l-Alanine Exporter AlaE Functions as One of the d-Alanine Exporters in Escherichia coli
Abstract
1. Introduction
2. Results and Discussion
2.1. Rationale of the Experimental Design
2.2. Screening of Candidate Clones Exporting d-Ala by a Bioassay Approach
2.3. Measurement of d-Ala Export Activity of Candidate Proteins for d-Ala Exporter
2.4. Accumulation of d-Ala in Intact Cells Expressing Plasmid-Borne alaE Gene
2.5. alaE Overexpression Promotes d-Ala Export
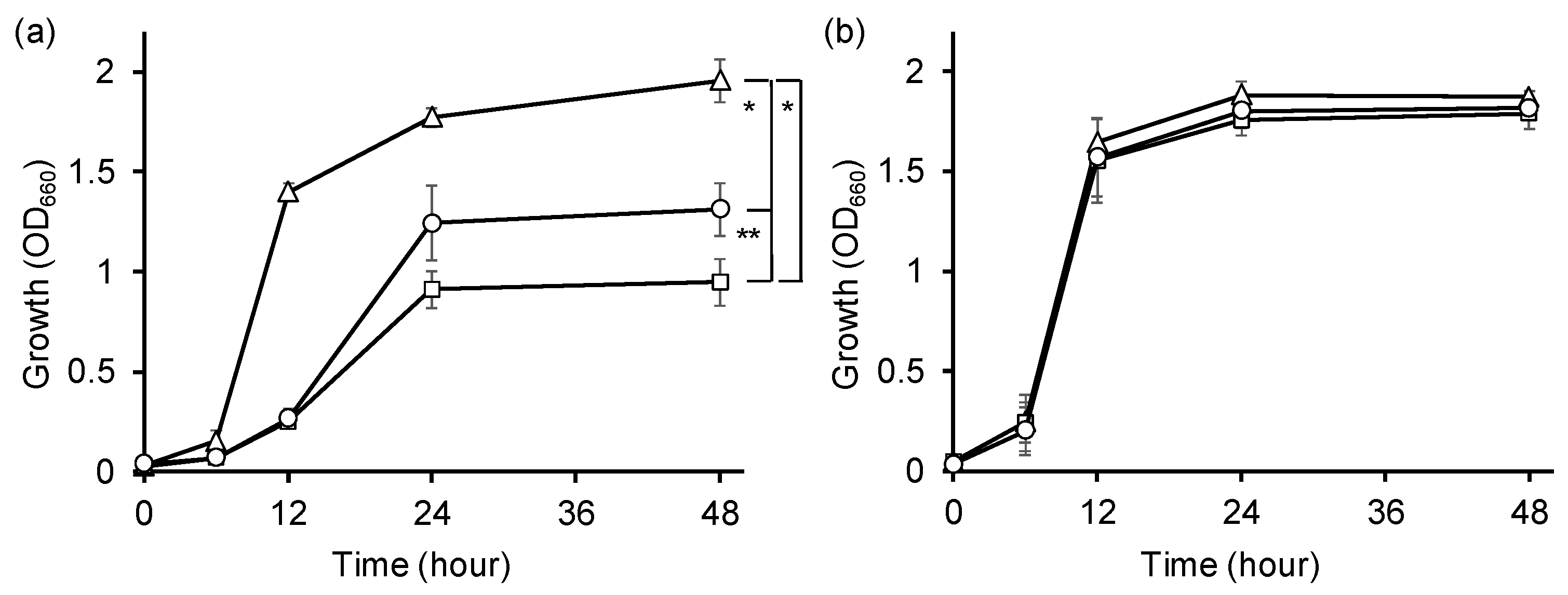
2.6. Impact of alaE Overexpression on the Growth of dadA-Deficient E. coli Cells in the Presence of d-Ala
3. Materials and Methods
3.1. Bacterial Strains and Plasmids
3.2. Bioassay Screening for Identification of d-Ala Exporter Candidates
3.3. d-Ala Accumulation in Intact Cells
3.4. Growth Measurements
3.5. Western Blotting Analysis
3.6. d-Ala Export Assay
3.7. Determination of an Extracellular d-Ala by HPLC
3.8. Determination of Protein Concentration
3.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genchi, G. An overview on d-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.D.; Hedges, J.I.; Benner, R. Major bacterial contribution to marine dissolved organic nitrogen. Science 1998, 281, 231–234. [Google Scholar] [CrossRef]
- Hashimoto, A.; Nishikawa, T.; Hayashi, T.; Fujii, N.; Harada, K.; Oka, T.; Takahashi, K. The presence of free d-serine in rat brain. FEBS Lett. 1992, 296, 33–36. [Google Scholar] [CrossRef]
- Shleper, M.; Kartvelishvily, E.; Wolosker, H. d-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J. Neurosci. 2005, 25, 9413–9417. [Google Scholar] [CrossRef]
- D'Aniello, A. d-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef]
- Radkov, A.D.; Moe, L.A. Bacterial synthesis of d-amino acids. Appl. Microbiol. Biotechnol. 2014, 98, 5363–5374. [Google Scholar] [CrossRef] [PubMed]
- Cava, F.; Lam, H.; de Pedro, M.A.; Waldor, M.K. Emerging knowledge of regulatory roles of d-amino acids in bacteria. Cell. Mol. Life Sci. 2011, 68, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.; Oh, D.C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. d-amino acids govern stationary phase cell wall remodeling in bacteria. Science 2009, 325, 1552–1555. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. d-amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef]
- Liu, X.; Cao, B.; Yang, L.; Gu, J.D. Biofilm control by interfering with c-di-GMP metabolism and signaling. Biotechnol. Adv. 2022, 56, 107915. [Google Scholar] [CrossRef]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New Insights Into the Mechanisms and Biological Roles of d-Amino Acids in Complex Eco-Systems. Front. Microbiol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Dassler, T.; Maier, T.; Winterhalter, C.; Böck, A. Identification of a major facilitator protein from Escherichia coli involved in efflux of metabolites of the cysteine pathway. Mol. Microbiol. 2000, 36, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Doroshenko, V.; Airich, L.; Vitushkina, M.; Kolokolova, A.; Livshits, V.; Mashko, S. YddG from Escherichia coli promotes export of aromatic amino acids. FEMS Microbiol. Lett. 2007, 275, 312–318. [Google Scholar] [CrossRef]
- Kennerknecht, N.; Sahm, H.; Yen, M.R.; Pátek, M.; Saier, M.H., Jr.; Eggeling, L. Export of L-isoleucine from Corynebacterium glutamicum: A two-gene-encoded member of a new translocator family. J. Bacteriol. 2002, 184, 3947–3956. [Google Scholar] [CrossRef]
- Kutukova, E.A.; Livshits, V.A.; Altman, I.P.; Ptitsyn, L.R.; Zyiatdinov, M.H.; Tokmakova, I.L.; Zakataeva, N.P. The yeaS (leuE) gene of Escherichia coli encodes an exporter of leucine, and the Lrp protein regulates its expression. FEBS Lett. 2005, 579, 4629–4634. [Google Scholar] [CrossRef]
- Livshits, V.A.; Zakataeva, N.P.; Aleshin, V.V.; Vitushkina, M.V. Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res. Microbiol. 2003, 154, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Nandineni, M.R.; Gowrishankar, J. Evidence for an arginine exporter encoded by yggA (argO) that is regulated by the LysR-type transcriptional regulator ArgP in Escherichia coli. J. Bacteriol. 2004, 186, 3539–3546. [Google Scholar] [CrossRef]
- Simic, P.; Sahm, H.; Eggeling, L. L-threonine export: Use of peptides to identify a new translocator from Corynebacterium glutamicum. J. Bacteriol. 2001, 183, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yoneyama, H.; Tobe, R.; Ando, T.; Isogai, E.; Katsumata, R. Inducible L-alanine exporter encoded by the novel gene ygaW (alaE) in Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Stäbler, N.; Oikawa, T.; Bott, M.; Eggeling, L. Corynebacterium glutamicum as a host for synthesis and export of d-Amino Acids. J. Bacteriol. 2011, 193, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ihara, K.; Katsube, S.; Hori, H.; Ando, T.; Isogai, E.; Yoneyama, H. Characterization of the l-alanine exporter AlaE of Escherichia coli and its potential role in protecting cells from a toxic-level accumulation of l-alanine and its derivatives. Microbiologyopen 2015, 4, 632–643. [Google Scholar] [CrossRef]
- Katsube, S.; Sato, K.; Ando, T.; Isogai, E.; Yoneyama, H. Secretion of d-alanine by Escherichia coli. Microbiology (Reading) 2016, 162, 1243–1252. [Google Scholar] [CrossRef]
- Kitagawa, M.; Ara, T.; Arifuzzaman, M.; Ioka-Nakamichi, T.; Inamoto, E.; Toyonaga, H.; Mori, H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): Unique resources for biological research. DNA Res. 2005, 12, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef] [PubMed]
- Strych, U.; Penland, R.L.; Jimenez, M.; Krause, K.L.; Benedik, M.J. Characterization of the alanine racemases from two mycobacteria. FEMS Microbiol. Lett. 2001, 196, 93–98. [Google Scholar] [CrossRef]
- Adibi, S.A.; Mercer, D.W. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J. Clin. Investig. 1973, 52, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kunisawa, A.; Hattori, T.; Kawana, S.; Kitada, Y.; Tamada, H.; Kawano, S.; Hayakawa, Y.; Iida, J.; Fukusaki, E. Free d-amino acids produced by commensal bacteria in the colonic lumen. Sci. Rep. 2018, 8, 17915. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, J.; Miyoshi, Y.; Rakoff-Nahoum, S.; Zhang, T.; Mita, M.; Davis, B.M.; Hamase, K.; Waldor, M.K. Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol. 2016, 1, 16125. [Google Scholar] [CrossRef]
- Suzuki, M.; Sujino, T.; Chiba, S.; Harada, Y.; Goto, M.; Takahashi, R.; Mita, M.; Hamase, K.; Kanai, T.; Ito, M.; et al. Host-microbe cross-talk governs amino acid chirality to regulate survival and differentiation of B cells. Sci. Adv. 2021, 7, eabd6480. [Google Scholar] [CrossRef]
- Fisher, R.; Tuli, R.; Haselkorn, R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc. Natl. Acad. Sci. USA 1981, 78, 3393–3397. [Google Scholar] [CrossRef]
- Horler, R.S.; Butcher, A.; Papangelopoulos, N.; Ashton, P.D.; Thomas, G.H. EchoLOCATION: An in silico analysis of the subcellular locations of Escherichia coli proteins and comparison with experimentally derived locations. Bioinformatics 2009, 25, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Nurva, S.; Ankeshwarapu, S.P. Mechanism of melibiose/cation symport of the melibiose permease of Salmonella typhimurium. J. Biol. Chem. 2011, 286, 6367–6374. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Akiba, K.; Hori, H.; Ando, T.; Nakae, T. Tat pathway-mediated translocation of the sec pathway substrate protein MexA, an inner membrane component of the MexAB-OprM xenobiotic extrusion pump in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2010, 54, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]



| Screening | Conditions | Genes Selected | |
|---|---|---|---|
| Ala-Ala (mM) | IPTG (mM) | ||
| First | 6 | 0.1 | ybiP, ymcE, ycjF, ydeA, ydeS, yebE, ydhU, ymfR, yeaQ, yhjX, ynfA, ybbV, yfdH, yfdG, yfbW, yfbV, ygaM, ygdD, ygfX, yqjE, yraM, yhiP, yrbK, yidH, yifL, yibN, yjfL, yijD, yjeT, yohO, ymcD, yjbO, yqjF, yiaB, ykgB, yihF, ybjT, yjdB, yjeM, yohJ, ybdJ, ybhQ, ybhL, ybjO, ykgH, ybjM, ydgG, ycfZ, yciC, yebZ, yggT, yqiJ, yfeZ, yicL, yidG, yeiS, ylaC, yigG, ycdZ, ymfA, yfbJ, yohC, ynjI, yfdI, alaE |
| Second | 1 | 0.1 | yeaQ, yfbV, yqjE, yraM, yidH, yifL, yjeT, ymcD, yjbO, yihF, ybdJ, ykgH, yciC, alaE |
| Third | 2 | 0.04 | yciC, ymcD, yidH, yraM, alaE |
| Strains and Plasmids | Characteristics | Reference |
|---|---|---|
| Strains | ||
| E. coli BW25113 | rrnB3, ∆lacZ4787, hsdR514, ∆(araBAD)567, ∆(rhaBAD)56 | [24] |
| E. coli JW1178 | ∆dadA::KMr derived from BW25113 | [24] |
| E. coli JW2645 | ∆alaE::KMr derived from BW25113 | [24] |
| E. coli JW1247 | ∆yciC::KMr derived from BW25113 | [24] |
| E. coli JW3652 | ∆yidH::KMr derived from BW25113 | [24] |
| E. coli JW5133 | ∆ymcD::KMr derived from BW25113 | [24] |
| E. coli JW3116 | ∆yraM::KMr derived from BW25113 | [24] |
| E. coli AG1 | F- endAl hsdRJ7 [rk− mk+] supE44 thi-J recAl gyrA96 relAl X | |
| E. coli MB2795 | d-Ala auxotroph (∆alr, ∆dadX) derived from MG1655 | [25] |
| Plasmids | ||
| pCA24N | CPr, lacIq | [23] |
| pCA24N-alaE | pCA24N harboring the alaE gene | [23] |
| pCA24N-yciC | pCA24N harboring the yciC gene | [23] |
| pCA24N-yidH | pCA24N harboring the yidH gene | [23] |
| pCA24N-ymcD | pCA24N harboring the yraM gene | [23] |
| pCA24N-yraM | pCA24N harboring the ymcD gene | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsube, S.; Sakai, K.; Ando, T.; Tobe, R.; Yoneyama, H. l-Alanine Exporter AlaE Functions as One of the d-Alanine Exporters in Escherichia coli. Int. J. Mol. Sci. 2023, 24, 10242. https://doi.org/10.3390/ijms241210242
Katsube S, Sakai K, Ando T, Tobe R, Yoneyama H. l-Alanine Exporter AlaE Functions as One of the d-Alanine Exporters in Escherichia coli. International Journal of Molecular Sciences. 2023; 24(12):10242. https://doi.org/10.3390/ijms241210242
Chicago/Turabian StyleKatsube, Satoshi, Keiichiro Sakai, Tasuke Ando, Ryuta Tobe, and Hiroshi Yoneyama. 2023. "l-Alanine Exporter AlaE Functions as One of the d-Alanine Exporters in Escherichia coli" International Journal of Molecular Sciences 24, no. 12: 10242. https://doi.org/10.3390/ijms241210242
APA StyleKatsube, S., Sakai, K., Ando, T., Tobe, R., & Yoneyama, H. (2023). l-Alanine Exporter AlaE Functions as One of the d-Alanine Exporters in Escherichia coli. International Journal of Molecular Sciences, 24(12), 10242. https://doi.org/10.3390/ijms241210242






