl Esters, Ethyl and Heptyl Protocatechuates, to Counteract UVB-Induced Oxidative Injuries and Photoaging in Fibroblasts L929 Cell Line. J. Photochem. Photobiol. B. 2020, 203, 111771. [Google Scholar] [CrossRef]
- Lin, P.; Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Yi, T.-H. Sambucus nigra L. Ameliorates UVB-Induced Photoaging and Inflammatory Response in Human Skin Keratinocytes. Cytotechnology 2019, 71, 1003–1017. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented Beverages with Health-Promoting Potential: Past and Future Perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In Vitro Metabolism of Anthocyanins by Human Gut Microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus: A Review on Kombucha. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Jung, H.; Kang, D.Y.; Sp, N.; Shin, W.; Lee, J.; Park, B.G.; Kang, Y.A.; Jang, K.-J.; Bae, S.W. The Skin-Whitening and Antioxidant Effects of Protocatechuic Acid (PCA) Derivatives in Melanoma and Fibroblast Cell Lines. Curr. Issues Mol. Biol. 2023, 45, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin Aging Properties of Protocatechuic Acid in Vitro and in Vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Wang, J.; Wang, Q.; Ma, Q.; Chen, Y. Protocatechuic Acid Inhibits Inflammatory Responses in LPS-Stimulated BV2 Microglia via NF-ΚB and MAPKs Signaling Pathways. Neurochem. Res. 2015, 40, 1655–1660. [Google Scholar] [CrossRef]
- Kaewmool, C.; Kongtawelert, P.; Phitak, T.; Pothacharoen, P.; Udomruk, S. Protocatechuic Acid Inhibits Inflammatory Responses in LPS-Activated BV2 Microglia via Regulating SIRT1/NF-ΚB Pathway Contributed to the Suppression of Microglial Activation-Induced PC12 Cell Apoptosis. J. Neuroimmunol. 2020, 341, 577164. [Google Scholar] [CrossRef]
- Salama, A.; Elgohary, R.; Amin, M.M.; Elwahab, S.A. Impact of Protocatechuic Acid on Alleviation of Pulmonary Damage Induced by Cyclophosphamide Targeting Peroxisome Proliferator Activator Receptor, Silent Information Regulator Type-1, and Fork Head Box Protein in Rats. Inflammopharmacology 2023, 31, 1361–1372. [Google Scholar] [CrossRef]
- Adeyanju, A.A.; Asejeje, F.O.; Molehin, O.R.; Owoeye, O.; Olatoye, E.O.; Ekpo, E.N. Protective Role of Protocatechuic Acid in Carbon Tetrachloride-Induced Oxidative Stress via Modulation of Proinflammatory Cytokines Levels in Brain and Liver of Wistar Rats. J. Basic. Clin. Physiol. Pharmacol. 2022, 33, 143–154. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells. Nutrients 2020, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Paixão, J.; Nunes, C.; Dinis, T.C.P.; Almeida, L.M. Cyanidin-3-Glucoside Suppresses Cytokine-Induced Inflammatory Response in Human Intestinal Cells: Comparison with 5-Aminosalicylic Acid. PLoS ONE 2013, 8, e73001. [Google Scholar] [CrossRef] [PubMed]
- Triebel, S.; Trieu, H.-L.; Richling, E. Modulation of Inflammatory Gene Expression by a Bilberry (Vaccinium Myrtillus L.) Extract and Single Anthocyanins Considering Their Limited Stability under Cell Culture Conditions. J. Agric. Food Chem. 2012, 60, 8902–8910. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H.; Andrade, J.M.; Rodrigues, M.J.; Custódio, L.; Bronze, M.R.; Duarte, N.; Baby, A.; Rocha, J.; Gaspar, M.M.; Simões, S.; et al. Synchronous Insight of in Vitro and in Vivo Biological Activities of Sambucus nigra L. Extracts for Industrial Uses. Ind. Crops Prod. 2020, 154, 112709. [Google Scholar] [CrossRef]
- Mota, A.H.; Andrade, J.M.; Ntungwe, E.N.; Pereira, P.; Cebola, M.J.; Bernardo-Gil, M.G.; Molpeceres, J.; Rijo, P.; Viana, A.S.; Ascensão, L.; et al. Green Extraction of Sambucus nigra L. for Potential Application in Skin Nanocarriers. Green. Mater. 2020, 8, 181–193. [Google Scholar] [CrossRef]
- Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago Officinalis Extracts on Skin Cells. Molecules 2023, 28, 868. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Wójciak, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Hoian, U.; Klimczak, K.; Szczepanek, D.; Sowa, I. Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra Chinensis Extracts and Their Applicability in Skin Care Product. Molecules 2022, 27, 8877. [Google Scholar] [CrossRef]
- Zofia, N.-Ł.; Martyna, Z.-D.; Aleksandra, Z.; Tomasz, B. Comparison of the Antiaging and Protective Properties of Plants from the Apiaceae Family. Oxid. Med. Cell. Longev. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
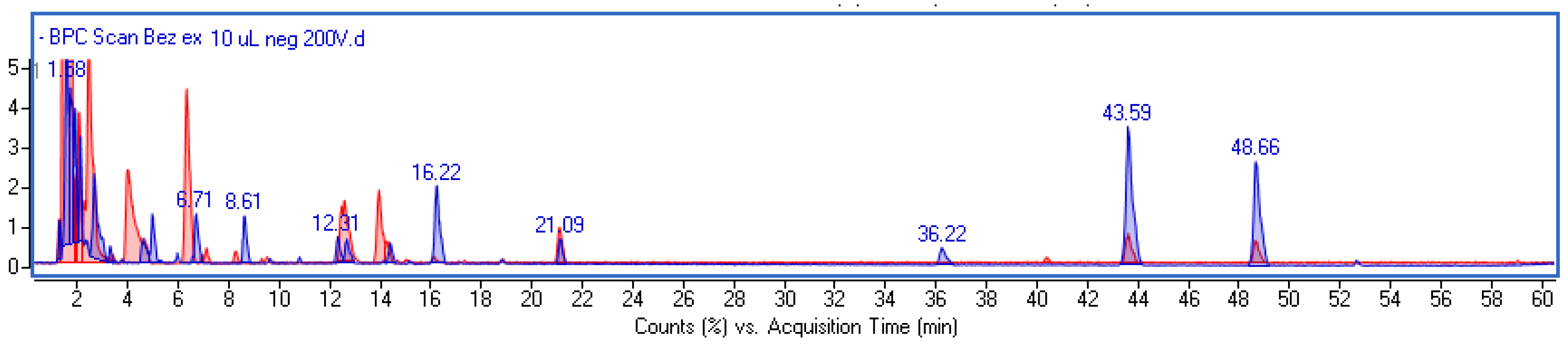


| Rt (min) | Observed Ion Mass [M-H]-/(Fragments) | Δ ppm | Formula | Identified | Extract (µg/g) | Ferment (µg/g) |
|---|---|---|---|---|---|---|
| 1.49 | 195.05182 | 4.05 | C6H12O7 | Gluconic acid * | - | +++ |
| 3.44 | 169.01431 (125) | 0.37 | C7H6O5 | Gallic acid * | - | 4.3 ± 0.3 |
| 4.32 | 299.0778 (137) | 1.86 | C13H16O8 | Hydroxybenzoic acid hexoside | - | + |
| 6.32 | 153.01946 | 0.83 | C7H6O4 | Protocatechuic acid * | 33.2 ± 1.5 | 181.1 ± 9.8 |
| 7.11 | 315.07(153) | 2.4 | C13H16O9 | Dihydroxybenzoic hexoside | - | 5.8 ± 0.2 |
| 8.69 | 353.08798 (191,179) | 0.49 | C16H18O9 | Neochlorogenic * | - | 12.8 ± 0.7 |
| 12.77 | 741.18864 (285) | 0.37 | C32H38O20 | Cyanidin 3-sambubioside-5-glucoside | + | - |
| 14.29 | 353.08804 (191,179) | 0.66 | C16H18O9 | Chlorogenic * | 0.4 ± 0.1 | 41.3 ± 2.1 |
| 13.05 | 289.07189 | 0.44 | C15H14O6 | Catechin * | - | + |
| 13.21 | 579.13567 (285) | 0.22 | C26H28O15 | Cyanidin 3-sambubioside | 42.3 ± 3.4 | 27.4 ± 1.8 |
| 18.29 | 447.09354 (285) | 0.57 | C21H20O11 | Cyanidin 3-glucoside * | 34.3 ± 1.9 | 37.4 ± 2.1 |
| 21.09 | 525.19764 (327) | −0.21 | C25H34O12 | unidentified | + | + |
| 29.26 | 609.14650 | 0.64 | C27H30O16 | Rutoside * | - | + |
| 36.22 | 610.42488 | 1.62 | C38H59O6 | unidentified | +++ | + |
| 43.59 | 723.50515 | −0.17 | C41H72O10 | unidentified | +++ | + |
| 48.66 | 836.58698 | 0.39 | C44H85O14 | unidentified | +++ | + |
| Method | Extract | Ferment |
|---|---|---|
| IC50 [ µg/mL] | IC50 [µg/mL] | |
| DPPH | 865 ± 1.2 **** | 909 ± 1.4 **** |
| ABTS | 91 ± 0.45 **** | 95 ± 0.65 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak, M.; Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Oniszczuk, T.; Sowa, I. Anti-Inflammatory and Protective Effects of Water Extract and Bioferment from Sambucus nigra Fruit in LPS-Induced Human Skin Fibroblasts. Int. J. Mol. Sci. 2023, 24, 10286. https://doi.org/10.3390/ijms241210286
Wójciak M, Ziemlewska A, Zagórska-Dziok M, Nizioł-Łukaszewska Z, Szczepanek D, Oniszczuk T, Sowa I. Anti-Inflammatory and Protective Effects of Water Extract and Bioferment from Sambucus nigra Fruit in LPS-Induced Human Skin Fibroblasts. International Journal of Molecular Sciences. 2023; 24(12):10286. https://doi.org/10.3390/ijms241210286
Chicago/Turabian StyleWójciak, Magdalena, Aleksandra Ziemlewska, Martyna Zagórska-Dziok, Zofia Nizioł-Łukaszewska, Dariusz Szczepanek, Tomasz Oniszczuk, and Ireneusz Sowa. 2023. "Anti-Inflammatory and Protective Effects of Water Extract and Bioferment from Sambucus nigra Fruit in LPS-Induced Human Skin Fibroblasts" International Journal of Molecular Sciences 24, no. 12: 10286. https://doi.org/10.3390/ijms241210286
APA StyleWójciak, M., Ziemlewska, A., Zagórska-Dziok, M., Nizioł-Łukaszewska, Z., Szczepanek, D., Oniszczuk, T., & Sowa, I. (2023). Anti-Inflammatory and Protective Effects of Water Extract and Bioferment from Sambucus nigra Fruit in LPS-Induced Human Skin Fibroblasts. International Journal of Molecular Sciences, 24(12), 10286. https://doi.org/10.3390/ijms241210286






