An All-Green Photo-Electrochemical Biosensor Using Microalgae Immobilized on Eco-Designed Lignin-Based Screen-Printed Electrodes to Detect Sustainable Nanoherbicides
Abstract
1. Introduction
2. Results and Discussion
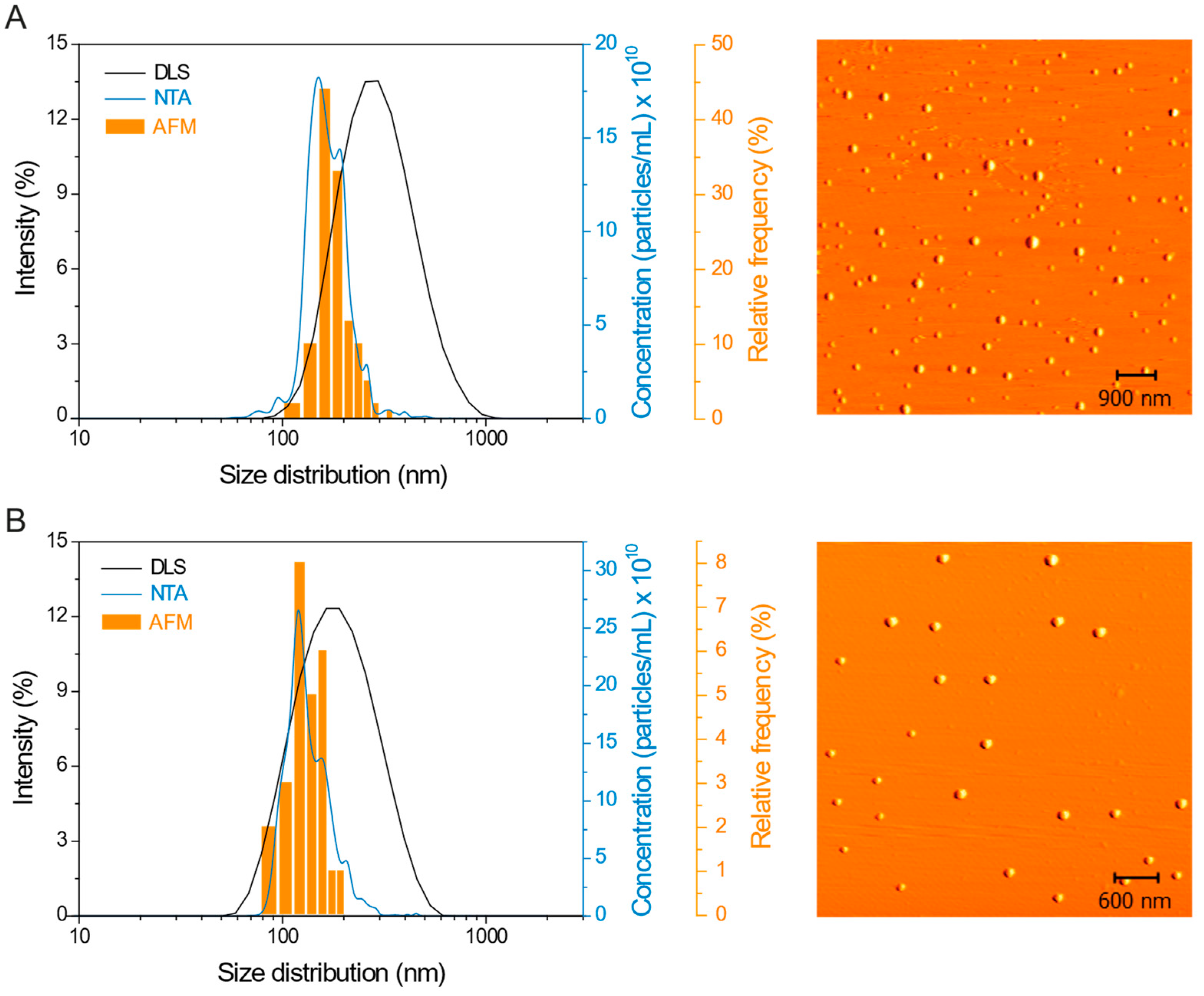
2.1. Nanoparticles Characterization
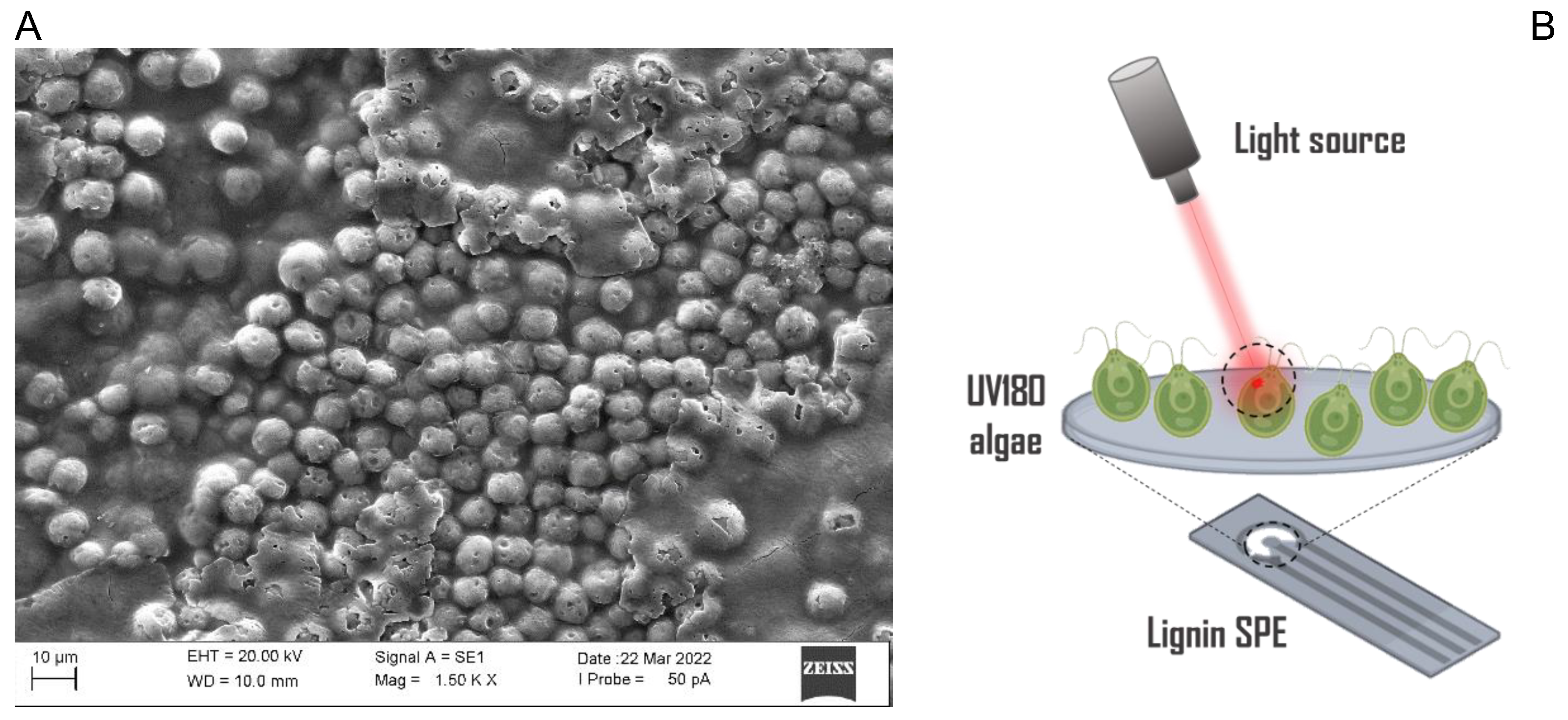
2.2. Physiological Characterization of UV180
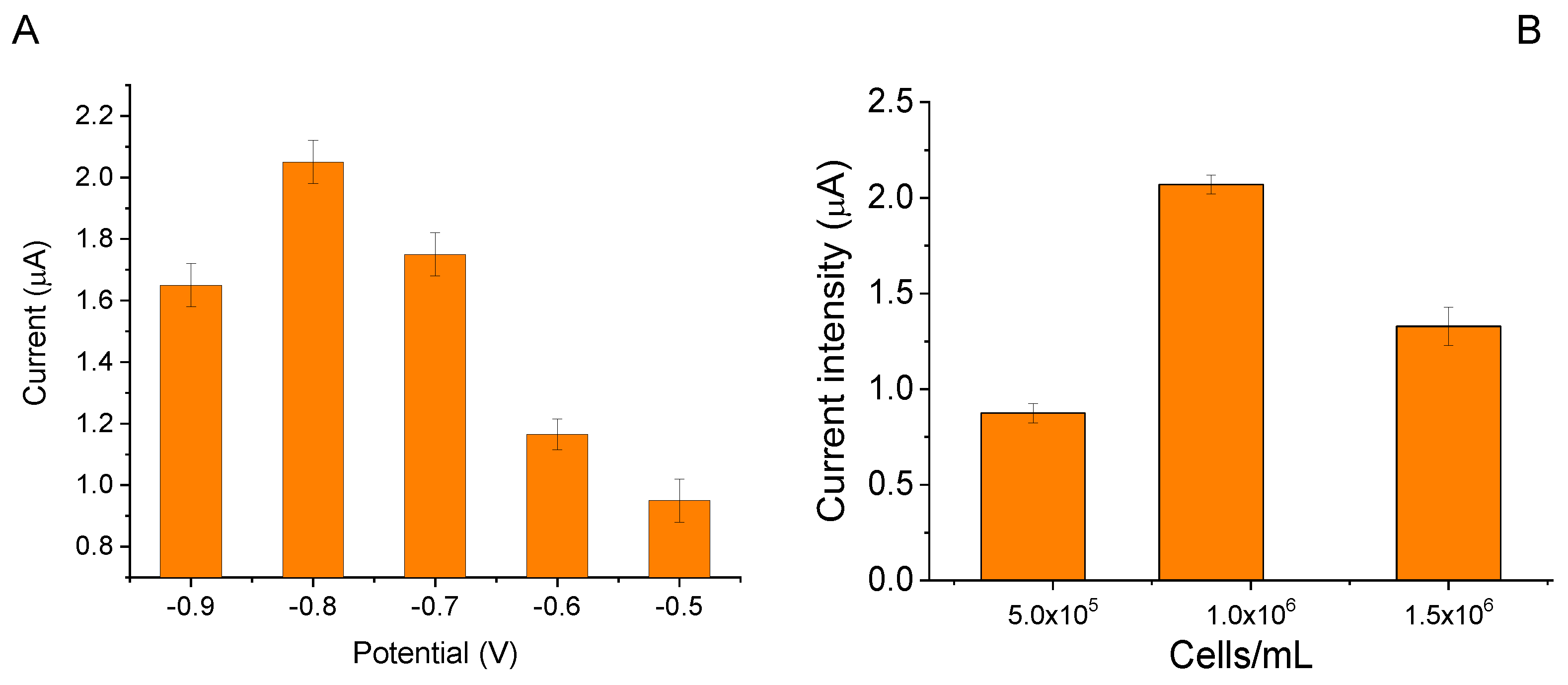
2.3. Electrochemical Characterization of UV180-LSPE Biosensor
2.4. Analytical Response of UV180-LSPE Biosensor
3. Materials and Methods
3.1. Chemicals
3.2. Nano-Formulations Preparation
3.3. Algae Growth Conditions and Characterization
3.4. UV180 and IL Immobilization on LSPEs
3.5. Photo-Electrochemical Array Set-Up and Analytical Parameters Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 1 May 2023).
- Sustainable Development Goal 6. Available online: https://sdgs.un.org/goals/goal6 (accessed on 1 May 2023).
- Bartolucci, C.; Scognamiglio, V.; Antonacci, A.; Fraceto, L.F. What makes nanotechnologies applied to agriculture green? Nano Today 2022, 43, 101389. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Nanopesticides: Preparation, Targeting, and Controlled Release. In New Pesticides and Soil Sensors; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128042991. [Google Scholar]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Rea, G.; Polticelli, F.; Antonacci, A.; Scognamiglio, V.; Katiyar, P.; Kulkarni, S.A.; Johanningmeier, U.; Giardi, M.T. Structure-based design of novel Chlamydomonas reinhardtii D1-D2 photosynthetic proteins for herbicide monitoring. Protein Sci. 2009, 18, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Hennig, T.B.; Bandeira, F.O.; Puerari, R.C.; Fraceto, L.F.; Matias, W.G. A systematic review of the toxic effects of a nanopesticide on non-target organisms: Estimation of protective concentrations using a species sensitivity distribution (SSD) approach—The case of atrazine. Sci. Total Environ. 2023, 871, 162094. [Google Scholar] [CrossRef] [PubMed]
- Scott-Fordsmand, J.J.; Fraceto, L.F.; Amorim, M.J.B. Nano-pesticides: The lunch-box principle—Deadly goodies (semio-chemical functionalised nanoparticles that deliver pesticide only to target species). J. Nanobiotechnol. 2022, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; do Pereira, E.S.A.; Aleksieienko, I.; do Carmo, G.C.; Gohari, G.; Santaella, C.; Fraceto, L.F.; Oliveira, H.C. Encapsulated plant growth regulators and associative microorganisms: Nature-based solutions to mitigate the effects of climate change on plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.; Stolf-Moreira, R.; Martinez, C.B.R.; Grillo, R.; De Jesus, M.B.; Fraceto, L.F. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS ONE 2015, 10, e0132971. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; dos Santos, N.Z.P.; Maruyama, C.R.; Rosa, A.H.; de Lima, R.; Fraceto, L.F. Poly(e{open}-caprolactone)nanocapsules as carrier systems for herbicides: Physico-chemical characterization and genotoxicity evaluation. J. Hazard. Mater. 2012, 231–232, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; McClements, D.J. Fabrication of surfactant-stabilized zein nanoparticles: A pH modulated antisolvent precipitation method. Food Res. Int. 2014, 64, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.A.; Camara, M.C.; de Oliveira, J.L.; Campos, E.V.R.; Carvalho, L.B.; Proença, P.L.; Guilger-Casagrande, M.; Lima, R.; do Nascimento, J.; Gonçalves, K.C.; et al. Zein based-nanoparticles loaded botanical pesticides in pest control: An enzyme stimuli-responsive approach aiming sustainable agriculture. J. Hazard. Mater. 2021, 417, 126004. [Google Scholar] [CrossRef] [PubMed]
- Pascoli, M.; de Albuquerque, F.P.; Calzavara, A.K.; Tinoco-Nunes, B.; Oliveira, W.H.C.; Gonçalves, K.C.; Polanczyk, R.A.; Vechia, J.F.D.; de Matos, S.T.S.; de Andrade, D.J.; et al. The potential of nanobiopesticide based on zein nanoparticles and neem oil for enhanced control of agricultural pests. J. Pest Sci. 2020, 93, 793–806. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Antonacci, A.; Arduini, F.; Moscone, D.; Campos, E.V.; Fraceto, L.F.; Palleschi, G. An eco-designed paper-based algal biosensor for nanoformulated herbicide optical detection. J. Hazard. Mater. 2019, 373, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Zappi, D.; Varani, G.; Cozzoni, E.; Iatsunskyi, I.; Laschi, S.; Giardi, M.T. Innovative eco-friendly conductive ink based on carbonized lignin for the production of flexible and stretchable bio-sensors. Nanomaterials 2021, 11, 3428. [Google Scholar] [CrossRef] [PubMed]
- Attaallah, R.; Antonacci, A.; Mazzaracchio, V.; Moscone, D.; Palleschi, G.; Arduini, F.; Amine, A.; Scognamiglio, V. Carbon black nanoparticles to sense algae oxygen evolution for herbicides detection: Atrazine as a case study. Biosens. Bioelectron. 2020, 159, 112203. [Google Scholar] [CrossRef] [PubMed]
- Johanningmeier, U.; Heiss, S. Construction of a Chlamydomonas reinhardtii mutant with an intronless psbA gene. Plant Mol. Biol. 1993, 22, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hoober, J.K. The Chlamydomonas Sourcebook. A Comprehensive Guide to Biology and Laboratory Use; Academic Press: Cambridge, MA, USA, 1989; Volume 246. [Google Scholar]
- Antonacci, A.; Attaallah, R.; Arduini, F.; Amine, A.; Giardi, M.T.; Scognamiglio, V. A dual electro-optical biosensor based on Chlamydomonas reinhardtii immobilised on paper-based nanomodified screen-printed electrodes for herbicide monitoring. J. Nanobiotechnol. 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonacci, A.; Frisulli, V.; Carvalho, L.B.; Fraceto, L.F.; Miranda, B.; De Stefano, L.; Johanningmeier, U.; Giardi, M.T.; Scognamiglio, V. An All-Green Photo-Electrochemical Biosensor Using Microalgae Immobilized on Eco-Designed Lignin-Based Screen-Printed Electrodes to Detect Sustainable Nanoherbicides. Int. J. Mol. Sci. 2023, 24, 10088. https://doi.org/10.3390/ijms241210088
Antonacci A, Frisulli V, Carvalho LB, Fraceto LF, Miranda B, De Stefano L, Johanningmeier U, Giardi MT, Scognamiglio V. An All-Green Photo-Electrochemical Biosensor Using Microalgae Immobilized on Eco-Designed Lignin-Based Screen-Printed Electrodes to Detect Sustainable Nanoherbicides. International Journal of Molecular Sciences. 2023; 24(12):10088. https://doi.org/10.3390/ijms241210088
Chicago/Turabian StyleAntonacci, Amina, Valeria Frisulli, Lucas Bragança Carvalho, Leonardo Fernandes Fraceto, Bruno Miranda, Luca De Stefano, Udo Johanningmeier, Maria Teresa Giardi, and Viviana Scognamiglio. 2023. "An All-Green Photo-Electrochemical Biosensor Using Microalgae Immobilized on Eco-Designed Lignin-Based Screen-Printed Electrodes to Detect Sustainable Nanoherbicides" International Journal of Molecular Sciences 24, no. 12: 10088. https://doi.org/10.3390/ijms241210088
APA StyleAntonacci, A., Frisulli, V., Carvalho, L. B., Fraceto, L. F., Miranda, B., De Stefano, L., Johanningmeier, U., Giardi, M. T., & Scognamiglio, V. (2023). An All-Green Photo-Electrochemical Biosensor Using Microalgae Immobilized on Eco-Designed Lignin-Based Screen-Printed Electrodes to Detect Sustainable Nanoherbicides. International Journal of Molecular Sciences, 24(12), 10088. https://doi.org/10.3390/ijms241210088











