Association of the rs3864283 Polymorphism Located in the HINT1 Gene with Cigarette Use and Personality Traits
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Psychometric Tests
4.3. Genotyping
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, P.; Peto, R. Global Effects of Smoking, of Quitting, and of Taxing Tobacco. N. Engl. J. Med. 2014, 370, 60–68. [Google Scholar] [CrossRef]
- Prochaska, J.J.; Benowitz, N.L. Current Advances in Research in Treatment and Recovery: Nicotine Addiction. Sci. Adv. 2019, 5, 9763–9779. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M. Effects of Isoarecolone, a Nicotinic Receptor Agonist in Rodent Models of Nicotine Dependence. Psychopharmacology 2006, 188, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Stolerman, I.P.; Jarvis, M.J. The Scientific Case That Nicotine Is Addictive. Psychopharmacology 1995, 117, 2–10. [Google Scholar] [CrossRef]
- Wills, L.; Kenny, P.J. Addiction-Related Neuroadaptations Following Chronic Nicotine Exposure. J. Neurochem. 2021, 157, 1652–1673. [Google Scholar] [CrossRef]
- McCrae, R.R.; John, O.P. An Introduction to the Five-Factor Model and Its Applications. J. Pers. 1992, 60, 175–215. [Google Scholar] [CrossRef]
- Benotsch, E.G.; Jeffers, A.J.; Snipes, D.J.; Martin, A.M.; Koester, S. The Five Factor Model of Personality and the Non-Medical Use of Prescription Drugs: Associations in a Young Adult Sample. Pers. Individ. Dif. 2013, 55, 852–855. [Google Scholar] [CrossRef]
- Terracciano, A.; Löckenhoff, C.E.; Crum, R.M.; Bienvenu, O.J.; Costa, P.T. Five-Factor Model Personality Profiles of Drug Users. BMC Psychiatry 2008, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.W.; Beutel, M.E.; Egloff, B.; Wölfling, K. Investigating Risk Factors for Internet Gaming Disorder: A Comparison of Patients with Addictive Gaming, Pathological Gamblers and Healthy Controls Regarding the Big Five Personality Traits. Eur. Addict. Res. 2014, 20, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bagby, R.M.; Vachon, D.D.; Bulmash, E.L.; Toneatto, T.; Quilty, L.C.; Costa, P.T. Pathological Gambling and the Five-Factor Model of Personality. Pers. Individ. Dif. 2007, 43, 873–880. [Google Scholar] [CrossRef]
- Khantzian, E.J. The Self-Medication Hypothesis of Addictive Disorders: Focus on Heroin and Cocaine Dependence. In The Cocaine Crisis; Springer: Boston, MA, USA, 1987; pp. 65–74. [Google Scholar] [CrossRef]
- Kuntsche, E.; Knibbe, R.; Gmel, G.; Engels, R. Who Drinks and Why? A Review of Socio-Demographic, Personality, and Contextual Issues behind the Drinking Motives in Young People. Addict. Behav. 2006, 31, 1844–1857. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Choi, J.S.; Gwak, A.R.; Jung, D.; Choi, S.W.; Lee, J.; Lee, J.Y.; Jung, H.Y.; Kim, D.J. Shared Psychological Characteristics That Are Linked to Aggression between Patients with Internet Addiction and Those with Alcohol Dependence. Ann. Gen. Psychiatry 2014, 13, 6. [Google Scholar] [CrossRef]
- Grekin, E.R.; Sher, K.J.; Wood, P.K. Personality and Substance Dependence Symptoms: Modeling Substance-Specific Traits. Psychol. Addict. Behav. 2006, 20, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Shin, Y.C.; Lim, S.W.; Park, H.Y.; Shin, N.Y.; Jang, J.H.; Park, H.Y.; Kwon, J.S. Multidimensional Comparison of Personality Characteristics of the Big Five Model, Impulsiveness, and Affect in Pathological Gambling and Obsessive-Compulsive Disorder. J. Gambl. Stud. 2012, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kotov, R.; Gamez, W.; Schmidt, F.; Watson, D. Linking “Big” Personality Traits to Anxiety, Depressive, and Substance Use Disorders: A Meta-Analysis. Psychol. Bull. 2010, 136, 768–821. [Google Scholar] [CrossRef]
- Kornør, H.; Nordvik, H. Five-Factor Model Personality Traits in Opioid Dependence. BMC Psychiatry 2007, 7, 37. [Google Scholar] [CrossRef]
- Cloninger, C.R. A Systematic Method for Clinical Description and Classification of Personality Variants. A Proposal. Arch. Gen. Psychiatry 1987, 44, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Cloninger, C.R.; Svrakic, D.M.; Przybeck, T.R. A Psychobiological Model of Temperament and Character. Arch. Gen. Psychiatry 1993, 50, 975–990. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Przybeck, T.R.; Svrakic, D.M.; Wetzel, R.D. The Temperament and Character Inventory (TCI): A Guide to Its Development and Use; Center for Psychobiology of Personality, Washington University: St. Louis, MO, USA, 1994; ISBN 0964291703. [Google Scholar]
- Mikołajczk, E.; Ziȩtek, J.; Samochowiec, A.; Samochowiec, J. Personality Dimensions Measured Using the Temperament and Character Inventory (TCI) and NEO-FFI on a Polish Sample. Int. J. Methods Psychiatr. Res. 2008, 17, 210–219. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Hossain, S.; O’Neill, F.A.; Walsh, D.; Pless, L.; Chowdari, K.V.; Nimgaonkar, V.L.; Schwab, S.G.; Wildenauer, D.B.; et al. Haplotypes Spanning SPEC2, PDZ-GEF2 and ACSL6 Genes Are Associated with Schizophrenia. Hum. Mol. Genet. 2006, 15, 3329–3342. [Google Scholar] [CrossRef]
- Straub, R.E.; MacLean, C.J.; O’Neill, F.A.; Walsh, D.; Kendler, K.S. Support for a Possible Schizophrenia Vulnerability Locus in Region 5q22-31 in Irish Families. Mol. Psychiatry 1997, 2, 148–155. [Google Scholar] [CrossRef]
- Schwab, S.G.; Eckstein, G.N.; Hallmayer, J.; Lerer, B.; Albus, M.; Borrmann, M.; Lichtermann, D.; Ertl, M.A.; Maier, W.; Wildenauer, D.B. Evidence Suggestive of a Locus on Chromosome 5q31 Contributing to Susceptibility for Schizophrenia in German and Israeli Families by Multipoint Affected Sib-Pair Linkage Analysis. Mol. Psychiatry 1997, 2, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Morel, V.; Campana-Salort, E.; Boyer, A.; Esselin, F.; Walther-Louvier, U.; Querin, G.; Latour, P.; Lia, A.S.; Magdelaine, C.; Beze-Beyrie, P.; et al. HINT1 Neuropathy: Expanding the Genotype and Phenotype Spectrum. Clin. Genet. 2022, 102, 379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Puche, A.C.; Wang, J.B. Distribution and Expression of Protein Kinase C Interactive Protein (PKCI/HINT1) in Mouse Central Nervous System (CNS). Neurochem. Res. 2008, 33, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Vawter, M.P.; Crook, J.M.; Hyde, T.M.; Kleinman, J.E.; Weinberger, D.R.; Becker, K.G.; Freed, W.J. Microarray Analysis of Gene Expression in the Prefrontal Cortex in Schizophrenia: A Preliminary Study. Schizophr. Res. 2002, 58, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Vawter, M.P.; Barrett, T.; Cheadle, C.; Sokolov, B.P.; Wood, W.H.; Donovan, D.M.; Webster, M.; Freed, W.J.; Becker, K.G. Application of CDNA Microarrays to Examine Gene Expression Differences in Schizophrenia. Brain Res. Bull. 2001, 55, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Vawter, M.P.; Weickert, C.S.; Ferran, E.; Matsumoto, M.; Overman, K.; Hyde, T.M.; Weinberger, D.R.; Bunney, W.E.; Kleinman, J.E. Gene Expression of Metabolic Enzymes and a Protease Inhibitor in the Prefrontal Cortex Are Decreased in Schizophrenia. Neurochem. Res. 2004, 29, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Guang, W.; Wang, H.; Su, T.; Weinstein, I.B.; Jia, B.W. Role of MPKCI, a Novel Mu-Opioid Receptor Interactive Protein, in Receptor Desensitization, Phosphorylation, and Morphine-Induced Analgesia. Mol. Pharm. 2004, 66, 1285–1292. [Google Scholar] [CrossRef]
- Barbier, E.; Zapata, A.; Oh, E.; Liu, Q.; Zhu, F.; Undie, A.; Shippenberg, T.; Wang, J.B. Supersensitivity to Amphetamine in Protein Kinase-C Interacting Protein/HINT1 Knockout Mice. Neuropsychopharmacology 2007, 32, 1774–1782. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; O’Neill, F.A.; Walsh, D.; Kendler, K.S.; Chen, X. Is the Histidine Triad Nucleotide-Binding Protein 1 (HINT1) Gene a Candidate for Schizophrenia? Schizophr. Res. 2008, 106, 200. [Google Scholar] [CrossRef]
- Jackson, K.J.; Chen, Q.; Chen, J.; Aggen, S.H.; Kendler, K.S.; Chen, X. Association of the Histidine-Triad Nucleotide-Binding Protein-1 (HINT1) Gene Variants with Nicotine Dependence. Pharm. J. 2011, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, X.; He, B. Association between Common Genetic Variants in the Opioid Pathway and Smoking Behaviors in Chinese Men. Behav. Brain Funct. 2014, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Waga, C.; Iwahashi, K. CYP2A6 Gene Polymorphism and Personality Traits for NEO-FFI on the Smoking Behavior of Youths. Drug. Chem. Toxicol. 2007, 30, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Parkar, S.; Kate, N.; Ninawe, K.; Limbachiya, R. Role of Personality in Tobacco Smoking Behavior in Corporate Sector: A Cross-Sectional Study. Ind. Psychiatry J. 2018, 27, 103. [Google Scholar] [CrossRef]
- Laviolette, S.R.; Lauzon, N.M.; Bishop, S.F.; Sun, N.; Tan, H. Dopamine Signaling through D1-Like versus D2-Like Receptors in the Nucleus Accumbens Core versus Shell Differentially Modulates Nicotine Reward Sensitivity. J. Neurosci. 2008, 28, 8025. [Google Scholar] [CrossRef]
- Sellings, L.H.L.; Baharnouri, G.; McQuade, L.E.; Clarke, P.B.S. Rewarding and Aversive Effects of Nicotine Are Segregated within the Nucleus Accumbens. Eur. J. Neurosci. 2008, 28, 342–352. [Google Scholar] [CrossRef]
- Brunzell, D.H.; Mineur, Y.S.; Neve, R.L.; Picciotto, M.R. Nucleus Accumbens CREB Activity Is Necessary for Nicotine Conditioned Place Preference. Neuropsychopharmacology 2009, 34, 1993. [Google Scholar] [CrossRef]
- Liechti, M.E.; Lhuillier, L.; Kaupmann, K.; Markou, A. Metabotropic Glutamate 2/3 Receptors in the Ventral Tegmental Area and the Nucleus Accumbens Shell Are Involved in Behaviors Relating to Nicotine Dependence. J. Neurosci. 2007, 27, 9077. [Google Scholar] [CrossRef]
- Jackson, K.J.; Wang, J.B.; Barbier, E.; Damaj, M.I.; Chen, X. The Histidine Triad Nucleotide Binding 1 Protein Is Involved in Nicotine Reward and Physical Nicotine Withdrawal in Mice. Neurosci. Lett. 2013, 550, 129–133. [Google Scholar] [CrossRef]
- Liu, P.; Chu, Z.; Lei, G.; Deng, L.S.; Yang, L.; Dang, Y.H. The Role of HINT1 Protein in Morphine Addiction: An Animal Model-Based Study. Addict. Biol. 2021, 26, e12897. [Google Scholar] [CrossRef]
- Costa, P.T.; McCrae, R.R. The Revised NEO Personality Inventory (NEO-PI-R). In The SAGE Handbook of Personality Theory and Assessment: Volume 2—Personality Measurement and Testing; Sage Publications, Inc.: London, UK, 2008; pp. 179–198. [Google Scholar] [CrossRef]
| Hardy-Weinberg Equilibrium Calculator Including Analysis for Ascertainment Bias | Observed (Expected) | Allele Freq | χ2 (p Value) | |
|---|---|---|---|---|
| rs3864283 Cigarette Users n = 371 | G/G | 19 (23.6) | p (ins) = 0.75 q (del) = 0.25 | 1.580 (0.2088) |
| A/A | 203 (207.6) | |||
| A/G | 149 (139.9) | |||
| rs3864283 control n = 151 | G/G | 16 (16.2) | p (ins) = 0.67 q (del) = 0.33 | 0.0070 (0.9332) |
| A/A | 68 (68.2) | |||
| A/G | 67 (66.5) | |||
| rs3864283 | |||||
|---|---|---|---|---|---|
| Genotypes | Alleles | ||||
| G/G n(%) | A/A n(%) | A/G n(%) | G n(%) | A n(%) | |
| Cigarette Users n = 371 | 19 (5.12%) | 203 (54.72%) | 149 (40.16%) | 187 (25.20%) | 555 (74.80%) |
| Control n = 151 | 16 (10.60%) | 68 (45.03%) | 67 (44.37%) | 99 (32.78%) | 203 (67.22%) |
| χ2 (p value) | 7.195 (0.0274) * | 6.200 (0.0128) * | |||
| STAI/NEO Five-Factor Inventory/ | Cigarette Users (n = 371) | Control (n = 151) | Z | (p-Value) |
|---|---|---|---|---|
| STAI trait/scale | 5.95 ± 2.22 | 5.64 ± 2.18 | 1.843 | 0.0652 |
| STAI state/scale | 5.56 ± 2.35 | 5.50 ± 2.23 | 0.562 | 0.5741 |
| Neuroticism/scale | 5.93 ± 2.22 | 5.71 ± 1.99 | 1.460 | 0.1442 |
| Extraversion/scale | 5.96 ± 2.09 | 5.25 ± 1.96 | 3.273 | 0.0011 * |
| Openness/scale | 5.20 ± 2.03 | 5.69 ± 2.01 | −2.750 | 0.0060 * |
| Agreeability/scale | 5.25 ± 2.25 | 6.35 ± 2.37 | −4.718 | 0.0000 * |
| Conscientiousness/scale | 5.82 ± 2.13 | 6.76 ± 2.25 | −4.251 | 0.0000 * |
| STAI/NEO Five-Factor Inventory | Group | rs3864283 | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| G/G n = 35 M ± SD | A/A N = 271 M ± SD | A/G n = 216 M ± SD | Factor | F (p Value) | ɳ2 | Power (alfa = 0.05) | ||
| STAI trait scale | Cigarette Users (CU); n = 371 | 5.53 ± 2.84 | 6.16 ± 2.50 | 5.73 ± 2.25 | intercept CU/control rs3864283 CU/control x rs3864283 | F1,516 = 1301.26 (p < 0.0001) F1,516 = 1.88 (p = 0.1710) F2,516 = 2.62 (p = 0.074) F2,516 = 0.49 (p = 0.6098) | 0.718 0.004 0.010 0.002 | 1.000 0.277 0.521 0.131 |
| Control; n = 151 | 4.63 ± 1.96 | 5.85 ± 2.07 | 5.67 ± 2.28 | |||||
| STAI state scale | Cigarette Users (CU); n = 371 | 5.73 ± 2.47 | 5.70 ± 2.44 | 5.33 ± 2.21 | intercept CU/control rs3864283 CU/control x rs3864283 | F1,516 = 1284.59 (p < 0.0001) F1,516 = 0.594 (p = 0.4411) F2,516 = 0.34 (p = 0.7113) F2,516 = 0.96 (p = 0.3829) | 0.713 0.001 0.001 0.004 | 1.000 0.120 0.104 0.217 |
| C: Control; n = 151 | 4.94 ± 2.32 | 5.53 ± 2.22 | 5.60 ± 221 | |||||
| Neuroticism scale | Cigarette Users (CU); n = 371 | 5.26 ± 2.84 | 5.89 ± 2.26 | 6.07 ± 2.07 | intercept CU/control rs3864283 CU/control x rs3864283 | F1,516 = 1596.18 (p < 0.0001) F1,516 = 0.19 (p = 0.6586) F2,516 = 1.59 (p = 0.2046) F2,516 = 0.07 (p = 0.9352) | 0.756 0.0004 0.006 0.0003 | 1.000 0.073 0.337 0.060 |
| Control; n = 151 | 5.31 ± 1.74 | 5.65 ± 1.84 | 5.88 ± 2.17 | |||||
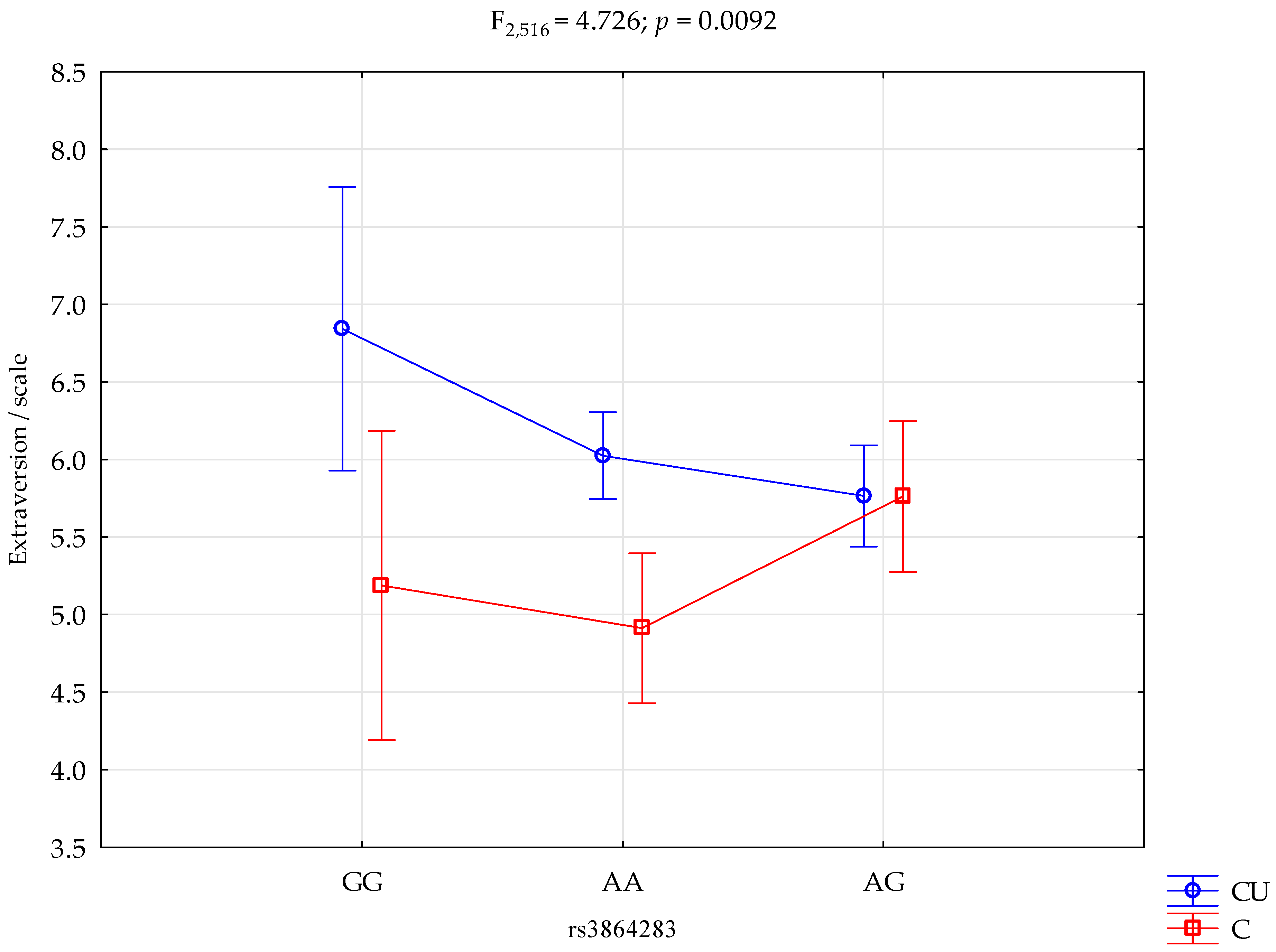
| Extraversion scale | Cigarette Users (CU); n = 371 | 6.84 ± 1.54 | 6.02 ± 2.17 | 5.76 ± 2.00 | intercept CU/control rs3864283 CU/control x rs3864283 | F1,516 = 1847.76 (p < 0.0001) F1,516 = 11.93 (p = 0.0006) * F2,516 = 1.66 (p = 0.1904) F2,516 = 4.73 (p = 0.0092) * | 0.782 0.023 0.006 0.018 | 1.000 0.931 0.351 0.789 |
| Control; n = 151 | 5.19 ± 1.64 | 4.91 ± 2.03 | 5.76 ± 1.80 | |||||
| Openness scale | Cigarette Users (CU); n = 371 | 5.21 ± 2.04 | 5.19 ± 1.91 | 5.21 ± 2.18 | intercept CU/control rs3864283 CU/control x rs3864283 | F1,516 = 1653.66 (p < 0.0001) F1,516 = 2.96 (p = 0.0858) F2,516 = 0.37 (p = 0.6931) F2,516 = 0.31 (p = 0.7367) | 0.762 0.006 0.001 0.001 | 1.000 0.404 0.109 0.099 |
| Control; n = 151 | 5.62 ± 2.53 | 5.51 ± 1.95 | 5.85 ± 1.95 | |||||
| Agreeability scale | Cigarette Users (CU); n = 371 | 5.53 ± 2.84 | 5.15 ± 2.20 | 5.36 ± 2.24 | intercept CU/control rs3864283 CU/control x rs3864283 | F1,516 = 1482.32 (p < 0.0001) F1,516 = 8.98 (p = 0.0028) * F2,516= 1.12 (p = 0.3256) F2,516 = 0.47 (p = 0.6234) | 0.741 0.017 0.004 0.002 | 1.000 0.848 0.248 0.127 |
| Control; n = 151 | 6.00 ± 2.42 | 6.13 ± 2.30 | 6.61 ± 2.42 | |||||
| Conscientiousness scale | Cigarette Users (CU); n = 371 | 5.58 ± 2.19 | 5.94 ± 2.21 | 5.72 ± 1.98 | intercept CU/control rs3864283 CU/control x rs3864283 | F1,516 = 1896.27 (p < 0.0001) F1,516 = 10.72 (p = 0.0011) * F2,516 = 0.32 (p = 0.7260) F2,516 = 0.18 (p = 0.8352) | 0.786 0.020 0.001 0.0007 | 1.000 0.904 0.101 0.078 |
| Control; n = 151 | 6.50 ± 1.93 | 6.75 ± 2.18 | 6.79 ± 2.43 | |||||
| rs3864283 and Extraversion Scale | ||||||
|---|---|---|---|---|---|---|
| {1} M = 6.84 | {2} M = 6.02 | {3} M = 5.76 | {4} M = 5.19 | {5} M = 4.91 | {6} M = 5.76 | |
| Cigarette Users G/G {1} | 0.0937 | 0.0298 | 0.0166 | 0.0003 * | 0.0409 | |
| Cigarette Users A/A {2} | 0.2363 | 0.1127 | 0.0001 * | 0.3572 | ||
| Cigarette Users A/G {3} | 0.2797 | 0.0042 | 0.9896 | |||
| Control G/G {4} | 0.6249 | 0.3100 | ||||
| Control A/A {5} | 0.0153 | |||||
| Control A/G {6} | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchanecka, A.; Boroń, A.; Chmielowiec, K.; Strońska-Pluta, A.; Masiak, J.; Lachowicz, M.; Chmielowiec, J.; Grzywacz, A. Association of the rs3864283 Polymorphism Located in the HINT1 Gene with Cigarette Use and Personality Traits. Int. J. Mol. Sci. 2023, 24, 10244. https://doi.org/10.3390/ijms241210244
Suchanecka A, Boroń A, Chmielowiec K, Strońska-Pluta A, Masiak J, Lachowicz M, Chmielowiec J, Grzywacz A. Association of the rs3864283 Polymorphism Located in the HINT1 Gene with Cigarette Use and Personality Traits. International Journal of Molecular Sciences. 2023; 24(12):10244. https://doi.org/10.3390/ijms241210244
Chicago/Turabian StyleSuchanecka, Aleksandra, Agnieszka Boroń, Krzysztof Chmielowiec, Aleksandra Strońska-Pluta, Jolanta Masiak, Milena Lachowicz, Jolanta Chmielowiec, and Anna Grzywacz. 2023. "Association of the rs3864283 Polymorphism Located in the HINT1 Gene with Cigarette Use and Personality Traits" International Journal of Molecular Sciences 24, no. 12: 10244. https://doi.org/10.3390/ijms241210244
APA StyleSuchanecka, A., Boroń, A., Chmielowiec, K., Strońska-Pluta, A., Masiak, J., Lachowicz, M., Chmielowiec, J., & Grzywacz, A. (2023). Association of the rs3864283 Polymorphism Located in the HINT1 Gene with Cigarette Use and Personality Traits. International Journal of Molecular Sciences, 24(12), 10244. https://doi.org/10.3390/ijms241210244








