Platelet-Derived Microparticles and Autoimmune Diseases
Abstract
1. Introduction
2. Overview of PMPs
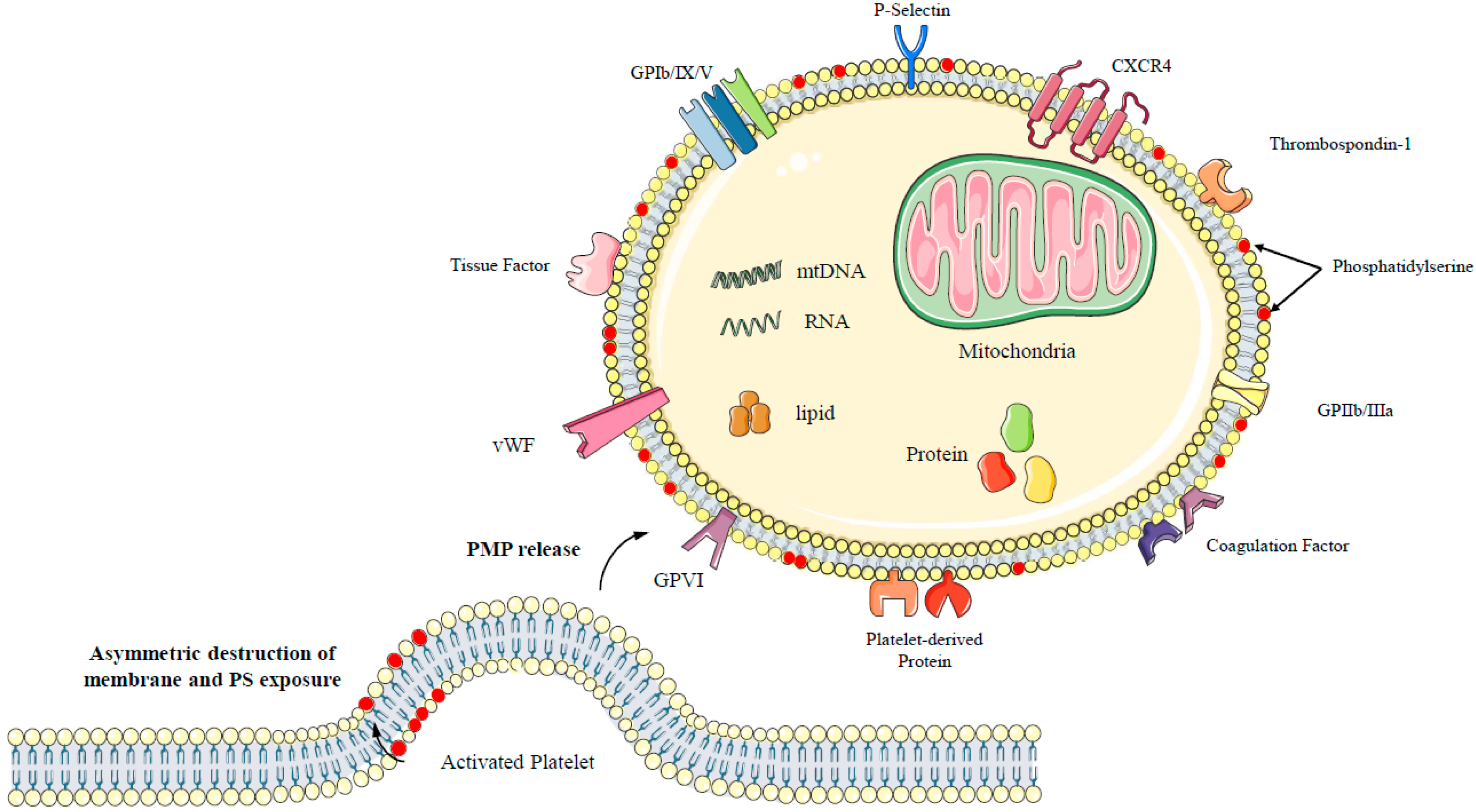
2.1. Formation of PMPs
2.2. Composition of PMPs
3. PMPs and Autoimmune Diseases
3.1. PMPs and Rheumatoid Arthritis
3.2. PMPs and Systemic Lupus Erythematosus
3.3. PMPs and Antiphospholipid Antibody Syndrome
3.4. PMP and Sjogren Syndrome
3.5. PMPs and Systemic Sclerosis
3.6. PMPs and Ankylosing Spondylitis
3.7. PMPs and Systemic Vasculitis
4. Summary
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Ma, K.L.; Gong, Y.X.; Wang, G.H.; Hu, Z.B.; Liu, L.; Lu, J.; Chen, P.P.; Lu, C.C.; Ruan, X.Z.; et al. Platelet Microparticles Mediate Glomerular Endothelial Injury in Early Diabetic Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2671–2695. [Google Scholar] [CrossRef]
- Taleb, R.S.Z.; Moez, P.; Younan, D.; Eisenacher, M.; Tenbusch, M.; Sitek, B.; Bracht, T. Protein Biomarker Discovery Using Human Blood Plasma Microparticles. In Proteomics for Biomarker Discovery: Methods and Protocols; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1959, pp. 51–64. [Google Scholar] [CrossRef]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet microvesicles in health and disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Badimon, L.; Suades, R.; Fuentes, E.; Palomo, I.; Padró, T. Role of Platelet-Derived Microvesicles As Crosstalk Mediators in Atherothrombosis and Future Pharmacology Targets: A Link between Inflammation, Atherosclerosis, and Thrombosis. Front. Pharmacol. 2016, 7, 293. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Kallenberg, C.; Shoenfeld, Y. Refractory disease in autoimmune diseases. Autoimmun. Rev. 2011, 10, 653–654. [Google Scholar] [CrossRef]
- Eilat, D. Introduction: Mechanisms of tissue injury in autoimmune diseases. Semin. Immunopathol. 2014, 36, 491–493. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.-S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Webber, A.J.; Johnson, S.A. Platelet participation in blood coagulation aspects of hemostasis. Am. J. Pathol. 1970, 60, 19–42. [Google Scholar] [PubMed]
- Gomes, F.G.; Andrade, A.C.; Wolf, M.; Hochmann, S.; Krisch, L.; Maeding, N.; Regl, C.; Poupardin, R.; Ebner-Peking, P.; Huber, C.G.; et al. Synergy of Human Platelet-Derived Extracellular Vesicles with Secretome Proteins Promotes Regenerative Functions. Biomedicines 2022, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Botha, J.; Handberg, A.; Simonsen, J.B. Lipid-based strategies used to identify extracellular vesicles in flow cytometry can be confounded by lipoproteins: Evaluations of annexin V, lactadherin, and detergent lysis. J. Extracell. Vesicles 2022, 11, e12200. [Google Scholar] [CrossRef]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet Extracellular Vesicles. Arterioscler. Thromb. Vasc. Biol. 2020, 41, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Guzera, M.; Miśkiewicz, A.; Jagielski, D.; Winnicka, A. The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumor Biol. 2016, 37, 14391–14401. [Google Scholar] [CrossRef] [PubMed]
- Gitz, E.; Pollitt, A.; Gitz-Francois, J.J.; AlShehri, O.; Mori, J.; Montague, S.; Nash, G.; Douglas, M.R.; Gardiner, E.; Andrews, R.K.; et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 2014, 124, 2262–2270. [Google Scholar] [CrossRef]
- Catani, M.V.; Savini, I.; Tullio, V.; Gasperi, V. The “Janus Face” of Platelets in Cancer. Int. J. Mol. Sci. 2020, 21, 788. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Conti, G.O.; Fiore, M.; Cangiano, F.; Zuccarello, P.; Gaudio, A.; Ferrante, M. Platelet-Derived Microparticles (MPs) and Thrombin Generation Velocity in Deep Vein Thrombosis (DVT): Results of a Case–Control Study. Vasc. Health Risk Manag. 2020, 16, 489–495. [Google Scholar] [CrossRef]
- Custodio-Chablé, S.J.; Lezama, R.A.; Reyes-Maldonado, E. Platelet activation as a trigger factor for inflammation and atherosclerosis. Cirugía Cir. 2020, 88, 233–243. [Google Scholar] [CrossRef]
- Mehdi-Alamdarlou, S.; Ahmadi, F.; Shahbazi, M.-A.; Azadi, A.; Ashrafi, H. Platelets and platelet-derived vesicles as an innovative cellular and subcellular platform for managing multiple sclerosis. Mol. Biol. Rep. 2023, 50, 4675–4686. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Bison, E.; Pontara, E.; Cattini, M.G.; Tonello, M.; Denas, G.; Pengo, V. Platelet- and endothelial-derived microparticles in the context of different antiphospholipid antibody profiles. Lupus 2022, 31, 1328–1334. [Google Scholar] [CrossRef]
- Thietart, S.; Rautou, P.-E. Extracellular vesicles as biomarkers in liver diseases: A clinician’s point of view. J. Hepatol. 2020, 73, 1507–1525. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.G.; Pink, R.C.; Erdbrügger, U.; Siljander, P.R.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology. J. Extracell. Vesicles 2022, 11, e12151. [Google Scholar] [CrossRef]
- Zaldivia, M.T.K.; McFadyen, J.D.; Lim, B.; Wang, X.; Peter, K. Platelet-Derived Microvesicles in Cardiovascular Diseases. Front. Cardiovasc. Med. 2017, 4, 74. [Google Scholar] [CrossRef]
- Vatter, F.A.P.; Cioffi, M.; Hanna, S.J.; Castarede, I.; Caielli, S.; Pascual, V.; Matei, I.; Lyden, D. Extracellular vesicle– and particle-mediated communication shapes innate and adaptive immune responses. J. Exp. Med. 2021, 218, e20202579. [Google Scholar] [CrossRef]
- Pagel, O.; Walter, E.; Jurk, K.; Zahedi, R.P. Taking the stock of granule cargo: Platelet releasate proteomics. Platelets 2016, 28, 119–128. [Google Scholar] [CrossRef]
- Marcoux, G.; Duchez, A.-C.; Cloutier, N.; Provost, P.; Nigrovic, P.A.; Boilard, E. Revealing the diversity of extracellular vesicles using high-dimensional flow cytometry analyses. Sci. Rep. 2016, 6, 35928. [Google Scholar] [CrossRef]
- Michael, B.N.R.; Kommoju, V.; Ganapathy, C.K.; Negi, V.S. Characterization of cell-derived microparticles in synovial fluid and plasma of patients with rheumatoid arthritis. Rheumatol. Int. 2019, 39, 1377–1387. [Google Scholar] [CrossRef]
- Laurenzana, I.; Trino, S.; Lamorte, D.; Girasole, M.; Dinarelli, S.; De Stradis, A.; Grieco, V.; Maietti, M.; Traficante, A.; Statuto, T.; et al. Analysis of Amount, Size, Protein Phenotype and Molecular Content of Circulating Extracellular Vesicles Identifies New Biomarkers in Multiple Myeloma. Int. J. Nanomed. 2021, 16, 3141–3160. [Google Scholar] [CrossRef] [PubMed]
- Latham, S.L.; Tiberti, N.; Gokoolparsadh, N.; Holdaway, K.; Couraud, P.O.; Grau, G.E.R.; Combes, V. Immuno-analysis of microparticles: Probing at the limits of detection. Sci. Rep. 2015, 5, 16314. [Google Scholar] [CrossRef] [PubMed]
- Spakova, T.; Janockova, J.; Rosocha, J. Characterization and Therapeutic Use of Extracellular Vesicles Derived from Platelets. Int. J. Mol. Sci. 2021, 22, 9701. [Google Scholar] [CrossRef] [PubMed]
- Rui, S.; Yuan, Y.; Du, C.; Song, P.; Chen, Y.; Wang, H.; Fan, Y.; Armstrong, D.G.; Deng, W.; Li, L. Comparison and Investigation of Exosomes Derived from Platelet-Rich Plasma Activated by Different Agonists. Cell Transplant. 2021, 30, 09636897211017833. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Baetta, R.; Mallia, A.; Banfi, C.; Tremoli, E. Platelets in Healthy and Disease States: From Biomarkers Discovery to Drug Targets Identification by Proteomics. Int. J. Mol. Sci. 2020, 21, 4541. [Google Scholar] [CrossRef]
- Léger, J.L.; Jougleux, J.-L.; Savadogo, F.; Pichaud, N.; Boudreau, L.H. Rapid isolation and purification of functional platelet mitochondria using a discontinuous Percoll gradient. Platelets 2019, 31, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rosińska, J.; Łukasik, M.; Kozubski, W. The Impact of Vascular Disease Treatment on Platelet-Derived Microvesicles. Cardiovasc. Drugs Ther. 2017, 31, 627–644. [Google Scholar] [CrossRef]
- Xu, L.; Liang, Y.; Xu, X.; Xia, J.; Wen, C.; Zhang, P.; Duan, L. Blood cell-derived extracellular vesicles: Diagnostic biomarkers and smart delivery systems. Bioengineered 2021, 12, 7929–7940. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Nakagomi, D.; Kobayashi, Y.; Hiraguri, M.; Sugiyama, T.; Amano, K.; Umibe, T.; Kono, H.; Kurasawa, K.; Kita, Y.; et al. Effect of Reduced-Dose vs High-Dose Glucocorticoids Added to Rituximab on Remission Induction in ANCA-Associated Vasculitis. JAMA 2021, 325, 2178–2187. [Google Scholar] [CrossRef]
- Jamal, O.; Sahel, N.; Saouab, R.; El, Q.M.; Zaizaa, M.; El, K.I.; Rkiouak, A.; Hammi, S.; Sekkach, Y. Fatal Sys-temic Vasculitis Associated with Chronic Active Epstein-Barr Virus Infection. Mo. Med. 2021, 118, 226–232. [Google Scholar]
- Catrina, A.; Krishnamurthy, A.; Rethi, B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 2021, 7, e001228. [Google Scholar] [CrossRef]
- Cafaro, G.; Bartoloni, E.; Alunno, A.; Gerli, R. Platelets: A potential target for rheumatoid arthritis treatment? Expert Rev. Clin. Immunol. 2018, 15, 1–3. [Google Scholar] [CrossRef]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.M.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets Amplify Inflammation in Arthritis via Collagen-Dependent Microparticle Production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef]
- Hu, S.; Cavanagh, B.L.; Harrington, R.; Ahmad, M.; Kearns, G.; Meaney, S.; Wynne, C. A Novel Pool of Microparticle Cholesterol Is Elevated in Rheumatoid Arthritis but Not in Systemic Lupus Erythematosus Patients. Int. J. Mol. Sci. 2020, 21, 9228. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Alperi-López, M.; Lopez-Suarez, P.; Alonso-Castro, S.; Carro-Esteban, S.R.; Ballina-Garcia, F.J.; Suarez-Diaz, A.M. Altered profile of circulating microparticles in rheumatoid arthritis patients. Clin. Sci. 2014, 128, 437–448. [Google Scholar] [CrossRef]
- Hsu, J.; Gu, Y.; Tan, S.-L.; Narula, S.; DeMartino, J.A.; Liao, C. Bruton’s Tyrosine Kinase mediates platelet receptor-induced generation of microparticles: A potential mechanism for amplification of inflammatory responses in rheumatoid arthritis synovial joints. Immunol. Lett. 2013, 150, 97–104. [Google Scholar] [CrossRef]
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; Albert, A.; Shnayder, R.; Gobezie, R.; Nigrovic, P.A.; et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2012, 5, 235–249. [Google Scholar] [CrossRef]
- Xu, M.; Du, R.; Xing, W.; Chen, X.; Wan, J.; Wang, S.; Xiong, L.; Nandakumar, K.S.; Holmdahl, R.; Geng, H. Platelets derived citrullinated proteins and microparticles are potential autoantibodies ACPA targets in RA patients. Front. Immunol. 2023, 14, 1084283. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, A.; Veselinovic, M.; Zong, Y.; Jakovljevic, V.; Pruner, I.; Antovic, A. Increased Expression of Extracellular Vesicles Is Associated With the Procoagulant State in Patients With Established Rheumatoid Arthritis. Front. Immunol. 2021, 12, 718845. [Google Scholar] [CrossRef] [PubMed]
- Villar-Vesga, J.; Grajales, C.; Burbano, C.; Vanegas–García, A.; Muñoz–Vahos, C.H.; Vásquez, G.; Rojas, M.; Castaño, D. Platelet-derived microparticles generated in vitro resemble circulating vesicles of patients with rheumatoid arthritis and activate monocytes. Cell. Immunol. 2018, 336, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; Deng, Z.; Liu, G.; Yang, J.; Zhou, W.; Zhang, C.; Shen, W.; Zhang, Y. Platelet-derived extracellular vesicles promote the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes via CXCR2 signaling. Exp. Ther. Med. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Knijff-Dutmer, E.A.J.; Koerts, J.; Nieuwland, R.; Kalsbeek-Batenburg, E.M.; Van De Laar, M.A.F.J. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 2002, 46, 1498–1503. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Tak, P.P.; Böing, A.N.; Romijn, F.; Kraan, M.C.; Breedveld, F.C.; Hack, C.E.; Sturk, A. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002, 46, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Rac1 regulates platelet microparticles formation and rheumatoid arthritis deterioration. Platelets 2019, 31, 112–119. [Google Scholar] [CrossRef]
- Lu, P.; Fleischmann, R.; Curtis, C.; Ignatenko, S.; Clarke, S.H.; Desai, M.; Wong, S.L.; Grebe, K.M.; Black, K.; Zeng, J.; et al. Safety and pharmacodynamics of venetoclax (ABT-199) in a randomized single and multiple ascending dose study in women with systemic lupus erythematosus. Lupus 2017, 27, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Ameer, M.A.; Chaudhry, H.; Mushtaq, J.; Khan, O.S.; Babar, M.; Hashim, T.; Zeb, S.; Tariq, M.A.; Patlolla, S.R.; Ali, J.; et al. An Overview of Systemic Lupus Erythematosus (SLE) Pathogenesis, Classification, and Management. Cureus 2022, 14, e30330. [Google Scholar] [CrossRef] [PubMed]
- Hanscombe, K.B.; Morris, D.L.; Noble, J.A.; Dilthey, A.T.; Tombleson, P.; Kaufman, K.M.; Comeau, M.; Langefeld, C.D.; Alarcon-Riquelme, M.E.; Gaffney, P.M.; et al. Genetic fine mapping of systemic lupus erythematosus MHC associations in Europeans and African Americans. Hum. Mol. Genet. 2018, 27, 3813–3824. [Google Scholar] [CrossRef]
- Li, H.; Tsokos, G.C. Gut viruses in the pathogenesis of systemic lupus erythematosus. Sci. Bull. 2023, 68, 664–665. [Google Scholar] [CrossRef]
- Illescas-Montes, R.; Corona-Castro, C.C.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Infectious processes and systemic lupus erythematosus. Immunology 2019, 158, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Pérez, L.; Rojas, M.; Muñoz-Vahos, C.; Vanegas-García, A.; Vásquez, G. Plasma microparticles from patients with systemic lupus erythematosus modulate the content of miRNAs in U937 cells. Immunology 2021, 164, 253–265. [Google Scholar] [CrossRef]
- Mobarrez, F.; Svenungsson, E.; Pisetsky, D.S. Microparticles as autoantigens in systemic lupus erythematosus. Eur. J. Clin. Investig. 2018, 48, e13010. [Google Scholar] [CrossRef]
- Plasín-Rodríguez, M.A.; Patricio, P.; Monteagudo, J.; García-Criado, A.; Cervera, R.; Reverter, J.C.; Espinosa, G.; Tàssies, D. Procoagulant microparticles are associated with arterial disease in patients with systemic lupus erythematosus. J. Thromb. Thrombolysis 2020, 52, 30–41. [Google Scholar] [CrossRef]
- Burbano, C.; Villar-Vesga, J.; Orejuela, J.; Muñoz, C.; Vanegas, A.; Vásquez, G.; Rojas, M.; Castaño, D. Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 322. [Google Scholar] [CrossRef]
- Hasse, S.; Julien, A.-S.; Duchez, A.-C.; Zhao, C.; Boilard, E.; Fortin, P.R.; Bourgoin, S.G. Red blood cell-derived phosphatidylserine positive extracellular vesicles are associated with past thrombotic events in patients with systemic erythematous lupus. Lupus Sci. Med. 2022, 9, e000605. [Google Scholar] [CrossRef]
- Cohen, H.; Cuadrado, M.J.; Erkan, D.; Duarte-Garcia, A.; Isenberg, D.A.; Knight, J.S.; Ortel, T.L.; Rahman, A.; Salmon, J.E.; Tektonidou, M.G.; et al. 16th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends. Lupus 2020, 29, 1571–1593. [Google Scholar] [CrossRef]
- Petri, M. Antiphospholipid syndrome. Transl. Res. 2020, 225, 70–81. [Google Scholar] [CrossRef]
- Serrano, A.; Cervera, R.; Gris, J.C. Editorial: Primary Antiphospholipid Syndrome. Front. Immunol. 2020, 11, 1993. [Google Scholar] [CrossRef]
- Khalil, D.N.; González-Albo, I.P.; Rosen, L.; Lillie, T.; Stacey, A.; Parfitt, L.; Soff, G.A. A tumor-selective adenoviral vector platform induces transient antiphospholipid antibodies, without increased risk of thrombosis, in phase 1 clinical studies. Investig. New Drugs 2023, 41, 317–323. [Google Scholar] [CrossRef]
- Akca, U.K.; Ayaz, N.A. Comorbidities of antiphospholipid syndrome and systemic lupus erythematosus in children. Curr. Rheumatol. Rep. 2020, 22, 21. [Google Scholar] [CrossRef]
- Štok, U.; Blokar, E.; Lenassi, M.; Holcar, M.; Frank-Bertoncelj, M.; Erman, A.; Resnik, N.; Sodin-Šemrl, S.; Čučnik, S.; Pirkmajer, K.P.; et al. Characterization of Plasma-Derived Small Extracellular Vesicles Indicates Ongoing Endothelial and Platelet Activation in Patients with Thrombotic Antiphospholipid Syndrome. Cells 2020, 9, 1211. [Google Scholar] [CrossRef]
- Breen, K.; Sanchez, K.; Kirkman, N.; Seed, P.; Parmar, K.; Moore, G.; Hunt, B. Endothelial and platelet microparticles in patients with antiphospholipid antibodies. Thromb. Res. 2014, 135, 368–374. [Google Scholar] [CrossRef]
- Zhou, Q.; Lian, Y.; Zhang, Y.; Li, L.; Li, H.; Shen, D.; Zhou, Y.; Zhang, M.; Lu, Y.; Liu, J.; et al. Platelet-derived microparticles from recurrent miscarriage associated with antiphospholipid antibody syndrome influence behaviours of trophoblast and endothelial cells. Mol. Hum. Reprod. 2019, 25, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Zha, C.; Liu, Y. Platelet-derived microparticles stimulated by anti-β2GPI/β2GPI complexes induce pyroptosis of endothelial cells in antiphospholipid syndrome. Platelets 2022, 34, 2156492. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhou, Q.; Yang, Y.; Li, L.; Yu, M.; Li, H.; Li, A.; Wang, X.; Jiang, Y. Identification and ultrasensitive photoelectrochemical detection of LncNR_040117: A biomarker of recurrent miscarriage and antiphospholipid antibody syndrome in platelet-derived microparticles. J. Nanobiotechnology 2022, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef]
- Wang, X.; Pang, K.; Wang, J.; Zhang, B.; Liu, Z.; Lu, S.; Xu, X.; Zhu, L.; Zhou, Z.; Niu, M.; et al. Microbiota dysbiosis in primary Sjögren’s syndrome and the ameliorative effect of hydroxychloroquine. Cell Rep. 2022, 40, 111352. [Google Scholar] [CrossRef] [PubMed]
- Maslinska, M.; Kostyra-Grabczak, K. The role of virus infections in Sjögren’s syndrome. Front. Immunol. 2022, 13, 823659. [Google Scholar] [CrossRef]
- Sun, Y.; Ieng, F.C.; Lai, Y.-H. Optic Neuritis and Immune Thrombocytopenia as the Initial Presentation of Primary Sjögren Syndrome. J. Neuro-Ophthalmology, 2021; online ahead of print. [Google Scholar] [CrossRef]
- Sellam, J.; Proulle, V.; Jüngel, A.; Ittah, M.; Richard, C.M.; Gottenberg, J.-E.; Toti, F.; Benessiano, J.; Gay, S.; Freyssinet, J.-M.; et al. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Thromb. Haemost. 2009, 11, R156. [Google Scholar] [CrossRef]
- Bartoloni, E.; Alunno, A.; Bistoni, O.; Caterbi, S.; Luccioli, F.; Santoboni, G.; Mirabelli, G.; Cannarile, F.; Gerli, R. Characterization of circulating endothelial microparticles and endothelial progenitor cells in primary Sjogren’s syndrome: New markers of chronic endothelial damage? Rheumatology 2014, 54, 536–544. [Google Scholar] [CrossRef]
- Frazzei, G.; van Vollenhoven, R.F.; de Jong, B.A.; Siegelaar, S.E.; van Schaardenburg, D. Preclinical Autoimmune Disease: A Comparison of Rheumatoid Arthritis, Systemic Lupus Erythematosus, Multiple Sclerosis and Type 1 Diabetes. Front. Immunol. 2022, 13, 899372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Wu, G.-R.; Zhou, Q.; Yue, H.; Rao, L.-Z.; Yuan, T.; Mo, B.; Wang, F.-X.; Chen, L.-M.; et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci. Adv. 2021, 7, eabb6075. [Google Scholar] [CrossRef]
- Marou, E.; Liaskos, C.; Efthymiou, G.; Dardiotis, E.; Daponte, A.; Scheper, T.; Meyer, W.; Hadjigeorgiou, G.; Bogdanos, D.P.; Sakkas, L.I. Increased immunoreactivity against human cytomegalovirus UL83 in systemic sclerosis. Ann. Rheum. Dis. 2017, 35 (Suppl. 106), 31–34. [Google Scholar]
- Houen, G.; Trier, N.H. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front. Immunol. 2021, 11, 587380. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Tashkin, D.P.; Denton, C.P.; Renzoni, E.A.; Desai, S.R.; Varga, J. Etiology, Risk Factors, and Biomarkers in Systemic Sclerosis with Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 650–660. [Google Scholar] [CrossRef]
- Čolić, J.; Cerinic, M.M.; Guiducci, S.; Damjanov, N. Microparticles in systemic sclerosis, targets or tools to control fibrosis: This is the question! J. Scleroderma Relat. Disord. 2019, 5, 6–20. [Google Scholar] [CrossRef]
- Ntelis, K.; Bogdanos, D.; Dimitroulas, T.; Sakkas, L.; Daoussis, D. Platelets in Systemic Sclerosis: The Missing Link Connecting Vasculopathy, Autoimmunity, and Fibrosis? Curr. Rheumatol. Rep. 2019, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Guillotin, V.; Truchetet, M.-E.; Contin-Bordes, C.; Sisirak, V.; Duffau, P.; Lazaro, E.; Richez, C.; Blanco, P. Systemic lupus erythematosus and systemic sclerosis: All roads lead to platelets. Autoimmun. Rev. 2018, 17, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Oyabu, C.; Morinobu, A.; Sugiyama, D.; Saegusa, J.; Tanaka, S.; Morinobu, S.; Tsuji, G.; Kasagi, S.; Kawano, S.; Kumagai, S. Plasma Platelet-derived Microparticles in Patients with Connective Tissue Diseases. J. Rheumatol. 2011, 38, 680–684. [Google Scholar] [CrossRef]
- Leleu, D.; Levionnois, E.; Laurent, P.; Lazaro, E.; Richez, C.; Duffau, P.; Blanco, P.; Sisirak, V.; Contin-Bordes, C.; Truchetet, M.-E. Elevated Circulatory Levels of Microparticles Are Associated to Lung Fibrosis and Vasculopathy During Systemic Sclerosis. Front. Immunol. 2020, 11, 532177. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.; Walia, A.; Ortiz, V.; Perry, C.; Woo, S.; Reeves, B.C.; Sun, H.; Winkler, J.; Kanyo, J.E.; Wang, W.; et al. Bioactive Plasma Mitochondrial DNA Is Associated With Disease Progression in Scleroderma-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2020, 72, 1905–1915. [Google Scholar] [CrossRef]
- Manfredi, A.A.; Ramirez, G.A.; Godino, C.; Capobianco, A.; Monno, A.; Franchini, S.; Tombetti, E.; Corradetti, S.; Distler, J.H.W.; Bianchi, M.E.; et al. Platelet Phagocytosis via P-selectin Glycoprotein Ligand 1 and Accumulation of Microparticles in Systemic Sclerosis. Arthritis Rheumatol. 2021, 74, 318–328. [Google Scholar] [CrossRef]
- Khan, M.A.; Braun, W.E.; Kushner, I.; Grecek, D.E.; Muir, W.A.; Steinberg, A.G. HLA B27 in Ankylosing Spondylitis: Differences in Frequency and Relative Risk in American Blacks and Caucasians. J. Rheumatol. 2023, 50, 39–43. [Google Scholar]
- Wang, C.; Liao, Q.; Hu, Y.; Zhong, D. T lymphocyte subset imbalances in patients contribute to ankylosing spondylitis. Exp. Ther. Med. 2014, 9, 250–256. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Diabetes mellitus in ankylosing spondylitis. Ann. Rheum. Dis. 2019, 80, e142. [Google Scholar] [CrossRef]
- Tsur, A.M.; David, P.; Watad, A.; Nissan, D.; Cohen, A.D.; Amital, H. Ankylosing Spondylitis and the Risk of Hip Fractures: A Matched Cohort Study. J. Gen. Intern. Med. 2022, 37, 3283–3288. [Google Scholar] [CrossRef]
- Qian, H.; Chen, R.; Wang, B.; Yuan, X.; Chen, S.; Liu, Y.; Shi, G. Associations of Platelet Count with Inflammation and Response to Anti-TNF-α Therapy in Patients with Ankylosing Spondylitis. Front. Pharmacol. 2020, 11, 559593. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Bozkaya, G.; Kirbiyik, H.; Alacacioglu, A.; Ates, H.; Sop, G.; Can, G.; Taylan, A.; Piskin, O.; Yildiz, Y.; et al. Evaluation of Circulating Endothelial and Platelet Microparticles in Men with Ankylosing Spondylitis. J. Rheumatol. 2012, 39, 594–599. [Google Scholar] [CrossRef]
- Wang, T.; Meng, S.; Chen, P.; Wei, L.; Liu, C.; Tang, D.; Liu, D.; Jiang, Z.; Hong, X. Comprehensive analysis of differentially expressed mRNA and circRNA in Ankylosing spondylitis patients’ platelets. Exp. Cell Res. 2021, 409, 112895. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Walsh, M.; Merkel, P.A.; Peh, C.A.; Szpirt, W.; Puéchal, X.; Fujimoto, S.; Hawley, C.; Khalidi, N.; Jones, R.; et al. Plasma exchange and glucocorticoids to delay death or end-stage renal disease in anti-neutrophil cytoplasm antibody-associated vasculitis: PEXIVAS non-inferiority factorial RCT. Health Technol. Assess. 2022, 26, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.; Sandoval, P.; Burgos, P.I. Central Nervous System and Systemic Vasculitis in an Immunocompetent Syphilitic Non-HIV Patient. Am. J. Clin. Oncol. 2020, 28, e684–e686. [Google Scholar] [CrossRef]
- Kronbichler, A.; Leierer, J.; Gauckler, P.; Shin, J.I. Comorbidities in ANCA-associated vasculitis. Rheumatology 2020, 59, iii79–iii83. [Google Scholar] [CrossRef]
- Yoon, C.W.; Park, H.-K.; Rha, J.-H. Yield of Screening Tests for Systemic Vasculitis in Young Adults with Ischemic Stroke. Eur. Neurol. 2018, 80, 245–248. [Google Scholar] [CrossRef]
- Brogan, P.A.; Shah, V.; Brachet, C.; Harnden, A.; Mant, D.; Klein, N.; Dillon, M.J. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum. 2004, 50, 927–936. [Google Scholar] [CrossRef]
- Berden, A.E.; Nolan, S.L.; Morris, H.L.; Bertina, R.M.; Erasmus, D.D.; Hagen, E.C.; Hayes, D.P.; van Tilburg, N.H.; Bruijn, J.A.; Savage, C.O.; et al. Anti-Plasminogen Antibodies Compromise Fibrinolysis and Associate with Renal Histology in ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2010, 21, 2169–2179. [Google Scholar] [CrossRef]
- Miao, D.; Ma, T.-T.; Chen, M.; Zhao, M.-H. Platelets release proinflammatory microparticles in anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatology 2019, 58, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Suzuki, H.; Onouchi, Y.; Ebata, R.; Terai, M.; Fuse, S.; Okajima, Y.; Kurotobi, S.; Hirai, K.; Soga, T.; et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): A randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet 2019, 393, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, J.; Lu, Y.; Fan, Z.; Huang, N.; Ma, L.; Yu, H. Platelet-Derived Microparticles: A New Index of Monitoring Platelet Activation and Inflammation in Kawasaki Disease. Indian J. Pediatr. 2018, 86, 250–255. [Google Scholar] [CrossRef] [PubMed]
| Exosomes | PMPs | |
|---|---|---|
| Size | 30~150 nm | 100~1000 nm |
| Density | 1.14–1.18 g/mL | >1.23 g/mL |
| Shape | Relatively uniform, round cup shape | Different sizes and shapes |
| Origin | Multivesicular bodies (MVB) | Plasma membrane |
| Release mechanism | Exocytosis | Ectocytosis |
| Markers |
|
|
| Reference | [15,16,17] | [20,21,22] |
| Autoimmune Disease | In-/Decrease of PMP | PMP Associated Molecules | Pathogenic Role of PMP | References |
|---|---|---|---|---|
| Rheumatoid arthritis | increase | P-selectin (CD62P), citrullinated fibrinogen, vimentin, CXCL5, CXCL4, and CXCL7 |
| [38,39,40,41,42,43,44,45,46,47,48,49,50,51] |
| Systemic Lupus Erythematosus | increase | CD41, CD61, P-selectin, IL-1β, IgG, and HMGB1 |
| [59,60,61] |
| Antiphospholipid antibody syndrome | not statistically significant | TNF-α, ICAM-1, VCAM-1, anti-β2GPI/β2GPI complex, and LncNR_040117 |
| [67,68,69,70,71] |
| Sjogren syndrome | increase | To be studied | To be studied | [75,76,77] |
| Systemic sclerosis | increase | P-selectin, HMGB1, and mtDNA (MT-ATP6) |
| [86,87,88,89] |
| Ankylosing Spondylitis | not statistically significant | To be studied |
| [95,96] |
| Systemic vasculitis | increase | chemokines, adhesion factors, growth factors, and apoptosis factors |
| [98,99,100,101,102,103,104,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Q. Platelet-Derived Microparticles and Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 10275. https://doi.org/10.3390/ijms241210275
Li X, Wang Q. Platelet-Derived Microparticles and Autoimmune Diseases. International Journal of Molecular Sciences. 2023; 24(12):10275. https://doi.org/10.3390/ijms241210275
Chicago/Turabian StyleLi, Xiaoshuai, and Qiushi Wang. 2023. "Platelet-Derived Microparticles and Autoimmune Diseases" International Journal of Molecular Sciences 24, no. 12: 10275. https://doi.org/10.3390/ijms241210275
APA StyleLi, X., & Wang, Q. (2023). Platelet-Derived Microparticles and Autoimmune Diseases. International Journal of Molecular Sciences, 24(12), 10275. https://doi.org/10.3390/ijms241210275





