Influence of Te-Incorporated LaCoO3 on Structural, Morphology and Magnetic Properties for Multifunctional Device Applications
Abstract
1. Introduction
2. Results and Discussion
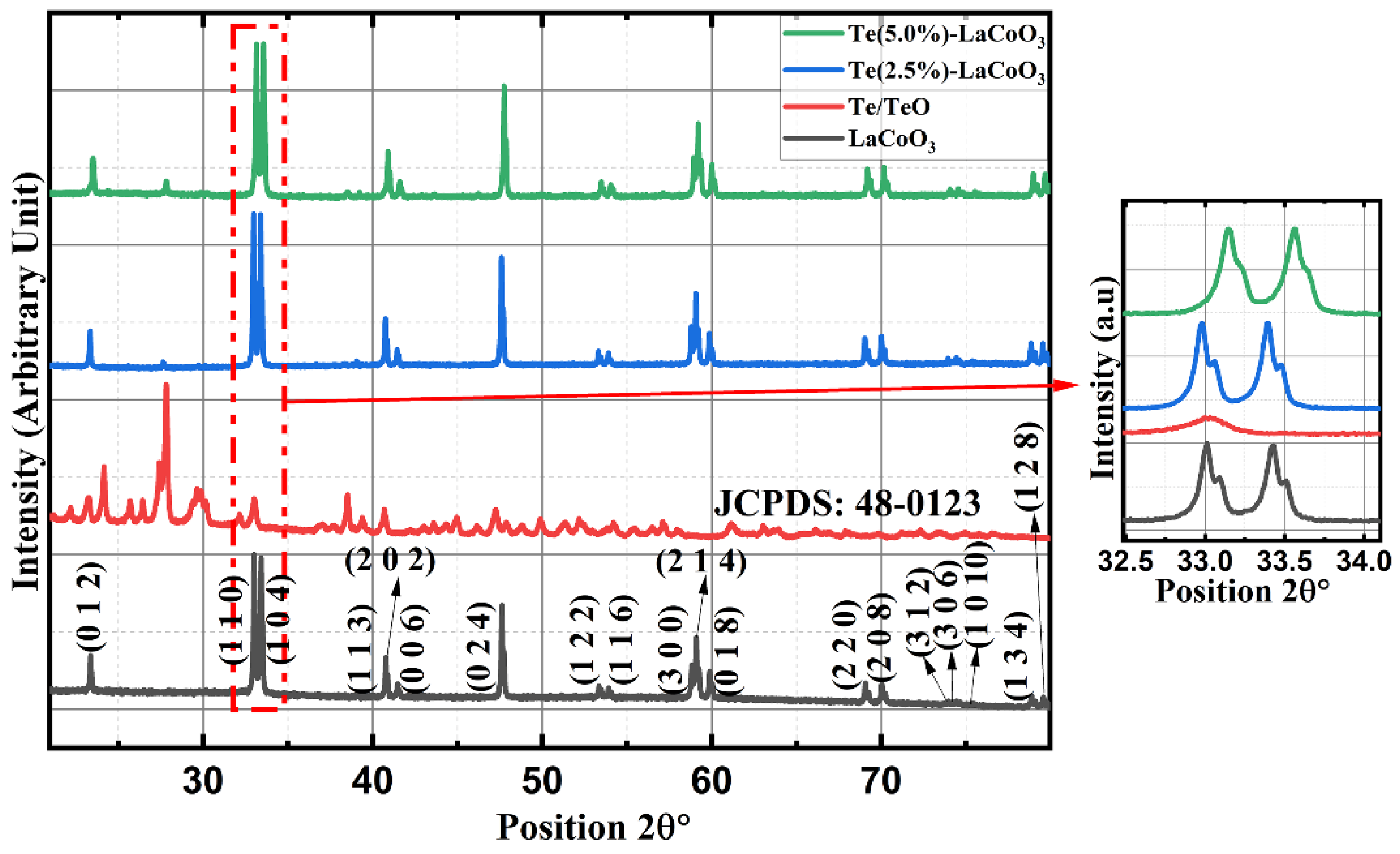
2.1. Powder X-ray Diffraction (P-XRD) of Te-Incorporated LaCoO3
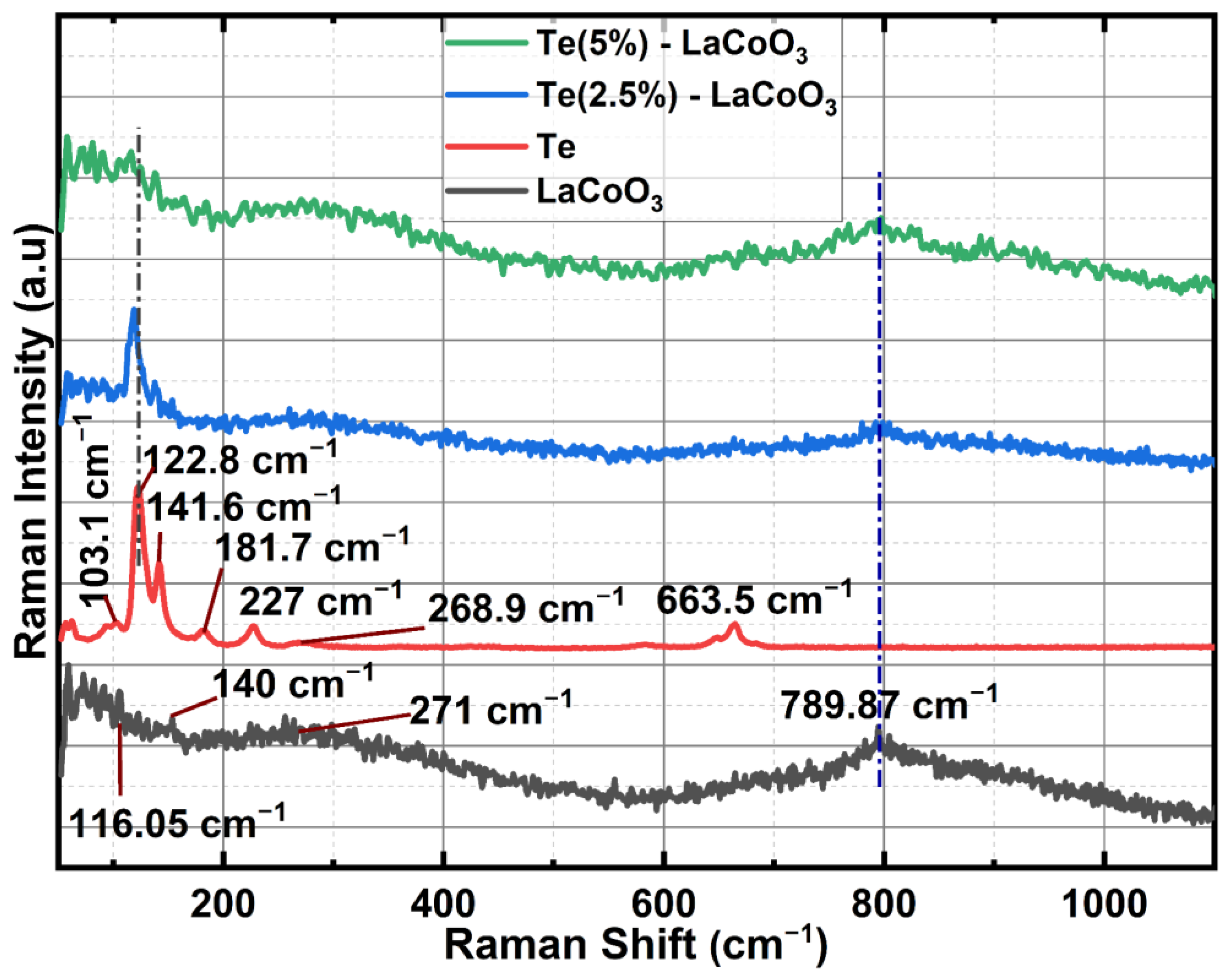
2.2. Raman Spectrum Analysis of Te-Incorporated LaCoO3
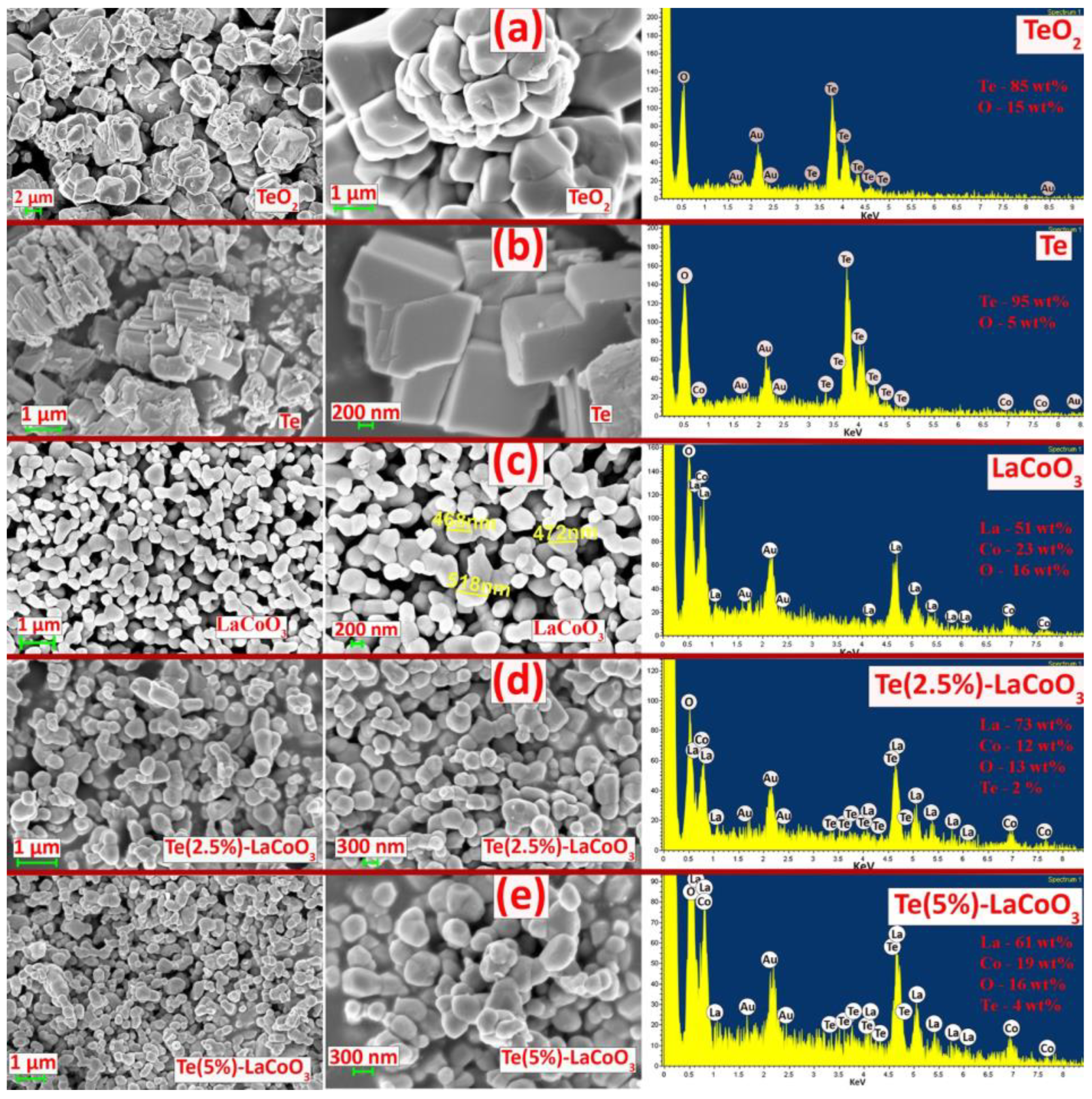
2.3. SEM and Energy-Dispersive X-ray (EDS) Analysis of Te-Incorporated LaCoO3
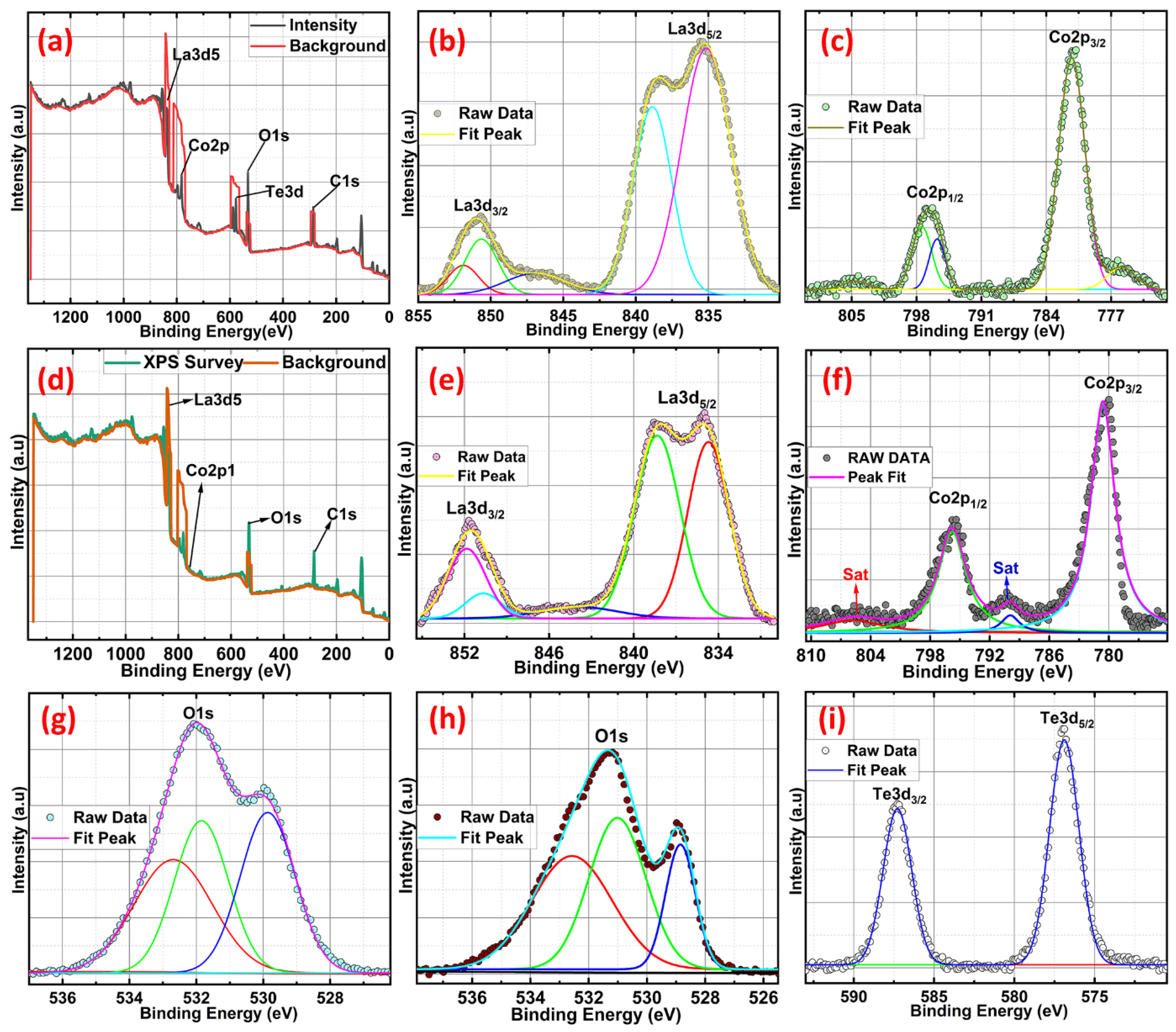
2.4. X-ray Photoelectron Spectroscopy (XPS) Studies of Te-Incorporated LaCoO3
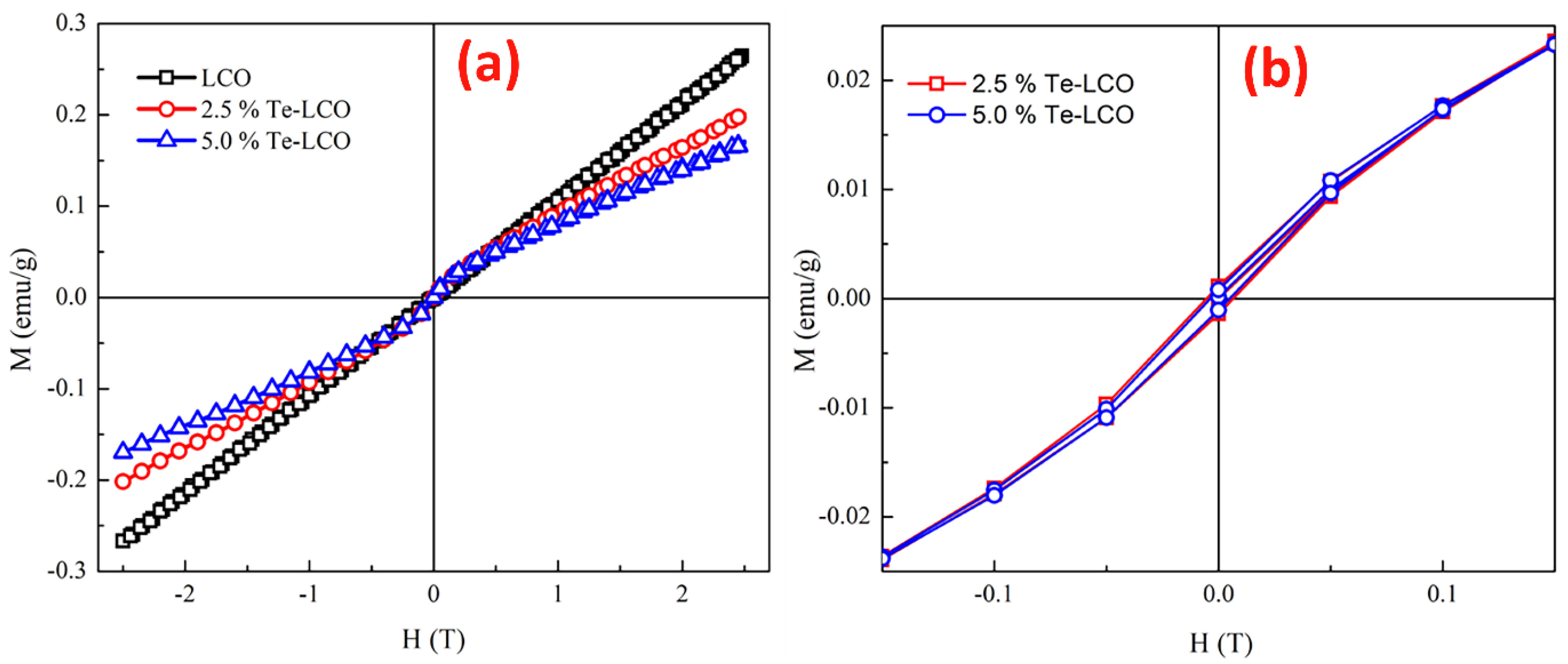
2.5. Magnetic Properties of Te-Incorporated LaCoO3
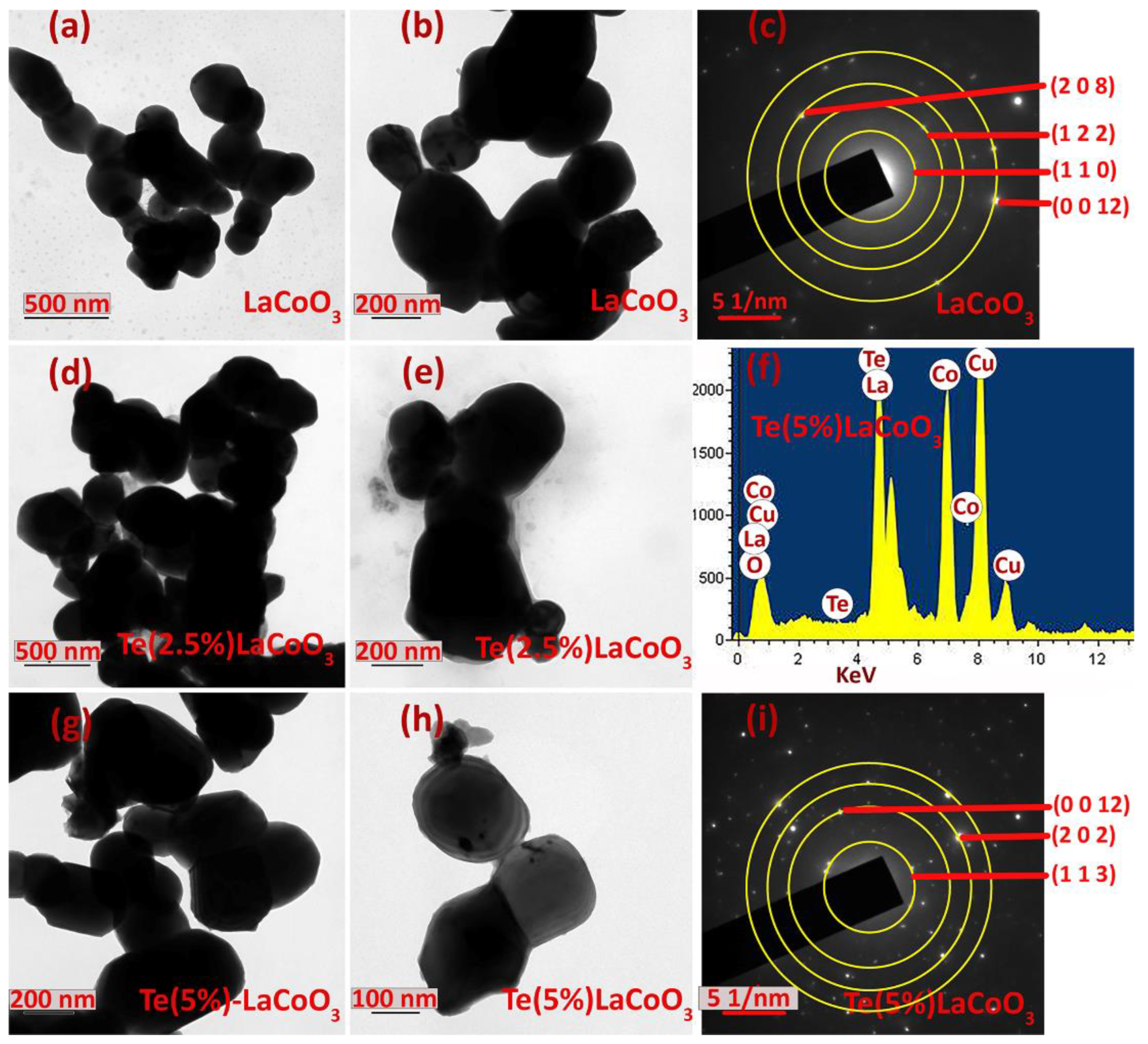
2.6. Transmission Analysis of Te-Incorporated LaCoO3
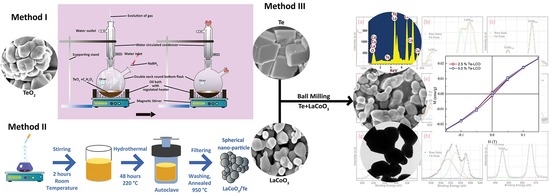
3. Materials and Methods
3.1. Materials
3.1.1. Materials for the Preparation of Te (Method 1)
3.1.2. Materials for the Preparation of LaCoO3 (Method 2)
3.1.3. Materials for the Preparation of Te (2.5%)-Impregnated LaCoO3 and Te-(5%) Impregnated LaCoO3 (Method 3)
3.2. Methods
3.2.1. Method 1: The Preparation of Te
3.2.2. Method 2: The Preparation of LaCoO3
3.2.3. Method 3: The Preparation of Te-Impregnated LaCoO3
3.3. Characterisation Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fist, N.; Dinan, J.; Stadelmann, R.; Orlovskaya, N. In situ three point bending device for measurements of vibrational response of ceramics under stress by microRaman spectroscopy. Adv. Appl. Ceram. 2012, 111, 433–439. [Google Scholar] [CrossRef]
- Samantaray, C.B.; Sim, H.; Hwang, H. Electronic structure and optical properties of barium strontium titanate (BaxSr1−xTiO3) using first-principles method. Phys. B Condens. Matter 2004, 351, 158–162. [Google Scholar] [CrossRef]
- Samantaray, C.B.; Sim, H.; Hwang, H. The electronic structures and optical properties of BaTiO3 and SrTiO3 using first-principles calculations. Microelectron. J. 2005, 36, 725–728. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Sr1−xCaxTiO3: An XY Quantum Ferroelectric with Transition to Randomness. Phys. Rev. Lett. 1984, 52, 2289–2292. [Google Scholar] [CrossRef]
- Frederikse, H.P.R.; Thurber, W.R.; Hosler, W.R. Electronic Transport in Strontium Titanate. Phys. Rev. 1964, 134, A442–A445. [Google Scholar] [CrossRef]
- Koonce, C.S.; Cohen, M.L.; Schooley, J.F.; Hosler, W.R.; Pfeiffer, E.R. Superconducting Transition Temperatures of Semiconducting SrTiO3. Phys. Rev. 1967, 163, 380–390. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Li, Q.; Zhu, Z.; Wang, R.; Woo, C.H. First-principles study of the cubic perovskitesBiMO3(M=Al, Ga, In, and Sc). Phys. Rev. B 2007, 75, 245209. [Google Scholar] [CrossRef]
- Baettig, P.; Schelle, C.F.; LeSar, R.; Waghmare, U.V.; Spaldin, N.A. Theoretical Prediction of New High-Performance Lead-Free Piezoelectrics. Chem. Mater. 2005, 17, 1376–1380. [Google Scholar] [CrossRef]
- Muta, H.; Kurosaki, K.; Yamanaka, S. Thermoelectric properties of rare earth doped SrTiO3. J. Alloys Compd. 2003, 350, 292–295. [Google Scholar] [CrossRef]
- Sivaprakash, P.; Divya, S.; Parameshwari, R.; Saravanan, C.; Sagadevan, S.; Arumugam, S.; Muthu, S.E. Influence of Zn2+ doping towards the structural, magnetic, and dielectric properties of NiFe2O4 composite. J. Mater. Sci. Mater. Electron. 2020, 31, 16369–16378. [Google Scholar] [CrossRef]
- Millis, A.J.; Shraiman, B.I.; Mueller, R. Dynamic Jahn-Teller Effect and Colossal Magnetoresistance inLa1−xSrxMnO3. Phys. Rev. Lett. 1996, 77, 175–178. [Google Scholar] [CrossRef]
- Divya, S.; Sivaprakash, P.; Raja, S.; Muthu, S.E.; Eed, E.M.; Arumugam, S.; Oh, T.H. Temperature-dependent dielectric and magnetic properties of NiFe2O4 nanoparticles. Appl. Nanosci. 2021, 13, 1327–1336. [Google Scholar] [CrossRef]
- Dragan, M.; Enache, S.; Varlam, M.; Petrov, K. Perovskite-Type Lanthanum Cobaltite LaCoO3: Aspects of Processing Route toward Practical Applications. In Cobalt Compounds and Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hilgenkamp, H.; Ariando, A.; Smilde, H.-J.H.; Blank, D.H.A.; Rijnders, G.; Rogalla, H.; Kirtley, J.R.; Tsuei, C.C. Ordering and manipulation of the magnetic moments in large-scale superconducting π-loop arrays. Nature 2003, 422, 50–53. [Google Scholar] [CrossRef]
- Raccah, P.M.; Goodenough, J.B. First-Order Localized-Electron ⇆ Collective-Electron Transition in LaCoO3. Phys. Rev. 1967, 155, 932–943. [Google Scholar] [CrossRef]
- Rodríguez, M.A.S.; Goodenough, J. Magnetic and Transport Properties of the System La1−xSrxCoO3−δ (0 < x ≤ 0.50). J. Solid State Chem. 1995, 118, 323–336. [Google Scholar] [CrossRef]
- Maignan, A.; Flahaut, D.; Hebert, S. Sign change of the thermoelectric power in LaCoO3. Eur. Phys. J. B 2004, 39, 145–148. [Google Scholar] [CrossRef]
- Guo, E.-J.; Desautels, R.; Keavney, D.; Roldan, M.A.; Kirby, B.J.; Lee, D.; Liao, Z.; Charlton, T.; Herklotz, A.; Ward, T.Z.; et al. Nanoscale ferroelastic twins formed in strained LaCoO3 films. Sci. Adv. 2019, 5, eaav5050. [Google Scholar] [CrossRef]
- Sahadevan, J.; Radhakrishnan, M.; Padmanathan, N.; Muthu, S.E.; Sivaprakash, P.; Kadiresan, M. Effect of Mn substitution on magnetic behaviour of oxygen defective LaCoO3 perovskite oxide. Mater. Sci. Eng. B 2022, 284, 115875. [Google Scholar] [CrossRef]
- Zhou, J.-S.; Yan, J.-Q.; Goodenough, J.B. Bulk modulus anomaly in RCoO3(R = La, Pr, and Nd). Phys. Rev. B 2005, 71, 220103. [Google Scholar] [CrossRef]
- Vogt, T.; Hriljac, J.A.; Hyatt, N.C.; Woodward, P. Pressure-induced intermediate-to-low spin state transition in LaCoO3. Phys. Rev. B 2003, 67, 140401. [Google Scholar] [CrossRef]
- Divya, S.; Sivaprakash, P.; Raja, S.; Muthu, S.E.; Kim, I.; Renuka, N.; Arumugam, S.; Oh, T.H. Impact of Zn doping on the dielectric and magnetic properties of CoFe2O4 nanoparticles. Ceram. Int. 2022, 48, 33208–33218. [Google Scholar] [CrossRef]
- Xu, Y.; Zielke, P.; Van Nong, N.; Pirou, S.; Reolon, R.; Si, X.; Simonsen, S.B.; Norby, P.; Lühmann, H.; Bensch, W.; et al. Hydrothermal Synthesis, Characterization, and Sintering Behavior of Core-Shell Particles: A Principle Study on Lanthanum Strontium Cobaltite Coated with Nanosized Gadolinium Doped Ceria. Ceramics 2018, 1, 246–260. [Google Scholar] [CrossRef]
- Ayyob, M.; Ahmad, I.; Hussain, F.; Bangash, M.K.; Awan, J.A.; Jaubert, J.-N. A new technique for the synthesis of lanthanum substituted nickel cobaltite nanocomposites for the photo catalytic degradation of organic dyes in wastewater. Arab. J. Chem. 2020, 13, 6341–6347. [Google Scholar] [CrossRef]
- Deeksha; Kour, P.; Ahmed, I.; Haldar, K.K.; Yadav, K. Tuning the Morphology of Lanthanum Cobaltite Using the Surfactant-Assisted Hydrothermal Approach for Enhancing Oxygen Evolution Catalysis. In Proceedings of the National Workshop on Recent Advances in Condensed Matter and High Energy Physics; Springer Proceedings in Physics. Springer: Singapore, 2022; Volume 278, pp. 15–24. [Google Scholar] [CrossRef]
- Tepech-Carrillo, L.; Escobedo-Morales, A.; Pérez-Centeno, A.; Chigo-Anota, E.; Sánchez-Ramírez, J.F.; López-Apreza, E.; Gutiérrez-Gutiérrez, J. Preparation of Nanosized LaCoO3 through Calcination of a Hydrothermally Synthesized Precursor. J. Nanomater. 2016, 2016, 6917950. [Google Scholar] [CrossRef]
- Popa, M. Characterization of LaMeO3 (Me: Mn, Co, Fe) perovskite powders obtained by polymerizable complex method. Solid State Ionics 2002, 154–155, 135–141. [Google Scholar] [CrossRef]
- Iliev, M.N.; Abrashev, M.V. Raman phonons and Raman Jahn-Teller bands in perovskite-like manganites. J. Raman Spectrosc. 2001, 32, 805–811. [Google Scholar] [CrossRef]
- Orlovskaya, N.; Steinmetz, D.; Yarmolenko, S.; Pai, D.; Sankar, J.; Goodenough, J. Detection of temperature- and stress-induced modifications of LaCoO3 by micro-Raman spectroscopy. Phys. Rev. B-Condens. Matter Mater. Phys. 2005, 72, 014122. [Google Scholar] [CrossRef]
- Martin, R.M.; Ucovsky, G.I.; Helliwell, K. Intermolecnlar bonding and lattice dynamics of Se and Te. Phys. Rev. B-Condens. Matter Mater. Phys. 1976, 13, 1383. [Google Scholar] [CrossRef]
- Pine, A.S.; Dresselhaus, G. Raman Scattering in Paratellurite, TeO2. Phys. Rev. B-Condens. Matter Mater. Phys. 1972, 5, 4087. [Google Scholar] [CrossRef]
- Marini, C.; Chermisi, D.; Lavagnini, M.; Di Castro, D.; Petrillo, C.; Degiorgi, L.; Scandolo, S.; Postorino, P. High-pressure phases of crystallite tellurium: A combined Raman and ab initio study. Phys. Rev. B-Condens. Matter Mater. Phys. 2012, 86, 064103. [Google Scholar] [CrossRef]
- He, J.; Lv, W.; Chen, Y.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. Tellurium-Impregnated Porous Cobalt-Doped Carbon Polyhedra as Superior Cathodes for Lithium-Tellurium Batteries. ACS Nano 2017, 11, 8144–8152. [Google Scholar] [CrossRef]
- Haber, J.; Ungier, L. On chemical shifts of ESCA and Auger lines in cobalt oxides. J. Electron. Spectrosc. Relat. Phenom. 1977, 12, 305–312. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Johnston, D.D.; Coatsworth, L.L.; Davidson, R.D.; Brown, J.R. X-ray photoelectron spectroscopic studies of thin film oxides of cobalt and molybdenum. Surf. Interface Anal. 1990, 15, 265–272. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Richins, S.; Liaw, K.; Yan, L.; Zhou, M.; Luo, H. Polymer-assisted approach to LaCo1-xNixO3 network nanostructures as bifunctional oxygen electrocatalysts. Electrochim. Acta 2019, 296, 945–953. [Google Scholar] [CrossRef]
- DFrost, C.; McDowell, C.A.; Woolsey, I.S. Evidence for multiplet splitting of 2p photoelectron lines of transition metal complexes. Chem. Phys. Lett. 1972, 17, 320–323. [Google Scholar] [CrossRef]
- Seim, H.; Nieminen, M.; Niinistö, L.; Fjellvåg, H.; Johansson, L.S. Growth of LaCoO3 thin films from β-diketonate precursors. Appl. Surf. Sci. 1997, 112, 243–250. [Google Scholar] [CrossRef]
- Ramana, C.V.; Vemuri, R.S.; Kaichev, V.V.; Kochubey, V.A.; Saraev, A.A.; Atuchin, V.V. X-ray photoelectron spectroscopy depth profiling of La2O3/Si thin films deposited by reactive magnetron sputtering. ACS Appl. Mater. Interfaces 2011, 3, 4370–4373. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, Y.; Zhang, Z.; Li, J.; Qian, J.; Gao, D. Cation substitution of B-site in LaCoO3 for bifunctional oxygen electrocatalytic activities. J. Alloys Compd. 2021, 878, 160433. [Google Scholar] [CrossRef]
- Armelao, L.; Barreca, D.; Bottaro, G.; Gasparotto, A.; Maragno, C.; Tondello, E. LaCoO3 Nanosystems by a Hybrid CVD/Sol-Gel Route: An XPS Investigation. Surf. Sci. Spectra 2003, 10, 143–149. [Google Scholar] [CrossRef]
- Vasquez, R.P. X-ray photoemission measurements of La1-xCaxCoO3(x = 0, 0.5). Phys. Rev. B-Condens. Matter Mater. Phys. 1996, 54, 14938. [Google Scholar] [CrossRef]
- Ricco, A.J.; White, H.S.; Wrighton, M.S. X-ray photoelectron and Auger electron spectroscopic study of the CdTe surface resulting from various surface pretreatments: Correlation of photoelectrochemical and capacitance-potential behavior with surface chemical composition. J. Vac. Sci. Technol. A Vac. Surf. Film. 1984, 2, 910–915. [Google Scholar] [CrossRef]
- Christie, A.B.; Sutherland, I.; Walls, J.M. Studies of the composition, ion-induced reduction and preferential sputtering of anodic oxide films on Hg0.8Cd0.2Te by XPS. Surf. Sci. 1983, 135, 225–242. [Google Scholar] [CrossRef]
- Hidetaka, K.; Yoshihisa, Y. Ylide-Metal Complexes. XIV. An X-Ray Photoelectron Spectroscopic Study on Tellurium Complexes of Methylenetriphenylphosphorane. Bull. Chem. Soc. Jpn. 1988, 61, 2990–2992. [Google Scholar] [CrossRef]
- Branford, W.; Green, M.A.; Neumann, D.A. Structure and ferromagnetism in Mn4+ spinels: AM0.5Mn1.5O4 (A = Li, Cu; M = Ni, Mg). Chem. Mater. 2002, 14, 1649–1656. [Google Scholar] [CrossRef]
- Thummer, K.P.; Chhantbar, M.C.; Modi, K.; Joshi, H. Effect of Mn4+ substitution on magnetic behaviour of cobalt ferrite. Indian J. Phys. 2005, 79, 41–45. [Google Scholar]
- Liang, H.; Hong, Y.; Zhu, C.; Li, S.; Chen, Y.; Liu, Z.; Ye, D. Influence of partial Mn-substitution on surface oxygen species of LaCoO3 catalysts. Catal. Today 2013, 201, 98–102. [Google Scholar] [CrossRef]
- Xiao, P.; Zhu, J.; Li, H.; Jiang, W.; Wang, T.; Zhu, Y.; Zhao, Y.; Li, J. Effect of textural structure on the catalytic performance of LaCoO3 for CO oxidation. ChemCatChem 2014, 6, 1774–1781. [Google Scholar] [CrossRef]
- Schmidt, R.; Wu, J.; Leighton, C.; Terry, I. Dielectric response to the low-temperature magnetic defect structure and spin state transition in polycrystalline LaCoO3. Phys. Rev. B-Condens. Matter Mater. Phys. 2009, 79, 125105. [Google Scholar] [CrossRef]
- Tomiyasu, K.; Sato, M.; Koyama, S.I.; Nojima, T.; Kajimoto, R.; Ji, S.; Iwasa, K. Magnetic properties of electron-doped LaCoO3. J. Phys. Soc. Jpn. 2017, 86, 094706. [Google Scholar] [CrossRef]
- Gignoux, D.; De Lacheisserie, E.D.T.; Schlenker, M. Magnetism: Materials and Applications; Springer: New York, NY, USA, 2005. [Google Scholar]
- Sivaprakash, P.; Nitthin Ananth, A.; Nagarajan, V.; Parameshwari, R.; Arumugam, S.; Jose, S.; Muthu, S.E. Role of Sm3+ dopant in the formation of La(1−x)SmxCrO3 solid state nanoperovskites–correlation of its augmented physical properties. Mater. Chem. Phys. 2020, 248, 122922. [Google Scholar] [CrossRef]
- Sahadevan, J.; Sojiya, R.; Padmanathan, N.; Kulathuraan, K.; Shalini, M.G.; Sivaprakash, P.; Esakki Muthu, S. Magnetic property of Fe2O3 and Fe3O4 nanoparticle prepared by solvothermal process. Mater. Today: Proc. 2022, 58, 895–897. [Google Scholar] [CrossRef]
- Sivaprakash, P.; Nitthin Ananth, A.; Nagarajan, V.; Jose, S.; Arumugam, S. Remarkable enhancement of La(1-x)SmxCrO3 nanoperovskite properties: An influence of its doping concentrations. Mater. Res. Bull. 2017, 95, 17–22. [Google Scholar] [CrossRef]
- Wang, R.; Ye, C.; Wang, H.; Jiang, F. Z-Scheme LaCoO3/g-C3N4 for Efficient Full-Spectrum Light-Simulated Solar Photocatalytic Hydrogen Generation. ACS Omega 2020, 5, 30373–30382. [Google Scholar] [CrossRef]

| Composition | Crystal System | Space Group | Crystallite Size (nm) | Lattice Parameter (Å) | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| TeO2 | Tetragonal | P41212 | 26.80514 | 5.40681 | 5.40865 | 13.20149 |
| Te | Hexagonal | P3121 | 28.33724 | 4.19097 | 4.19123 | 5.98099 |
| LaCoO3 | Rhombohedral | c | 43.42469 | 5.41768 | 5.39366 | 13.13084 |
| 2.5% Te/LCO | Rhombohedral | c | 31.59925 | 5.42428 | 5.39256 | 13.1579 |
| 5% Te/LCO | Rhombohedral | c | 31.59925 | 5.42681 | 5.39434 | 13.20149 |
| Composition | HC (T) | M (emu/g) @300 K | Mr (emu/g) |
|---|---|---|---|
| LCO | -- | --- | ----- |
| 2.5% Te-LCO | 0.0065 | 0.20166 | 0.00128 |
| 5% Te-LCO | 0.0049 | 0.17051 | 0.00094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahadevan, J.; Sivaprakash, P.; Esakki Muthu, S.; Kim, I.; Padmanathan, N.; Eswaramoorthi, V. Influence of Te-Incorporated LaCoO3 on Structural, Morphology and Magnetic Properties for Multifunctional Device Applications. Int. J. Mol. Sci. 2023, 24, 10107. https://doi.org/10.3390/ijms241210107
Sahadevan J, Sivaprakash P, Esakki Muthu S, Kim I, Padmanathan N, Eswaramoorthi V. Influence of Te-Incorporated LaCoO3 on Structural, Morphology and Magnetic Properties for Multifunctional Device Applications. International Journal of Molecular Sciences. 2023; 24(12):10107. https://doi.org/10.3390/ijms241210107
Chicago/Turabian StyleSahadevan, Jhelai, P. Sivaprakash, S. Esakki Muthu, Ikhyun Kim, N. Padmanathan, and V. Eswaramoorthi. 2023. "Influence of Te-Incorporated LaCoO3 on Structural, Morphology and Magnetic Properties for Multifunctional Device Applications" International Journal of Molecular Sciences 24, no. 12: 10107. https://doi.org/10.3390/ijms241210107
APA StyleSahadevan, J., Sivaprakash, P., Esakki Muthu, S., Kim, I., Padmanathan, N., & Eswaramoorthi, V. (2023). Influence of Te-Incorporated LaCoO3 on Structural, Morphology and Magnetic Properties for Multifunctional Device Applications. International Journal of Molecular Sciences, 24(12), 10107. https://doi.org/10.3390/ijms241210107