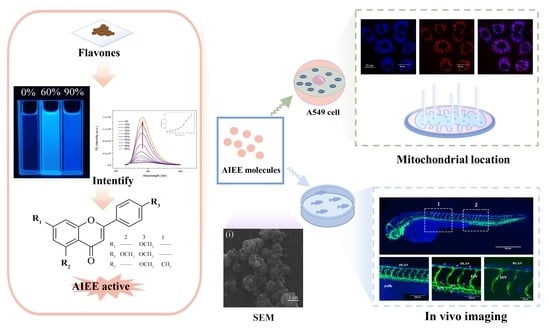
AIEE-Active Flavones as a Promising Tool for the Real-Time Tracking of Uptake and Distribution in Live Zebrafish
Abstract
1. Introduction
2. Results and Discussion
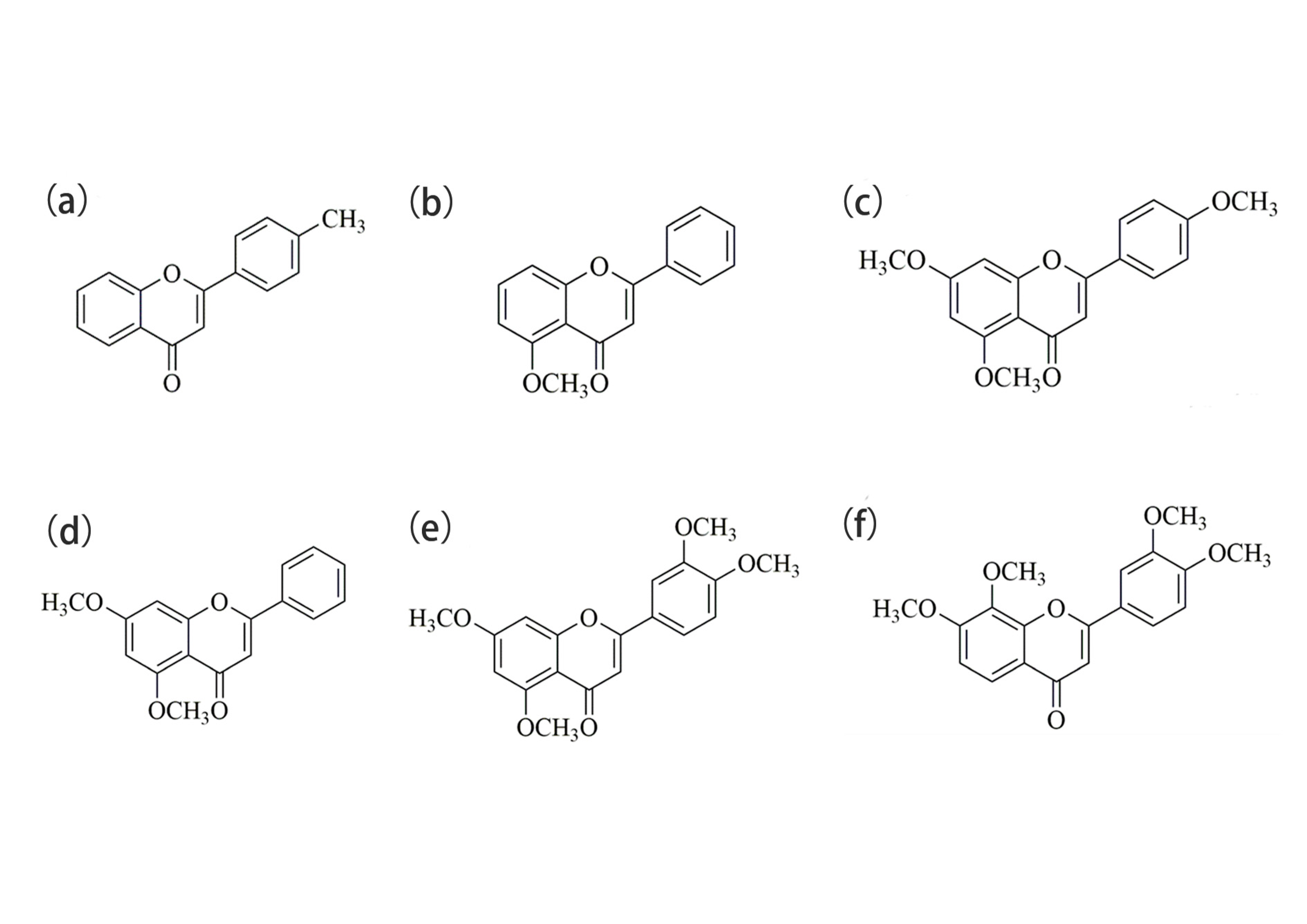
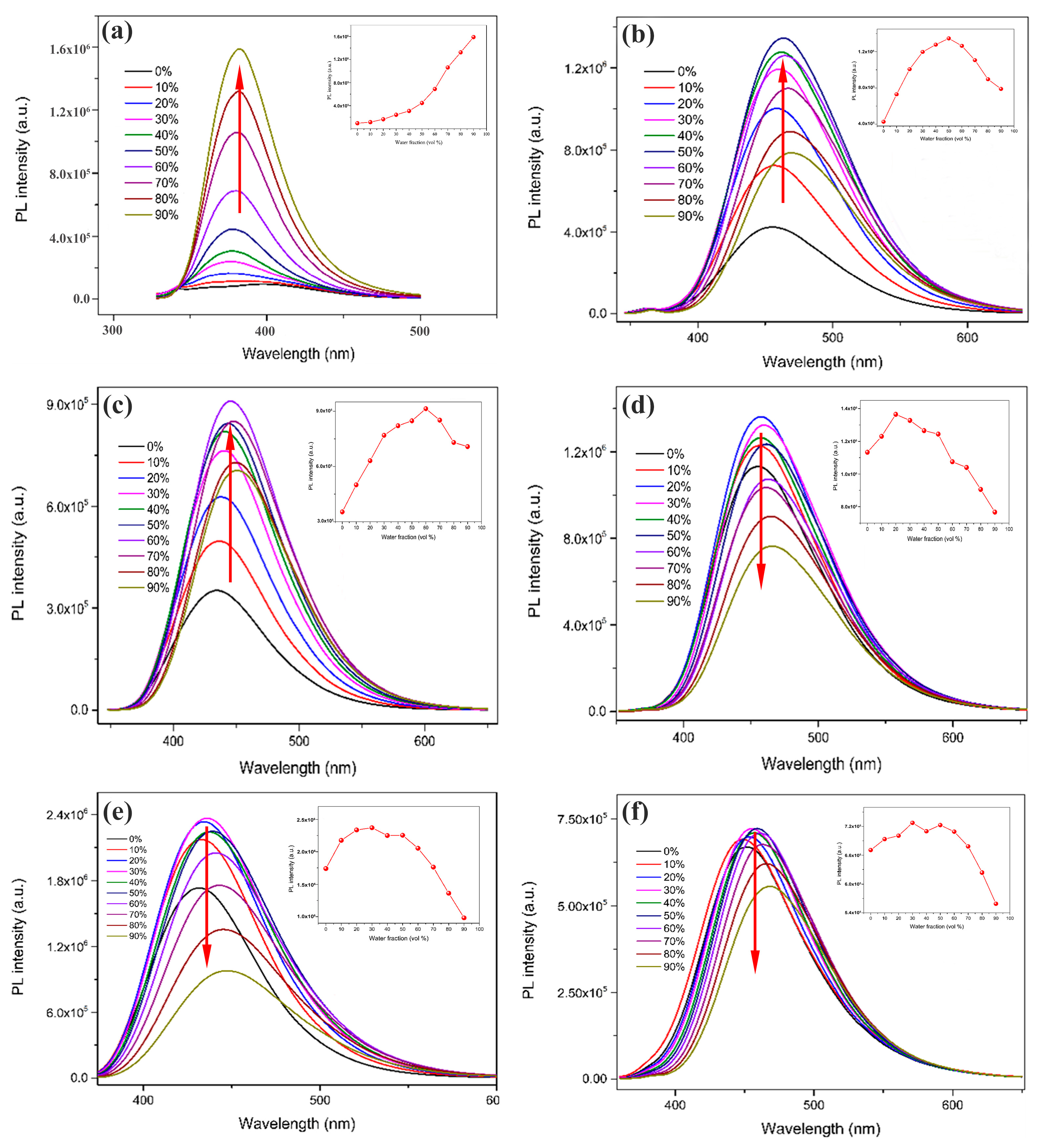
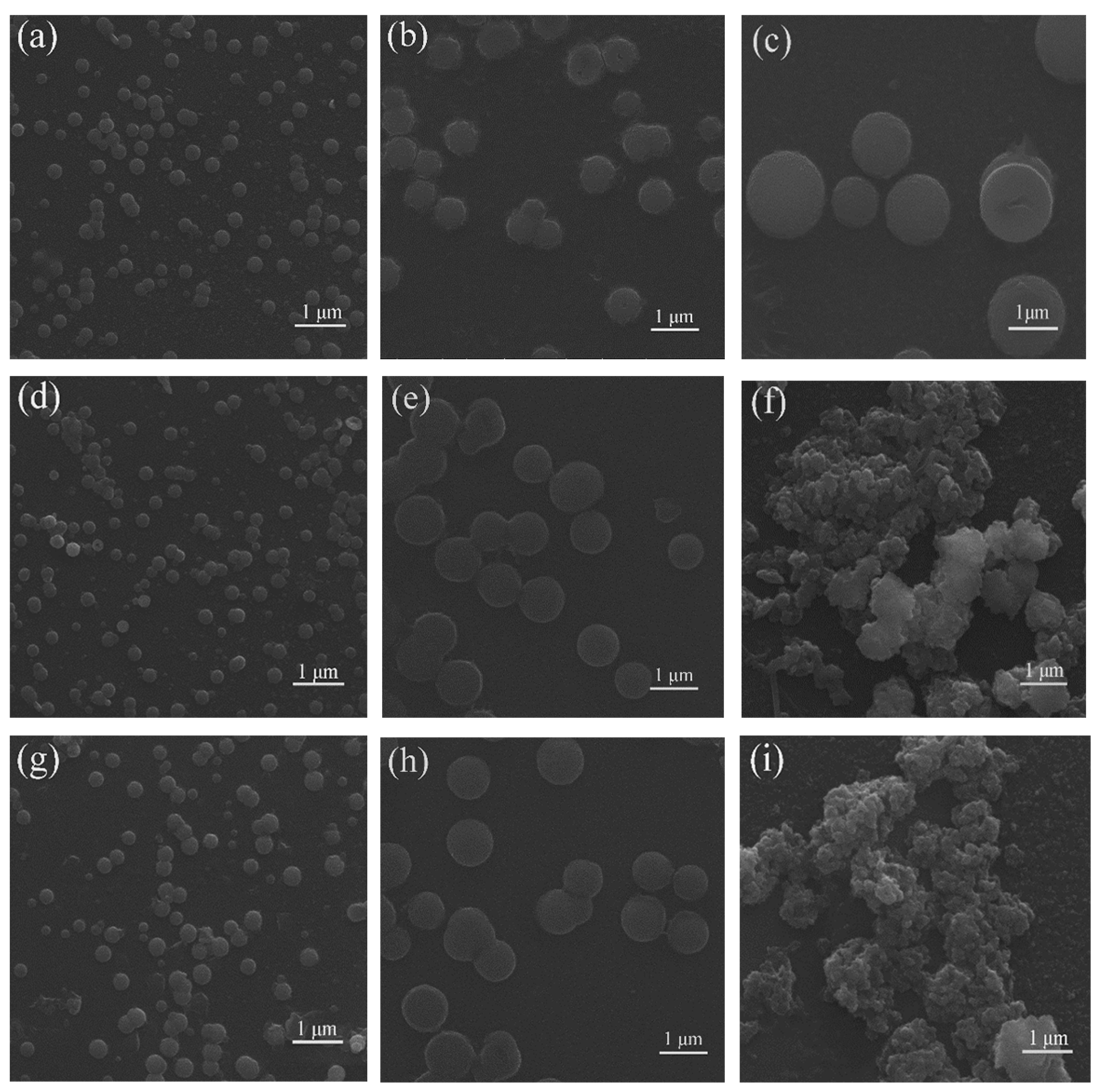
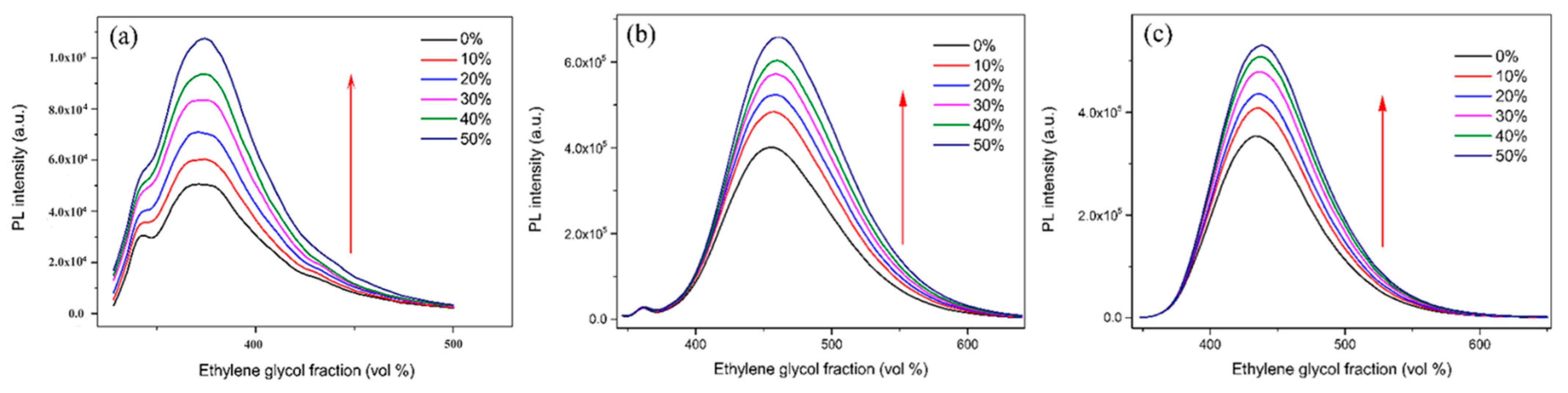
2.1. Optical Properties and Aggregation-Induced Emission Enhancement Properties
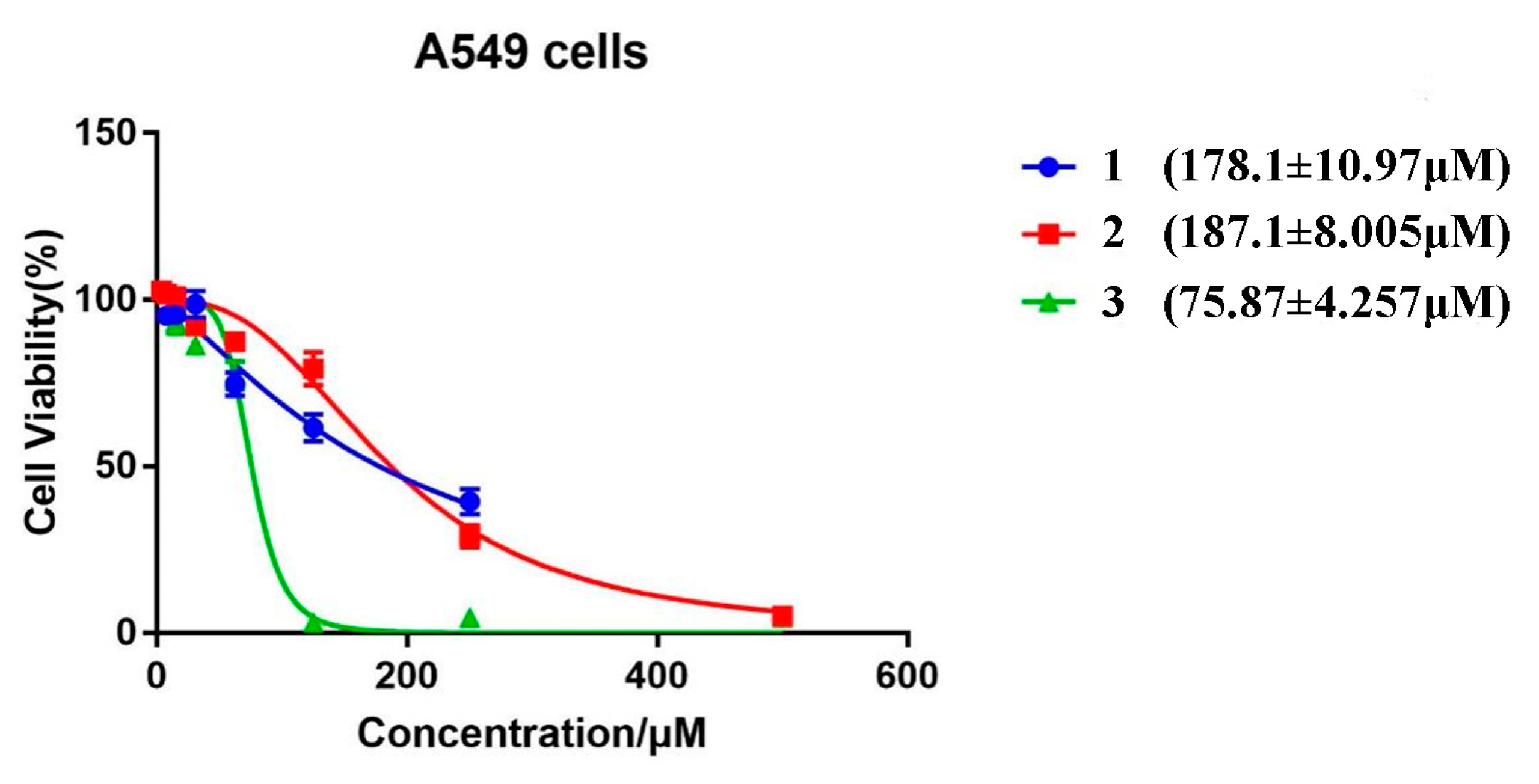
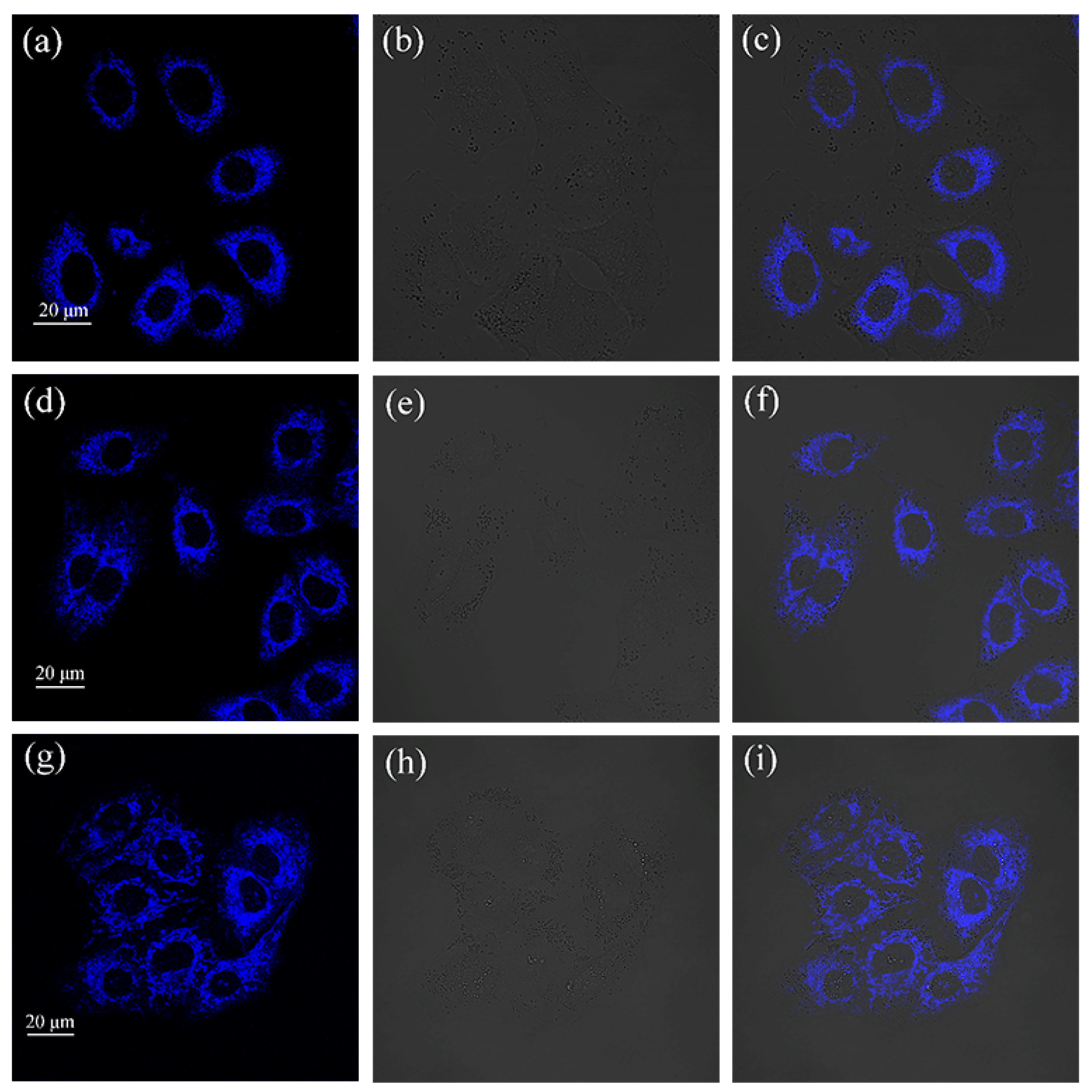
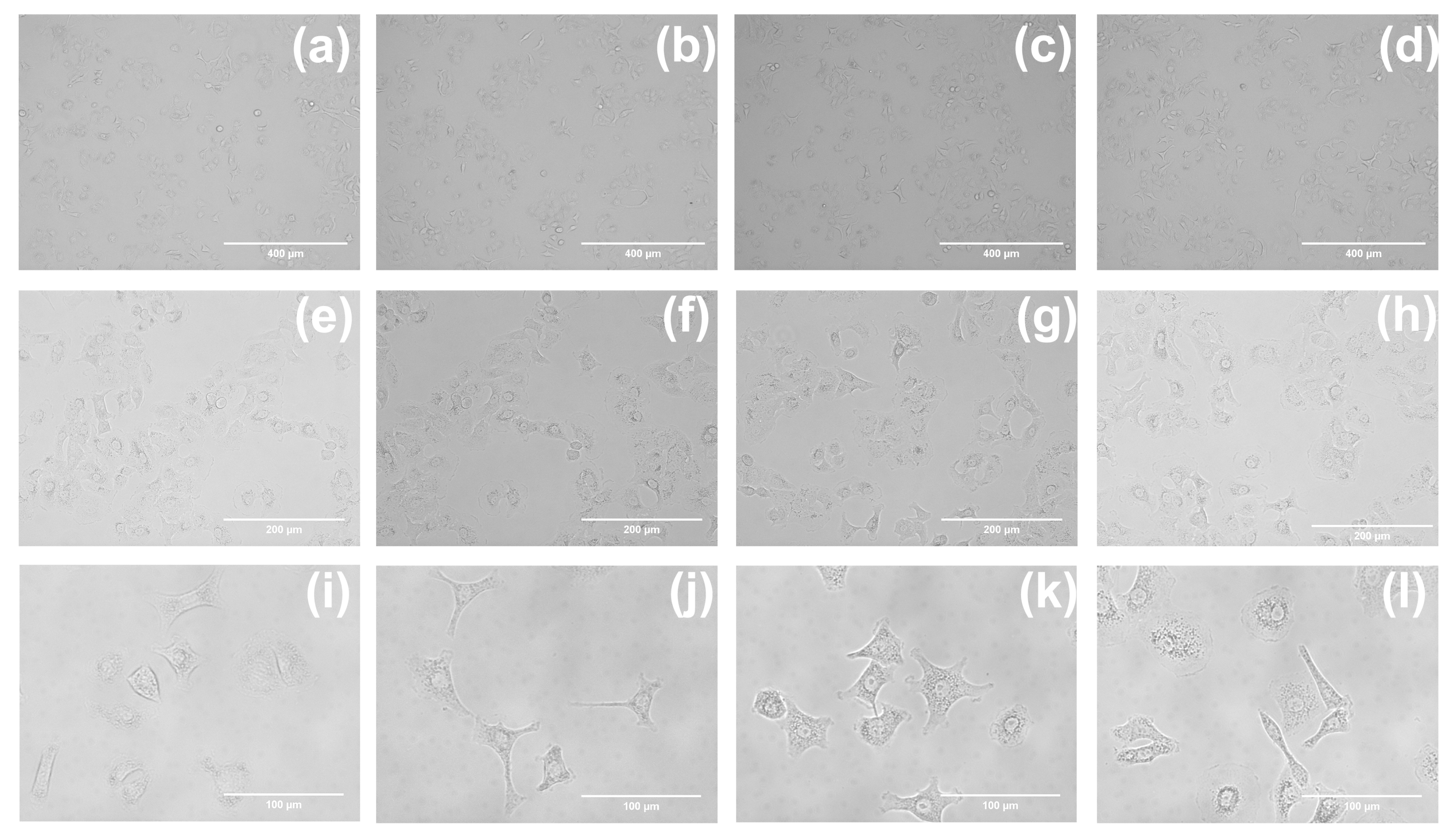
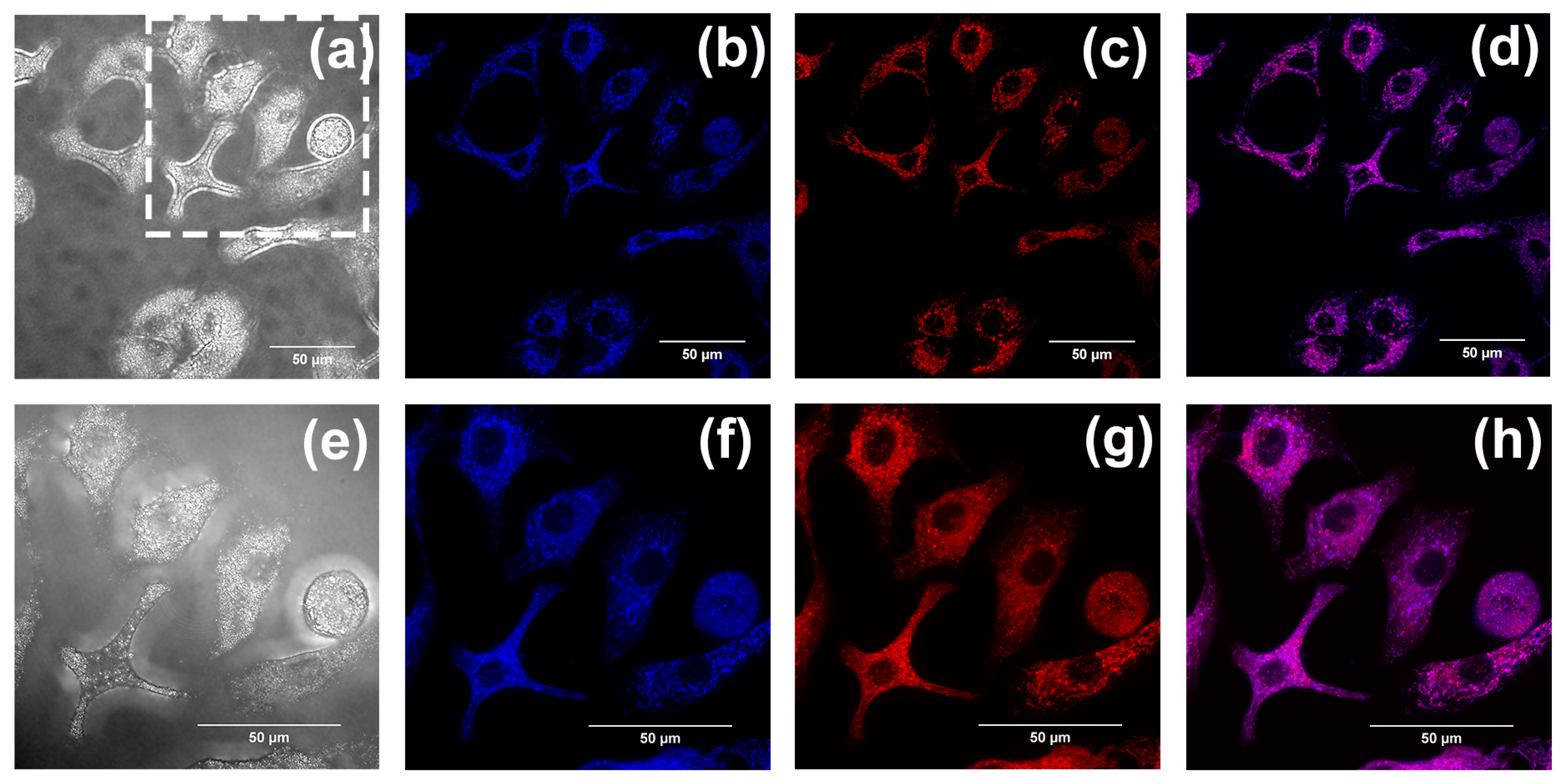
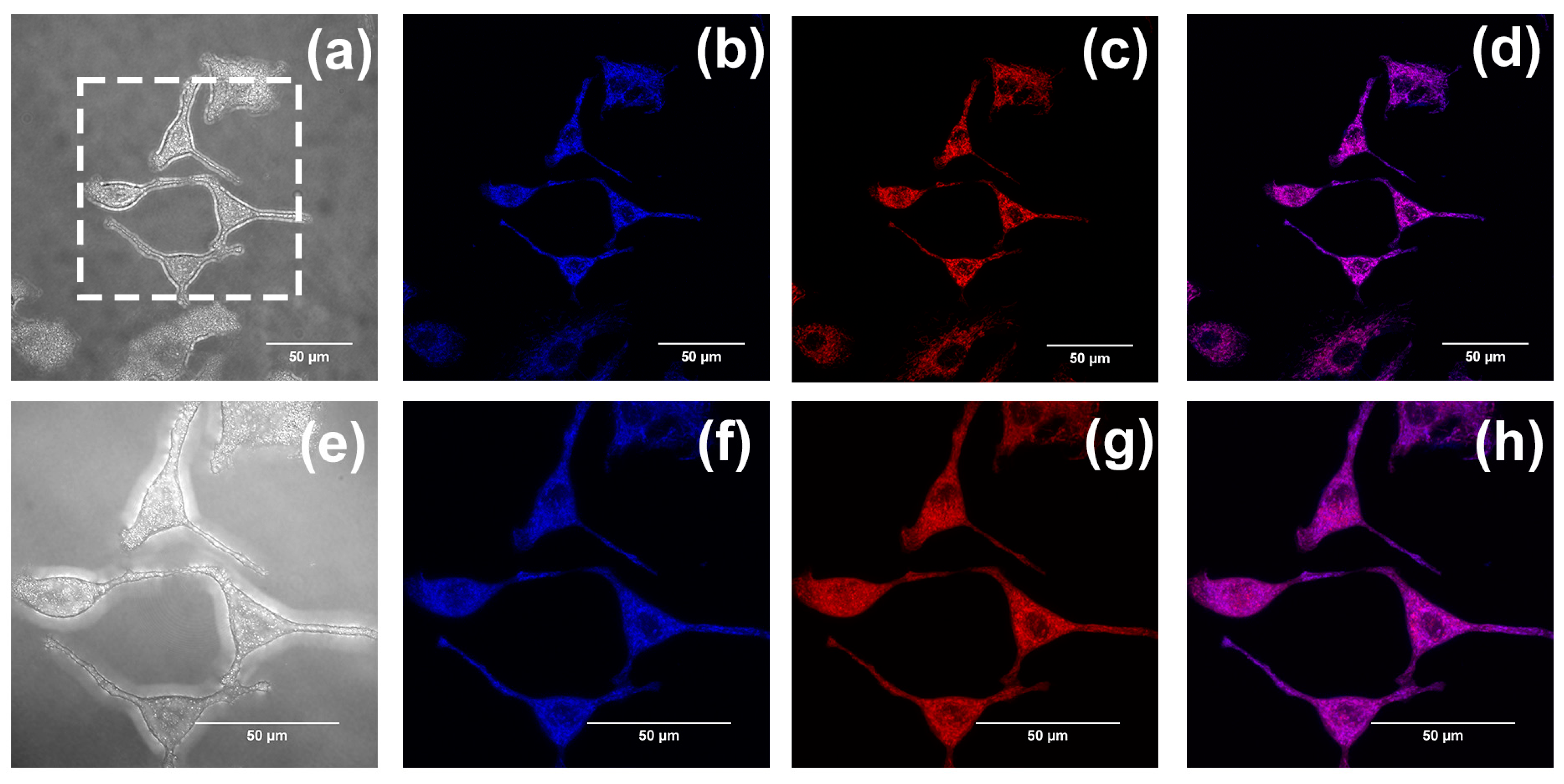
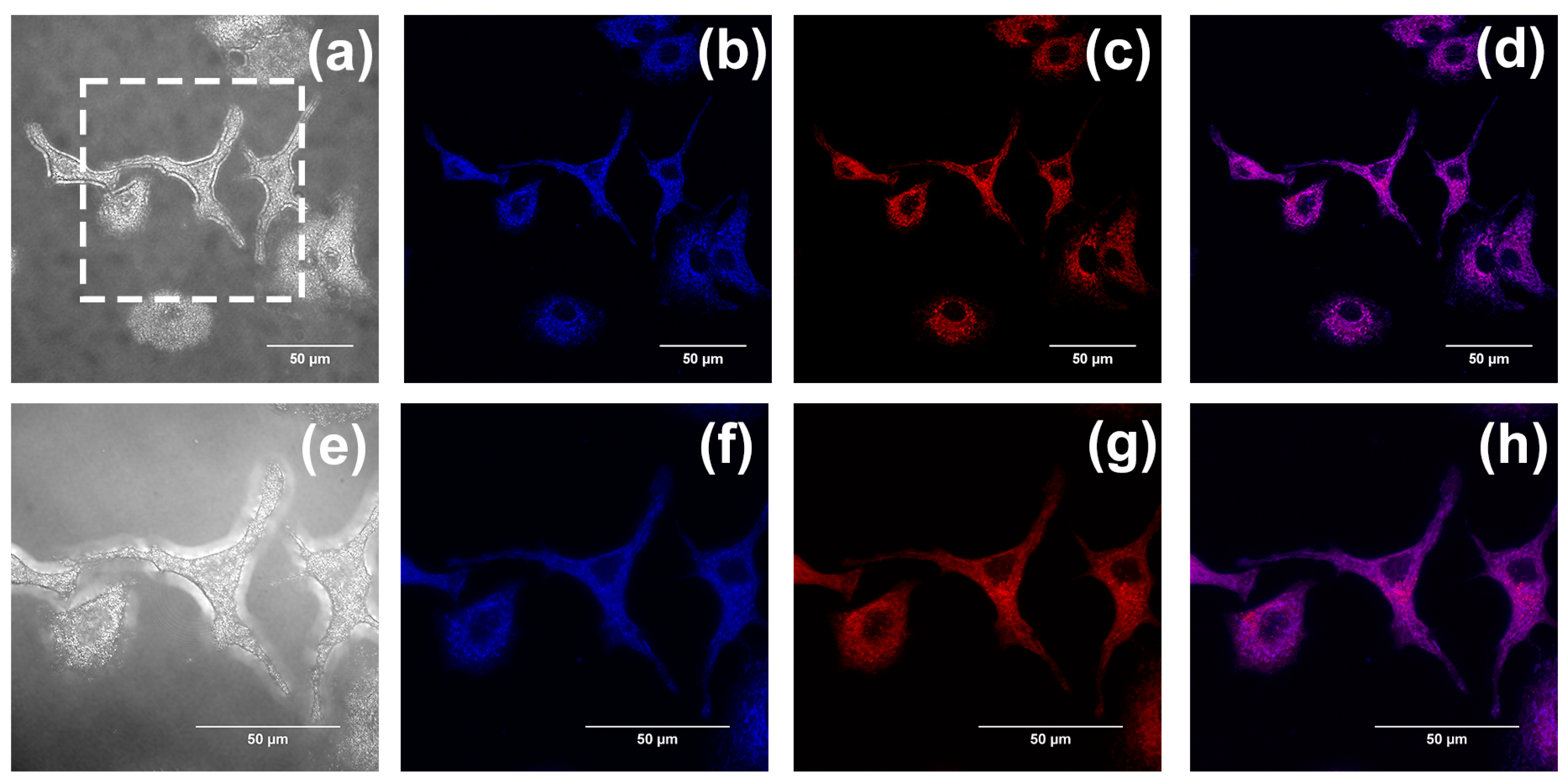
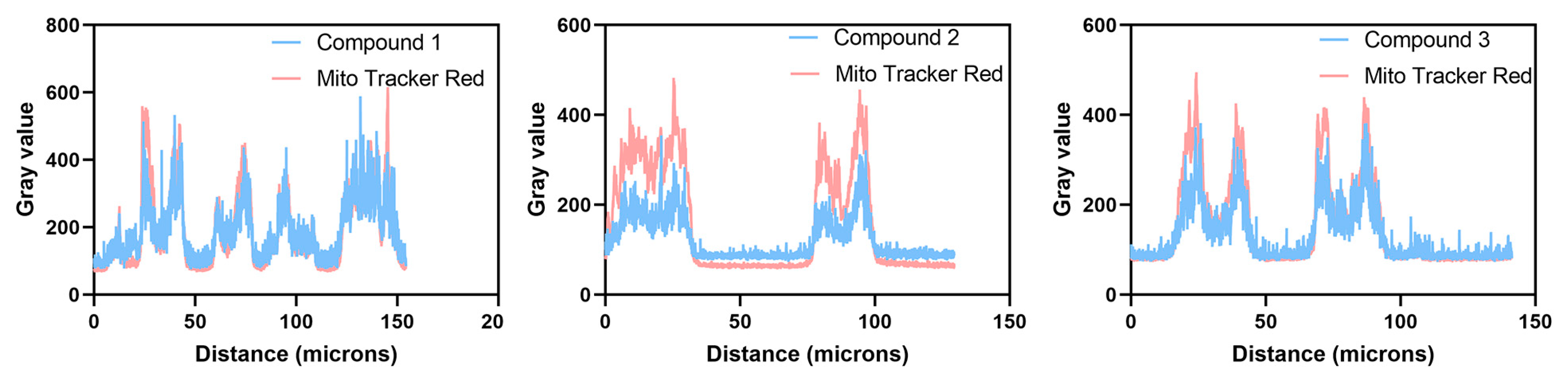
2.2. In Vitro Imaging of A549 Cells
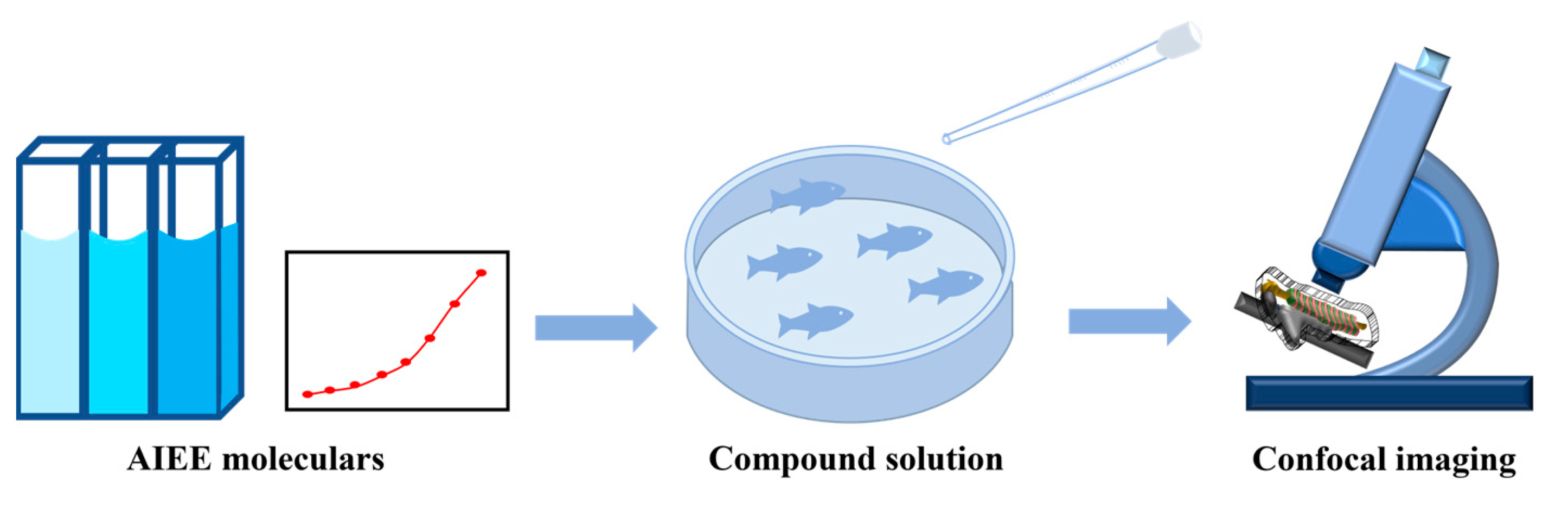
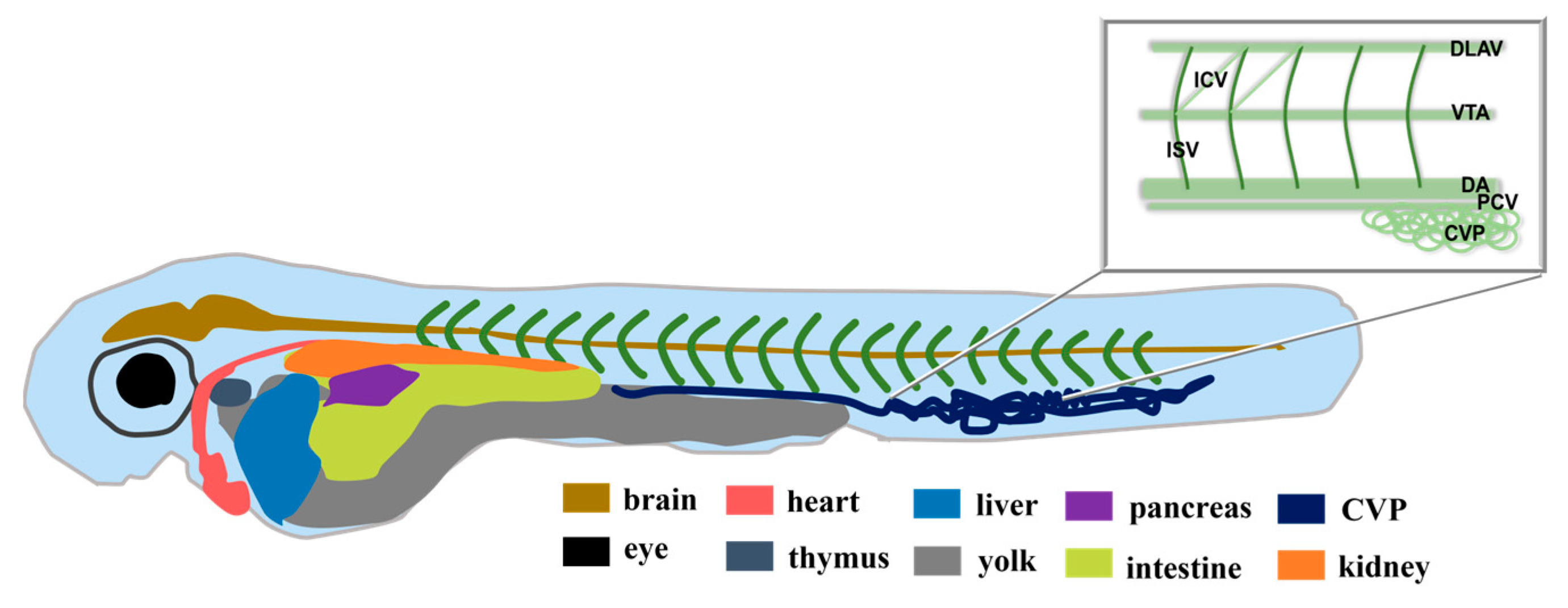
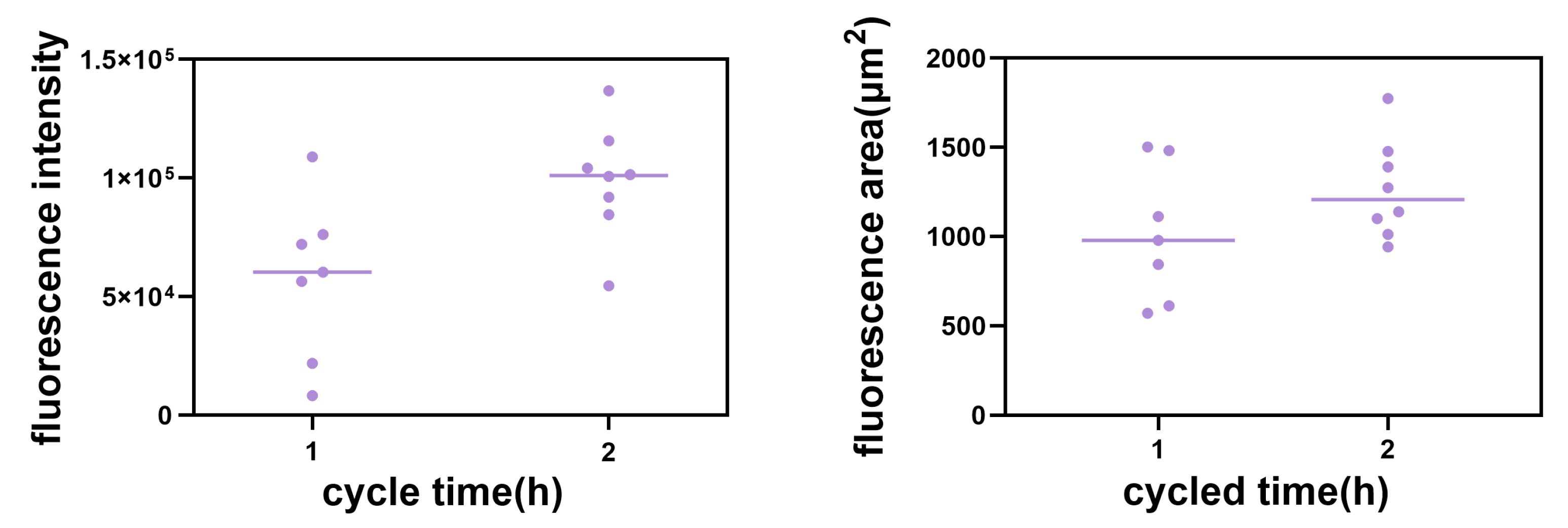
2.3. Real-Time Tracking in Zebrafish
3. Materials and Methods
3.1. Materials and Instruments
3.1.1. Chemical Compounds Used
3.1.2. Cell
3.1.3. Zebrafish
3.1.4. Scientific Instruments
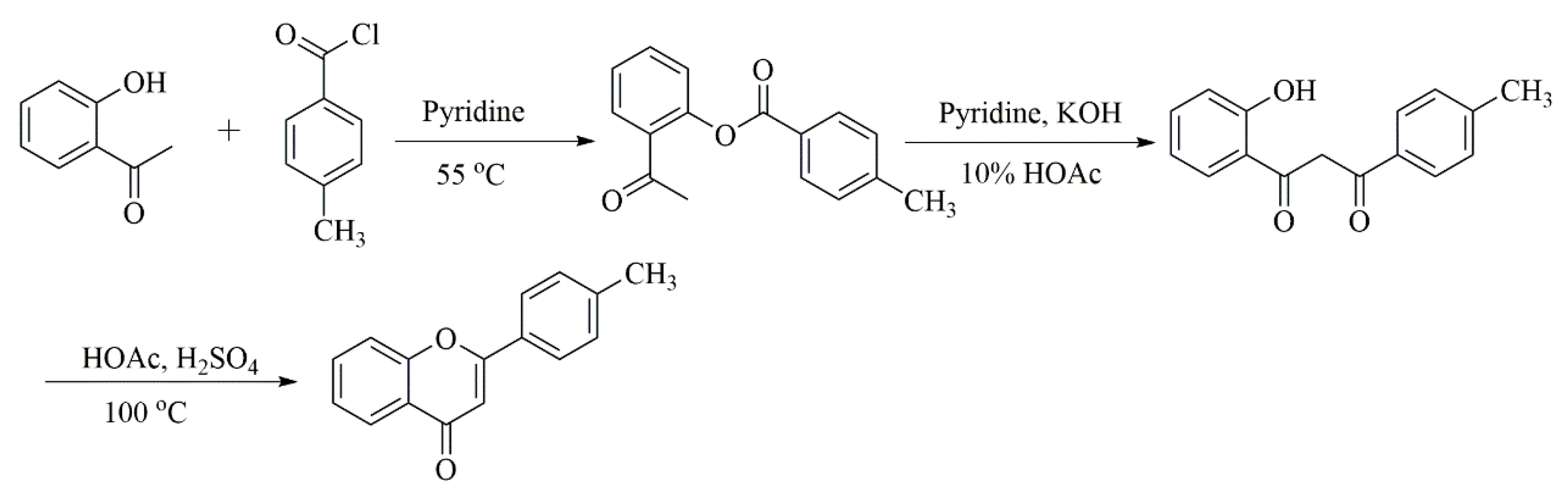
3.2. Synthesis of 4′-Methyflavanone
3.2.1. Synthesis of Compound 1
3.2.2. Characterization of 4′-Methyflavanone
3.3. Preparation for UV–Vis Spectra, PL Spectra, and SEM Measurements
3.3.1. Preparatory Work before UV–Vis Spectra and PL Spectra Measurements
3.3.2. Preparatory Work before SEM Measurements
3.4. Preparatory Work before Ethylene Glycol (EG) Measurements
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. In Vitro Imaging of A549 Cells
3.8. Real-Time Tracking in Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Ren, T.B.; Huan, S.; Yuan, L.; Zhang, X.B. Progress and Perspective of Solid-State Organic Fluorophores for Biomedical Applications. J. Am. Chem. Soc. 2021, 143, 21143–21160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Sun, F.; Zhu, X.; Zhang, J.; Ou, X.; Zhang, J.; Xu, C.; Sung, H.H.Y.; Williams, I.D.; Chen, S.; et al. Rational Design of NIR-II AIEgens with Ultrahigh Quantum Yields for Photo- and Chemiluminescence Imaging. J. Am. Chem. Soc. 2022, 144, 15391–15402. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Liu, M.; Zhang, X.; Tao, L.; Chen, Y.; Wei, Y. Polymeric AIE-based nanoprobes for biomedical applications: Recent advances and perspectives. Nanoscale 2015, 7, 11486–11508. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights. Acc. Chem. Res. 2018, 51, 1404–1414. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Leung, C.W.; Hong, Y.; Hanske, J.; Zhao, E.; Chen, S.; Pletneva, E.V.; Tang, B.Z. Superior fluorescent probe for detection of cardiolipin. Anal. Chem. 2014, 86, 1263–1268. [Google Scholar] [CrossRef]
- Wang, M.; Gu, X.; Zhang, G.; Zhang, D.; Zhu, D. Convenient and continuous fluorometric assay method for acetylcholinesterase and inhibitor screening based on the aggregation-induced emission. Anal. Chem. 2009, 81, 4444–4449. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Chi, W.; Zhao, Y.; Tang, Y.; Jing, X.; Wang, Z.; Zhou, Y.; Shen, Q.; Zhang, J.; Yang, Z.; et al. All-in-One Theranostic Platforms: Deep-Red AIE Nanocrystals to Target Dual-Organelles for Efficient Photodynamic Therapy. ACS Nano 2022, 16, 20151–20162. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Kang, F.; Yang, W.; Zhang, M.; Dang, R.; Jiang, P.; Wang, J. Molecular engineering of a high quantum yield NIR-II molecular fluorophore with aggregation-induced emission (AIE) characteristics for in vivo imaging. Nanoscale 2020, 12, 5084–5090. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Shen, Z.; Wang, D.; Dong, R.; Zhang, J.; Pan, Y.; Li, Y.; Wang, D.; Tang, B.Z. Double-pronged Antimicrobial Agents based on a Donor-π-Acceptor Type Aggregation-Induced Emission Luminogen. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212386. [Google Scholar] [CrossRef]
- Liu, X.S.; Tang, Z.; Li, Z.; Li, M.; Xu, L.; Liu, L. Modular and stereoselective synthesis of tetrasubstituted vinyl sulfides leading to a library of AIEgens. Nat. Commun. 2021, 12, 7298. [Google Scholar] [CrossRef]
- Suzuki, S.; Sasaki, S.; Sairi, A.S.; Iwai, R.; Tang, B.Z.; Konishi, G.I. Principles of Aggregation-Induced Emission: Design of Deactivation Pathways for Advanced AIEgens and Applications. Angew. Chem. Int. Ed. Engl. 2020, 59, 9856–9867. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Li, Z. Aggregation-Induced Emission Luminogens with Photoresponsive Behaviors for Biomedical Applications. Adv. Healthc. Mater. 2021, 10, e2101169. [Google Scholar] [CrossRef]
- Xia, F.; Wu, J.; Wu, X.; Hu, Q.; Dai, J.; Lou, X. Modular Design of Peptide- or DNA-Modified AIEgen Probes for Biosensing Applications. Acc. Chem. Res. 2019, 52, 3064–3074. [Google Scholar] [CrossRef]
- Qi, J.; Sun, C.; Zebibula, A.; Zhang, H.; Kwok, R.T.K.; Zhao, X.; Xi, W.; Lam, J.W.Y.; Qian, J.; Tang, B.Z. Real-Time and High-Resolution Bioimaging with Bright Aggregation-Induced Emission Dots in Short-Wave Infrared Region. Adv. Mater. 2018, 30, e1706856. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Chen, H.; Zang, K.; Liu, S.H.; Xie, Y.; Tan, Y.; Yin, J. Near-Infrared Thienoisoindigos with Aggregation-Induced Emission: Molecular Design, Optical Performance, and Bioimaging Application. Anal. Chem. 2021, 93, 3378–3385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Trépout, S.; Chen, H.; Li, M.H. AIE Polymer Micelle/Vesicle Photocatalysts Combined with Native Enzymes for Aerobic Photobiocatalysis. J. Am. Chem. Soc. 2023, 145, 288–299. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Zhang, J.; Wang, H.; Liu, G.; Bu, Y.; Yu, J.; Tian, Y.; Zhou, H. AIE-Based Theranostic Agent: In Situ Tracking Mitophagy Prior to Late Apoptosis To Guide the Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef]
- Vissenaekens, H.; Criel, H.; Grootaert, C.; Raes, K.; Smagghe, G.; Van Camp, J. Flavonoids and cellular stress: A complex interplay affecting human health. Crit. Rev. Food Sci. Nutr. 2022, 62, 8535–8566. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Coombs, M.R.P. Editorial: Immune Modulation by Flavonoids. Front. Immunol. 2022, 13, 899577. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Guan, L.P.; Liu, B.Y. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016, 121, 47–57. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Liu, L.; Lei, Y.; Zhang, J.; Li, N.; Zhang, F.; Wang, H.; He, F. Rational Design for Multicolor Flavone-Based Fluorophores with Aggregation-Induced Emission Enhancement Characteristics and Applications in Mitochondria-Imaging. Molecules 2018, 23, 2290. [Google Scholar] [CrossRef]
- Li, N.; Liu, L.; Luo, H.; Wang, H.; Yang, D.; He, F. Flavanone-Based Fluorophores with Aggregation-Induced Emission Enhancement Characteristics for Mitochondria-Imaging and Zebrafish-Imaging. Molecules 2020, 25, 3298. [Google Scholar] [CrossRef]
- Luo, H.; Li, N.; Liu, L.; Wang, H.; He, F. Synthesis of New AIEE-Active Chalcones for Imaging of Mitochondria in Living Cells and Zebrafish In Vivo. Int. J. Mol. Sci. 2021, 22, 8949. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Flores-Toro, J.A.; Go, K.L.; Leeuwenburgh, C.; Kim, J.S. Autophagy in the liver: Cell’s cannibalism and beyond. Arch. Pharm. Res. 2016, 39, 1050–1061. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology 2014, 147, 765–783. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Zhao, H.; Yu, K.; Song, M.; Zhu, Y.; Li, Y. Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J. Inorg. Biochem. 2017, 174, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Li, W.; Zhou, H.; Li, Y.; Yu, C. Nucleic acid-induced tetraphenylethene probe noncovalent self-assembly and the superquenching of aggregation-induced emission. Anal. Chem. 2014, 86, 9866–9872. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, L.; Miao, W.; Wang, X.; Guo, G. Aggregation-Induced Electrochemiluminescence of the Dichlorobis(1,10-phenanthroline)ruthenium(II) (Ru(phen)2Cl2)/Tri-n-propylamine (TPrA) System in H2O–MeCN Mixtures for Identification of Nucleic Acids. Anal. Chem. 2020, 92, 9613–9619. [Google Scholar] [CrossRef]
- Borjihan, Q.; Wu, H.; Dong, A.; Gao, H.; Yang, Y.W. AIEgens for Bacterial Imaging and Ablation. Adv. Healthc. Mater. 2021, 10, e2100877. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yu, Z.; Yang, M.; Jin, F.; Zhang, Q.; Zhou, H.; Wu, J.; Tian, Y. Substituent group variations directing the molecular packing, electronic structure, and aggregation-induced emission property of isophorone derivatives. J. Org. Chem. 2013, 78, 3222–3234. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, T.; Miao, X.; Yi, X.; Wang, X.; Zhao, H.; Lee, S.M.; Zheng, Y. Zebrafish: A promising in vivo model for assessing the delivery of natural products, fluorescence dyes and drugs across the blood-brain barrier. Pharmacol. Res. 2017, 125 Pt B, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Dekoninck, S.; Hannezo, E.; Sifrim, A.; Miroshnikova, Y.A.; Aragona, M.; Malfait, M.; Gargouri, S.; de Neunheuser, C.; Dubois, C.; Voet, T.; et al. Defining the Design Principles of Skin Epidermis Postnatal Growth. Cell 2020, 181, 604–620.e22. [Google Scholar] [CrossRef]
- Fan, X.; He, C.; Ji, M.; Sun, X.; Luo, H.; Li, C.; Tong, H.; Zhang, W.; Sun, Z.; Chu, W. Visible light-induced deoxygenation/cyclization of salicylic acid derivatives and aryl acetylene for the synthesis of flavonoids. Chem. Commun. 2022, 58, 6348–6351. [Google Scholar] [CrossRef]
- Narwal, M.; Koivunen, J.; Haikarainen, T.; Obaji, E.; Legala, O.E.; Venkannagari, H.; Joensuu, P.; Pihlajaniemi, T.; Lehtiö, L. Discovery of tankyrase inhibiting flavones with increased potency and isoenzyme selectivity. J. Med. Chem. 2013, 56, 7880–7889. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]





| Compound | Solvent | Quantum Yield (ϕF) |
|---|---|---|
| 1 | CH3OH | 0.02 |
| CH3OH/H2O (5:5) | 0.16 | |
| CH3OH/H2O (1:9) | 0.29 | |
| 2 | CH3OH | 0.06 |
| CH3OH/H2O (5:5) | 0.30 | |
| CH3OH/H2O (1:9) | 0.17 | |
| 3 | CH3OH | 0.23 |
| CH3OH/H2O (4:6) | 0.68 | |
| CH3OH/H2O (1:9) | 0.51 |
| Compound | Corresponding R Values (Pearson Correlation Coefficient) |
|---|---|
| 1 | 0.86 |
| 2 | 0.87 |
| 3 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; He, Y.; Luo, H.; Jin, T.; He, F. AIEE-Active Flavones as a Promising Tool for the Real-Time Tracking of Uptake and Distribution in Live Zebrafish. Int. J. Mol. Sci. 2023, 24, 10183. https://doi.org/10.3390/ijms241210183
Wu Y, He Y, Luo H, Jin T, He F. AIEE-Active Flavones as a Promising Tool for the Real-Time Tracking of Uptake and Distribution in Live Zebrafish. International Journal of Molecular Sciences. 2023; 24(12):10183. https://doi.org/10.3390/ijms241210183
Chicago/Turabian StyleWu, Yi, Ying He, Huiqing Luo, Tingting Jin, and Feng He. 2023. "AIEE-Active Flavones as a Promising Tool for the Real-Time Tracking of Uptake and Distribution in Live Zebrafish" International Journal of Molecular Sciences 24, no. 12: 10183. https://doi.org/10.3390/ijms241210183
APA StyleWu, Y., He, Y., Luo, H., Jin, T., & He, F. (2023). AIEE-Active Flavones as a Promising Tool for the Real-Time Tracking of Uptake and Distribution in Live Zebrafish. International Journal of Molecular Sciences, 24(12), 10183. https://doi.org/10.3390/ijms241210183