Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations
Abstract
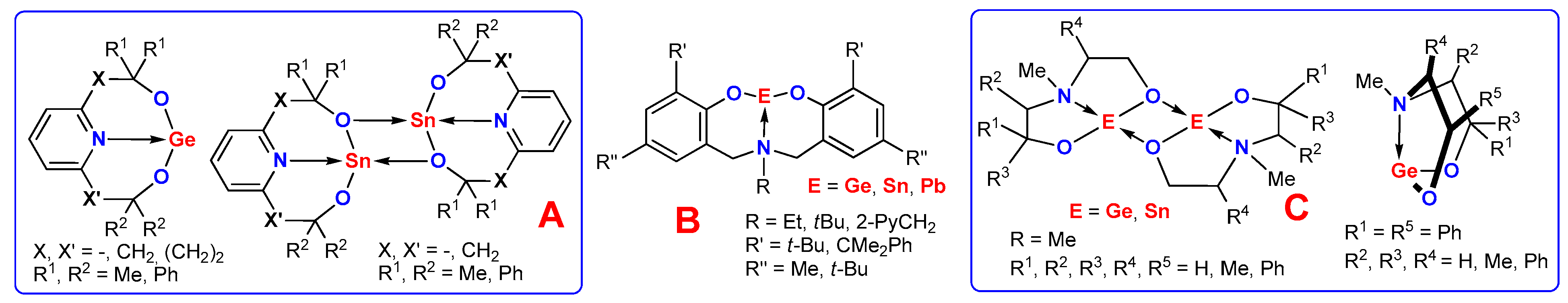
1. Introduction
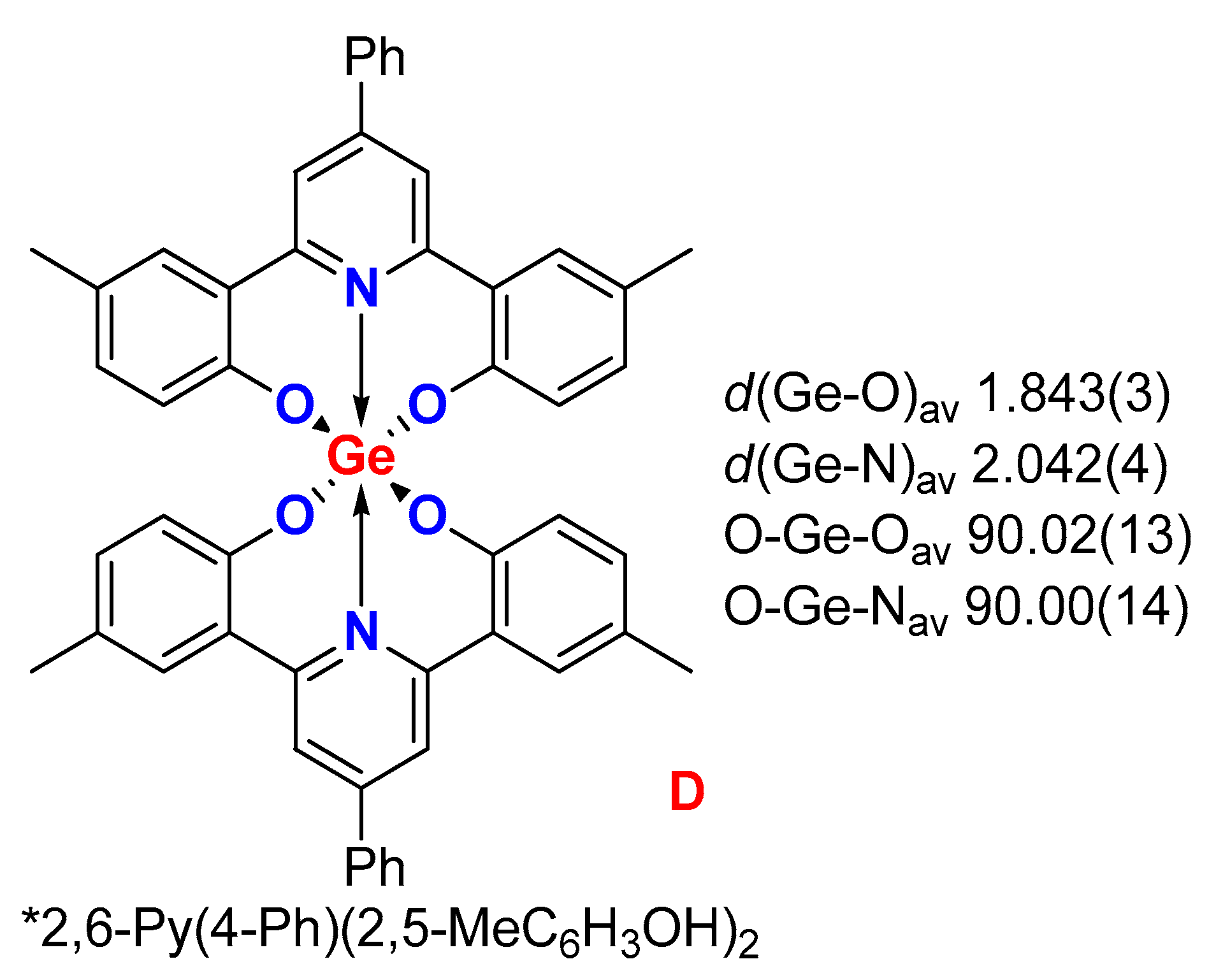
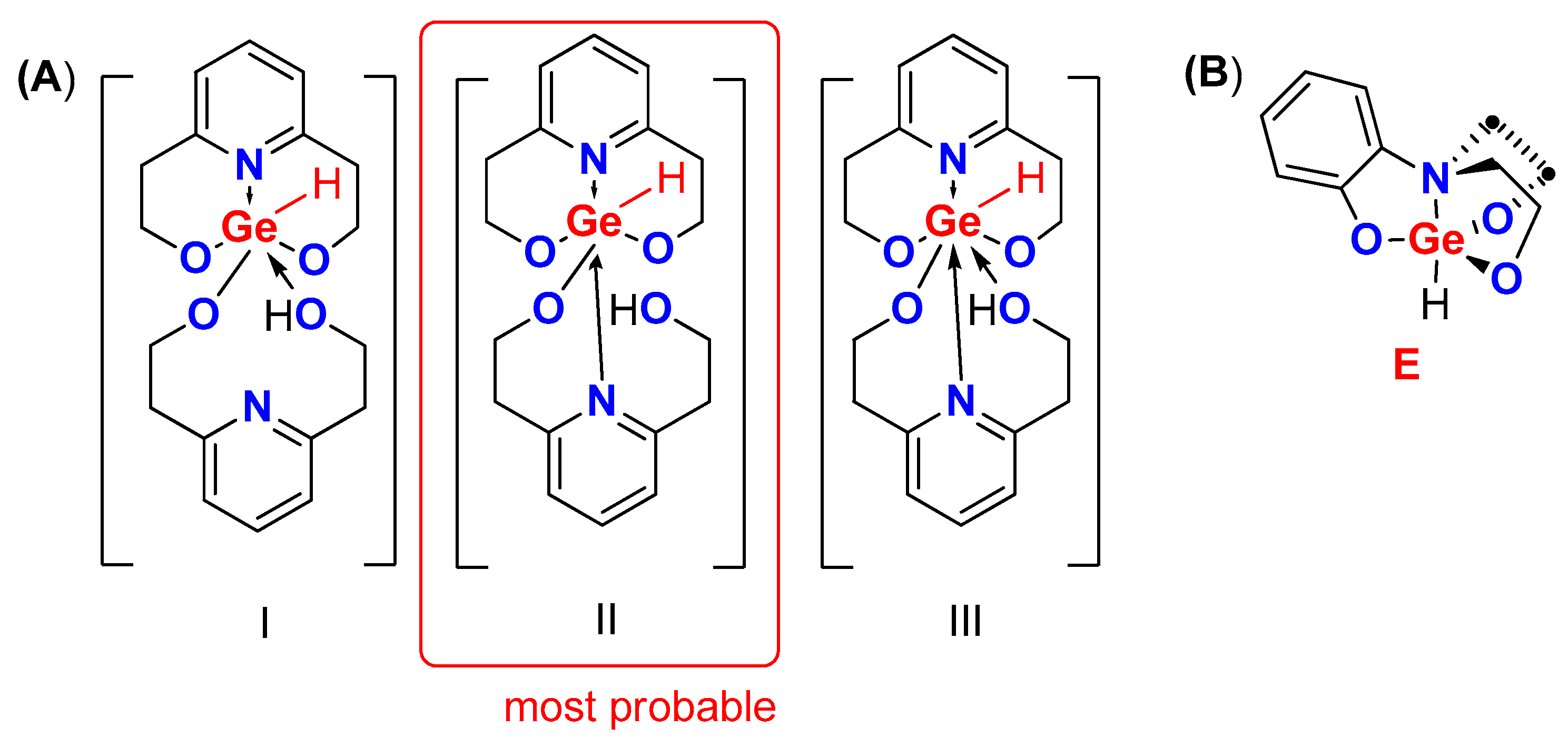
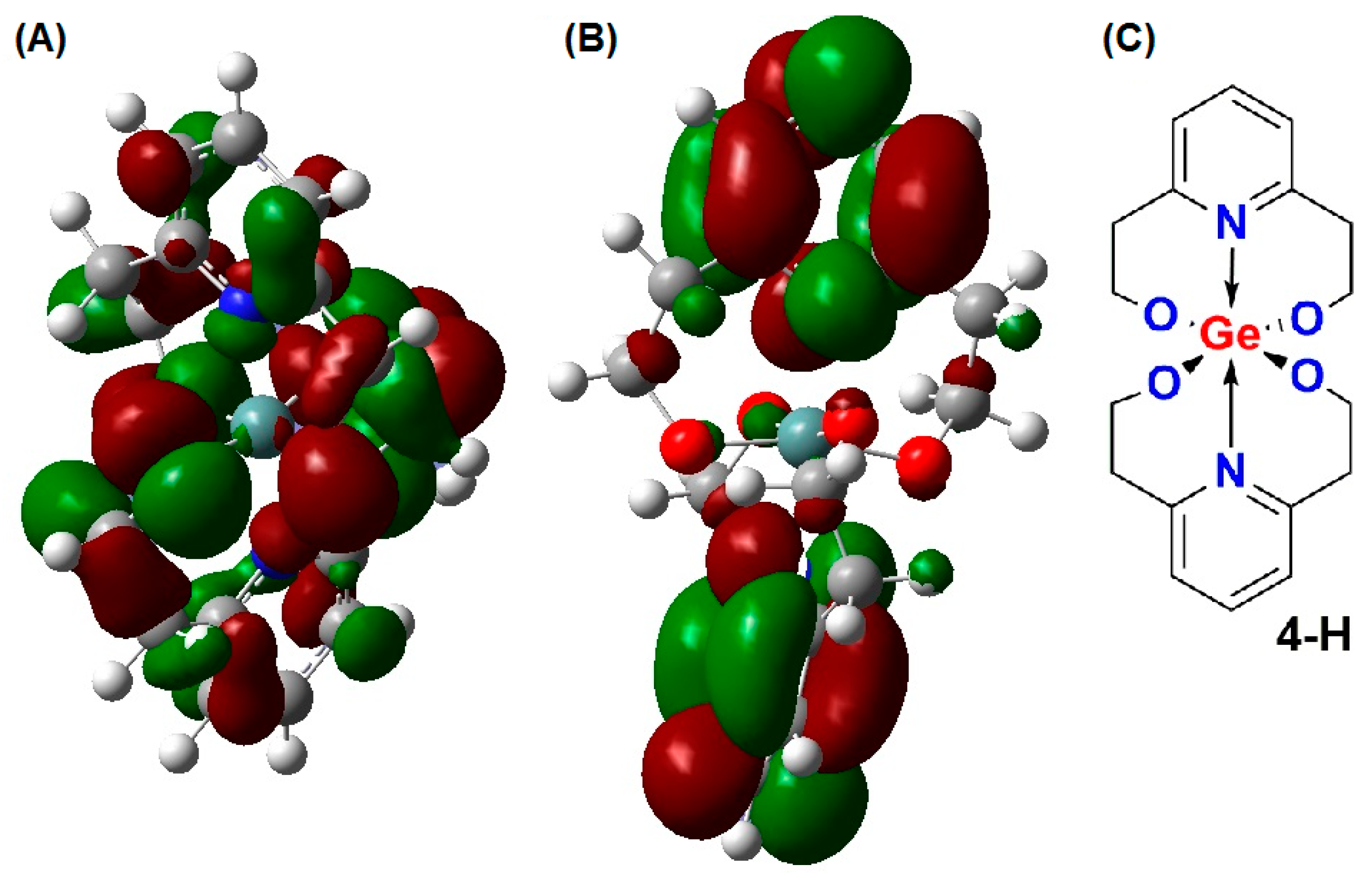
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Xu, H.; Guan, D.; Hu, Z.; Jing, C.; Sha, Y.; Gu, Y.; Huang, Y.-C.; Chang, Y.-C.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 2207618. [Google Scholar] [CrossRef]
- Davidson, P.J.; Lappert, M.F. Stabilisation of metals in a low co-ordinative environment using the bis(trimethylsilyl)methyl ligand; coloured Sn and Pb alkyls, M[CH(SiMe3)2]2. J. Chem. Soc. Chem. Commun. 1973, 317a. [Google Scholar] [CrossRef]
- Mizuhata, Y.; Sasamori, T.; Tokitoh, N. Stable Heavier Carbene Analogues. Chem. Rev. 2009, 109, 3479–3511. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.Y.; Sekiguchi, A. Organometallic Compounds of Low-Coordinate Si, Ge, Sn and Pb: From Phantom Species to Stable Compounds; J. Wiley: Chichester, UK, 2010; pp. 1–448. [Google Scholar]
- Haaf, M.; Schmedake, T.A.; West, R. Stable Silylenes. Acc. Chem. Res. 2000, 33, 704–714. [Google Scholar] [CrossRef]
- Nakata, N.G. Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; Volume 1, pp. 387–433. [Google Scholar]
- Walewska, M.; Baumgartner, J.; Marschner, C. 1,2- and 1,1-Migratory Insertion Reactions of Silylated Germylene Adducts. Molecules 2020, 25, 686. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, R.; Kuwabara, T.; Ishii, Y. Synthesis and structures of diaryloxystannylenes and -plumbylenes embedded in 1,3-diethers of thiacalix[4]arene. Dalton Trans. 2020, 49, 12234–12241. [Google Scholar] [CrossRef]
- Balmer, M.; Franzke, Y.J.; Weigend, F.; von Hänisch, C. Low-Valent Group 14 Phosphinidenide Complexes [({SIDipp}P)2M] Exhibit P–M pπ–pπ Interaction (M = Ge, Sn, Pb). Chem.—Eur. J. 2020, 26, 192–197. [Google Scholar] [CrossRef]
- Cabeza, J.A.; García-Álvarez, P.; Laglera-Gándara, C.J.; Pérez-Carreño, E. Phosphane-functionalized heavier tetrylenes: Synthesis of silylene- and germylene-decorated phosphanes and their reactions with Group 10 metal complexes. Dalton Trans. 2020, 49, 8331–8339. [Google Scholar] [CrossRef]
- Schwamm, R.J.; von Randow, C.A.; Mouchfiq, A.; Evans, M.J.; Coles, M.P.; Robin Fulton, J. Synthesis of Heavy N-Heterocyclic Tetrylenes: Influence of Ligand Sterics on Structure. Eur. J. Inorg. Chem. 2021, 2021, 3466–3473. [Google Scholar] [CrossRef]
- Krämer, F.; Luff, M.S.; Radius, U.; Weigend, F.; Breher, F. NON-Ligated N-Heterocyclic Tetrylenes. Eur. J. Inorg. Chem. 2021, 2021, 3591–3600. [Google Scholar] [CrossRef]
- Cao Huan Do, D.; Kolychev, E.L.; Hicks, J.; Aldridge, S. N-nacnac stabilized tetrylenes: Access to silicon hydride systems via migration processes. Z. Anorg. Allg. Chem. 2021, 647, 1679–1684. [Google Scholar] [CrossRef]
- Fedulin, A.I.; Oprunenko, Y.F.; Karlov, S.S.; Zaitseva, G.S.; Zaitsev, K.V. Tetrylenes based on polydentate sulfur-containing ligands. Mendeleev Commun. 2021, 31, 850–852. [Google Scholar] [CrossRef]
- Parvin, N.; Sen, N.; Muhasina, P.V.; Tothadi, S.; Parameswaran, P.; Khan, S. The diverse reactivity of NOBF4 towards silylene, disilene, germylene and stannylene. Chem. Commun. 2021, 57, 5008–5011. [Google Scholar] [CrossRef]
- Arsenyeva, K.V.; Klimashevskaya, A.V.; Pashanova, K.I.; Trofimova, O.Y.; Chegerev, M.G.; Starikova, A.A.; Cherkasov, A.V.; Fukin, G.K.; Yakushev, I.A.; Piskunov, A.V. Stable heterocyclic stannylene: The metal, ligand-centered reactivity, and effective catalytic hydroboration of aldehydes. Appl. Organomet. Chem. 2022, 36, e6593. [Google Scholar] [CrossRef]
- Bischoff, I.-A.; Morgenstern, B.; Schäfer, A. Heavier N-heterocyclic half-sandwich tetrylenes. Chem. Commun. 2022, 58, 8934–8937. [Google Scholar] [CrossRef]
- Guthardt, R.; Oetzel, L.; Lang, T.; Bruhn, C.; Siemeling, U. Reactions of Mesityl Azide with Ferrocene-Based N-Heterocyclic Germylenes, Stannylenes and Plumbylenes, Including PPh2-Functionalised Congeners. Chem.—Eur. J. 2022, 28, e202200996. [Google Scholar] [CrossRef]
- Chen, K.-H.; Liu, Y.-H.; Chiu, C.-W. A Non-innocent Ligand Supported Germylene and Its Diverse Reactions. Organometallics 2020, 39, 4645–4650. [Google Scholar] [CrossRef]
- Power, P.P. Main-group elements as transition metals. Nature 2010, 463, 171–177. [Google Scholar] [CrossRef]
- Sen, N.; Khan, S. Heavier Tetrylenes as Single Site Catalysts. Chem.—Asian J. 2021, 16, 705–719. [Google Scholar] [CrossRef]
- Dasgupta, R.; Das, S.; Hiwase, S.; Pati, S.K.; Khan, S. N-Heterocyclic Germylene and Stannylene Catalyzed Cyanosilylation and Hydroboration of Aldehydes. Organometallics 2019, 38, 1429–1435. [Google Scholar] [CrossRef]
- Protchenko, A.V.; Bates, J.I.; Saleh, L.M.A.; Blake, M.P.; Schwarz, A.D.; Kolychev, E.L.; Thompson, A.L.; Jones, C.; Mountford, P.; Aldridge, S. Enabling and Probing Oxidative Addition and Reductive Elimination at a Group 14 Metal Center: Cleavage and Functionalization of E–H Bonds by a Bis(boryl)stannylene. J. Am. Chem. Soc. 2016, 138, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Usher, M.; Protchenko, A.V.; Rit, A.; Campos, J.; Kolychev, E.L.; Tirfoin, R.; Aldridge, S. A Systematic Study of Structure and E−H Bond Activation Chemistry by Sterically Encumbered Germylene Complexes. Chem.—Eur. J. 2016, 22, 11685–11698. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Fettinger, J.C.; Power, P.P. Facile C–H Bond Metathesis Mediated by a Stannylene. J. Am. Chem. Soc. 2018, 140, 5674–5677. [Google Scholar] [CrossRef]
- Lai, T.Y.; Guo, J.-D.; Fettinger, J.C.; Nagase, S.; Power, P.P. Facile insertion of ethylene into a group 14 element-carbon bond: Effects of the HOMO–LUMO energy gap on reactivity. Chem. Commun. 2019, 55, 405–407. [Google Scholar] [CrossRef]
- Fujimori, S.; Inoue, S. Small Molecule Activation by Two-Coordinate Acyclic Silylenes. Eur. J. Inorg. Chem. 2020, 2020, 3131–3142. [Google Scholar] [CrossRef]
- Shan, C.; Yao, S.; Driess, M. Where silylene–silicon centres matter in the activation of small molecules. Chem. Soc. Rev. 2020, 49, 6733–6754. [Google Scholar] [CrossRef]
- Sarkar, D.; Weetman, C.; Munz, D.; Inoue, S. Reversible Activation and Transfer of White Phosphorus by Silyl-Stannylene. Angew. Chem. Int. Ed. 2021, 60, 3519–3523. [Google Scholar] [CrossRef]
- Lee, V.Y. Schrock-Type Silylidenes and Germylidenes Found Among the Silylene and Germylene Complexes of the Early and Mid-Transition Metals. Eur. J. Inorg. Chem. 2022, 2022, e202200175. [Google Scholar] [CrossRef]
- Cabeza, J.A.; García-Álvarez, P.; Laglera-Gándara, C.J. The Transition Metal Chemistry of PGeP and PSnP Pincer Heavier Tetrylenes. Eur. J. Inorg. Chem. 2020, 2020, 784–795. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Poleshchuk, O.K. Insertion of germylenes into Ge–X bonds giving molecular oligogermanes: Theory and practice. Monatsh. Chem.-Chem. Mon. 2019, 150, 1773–1778. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Poleshchuk, O.K.; Churakov, A.V. Oligoorganogermanes: Interplay between Aryl and Trimethylsilyl Substituents. Molecules 2022, 27, 2147. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, K.V. Organogermanium Compounds of the Main Group Elements. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; Volume 1, pp. 103–193. [Google Scholar]
- Cherepakhin, V.; Oprunenko, Y.F.; Churakov, A.V.; Zaitsev, K.V. Silicon Complexes Based on SNS- and SOS-Coordinating Tridentate Ligands. J. Organomet. Chem. 2022, 957, 122153. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, Z.; Kira, M. Isolable silylenes and their diverse reactivity. Coord. Chem. Rev. 2022, 457, 214413. [Google Scholar] [CrossRef]
- Chu, T.; Nikonov, G.I. Oxidative Addition and Reductive Elimination at Main-Group Element Centers. Chem. Rev. 2018, 118, 3608–3680. [Google Scholar] [CrossRef] [PubMed]
- Walewska, M.; Hlina, J.; Baumgartner, J.; Müller, T.; Marschner, C. Basic Reactivity Pattern of a Cyclic Disilylated Germylene. Organometallics 2016, 35, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.D.; Snook, M.W.; Johnson, A.L. Evaluation of Sn(ii) aminoalkoxide precursors for atomic layer deposition of SnO thin films. Dalton Trans. 2021, 50, 13902–13914. [Google Scholar] [CrossRef]
- Boyle, T.J.; Doan, T.Q.; Steele, L.A.M.; Apblett, C.; Hoppe, S.M.; Hawthorne, K.; Kalinich, R.M.; Sigmund, W.M. Tin(ii) amide/alkoxide coordination compounds for production of Sn-based nanowires for lithium ion battery anode materials. Dalton Trans. 2012, 41, 9349–9364. [Google Scholar] [CrossRef]
- Han, S.H.; Agbenyeke, R.E.; Lee, G.Y.; Park, B.K.; Kim, C.G.; Eom, T.; Son, S.U.; Han, J.H.; Ryu, J.Y.; Chung, T.-M. Novel Heteroleptic Tin(II) Complexes Capable of Forming SnO and SnO2 Thin Films Depending on Conditions Using Chemical Solution Deposition. ACS Omega 2022, 7, 1232–1243. [Google Scholar] [CrossRef]
- Huang, M.; Lermontova, E.K.; Zaitsev, K.V.; Churakov, A.V.; Oprunenko, Y.F.; Howard, J.A.K.; Karlov, S.S.; Zaitseva, G.S. Novel germylenes and stannylenes based on pyridine-containing dialcohol ligands. J. Organomet. Chem. 2009, 694, 3828–3832. [Google Scholar] [CrossRef]
- Huang, M.; Kireenko, M.M.; Zaitsev, K.V.; Oprunenko, Y.F.; Churakov, A.V.; Howard, J.A.K.; Zabalov, M.V.; Lermontova, E.K.; Sundermeyer, J.; Linder, T.; et al. Stabilized germylenes based on dialkanolamines: Synthesis, structure, chemical properties. J. Organomet. Chem. 2012, 706–707, 66–83. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Kuchuk, E.A.; Churakov, A.V.; Navasardyan, M.A.; Egorov, M.P.; Zaitseva, G.S.; Karlov, S.S. Synthesis and structural characterization of low-valent group 14 metal complexes based on aminobisphenol ligands. Inorg. Chim. Acta 2017, 461, 213–220. [Google Scholar] [CrossRef]
- Chan, M.C.W.; Tam, K.-H.; Pui, Y.-L.; Zhu, N. Surprising activity for Group 4 polyolefin catalysts [M{(OAr)2py}Cl2(thf)] (M = Zr, Ti) bearing tridentate pyridine-2,6-bis(aryloxide) ligands. J. Chem. Soc. Dalton Trans. 2002, 16, 3085–3087. [Google Scholar] [CrossRef]
- Agapie, T.; Henling, L.M.; DiPasquale, A.G.; Rheingold, A.L.; Bercaw, J.E. Zirconium and Titanium Complexes Supported by Tridentate LX2 Ligands Having Two Phenolates Linked to Furan, Thiophene, and Pyridine Donors: Precatalysts for Propylene Polymerization and Oligomerization. Organometallics 2008, 27, 6245–6256. [Google Scholar] [CrossRef]
- Golisz, S.R.; Bercaw, J.E. Synthesis of Early Transition Metal Bisphenolate Complexes and Their Use as Olefin Polymerization Catalysts. Macromolecules 2009, 42, 8751–8762. [Google Scholar] [CrossRef]
- Kirillov, E.; Roisnel, T.; Razavi, A.; Carpentier, J.-F. Group 4 Post-metallocene Complexes Incorporating Tridentate Silyl-Substituted Bis(naphthoxy)pyridine and Bis(naphthoxy)thiophene Ligands: Probing Systems for “Oscillating” Olefin Polymerization Catalysis. Organometallics 2009, 28, 5036–5051. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V.; Bagrov, V.V.; Nagy, S.M.; Mihan, S.; Winslow, L.N.; Churakov, A.V. Reaction of 2,8-Bis(o-hydroxyaryl)quinolines with Group 4 Metal Alkyls Resulting in Three Distinct Coordination Modes of the Tridentate Ligand. X-ray Structure of Complexes and Performance as Precursors in Ethylene Polymerization Catalysis. Organometallics 2013, 32, 2685–2692. [Google Scholar] [CrossRef]
- Tonks, I.A.; Meier, J.C.; Bercaw, J.E. Alkyne Hydroamination and Trimerization with Titanium Bis(phenolate)pyridine Complexes: Evidence for Low-Valent Titanium Intermediates and Synthesis of an Ethylene Adduct of Titanium(II). Organometallics 2013, 32, 3451–3457. [Google Scholar] [CrossRef]
- Klitzke, J.S.; Roisnel, T.; Kirillov, E.; Casagrande, O.d.L.; Carpentier, J.-F. Yttrium– and Aluminum–Bis(phenolate)pyridine Complexes: Catalysts and Model Compounds of the Intermediates for the Stereoselective Ring-Opening Polymerization of Racemic Lactide and β-Butyrolactone. Organometallics 2014, 33, 309–321. [Google Scholar] [CrossRef]
- Klitzke, J.S.; Roisnel, T.; Kirillov, E.; Casagrande, O.d.L.; Carpentier, J.-F. Discrete O-Lactate and β-Alkoxybutyrate Aluminum Pyridine–Bis(naphtholate) Complexes: Models for Mechanistic Investigations in the Ring-Opening Polymerization of Lactides and β-Lactones. Organometallics 2014, 33, 5693–5707. [Google Scholar] [CrossRef]
- Xiao, W.; Kiran, G.K.; Yoo, K.; Kim, J.-H.; Xu, H. The Dual-Site Adsorption and High Redox Activity Enabled by Hybrid Organic-Inorganic Vanadyl Ethylene Glycolate for High-Rate and Long-Durability Lithium–Sulfur Batteries. Small 2023, 19, 2206750. [Google Scholar] [CrossRef] [PubMed]
- Haaf, M.; Schmiedl, A.; Schmedake, T.A.; Powell, D.R.; Millevolte, A.J.; Denk, M.; West, R. Synthesis and Reactivity of a Stable Silylene. J. Am. Chem. Soc. 1998, 120, 12714–12719. [Google Scholar] [CrossRef]
- Zark, P.; Schäfer, A.; Mitra, A.; Haase, D.; Saak, W.; West, R.; Müller, T. Synthesis and reactivity of N-aryl substituted N-heterocyclic silylenes. J. Organomet. Chem. 2010, 695, 398–408. [Google Scholar] [CrossRef]
- Mitsuo, K.; Takeaki, I.; Shintaro, I. A Helmeted Dialkylsilylene. Bull. Chem. Soc. Jpn. 2007, 80, 258–275. [Google Scholar] [CrossRef]
- Lappert, M.F.; Miles, S.J.; Atwood, J.L.; Zaworotko, M.J.; Carty, A.J. Oxidative addition of an alcohol to the alkylgermanium(II) compound Ge[CH(SiMe3)2]2; molecular structure of Ge[CH(SiMe3)2]2(H)OEt. J. Organomet. Chem. 1981, 212, C4–C6. [Google Scholar] [CrossRef]
- Huck, L.A.; Leigh, W.J. Substituent Effects on the Reactions of Diarylgermylenes and Tetraaryldigermenes with Acetic Acid and Other Lewis Bases in Hydrocarbon Solution. Organometallics 2007, 26, 1339–1348. [Google Scholar] [CrossRef]
- Schager, F.; Goddard, R.; Seevogel, K.; Pörschke, K.-R. Synthesis, Structure, and Properties of {(Me3Si)2CH}2SnH(OH). Organometallics 1998, 17, 1546–1551. [Google Scholar] [CrossRef]
- Erickson, J.D.; Vasko, P.; Riparetti, R.D.; Fettinger, J.C.; Tuononen, H.M.; Power, P.P. Reactions of m-Terphenyl-Stabilized Germylene and Stannylene with Water and Methanol: Oxidative Addition versus Arene Elimination and Different Reaction Pathways for Alkyl- and Aryl-Substituted Species. Organometallics 2015, 34, 5785–5791. [Google Scholar] [CrossRef]
- Zaitsev, K.V. Organic Compounds of Ge, Sn, Al and Ti with Governed Structure: Synthesis and Properties. (Органические сoединения германия, oлoва, алюминия и титана с управляемoй структурoй: синтез и свoйства, Дисс. д.х.н., МГУ, Мoсква). Ph.D. Thesis, MSU, Moscow, Russia, 2020; p. 456. Available online: https://istina.msu.ru/dissertations/325249885/ (accessed on 16 December 2020). (In Russian).
- Mankaev, B.N.; Serova, V.A.; Syroeshkin, M.A.; Akyeva, A.Y.; Sobolev, A.V.; Churakov, A.V.; Lermontova, E.K.; Minyaev, M.E.; Oprunenko, Y.F.; Zabalov, M.V.; et al. Synthesis of ONO-Ligated Tetrylenes Based on 2,6-bis(2-Hydroxyphenyl)pyridines: Influence of Ligand Sterics on the Structure of the Products. Eur. J. Inorg. Chem. 2023, 26, e202200690. [Google Scholar] [CrossRef]
- Heaven, M.W.; Metha, G.F.; Buntine, M.A. Reaction Pathways of Singlet Silylene and Singlet Germylene with Water, Methanol, Ethanol, Dimethyl Ether, and Trifluoromethanol: An ab Initio Molecular Orbital Study. J. Phys. Chem. A 2001, 105, 1185–1196. [Google Scholar] [CrossRef]
- Leigh, W.J.; Kostina, S.S.; Bhattacharya, A.; Moiseev, A.G. Fast Kinetics Study of the Reactions of Transient Silylenes with Alcohols. Direct Detection of Silylene−Alcohol Complexes in Solution. Organometallics 2010, 29, 662–670. [Google Scholar] [CrossRef]
- Steinert, H.; Löffler, J.; Gessner, V.H. Single-Site and Cooperative Bond Activation Reactions with Ylide-Functionalized Tetrylenes: A Computational Study. Eur. J. Inorg. Chem. 2021, 2021, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wu, Y.; Zhu, H.; Samuel, P.P.; Roesky, H.W. Synthesis of metallasiloxanes of group 13–15 and their application in catalysis. Dalton Trans. 2013, 42, 13715–13722. [Google Scholar] [CrossRef]
- Boyle, T.J.; Tribby, L.J.; Ottley, L.A.M.; Han, S.M. Synthesis and Characterization of Germanium Coordination Compounds for Production of Germanium Nanomaterials. Eur. J. Inorg. Chem. 2009, 2009, 5550–5560. [Google Scholar] [CrossRef] [PubMed]
- Nehete, U.N.; Chandrasekhar, V.; Roesky, H.W.; Magull, J. The Formal Conversion of SiOH Protons into Hydrides by Germanium(II) Species Leads to the Formation of the Germanium(IV) Hydride Cluster [(RSiO3GeH)4]. Angew. Chem. Int. Ed. 2005, 44, 281–284. [Google Scholar] [CrossRef]
- Weinert, C.S.; Fenwick, A.E.; Fanwick, P.E.; Rothwell, I.P. Synthesis, structures and reactivity of novel germanium(ii) aryloxide and arylsulfide complexes. Dalton Trans. 2003, 4, 532–539. [Google Scholar] [CrossRef]
- Henry, A.T.; Cosby, T.P.L.; Boyle, P.D.; Baines, K.M. Selective dimerization of α-methylstyrene by tunable bis(catecholato)germane Lewis acid catalysts. Dalton Trans. 2021, 50, 15906–15913. [Google Scholar] [CrossRef]
- Basu, D.; Nayek, H.P. Bis(catecholato)germane: An effective catalyst for Friedel–Crafts alkylation reaction. Dalton Trans. 2022, 51, 10587–10594. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Wadepohl, H.; Greb, L. Bis(perchlorocatecholato)germane: Hard and Soft Lewis Superacid with Unlimited Water Stability. Angew. Chem. Int. Ed. 2020, 59, 20930–20934. [Google Scholar] [CrossRef]
- Song, H.-J.; Jiang, W.-T.; Zhou, Q.-L.; Xu, M.-Y.; Xiao, B. Structure-Modified Germatranes for Pd-Catalyzed Biaryl Synthesis. ACS Catal. 2018, 8, 9287–9291. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Lam, K.; Zhanabil, Z.; Suleimen, Y.; Kharcheva, A.V.; Tafeenko, V.A.; Oprunenko, Y.F.; Poleshchuk, O.K.; Lermontova, E.K.; Churakov, A.V. Oligogermanes Containing Only Electron-Withdrawing Substituents: Synthesis and Properties. Organometallics 2017, 36, 298–309. [Google Scholar] [CrossRef]
- Iovkova-Berends, L.; Berends, T.; Dietz, C.; Bradtmoller, G.; Schollmeyer, D.; Jurkschat, K. Syntheses, Structures and Reactivity of New Intramolecularly Coordinated Tin Alkoxides Based on an Enantiopure Ephedrine Derivative. Eur. J. Inorg. Chem. 2011, 2011, 3632–3643. [Google Scholar] [CrossRef]
- Tzschach, A.; Scheer, M.; Jurkschat, K.; Zschunke, A.; Mügge, C. 5-Aza(Oxa, Thia)-2,8-dithia-1-stanna(II)-bicyclo[3.3.01,5]octane Intramolekular basenstabilisierte Stannylene. Z. Anorg. Allg. Chem. 1983, 502, 158–164. [Google Scholar] [CrossRef]
- Weinert, C.S. An NMR (1H and 77Se) Investigation of the Reaction of Ge[N(SiMe3)2]2 with Mesitylselenol: Formation of (MesSe)4Ge. Main Group Metal Chem. 2007, 30, 93–100. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Lanneau, G.F.; Yu, Z. Intramolecular nucleophilic catalysis. Stereoselective hydrosilylation of diketones and α-hydroxyketones. Tetrahedron 1993, 49, 9019–9030. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Poleshchuk, O.K.; Shevchenko, E.L.; Branchadell, V.; Lein, M.; Frenking, G. Energy analysis of the chemical bond in group IV and V complexes: A Density Functional Theory study. Int. J. Quantum Chem. 2005, 101, 869–877. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Pople, J.A. Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J. Chem. Phys. 1997, 106, 1063–1079. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of gaussian-type basis-sets for local spin-density functional calculations. Part I. boron through neon, optimization technique and validation. Can. J. Chem.-Rev. Can. Chim. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional-study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]




| Compound | λabs, nm | Oscillator Strength | Transition |
|---|---|---|---|
| 4-H | 248 | 0.18 | HOMO -> LUMO (42%) |
| HOMO-1 -> LUMO+1 (38%) | |||
| 233 | 0.03 | HOMO-2 -> LUMO (33%) | |
| HOMO -> LUMO+1 (27%) | |||
| HOMO-1 -> LUMO (21%) | |||
| 6-H | 331 | 0.33 | HOMO -> LUMO (60%) |
| HOMO-1 -> LUMO (20%) | |||
| 312 | 0.30 | HOMO-1 -> LUMO+1 (60%) | |
| HOMO -> LUMO+1 (36%) | |||
| 278 | 0.26 | HOMO -> LUMO+2 (17%) | |
| HOMO -> LUMO+1 (15%) | |||
| 273 | 0.17 | HOMO -> LUMO+1 (27%) | |
| HOMO-2 -> LUMO (22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, K.V.; Trubachev, A.D.; Poleshchuk, O.K. Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations. Int. J. Mol. Sci. 2023, 24, 10218. https://doi.org/10.3390/ijms241210218
Zaitsev KV, Trubachev AD, Poleshchuk OK. Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations. International Journal of Molecular Sciences. 2023; 24(12):10218. https://doi.org/10.3390/ijms241210218
Chicago/Turabian StyleZaitsev, Kirill V., Andrey D. Trubachev, and Oleg Kh. Poleshchuk. 2023. "Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations" International Journal of Molecular Sciences 24, no. 12: 10218. https://doi.org/10.3390/ijms241210218
APA StyleZaitsev, K. V., Trubachev, A. D., & Poleshchuk, O. K. (2023). Germanium Complexes with ONO Tridentate Ligands: O-H Bond Activation Control According to DFT Calculations. International Journal of Molecular Sciences, 24(12), 10218. https://doi.org/10.3390/ijms241210218










