7-Ketocholesterol Promotes Retinal Pigment Epithelium Senescence and Fibrosis of Choroidal Neovascularization via IQGAP1 Phosphorylation-Dependent Signaling
Abstract
1. Introduction
2. Results
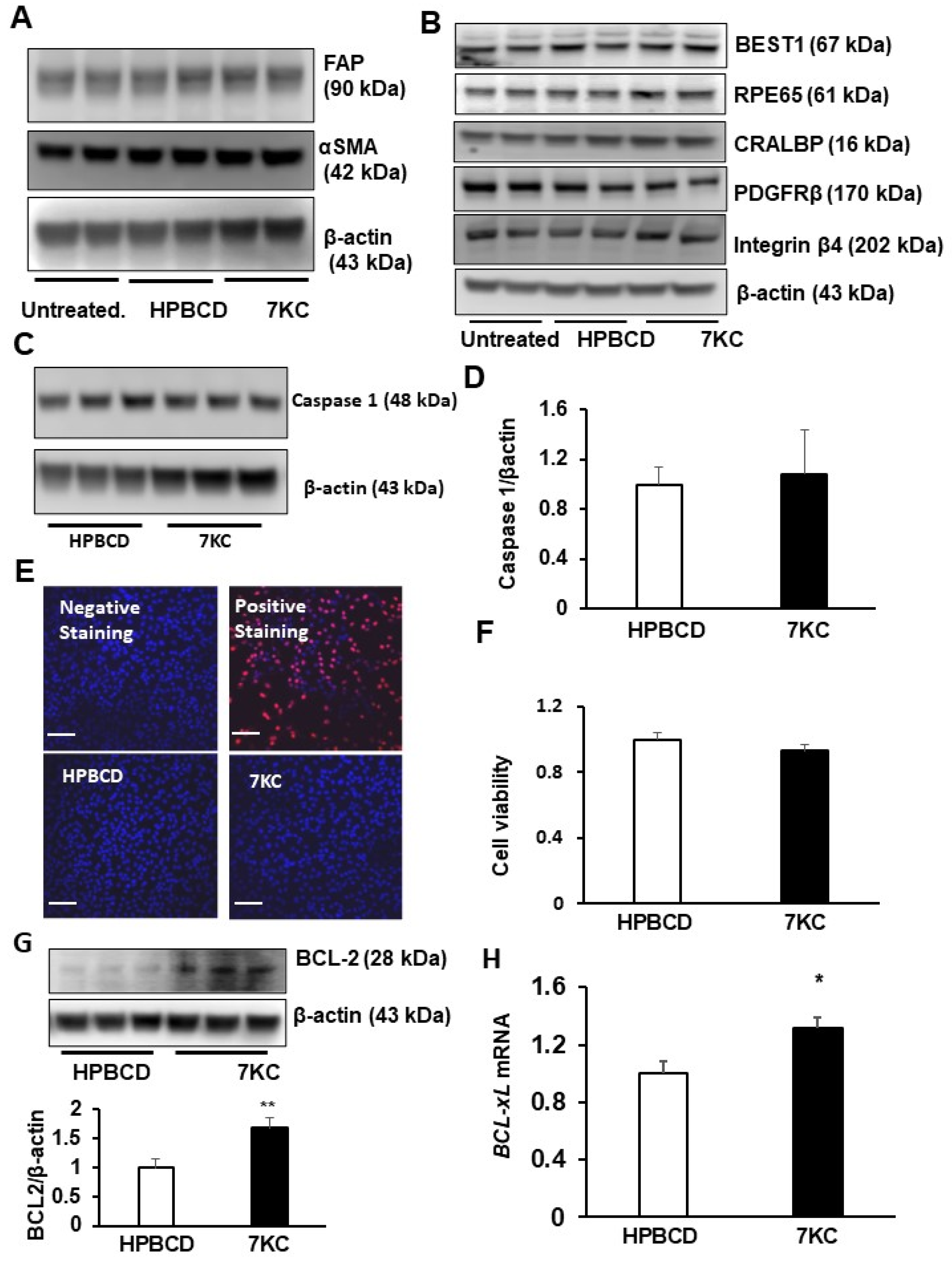
2.1. 7-Ketocholesterol (7KC) Does Not Increase Mesenchymal Transition or Death of hRPE
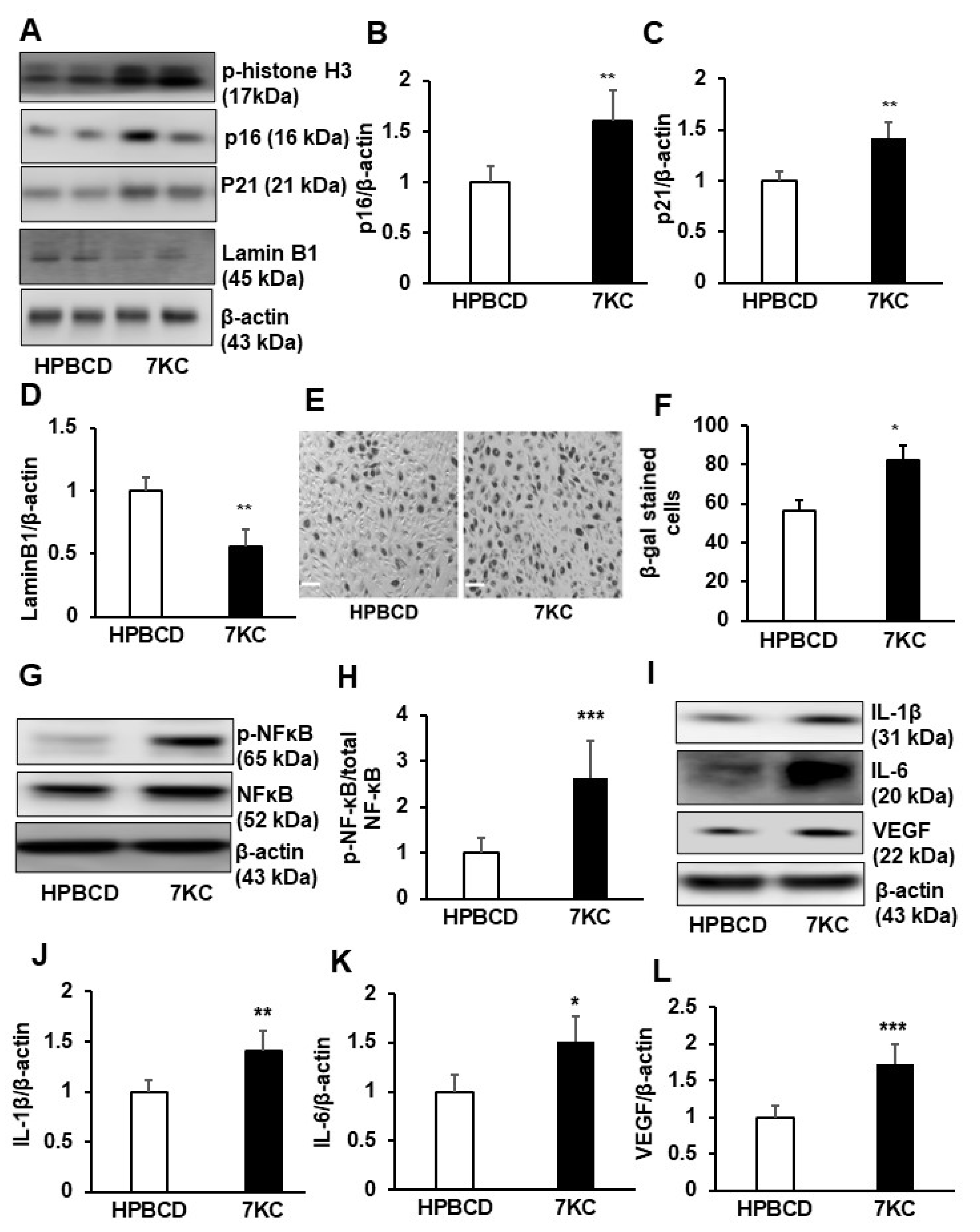
2.2. 7KC Induces Cell Cycle Arrest at Mitosis and Promotes Senescence and SASP in hRPE
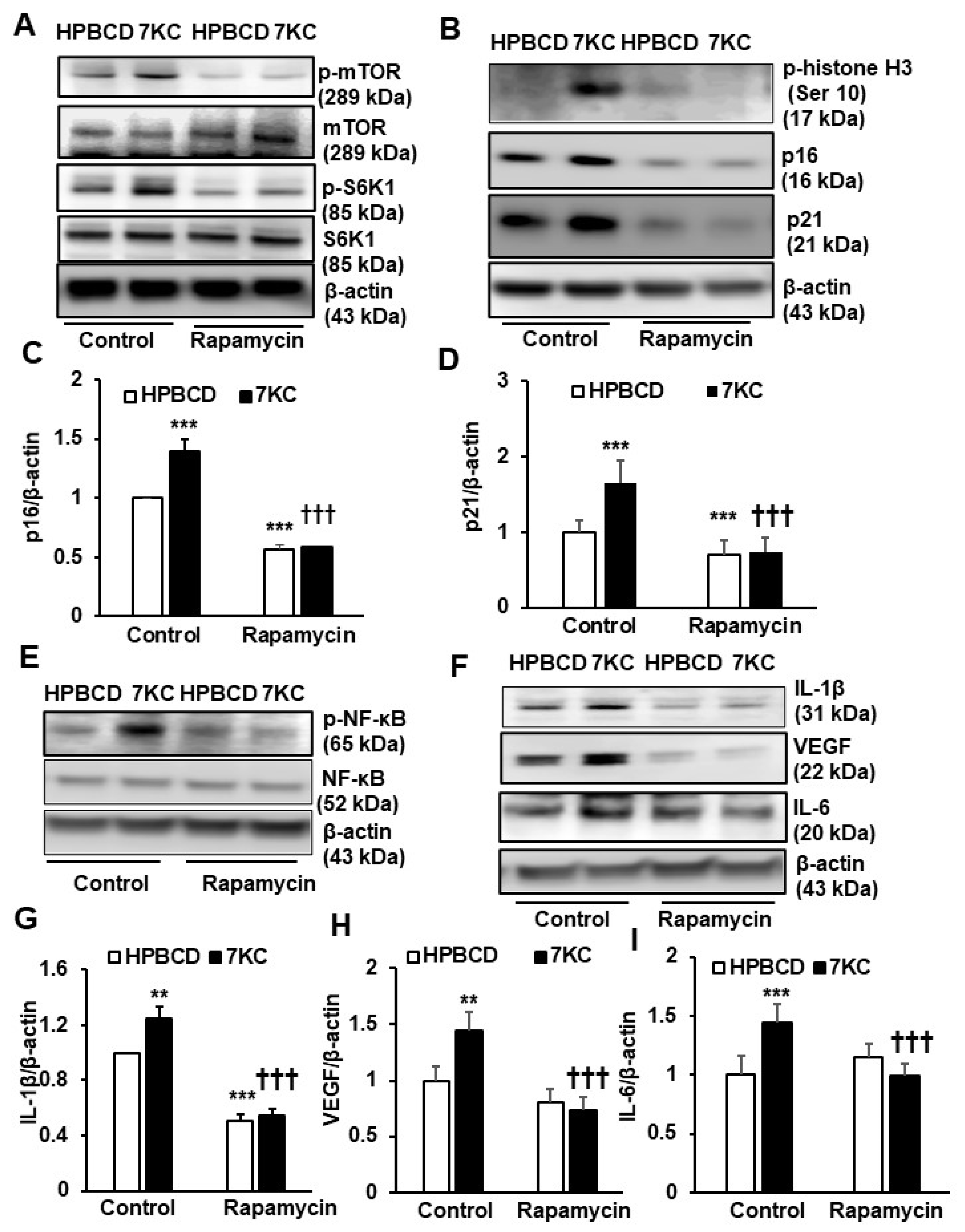
2.3. Inhibition of mTOR Signaling Prevents 7KC-Mediated Senescence and SASP in hRPE
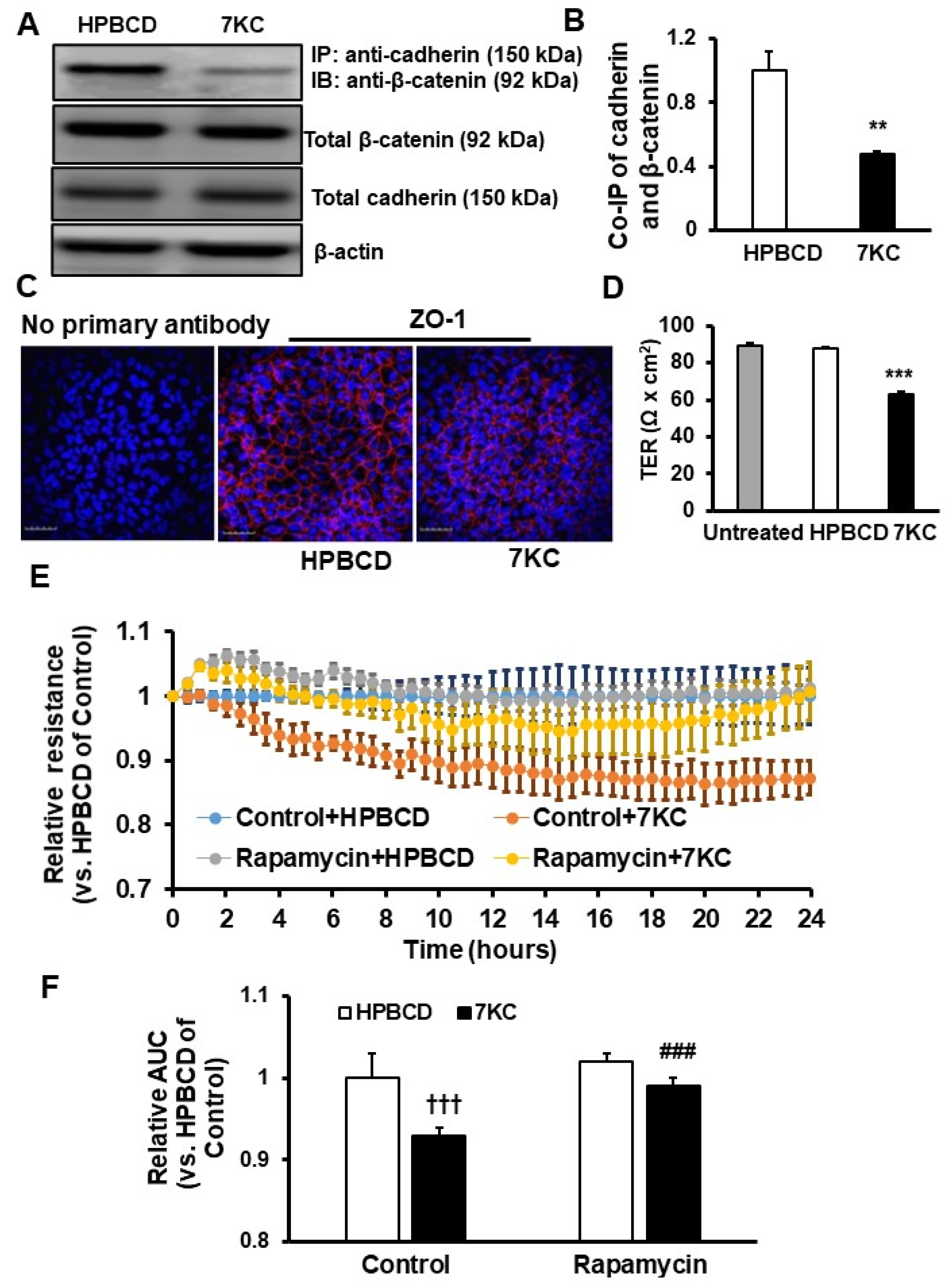
2.4. Reduced Barrier Resistance in Senescent 7KC-Treated hRPE
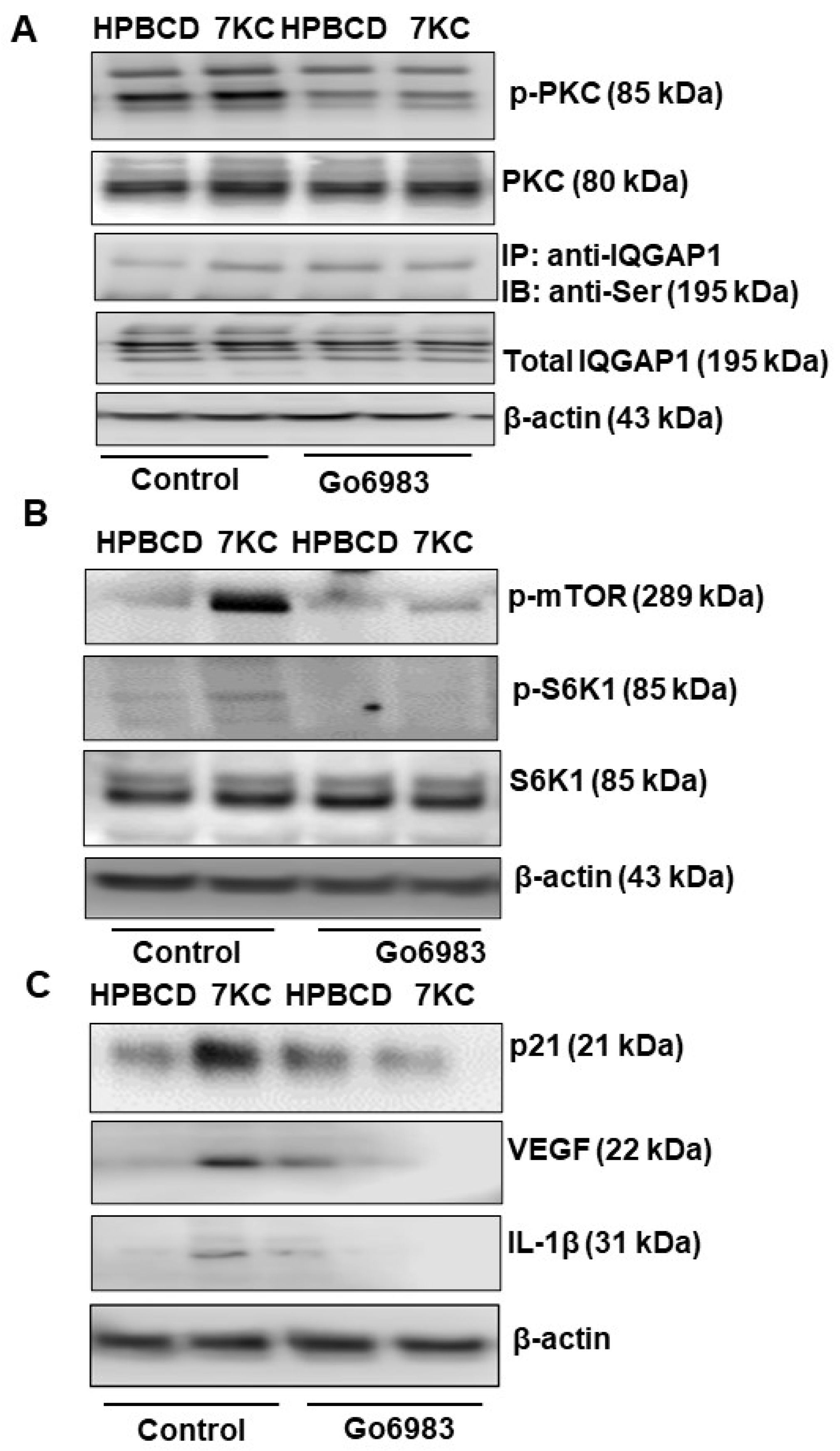
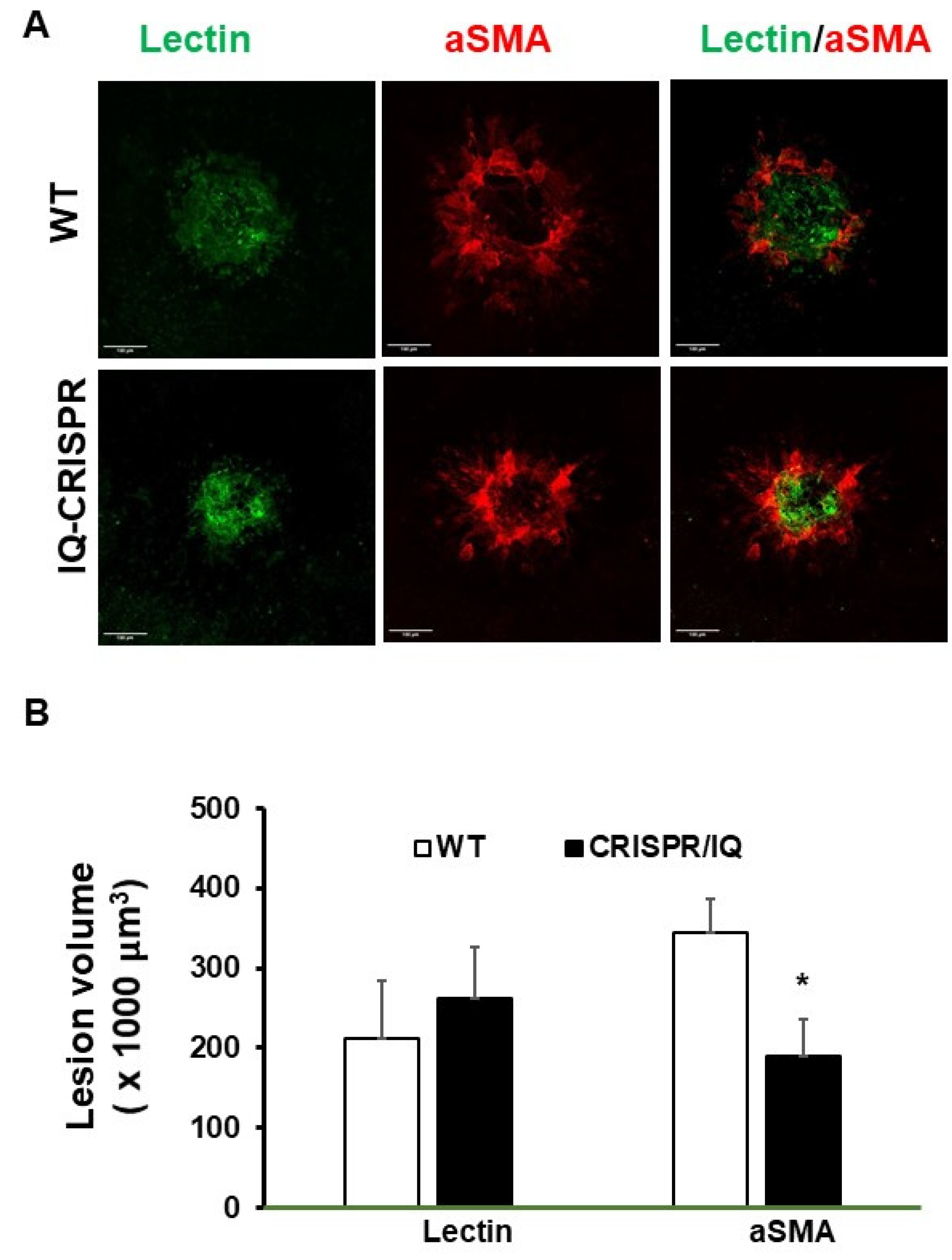
2.5. Serine Phosphorylation of IQGAP Regulates 7KC-Mediated RPE Senescence and SASP and Fibrosis of CNV Lesion
3. Discussion
4. Materials and Methods
4.1. Animals and Procedures
4.2. Cell Culture and Treatments
4.3. Cell Senescence Measurement by β-Galactosidase Staining
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Immunoprecipitation and Western Blots
4.6. RPE Cell Death Measured by TUNEL Staining
4.7. hRPE Viability Assay
4.8. Immunostaining of Junctional Protein ZO-1 in hRPE
4.9. Transepithelial Resistance (TER) and Electrical Cell-Substrate Impedance Sensing (ECIS) of RPE Barrier Resistance
4.10. Generation of the IQGAP1 Serine 1441A Mouse Line
4.11. Intravitreal Injections of 7KC and Laser-Induced Injury
4.12. RPE/Choroidal Flat Mount Preparation, Staining, and Lesion Volume Quantification
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration—Emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000, 14, 835–846. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Rodriguez, I.R.; Clark, M.E.; Lee, J.W.; Curcio, C.A. 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp. Eye Res. 2014, 128, 151–155. [Google Scholar] [CrossRef]
- Anderson, A.; Campo, A.; Fulton, E.; Corwin, A.; Jerome, W.G., 3rd; O’Connor, M.S. 7-Ketocholesterol in disease and aging. Redox Biol. 2020, 29, 101380. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, S.J. Cholesterol homeostasis in the retina: Seeing is believing. J. Lipid Res. 2015, 56, 1–4. [Google Scholar] [CrossRef]
- Dugas, B.; Charbonnier, S.; Baarine, M.; Ragot, K.; Delmas, D.; Ménétrier, F.; Lherminier, J.; Malvitte, L.; Khalfaoui, T.; Bron, A.; et al. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: Cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010, 49, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef]
- Indaram, M.; Ma, W.; Zhao, L.; Fariss, R.N.; Rodriguez, I.R.; Wong, W.T. 7-Ketocholesterol increases retinal microglial migration, activation, and angiogenicity: A potential pathogenic mechanism underlying age-related macular degeneration. Sci. Rep. 2015, 5, 9144. [Google Scholar] [CrossRef]
- Neekhra, A.; Tran, J.; Esfahani, P.R.; Schneider, K.; Pham, K.; Sharma, A.; Chwa, M.; Luthra, S.; Gramajo, A.L.; Mansoor, S.; et al. Memantine, Simvastatin, and Epicatechin Inhibit 7-Ketocholesterol-induced Apoptosis in Retinal Pigment Epithelial Cells But Not Neurosensory Retinal Cells In Vitro. J. Ophthalmic Vis. Res. 2020, 15, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, X.; Kunz, E.; Hartnett, M.E. Thy-1 Regulates VEGF-Mediated Choroidal Endothelial Cell Activation and Migration: Implications in Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5525–5534. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.R.; Larrayoz, I.M. Cholesterol oxidation in the retina: Implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J. Lipid Res. 2010, 51, 2847–2862. [Google Scholar] [CrossRef]
- Huang, J.D.; Amaral, J.; Lee, J.W.; Rodriguez, I.R. 7-Ketocholesterol-induced inflammation signals mostly through the TLR4 receptor both in vitro and in vivo. PLoS ONE 2014, 9, e100985. [Google Scholar] [CrossRef]
- Rezig, L.; Ghzaiel, I.; Ksila, M.; Yammine, A.; Nury, T.; Zarrouk, A.; Samadi, M.; Chouaibi, M.; Vejux, A.; Lizard, G. Cytoprotective activities of representative nutrients from the Mediterranean diet and of Mediterranean oils against 7-ketocholesterol- and 7β-hydroxycholesterol-induced cytotoxicity: Application to age-related diseases and civilization diseases. Steroids 2022, 187, 109093. [Google Scholar] [CrossRef]
- Wang, H.; Ramshekar, A.; Kunz, E.; Hartnett, M.E. 7-ketocholesterol induces endothelial-mesenchymal transition and promotes fibrosis: Implications in neovascular age-related macular degeneration and treatment. Angiogenesis 2021, 24, 583–595. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef]
- Freund, A.; Laberge, R.-M.; Demaria, M.; Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef]
- Plaza Reyes, A.; Petrus-Reurer, S.; Padrell Sánchez, S.; Kumar, P.; Douagi, I.; Bartuma, H.; Aronsson, M.; Westman, S.; Lardner, E.; André, H.; et al. Identification of cell surface markers and establishment of monolayer differentiation to retinal pigment epithelial cells. Nat. Commun. 2020, 11, 1609. [Google Scholar] [CrossRef]
- Palmer, A.K.; Tchkonia, T.; Kirkland, J.L. Targeting cellular senescence in metabolic disease. Mol. Metab. 2022, 66, 101601. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.C.; Morton, J.P.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Cai, J.; Sternberg, P. Altered mTOR signaling in senescent retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5314–5319. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Tullet, J.M.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Bretz, C.A.; Becker, S.; Gambhir, D.; Smith, G.W.; Samulski, R.J.; Wittchen, E.S.; Quilliam, L.A.; Chrzanowska-Wodnicka, M.; et al. Retinal pigment epithelial cell expression of active Rap 1a by scAAV2 inhibits choroidal neovascularization. Mol. Ther. Methods Clin. Dev. 2016, 3, 16056. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; McNulty, D.E.; Marler, K.J.; Lim, L.; Hall, C.; Annan, R.S.; Sacks, D.B. IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J. Biol. Chem. 2005, 280, 13871–13878. [Google Scholar] [CrossRef]
- Seddon, J.M.; George, S.; Rosner, B.; Klein, M.L. CFH Gene Variant, Y402H, and Smoking, Body Mass Index, Environmental Associations with Advanced Age-Related Macular Degeneration. Hum. Hered. 2006, 61, 157–165. [Google Scholar] [CrossRef]
- Telander, D.G. Inflammation and age-related macular degeneration (AMD). Semin. Ophthalmol. 2011, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hartnett, M.E. Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol. Vis. 2016, 22, 189–202. [Google Scholar]
- Chakravarthy, U.; Bailey, C.C.; Scanlon, P.H.; McKibbin, M.; Khan, R.S.; Mahmood, S.; Downey, L.; Dhingra, N.; Brand, C.; Brittain, C.J.; et al. Progression from Early/Intermediate to Advanced Forms of Age-Related Macular Degeneration in a Large UK Cohort: Rates and Risk Factors. Ophthalmol. Retin. 2020, 4, 662–672. [Google Scholar] [CrossRef]
- Kuny, S.; Cho, W.J.; Dimopoulos, I.S.; Sauvé, Y. Early Onset Ultrastructural and Functional Defects in RPE and Photoreceptors of a Stargardt-Like Macular Dystrophy (STGD3) Transgenic Mouse Model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7109–7121. [Google Scholar] [CrossRef]
- Blasiak, J.; Petrovski, G.; Vereb, Z.; Facsko, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. BioMed Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Ioannidou, A.; Goulielmaki, E.; Garinis, G.A. DNA Damage: From Chronic Inflammation to Age-Related Deterioration. Front. Genet. 2016, 7, 187. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Hubackova, S.; Krejcikova, K.; Bartek, J.; Hodny, Z. IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘bystander senescence’. Aging 2012, 4, 932–951. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Smith, L.E.H. Cellular senescence in pathologic retinal angiogenesis. Trends Endocrinol. Metab. 2021, 32, 415–416. [Google Scholar] [CrossRef]
- Zhu, D.; Wu, J.; Spee, C.; Ryan, S.J.; Hinton, D.R. BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J. Biol. Chem. 2009, 284, 9529–9539. [Google Scholar] [CrossRef] [PubMed]
- Radspieler, M.M.; Schindeldecker, M.; Stenzel, P.; Försch, S.; Tagscherer, K.E.; Herpel, E.; Hohenfellner, M.; Hatiboglu, G.; Roth, W.; Macher-Goeppinger, S. Lamin-B1 is a senescence-associated biomarker in clear-cell renal cell carcinoma. Oncol. Lett. 2019, 18, 2654–2660. [Google Scholar] [CrossRef]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Dalal, M.; Jacobs-El, N.; Nicholson, B.; Tuo, J.; Chew, E.; Chan, C.C.; Nussenblatt, R.; Ferris, F.; Meyerle, C. Subconjunctival Palomid 529 in the treatment of neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2705–2709. [Google Scholar] [CrossRef]
- Gensler, G.; Clemons, T.E.; Domalpally, A.; Danis, R.P.; Blodi, B.; Wells, J., 3rd; Rauser, M.; Hoskins, J.; Hubbard, G.B.; Elman, M.J.; et al. Treatment of Geographic Atrophy with Intravitreal Sirolimus: The Age-Related Eye Disease Study 2 Ancillary Study. Ophthalmol. Retin. 2018, 2, 441–450. [Google Scholar] [CrossRef]
- Ishikawa, K.; Kannan, R.; Hinton, D.R. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye Res. 2016, 142, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ayee, M.A.A.; Levitan, I. Lipoprotein-Induced Increases in Cholesterol and 7-Ketocholesterol Result in Opposite Molecular-Scale Biophysical Effects on Membrane Structure. Front. Cardiovasc. Med. 2021, 8, 715932. [Google Scholar] [CrossRef]
- Shentu, T.P.; Singh, D.K.; Oh, M.J.; Sun, S.; Sadaat, L.; Makino, A.; Mazzone, T.; Subbaiah, P.V.; Cho, M.; Levitan, I. The role of oxysterols in control of endothelial stiffness. J. Lipid Res. 2012, 53, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Shi, D.; Quilliam, L.A.; Chrzanowska-Wodnicka, M.; Wittchen, E.S.; Li, D.Y.; Hartnett, M.E. Activation of Rap1 inhibits NADPH oxidase-dependent ROS generation in retinal pigment epithelium and reduces choroidal neovascularization. FASEB J. 2014, 28, 265–274. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ramshekar, A.; Cung, T.; Wallace-Carrete, C.; Zaugg, C.; Nguyen, J.; Stoddard, G.J.; Hartnett, M.E. 7-Ketocholesterol Promotes Retinal Pigment Epithelium Senescence and Fibrosis of Choroidal Neovascularization via IQGAP1 Phosphorylation-Dependent Signaling. Int. J. Mol. Sci. 2023, 24, 10276. https://doi.org/10.3390/ijms241210276
Wang H, Ramshekar A, Cung T, Wallace-Carrete C, Zaugg C, Nguyen J, Stoddard GJ, Hartnett ME. 7-Ketocholesterol Promotes Retinal Pigment Epithelium Senescence and Fibrosis of Choroidal Neovascularization via IQGAP1 Phosphorylation-Dependent Signaling. International Journal of Molecular Sciences. 2023; 24(12):10276. https://doi.org/10.3390/ijms241210276
Chicago/Turabian StyleWang, Haibo, Aniket Ramshekar, Thaonhi Cung, Chris Wallace-Carrete, Chandler Zaugg, Jasmine Nguyen, Gregory J. Stoddard, and M. Elizabeth Hartnett. 2023. "7-Ketocholesterol Promotes Retinal Pigment Epithelium Senescence and Fibrosis of Choroidal Neovascularization via IQGAP1 Phosphorylation-Dependent Signaling" International Journal of Molecular Sciences 24, no. 12: 10276. https://doi.org/10.3390/ijms241210276
APA StyleWang, H., Ramshekar, A., Cung, T., Wallace-Carrete, C., Zaugg, C., Nguyen, J., Stoddard, G. J., & Hartnett, M. E. (2023). 7-Ketocholesterol Promotes Retinal Pigment Epithelium Senescence and Fibrosis of Choroidal Neovascularization via IQGAP1 Phosphorylation-Dependent Signaling. International Journal of Molecular Sciences, 24(12), 10276. https://doi.org/10.3390/ijms241210276




