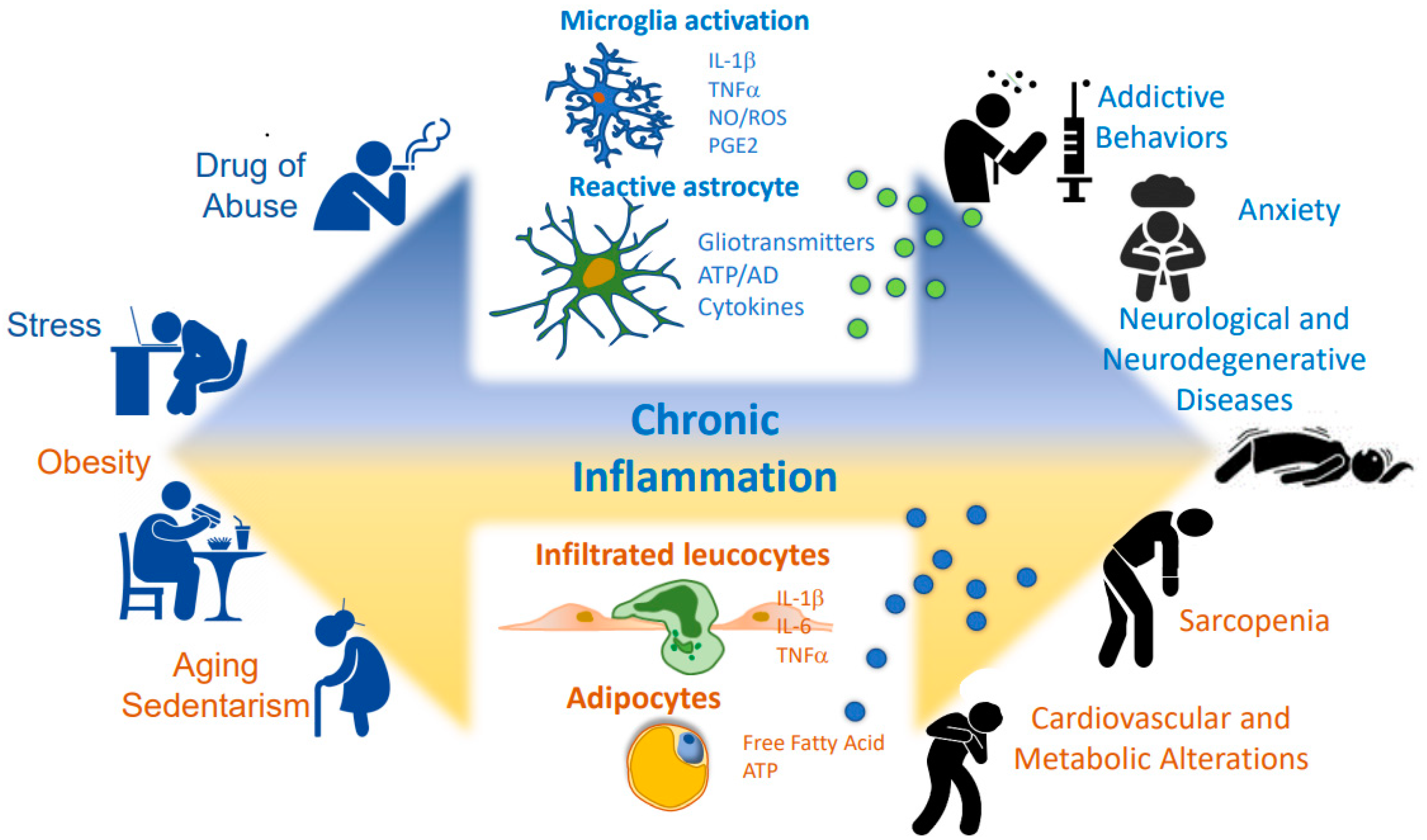
Central and Peripheral Inflammation: A Common Factor Causing Addictive and Neurological Disorders and Aging-Related Pathologies
Abstract
1. Introduction: Cellular and Molecular Mechanisms of Brain and Peripheral Inflammation
2. Factors Triggering Inflammation: Lifestyle
2.1. Diet and Body Weight
2.2. Stress: Effects of Glucocorticoids on Inflammation in the Context of Eustress and Distress
3. Consequences of Inflammation Inducing Central and Peripheral Diseases
3.1. Neuroinflammation as a Key Mediator in Addictive Behavior
3.1.1. Hypercaloric Diet Induces Neuroinflammation in Brain Areas Associated with Addictive Behaviors
3.1.2. Drugs of Abuse Induce Neuroinflammation in Brain Nuclei Associated with Addictive Behavior
3.2. Inflammaging, Gut Dysbiosis, and Highly Prevalent Diseases among Older Adults
3.3. Neuroinflammation as a Common Feature of Neurological and Neurodegenerative Diseases
3.3.1. Neuroinflammation as a Pathological Mechanism for Cognitive Impairment in Alzheimer’s Disease
3.3.2. Neuroinflammation as a Driver of Epilepsy
3.3.3. Neuroinflammation Is a Common Mechanism for Different Factors in Exacerbated Anxiety
3.4. Peripheric Inflammation Contributing to the Generation of Cardiovascular Alterations
4. Clinical Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Calderón, J.R.; Castillo, E.C.; Cuellar-Tamez, R.X.; García-Garza, M.; Elizondo-Montemayor, L.; García-Rivas, G. Reduced Th1 response is associated with lower glycolytic activity in asctivated peripheral blood mononuclear cells after metabolic and bariatric surgery. J. Endocrinol. Investig. 2021, 44, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Farrar, J.D. Adrenergic regulation of immune cell function and inflammation. Semin. Immunopathol. 2020, 42, 709–717. [Google Scholar] [CrossRef]
- Tarnawski, L.; Olofsson, P.S. Inflammation neuroscience: Neuro-immune crosstalk and interfaces. Clin. Transl. Immunol. 2021, 10, e1352. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, G.K.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Lifestyle Modifications and Nutritional Interventions in Aging-Associated Cognitive Decline and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 369. [Google Scholar] [CrossRef]
- Firth, J.; Veronese, N.; Cotter, J.; Shivappa, N.; Hebert, J.R.; Ee, C.; Smith, L.; Stubbs, B.; Jackson, S.E.; Sarris, J. What Is the Role of Dietary Inflammation in Severe Mental Illness? A Review of Observational and Experimental Findings. Front. Psychiatry 2019, 10, 350. [Google Scholar] [CrossRef]
- Matejuk, A.; Vandenbark, A.A.; Offner, H. Cross-talk of the CNS with immune cells and functions in health and disease. Front. Neurol. 2021, 12, 672455. [Google Scholar] [CrossRef]
- Mohamed, W.; Kumar, J.; Alghamdi, B.S.; Soliman, A.H.; Toshihide, Y. Neurodegeneration and inflammation crosstalk: Therapeutic targets and perspectives. IBRO Neurosci. Rep. 2023, 14, 95–110. [Google Scholar] [CrossRef]
- Durkee, C.A.; Araque, A. Diversity and Specificity of Astrocyte-neuron Communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Eugenín-von Bernhardi, J.; Flores, B.; Eugenín León, J. Glial Cells and Integrity of the Nervous System. Adv. Exp. Med. Biol. 2016, 949, 1–24. [Google Scholar]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-astrocyte crosstalk: An intimate molecular conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.; Kipp, M. What Guides Peripheral Immune Cells into the Central Nervous System? Cells 2021, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef] [PubMed]
- Mapunda, J.A.; Tibar, H.; Regragui, W.; Engelhardt, B. How Does the Immune System Enter the Brain? Front. Immunol. 2022, 13, 805657. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Gate, D.; Town, T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J. Neuroimmune Pharmacol. 2009, 4, 462–475. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Riazi, K.; Galic, M.A.; Kentner, A.C.; Reid, A.Y.; Sharkey, K.A.; Pittman, Q.J. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J. Neurosci. 2015, 35, 4942–4952. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.L. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology 2015, 96, 11–18. [Google Scholar] [CrossRef]
- McAllister, A.K. Major histocompatibility complex I in brain development and schizophrenia. Biol. Psychiatry 2014, 75, 262–268. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Gross, C.T. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Aizenman, C.D.; Pratt, K.G. There’s more than one way to scale a synapse. Neuron 2008, 58, 651–653. [Google Scholar] [CrossRef]
- Di Filippo, M.; Sarchielli, P.; Picconi, B.; Calabresi, P. Neuroinflammation and synaptic plasticity: Theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol. Sci. 2008, 29, 402–412. [Google Scholar] [CrossRef]
- Bourgognon, J.M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020, 4, 2398212820979802. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef]
- O’Callaghan, J.P.; Kelly, K.A.; VanGilder, R.L.; Sofroniew, M.V.; Miller, D.B. Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PLoS ONE 2014, 9, e102003. [Google Scholar] [CrossRef] [PubMed]
- Guarner, V.; Rubio-Ruiz, M.E. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip. Top. Gerontol. 2015, 40, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef]
- Engin, A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. Adv. Exp. Med. Biol. 2017, 960, 221–245. [Google Scholar] [CrossRef]
- Nativel, B.; Marimoutou, M.; Thon-Hon, V.G.; Gunasekaran, M.K.; Andries, J.; Stanislas, G.; Planesse, C.; Da Silva, C.R.; Césari, M.; Iwema, T.; et al. Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: Contribution to chronic inflammation in fat tissue. PLoS ONE 2013, 8, e76039. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Bijnen, M.; Josefs, T.; Cuijpers, I.; Maalsen, C.J.; van de Gaar, J.; Vroomen, M.; Wijnands, E.; Rensen, S.S.; Greve, J.W.M.; Hofker, M.H.; et al. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut 2018, 67, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Luci, C.; Bourinet, M.; Leclère, P.S.; Anty, R.; Gual, P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies. Front. Endocrinol. 2020, 11, 597648. [Google Scholar] [CrossRef]
- Ghareeb, D.A.; Hafez, H.S.; Hussien, H.M.; Kabapy, N.F. Non-alcoholic fatty liver induces insulin resistance and metabolic disorders with development of brain damage and dysfunction. Metab. Brain Dis. 2011, 26, 253–267. [Google Scholar] [CrossRef]
- Kim, D.G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflammation 2016, 13, 1. [Google Scholar] [CrossRef]
- George, E.S.; Sood, S.; Daly, R.M.; Tan, S.Y. Is there an association between non-alcoholic fatty liver disease and cognitive function? A systematic review. BMC Geriatr. 2022, 22, 47. [Google Scholar] [CrossRef]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.; Velloso, L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef]
- Grayson, B.E.; Levasseur, P.R.; Williams, S.M.; Smith, M.S.; Marks, D.L.; Grove, K.L. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010, 151, 1622–1632. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell. Metab. 2017, 26, 185–197.e183. [Google Scholar] [CrossRef]
- Douglass, J.D.; Dorfman, M.D.; Fasnacht, R.; Shaffer, L.D.; Thaler, J.P. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 2017, 6, 366–373. [Google Scholar] [CrossRef]
- Schur, E.A.; Melhorn, S.J.; Oh, S.K.; Lacy, J.M.; Berkseth, K.E.; Guyenet, S.J.; Sonnen, J.A.; Tyagi, V.; Rosalynn, M.; De Leon, B.; et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity 2015, 23, 2142–2148. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.A.; Clegg, D.J.; Printz, R.L.; Byun, J.; Morton, G.J.; Vivekanandan-Giri, A.; Pennathur, S.; Baskin, D.G.; Heinecke, J.W.; Woods, S.C.; et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1003–E1012. [Google Scholar] [CrossRef]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Y.; Cai, D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell. Biol. 2012, 14, 999–1012. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Waise, T.M.Z.; Toshinai, K.; Naznin, F.; NamKoong, C.; Md Moin, A.S.; Sakoda, H.; Nakazato, M. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem. Biophys. Res. Commun. 2015, 464, 1157–1162. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Robb, J.L.; Morrissey, N.A.; Weightman Potter, P.G.; Smithers, H.E.; Beall, C.; Ellacott, K.L.J. Immunometabolic Changes in Glia–A Potential Role in the Pathophysiology of Obesity and Diabetes. Neuroscience 2020, 447, 167–181. [Google Scholar] [CrossRef]
- Mendes, N.F.; Kim, Y.B.; Velloso, L.A.; Araújo, E.P. Hypothalamic Microglial Activation in Obesity: A Mini-Review. Front. Neurosci. 2018, 12, 846. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Buckman, L.B.; Thompson, M.M.; Lippert, R.N.; Blackwell, T.S.; Yull, F.E.; Ellacott, K.L. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol. Metab. 2015, 4, 58–63. [Google Scholar] [CrossRef]
- Dinel, A.L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef]
- André, C.; Dinel, A.L.; Ferreira, G.; Layé, S.; Castanon, N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: Focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav. Immun. 2014, 41, 10–21. [Google Scholar] [CrossRef]
- Hao, S.; Dey, A.; Yu, X.; Stranahan, A.M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 2016, 51, 230–239. [Google Scholar] [CrossRef] [PubMed]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Sinacore, D.R.; Gulve, E.A. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: Implications for physical therapy. Phys. Ther. 1993, 73, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Fink, L.N.; Costford, S.R.; Lee, Y.S.; Jensen, T.E.; Bilan, P.J.; Oberbach, A.; Blüher, M.; Olefsky, J.M.; Sams, A.; Klip, A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 2014, 22, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Perrard, X.Y.; Brunner, G.; Lui, H.; Sparks, L.M.; Smith, S.R.; Wang, X.; Shi, Z.Z.; Lewis, D.E.; Wu, H.; et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int. J. Obes. 2015, 39, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Pedersen, M.; Pedersen, B.K.; Scheele, C. Elevated NF-κB activation is conserved in human myocytes cultured from obese type 2 diabetic patients and attenuated by AMP-activated protein kinase. Diabetes 2011, 60, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, G.; Meneses-Valdés, R.; Rosales-Soto, G.; Valladares-Ide, D.; Campos, C.; Silva-Monasterio, M.; Llanos, P.; Cruz, G.; Jaimovich, E.; Casas, M. High extracellular ATP levels released through pannexin-1 channels mediate inflammation and insulin resistance in skeletal muscle fibres of diet-induced obese mice. Diabetologia 2021, 64, 1389–1401. [Google Scholar] [CrossRef]
- Erion, J.R.; Wosiski-Kuhn, M.; Dey, A.; Hao, S.; Davis, C.L.; Pollock, N.K.; Stranahan, A.M. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J. Neurosci. 2014, 34, 2618–2631. [Google Scholar] [CrossRef]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J. Clin. Investig. 2020, 130, 1961–1976. [Google Scholar] [CrossRef]
- Lee, T.H.; Yau, S.Y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2020, 22, 201. [Google Scholar] [CrossRef]
- Lu, S.; Wei, F.; Li, G. The evolution of the concept of stress and the framework of the stress system. Cell. Stress. 2021, 5, 76–85. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Bernardini, R. Psychoneuroendocrinological links between chronic stress and depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- McEwen, B.S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Smolovich, J. Psychoneuroendocrinological studies on chronic stress and depression. Ann. N. Y. Acad. Sci. 2004, 1032, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Mucci, F.; Marazziti, D.; Della Vecchia, A.; Baroni, S.; Morana, P.; Carpita, B.; Mangiapane, P.; Morana, F.; Morana, B.; Dell’Osso, L. State-of-the-Art: Inflammatory and Metabolic Markers in Mood Disorders. Life 2020, 10, 82. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef]
- Loftis, J.M.; Huckans, M.; Morasco, B.J. Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiol. Dis. 2010, 37, 519–533. [Google Scholar] [CrossRef]
- Sasayama, D.; Hattori, K.; Wakabayashi, C.; Teraishi, T.; Hori, H.; Ota, M.; Yoshida, S.; Arima, K.; Higuchi, T.; Amano, N.; et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 2013, 47, 401–406. [Google Scholar] [CrossRef]
- Devorak, J.; Torres-Platas, S.G.; Davoli, M.A.; Prud’homme, J.; Turecki, G.; Mechawar, N. Cellular and Molecular Inflammatory Profile of the Choroid Plexus in Depression and Suicide. Front. Psychiatry 2015, 6, 138. [Google Scholar] [CrossRef]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef]
- Engler, H.; Doenlen, R.; Engler, A.; Riether, C.; Prager, G.; Niemi, M.B.; Pacheco-López, G.; Krügel, U.; Schedlowski, M. Acute amygdaloid response to systemic inflammation. Brain Behav. Immun. 2011, 25, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Manasian, Y.; Kim, W.; Kim, K.S.; et al. Maternal and Early Postnatal Immune Activation Produce Dissociable Effects on Neurotransmission in mPFC-Amygdala Circuits. J. Neurosci. 2018, 38, 3358–3372. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Meijer, O.C.; de Nicola, A.F.; de Rijk, R.H.; Joels, M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 2018, 49, 124–145. [Google Scholar] [CrossRef] [PubMed]
- Reul, J.M.; de Kloet, E.R. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology 1985, 117, 2505–2511. [Google Scholar] [CrossRef]
- Reul, J.M.; van den Bosch, F.R.; de Kloet, E.R. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: Functional implications. J. Endocrinol. 1987, 115, 459–467. [Google Scholar] [CrossRef]
- Dallman, M.F.; Akana, S.F.; Levin, N.; Walker, C.D.; Bradbury, M.J.; Suemaru, S.; Scribner, K.S. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann. N. Y. Acad. Sci. 1994, 746, 22–31, discussion 31–22, 64–27. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Vreugdenhil, E.; Oitzl, M.S.; Joels, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998, 19, 269–301. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Gemma, C.; Bachstetter, A.D. The role of microglia in adult hippocampal neurogenesis. Front. Cell. Neurosci. 2013, 7, 229. [Google Scholar] [CrossRef]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef]
- Monje, F.J.; Cabatic, M.; Divisch, I.; Kim, E.J.; Herkner, K.R.; Binder, B.R.; Pollak, D.D. Constant darkness induces IL-6-dependent depression-like behavior through the NF-κB signaling pathway. J. Neurosci. 2011, 31, 9075–9083. [Google Scholar] [CrossRef]
- Tanaka, J.; Fujita, H.; Matsuda, S.; Toku, K.; Sakanaka, M.; Maeda, N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids. Glia 1997, 20, 23–37. [Google Scholar] [CrossRef]
- Chantong, B.; Kratschmar, D.V.; Nashev, L.G.; Balazs, Z.; Odermatt, A. Mineralocorticoid and glucocorticoid receptors differentially regulate NF-kappaB activity and pro-inflammatory cytokine production in murine BV-2 microglial cells. J. Neuroinflammation 2012, 9, 260. [Google Scholar] [CrossRef]
- Mifsud, K.R.; Kennedy, C.L.M.; Salatino, S.; Sharma, E.; Price, E.M.; Haque, S.N.; Gialeli, A.; Goss, H.M.; Panchenko, P.E.; Broxholme, J.; et al. Distinct regulation of hippocampal neuroplasticity and ciliary genes by corticosteroid receptors. Nat. Commun. 2021, 12, 4737. [Google Scholar] [CrossRef]
- Polman, J.A.; de Kloet, E.R.; Datson, N.A. Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome. Endocrinology 2013, 154, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Almawi, W.Y.; Beyhum, H.N.; Rahme, A.A.; Rieder, M.J. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J. Leukoc. Biol. 1996, 60, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.A.; Kanagawa, O.; Sleckman, B.P.; Muglia, L.J. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J. Immunol. 2002, 169, 1837–1843. [Google Scholar] [CrossRef]
- Fushimi, T.; Shimura, S.; Suzuki, S.; Saitoh, H.; Okayama, H.; Shirato, K. Suppression of gene expression and production of interleukin 13 by dexamethasone in human peripheral blood mononuclear cells. Tohoku J. Exp. Med. 1998, 185, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Kunicka, J.E.; Talle, M.A.; Denhardt, G.H.; Brown, M.; Prince, L.A.; Goldstein, G. Immunosuppression by glucocorticoids: Inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell. Immunol. 1993, 149, 39–49. [Google Scholar] [CrossRef]
- Banuelos, J.; Lu, N.Z. A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor. Rev. 2016, 31, 27–35. [Google Scholar] [CrossRef]
- Paolino, S.; Cutolo, M.; Pizzorni, C. Glucocorticoid management in rheumatoid arthritis: Morning or night low dose? Reumatologia 2017, 55, 189–197. [Google Scholar] [CrossRef]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef]
- Ito, N.; Sasaki, K.; Takemoto, H.; Kobayashi, Y.; Isoda, H.; Odaguchi, H. Emotional Impairments and Neuroinflammation are Induced in Male Mice Invulnerable to Repeated Social Defeat Stress. Neuroscience 2020, 443, 148–163. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflammation 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Finnell, J.E.; Wood, S.K. Putative Inflammatory Sensitive Mechanisms Underlying Risk or Resilience to Social Stress. Front. Behav. Neurosci. 2018, 12, 240. [Google Scholar] [CrossRef]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol. Stress. 2016, 4, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Murrough, J.W.; Han, M.H.; Charney, D.S.; Nestler, E.J. Neurobiology of resilience. Nat. Neurosci. 2012, 15, 1475–1484. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E., 3rd; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Hanke, M.L.; Corona, A.W.; Powell, N.D.; Stiner, L.M.; Bailey, M.T.; Nelson, R.J.; Godbout, J.P.; Sheridan, J.F. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 2011, 31, 6277–6288. [Google Scholar] [CrossRef]
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol. Psychiatry 2018, 83, 61–69. [Google Scholar] [CrossRef]
- Cathomas, F.; Murrough, J.W.; Nestler, E.J.; Han, M.H.; Russo, S.J. Neurobiology of Resilience: Interface Between Mind and Body. Biol. Psychiatry 2019, 86, 410–420. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef]
- Bravo-Tobar, I.D.; Fernandez, P.; Saez, J.C.; Dagnino-Subiabre, A. Long-term effects of stress resilience: Hippocampal neuroinflammation and behavioral approach in male rats. J. Neurosci. Res. 2021, 99, 2493–2510. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Lv, J.; Wang, H.; Huang, H.; Wang, Q.; Lu, C.; Zeng, G.; Liu, X.M. Ginsenoside Rg1 ameliorates chronic social defeat stress-induced depressive-like behaviors and hippocampal neuroinflammation. Life Sci. 2020, 252, 117669. [Google Scholar] [CrossRef]
- Velloso, L.A.; Araujo, E.P.; de Souza, C.T. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation 2008, 15, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Corbin, W.R. Body mass index and alcohol consumption: Family history of alcoholism as a moderator. Psychol. Addict. Behav. 2009, 23, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Rapuano, K.M.; Laurent, J.S.; Hagler, D.J., Jr.; Hatton, S.N.; Thompson, W.K.; Jernigan, T.L.; Dale, A.M.; Casey, B.J.; Watts, R. Nucleus accumbens cytoarchitecture predicts weight gain in children. Proc. Natl. Acad. Sci. USA 2020, 117, 26977–26984. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Martos, M.; Girard, B.; Mendonca-Netto, S.; Perroy, J.; Valjent, E.; Maldonado, R.; Martin, M. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addict. Biol. 2018, 23, 735–749. [Google Scholar] [CrossRef]
- Decarie-Spain, L.; Sharma, S.; Hryhorczuk, C.; Issa-Garcia, V.; Barker, P.A.; Arbour, N.; Alquier, T.; Fulton, S. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol. Metab. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Lenoir, M.; Serre, F.; Cantin, L.; Ahmed, S.H. Intense sweetness surpasses cocaine reward. PLoS ONE 2007, 2, e698. [Google Scholar] [CrossRef]
- Bayol, S.A.; Farrington, S.J.; Stickland, N.C. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br. J. Nutr. 2007, 98, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Bocarsly, M.E.; Barson, J.R.; Hauca, J.M.; Hoebel, B.G.; Leibowitz, S.F.; Avena, N.M. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiol. Behav. 2012, 107, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Raibstein, D.; Sarker, G.; Litwan, K.; Kramer, S.D.; Ametamey, S.M.; Schibli, R.; Wolfrum, C. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Transl. Psychiatry 2016, 6, e911. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Ruiz, R.; Cardenas-Tueme, M.; Montalvo-Martinez, L.; Vidaltamayo, R.; Garza-Ocanas, L.; Resendez-Perez, D.; Camacho, A. Priming of Hypothalamic Ghrelin Signaling and Microglia Activation Exacerbate Feeding in Rats’ Offspring Following Maternal Overnutrition. Nutrients 2019, 11, 1241. [Google Scholar] [CrossRef]
- Molina, J.; Joaquim, A.; Bonamin, L.V.; Martins, M.F.M.; Kirsten, T.B.; Cardoso, C.V.; Bernardi, M.M.; Bondan, E.F. Reduced astrocytic expression of GFAP in the offspring of female rats that received hypercaloric diet. Nutr. Neurosci. 2020, 23, 411–421. [Google Scholar] [CrossRef]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Lijffijt, M.; Hu, K.; Swann, A.C. Stress modulates illness-course of substance use disorders: A translational review. Front. Psychiatry 2014, 5, 83. [Google Scholar] [CrossRef]
- Montesinos, J.; Gil, A.; Guerri, C. Nalmefene Prevents Alcohol-Induced Neuroinflammation and Alcohol Drinking Preference in Adolescent Female Mice: Role of TLR4. Alcohol. Clin. Exp. Res. 2017, 41, 1257–1270. [Google Scholar] [CrossRef]
- Alasmari, F.; Alhaddad, H.; Wong, W.; Bell, R.L.; Sari, Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules 2020, 10, 1030. [Google Scholar] [CrossRef]
- Aghaie, C.I.; Hausknecht, K.A.; Wang, R.; Dezfuli, P.H.; Haj-Dahmane, S.; Kane, C.J.M.; Sigurdson, W.J.; Shen, R.Y. Prenatal Ethanol Exposure and Postnatal Environmental Intervention Alter Dopaminergic Neuron and Microglia Morphology in the Ventral Tegmental Area During Adulthood. Alcohol. Clin. Exp. Res. 2020, 44, 435–444. [Google Scholar] [CrossRef]
- Juarez-Rodriguez, P.; Godinez-Rubi, M.; Guzman-Brambila, C.; Padilla-Velarde, E.; Orozco-Barocio, A.; Ortuno-Sahagun, D.; Rojas-Mayorquin, A.E. Prenatal Alcohol Exposure in Rats Diminishes Postnatal Cxcl16 Chemokine Ligand Brain Expression. Brain Sci. 2020, 10, 987. [Google Scholar] [CrossRef]
- Abel, S.; Hundhausen, C.; Mentlein, R.; Schulte, A.; Berkhout, T.A.; Broadway, N.; Hartmann, D.; Sedlacek, R.; Dietrich, S.; Muetze, B.; et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 2004, 172, 6362–6372. [Google Scholar] [CrossRef]
- Wakida, N.; Kiguchi, N.; Saika, F.; Nishiue, H.; Kobayashi, Y.; Kishioka, S. CC-chemokine ligand 2 facilitates conditioned place preference to methamphetamine through the activation of dopamine systems. J. Pharmacol. Sci. 2014, 125, 68–73. [Google Scholar] [CrossRef]
- Glabinski, A.R.; Balasingam, V.; Tani, M.; Kunkel, S.L.; Strieter, R.M.; Yong, V.W.; Ransohoff, R.M. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J. Immunol. 1996, 156, 4363–4368. [Google Scholar] [CrossRef]
- Wang, X.; Northcutt, A.L.; Cochran, T.A.; Zhang, X.; Fabisiak, T.J.; Haas, M.E.; Amat, J.; Li, H.; Rice, K.C.; Maier, S.F.; et al. Methamphetamine Activates Toll-Like Receptor 4 to Induce Central Immune Signaling within the Ventral Tegmental Area and Contributes to Extracellular Dopamine Increase in the Nucleus Accumbens Shell. ACS Chem. Neurosci. 2019, 10, 3622–3634. [Google Scholar] [CrossRef]
- Wang, B.; Chen, T.; Xue, L.; Wang, J.; Jia, Y.; Li, G.; Ren, H.; Wu, F.; Wu, M.; Chen, Y. Methamphetamine exacerbates neuroinflammatory response to lipopolysaccharide by activating dopamine D1-like receptors. Int. Immunopharmacol. 2019, 73, 1–9. [Google Scholar] [CrossRef]
- Frank, M.G.; Adhikary, S.; Sobesky, J.L.; Weber, M.D.; Watkins, L.R.; Maier, S.F. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav. Immun. 2016, 51, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Porceddu, P.F.; Serra, M.; Casu, M.A.; Schiano, V.; Napolitano, F.; Pinna, A.; Usiello, A.; Morelli, M. Lack of Rhes Increases MDMA-Induced Neuroinflammation and Dopamine Neuron Degeneration: Role of Gender and Age. Int. J. Mol. Sci. 2019, 20, 1556. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.T.; Levis, S.C.; O’Neill, C.E.; Northcutt, A.L.; Fabisiak, T.J.; Watkins, L.R.; Bachtell, R.K. Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav. Immun. 2018, 67, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Goins, E.C.; Bajic, D. Astrocytic hypertrophy in the rat ventral tegmental area following chronic morphine differs with age. J. Neurol. Neurorehabilit. Res. 2018, 3, 14–21. [Google Scholar] [CrossRef]
- Torkzadeh-Mahani, S.; Esmaeili-Mahani, S.; Nasri, S.; Darvishzadeh, F.; Naderi, R. Ginger Extract Reduces Chronic Morphine-Induced Neuroinflammation and Glial Activation in Nucleus Accumbens of Rats. Addict. Health 2019, 11, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rainville, J.R.; Williams, K.; Lile, J.A.; Hodes, G.E.; Vassoler, F.M.; Turner, J.R. Sexually dimorphic neuroimmune response to chronic opioid treatment and withdrawal. Neuropharmacology 2021, 186, 108469. [Google Scholar] [CrossRef]
- Steinegger, C.A.; Zoelch, N.; Hock, A.; Henning, A.; Engeli, E.J.E.; Seifritz, E.; Hulka, L.M.; Herdener, M. Neurometabolic alterations in the nucleus accumbens of smokers assessed with (1) H magnetic resonance spectroscopy: The role of glutamate and neuroinflammation. Addict. Biol. 2021, 26, e13027. [Google Scholar] [CrossRef]
- Hammad, A.M.; Swiss, G.M.S.; Hall, F.S.; Hikmat, S.; Sari, Y.; Al-Qirim, T.M.; Amawi, H.A. Ceftriaxone Reduces Waterpipe Tobacco Smoke Withdrawal-induced Anxiety in rats via Modulating the Expression of TNF-alpha/NFkB, Nrf2, and GLT-1. Neuroscience 2021, 463, 128–142. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediators Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Ko, F.; Abadir, P.; Marx, R.; Westbrook, R.; Cooke, C.; Yang, H.; Walston, J. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp. Gerontol. 2016, 73, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Kny, M.; Riediger, F.; Busch, K.; Schmidt, S.; Luft, F.C.; Slevogt, H.; Fielitz, J. Deletion of Nlrp3 protects from inflammation-induced skeletal muscle atrophy. Intensive Care Med. Exp. 2017, 5, 3. [Google Scholar] [CrossRef]
- McBride, M.J.; Foley, K.P.; D’Souza, D.M.; Li, Y.E.; Lau, T.C.; Hawke, T.J.; Schertzer, J.D. The NLRP3 inflammasome contributes to sarcopenia and lower muscle glycolytic potential in old mice. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E222–E232. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.A.; Fernández-Ortiz, M.; Diaz-Casado, M.E.; Aranda-Martínez, P.; Fernández-Martínez, J.; Guerra-Librero, A.; Escames, G.; López, L.C.; Alsaadawy, R.M.; Acuña-Castroviejo, D. Lack of NLRP3 Inflammasome Activation Reduces Age-Dependent Sarcopenia and Mitochondrial Dysfunction, Favoring the Prophylactic Effect of Melatonin. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31. [Google Scholar] [CrossRef]
- Bian, F.; Xiao, Y.; Zaheer, M.; Volpe, E.A.; Pflugfelder, S.C.; Li, D.Q.; de Paiva, C.S. Inhibition of NLRP3 Inflammasome Pathway by Butyrate Improves Corneal Wound Healing in Corneal Alkali Burn. Int. J. Mol. Sci. 2017, 18, 562. [Google Scholar] [CrossRef]
- Wang, X.; He, G.; Peng, Y.; Zhong, W.; Wang, Y.; Zhang, B. Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci. Rep. 2015, 5, 12676. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, N.; Nam, R.H.; Park, J.H.; Choi, S.I.; Park, Y.T.; Kim, Y.R.; Seok, Y.J.; Shin, C.M.; Lee, D.H. Gut microbiota and butyrate level changes associated with the long-term administration of proton pump inhibitors to old rats. Sci. Rep. 2019, 9, 6626. [Google Scholar] [CrossRef]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Luc, M.; Misiak, B.; Pawlowski, M.; Stanczykiewicz, B.; Zablocka, A.; Szczesniak, D.; Palega, A.; Rymaszewska, J. Gut microbiota in dementia. Critical review of novel findings and their potential application. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 104, 110039. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kaneko, Y.; Sato, T.; Shimizu, S.; Kanetaka, H.; Hanyu, H. Sarcopenia and Muscle Functions at Various Stages of Alzheimer Disease. Front. Neurol. 2018, 9, 710. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Engertsberger, L.; Komarova, I.; Feldbacher, N.; Leber, B.; Pichler, G.; Fink, N.; Scarpatetti, M.; Schippinger, W.; Schmidt, R.; et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Murotani, K.; Hisada, T.; Kunihiro, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. Relationship between dementia and gut microbiome-associated metabolites: A cross-sectional study in Japan. Sci. Rep. 2020, 10, 8088. [Google Scholar] [CrossRef]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow. Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Lyra, E.S.N.M.; Goncalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Rios, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernandez, J.; Campos-Pena, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1beta, IL-6, TNF- alpha and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ajnakina, O.; Steptoe, A.; Cadar, D. Higher risk of dementia in English older individuals who are overweight or obese. Int. J. Epidemiol. 2020, 49, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Fu, W.; Wang, C.; Mao, J.; Liu, B.; Zou, L.; Lv, C. Association between sedentary behavior and the risk of dementia: A systematic review and meta-analysis. Transl. Psychiatry 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Falconer, C.L.; Cooper, A.R.; Walhin, J.P.; Thompson, D.; Page, A.S.; Peters, T.J.; Montgomery, A.A.; Sharp, D.J.; Dayan, C.M.; Andrews, R.C. Sedentary time and markers of inflammation in people with newly diagnosed type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Rodas, L.; Riera-Sampol, A.; Aguilo, A.; Martinez, S.; Tauler, P. Effects of Habitual Caffeine Intake, Physical Activity Levels, and Sedentary Behavior on the Inflammatory Status in a Healthy Population. Nutrients 2020, 12, 2325. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Zeba, Z.; Hosen, I.; Al-Mamun, F.; Mamun, M.A. Impact of the COVID-19 pandemic on BMI: Its changes in relation to socio-demographic and physical activity patterns based on a short period. PLoS ONE 2022, 17, e0266024. [Google Scholar] [CrossRef]
- Restrepo, B.J. Obesity Prevalence Among U.S. Adults During the COVID-19 Pandemic. Am. J. Prev. Med. 2022, 63, 102–106. [Google Scholar] [CrossRef]
- Stockwell, S.; Trott, M.; Tully, M.; Shin, J.; Barnett, Y.; Butler, L.; McDermott, D.; Schuch, F.; Smith, L. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open. Sport. Exerc. Med. 2021, 7, e000960. [Google Scholar] [CrossRef]
- Liu, Y.H.; Chen, Y.; Wang, Q.H.; Wang, L.R.; Jiang, L.; Yang, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; et al. One-Year Trajectory of Cognitive Changes in Older Survivors of COVID-19 in Wuhan, China: A Longitudinal Cohort Study. JAMA Neurol. 2022, 79, 509–517. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017.
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Goodman, M.S.; Kumar, S.; Zomorrodi, R.; Ghazala, Z.; Cheam, A.S.M.; Barr, M.S.; Daskalakis, Z.J.; Blumberger, D.M.; Fischer, C.; Flint, A.; et al. Theta-Gamma Coupling and Working Memory in Alzheimer’s Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 101. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Neural oscillations and brain stimulation in Alzheimer’s disease. Prog. Neurobiol. 2020, 194, 101878. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, L.; Tsvetanov, K.A.; Jones, P.S.; Bevan-Jones, W.R.; Arnold, R.; Borchert, R.J.; Mak, E.; Su, L.; O’Brien, J.T.; Rowe, J.B. Neuroinflammation and Functional Connectivity in Alzheimer’s Disease: Interactive Influences on Cognitive Performance. J. Neurosci. 2019, 39, 7218–7226. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Bramian, A.; Soma, S.; Saito, T.; Saido, T.C.; Igarashi, K.M. Disrupted Place Cell Remapping and Impaired Grid Cells in a Knockin Model of Alzheimer’s Disease. Neuron 2020, 107, 1095–1112.e1096. [Google Scholar] [CrossRef] [PubMed]
- Lison, A.E. Treatment of glomerulonephritis. Verh. Dtsch. Ges. Inn. Med. 1989, 95, 581–586. [Google Scholar]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Nebes, R.D.; Saxton, J.A.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Ziolko, S.K.; James, J.A.; Snitz, B.E.; Houck, P.R.; et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008, 65, 1509–1517. [Google Scholar] [CrossRef]
- Chetelat, G. Alzheimer disease: Abeta-independent processes-rethinking preclinical AD. Nat. Rev. Neurol. 2013, 9, 123–124. [Google Scholar] [CrossRef]
- Asih, P.R.; Chatterjee, P.; Verdile, G.; Gupta, V.B.; Trengove, R.D.; Martins, R.N. Clearing the amyloid in Alzheimer’s: Progress towards earlier diagnosis and effective treatments–an update for clinicians. Neurodegener. Dis. Manag. 2014, 4, 363–378. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Giardina, G.A. gamma-secretase inhibitors and modulators for the treatment of Alzheimer’s disease: Disappointments and hopes. Curr. Top. Med. Chem. 2011, 11, 1555–1570. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; Scheltens, P. Treating Alzheimer’s disease with monoclonal antibodies: Current status and outlook for the future. Alzheimers Res. Ther. 2013, 5, 56. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef]
- Varnum, M.M.; Ikezu, T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch. Immunol. Ther. Exp. (Warsz) 2012, 60, 251–266. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Tago, H.; McGeer, E.G. Occurrence of HLA-DR reactive microglia in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1988, 540, 319–323. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Sastre, M.; Klockgether, T.; Heneka, M.T. Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Latif-Hernandez, A.; McReynolds, M.R.; Durairaj, A.S.; Wang, Q.; Rubin, A.; Joshi, A.U.; He, J.Q.; Gauba, E.; Liu, L.; et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 2021, 590, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Itagaki, S.; McGeer, P.L.; Akiyama, H.; Zhu, S.; Selkoe, D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 1989, 24, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Wisniewski, H.M. The complex of microglial cells and amyloid star in three-dimensional reconstruction. Acta Neuropathol. 1990, 81, 116–124. [Google Scholar] [CrossRef]
- Barger, S.W.; Harmon, A.D. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 1997, 388, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.M.; Goate, A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 2015, 77, 43–51. [Google Scholar] [CrossRef]
- Hickman, S.E.; El Khoury, J. The neuroimmune system in Alzheimer’s disease: The glass is half full. J. Alzheimers Dis. 2013, 33 (Suppl. 1), S295–S302. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Tan, M.S.; Gu, L.Z.; Zhang, Y.D.; Tan, L. Triggering receptor expressed on myeloid cells 2 knockdown exacerbates aging-related neuroinflammation and cognitive deficiency in senescence-accelerated mouse prone 8 mice. Neurobiol. Aging 2014, 35, 1243–1251. [Google Scholar] [CrossRef]
- Jiang, T.; Tan, L.; Zhu, X.C.; Zhang, Q.Q.; Cao, L.; Tan, M.S.; Gu, L.Z.; Wang, H.F.; Ding, Z.Z.; Zhang, Y.D.; et al. Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology 2014, 39, 2949–2962. [Google Scholar] [CrossRef]
- Fassler, M.; Rappaport, M.S.; Cuno, C.B.; George, J. Engagement of TREM2 by a novel monoclonal antibody induces activation of microglia and improves cognitive function in Alzheimer’s disease models. J. Neuroinflamm. 2021, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Candore, G.; Balistreri, C.R.; Grimaldi, M.P.; Listi, F.; Vasto, S.; Chiappelli, M.; Licastro, F.; Colonna-Romano, G.; Lio, D.; Caruso, C. Polymorphisms of pro-inflammatory genes and Alzheimer’s disease risk: A pharmacogenomic approach. Mech. Ageing Dev. 2007, 128, 67–75. [Google Scholar] [CrossRef]
- Megiddo, I.; Colson, A.; Chisholm, D.; Dua, T.; Nandi, A.; Laxminarayan, R. Health and economic benefits of public financing of epilepsy treatment in India: An agent-based simulation model. Epilepsia 2016, 57, 464–474. [Google Scholar] [CrossRef]
- Qu, L.; Boyce, R.; Leung, L.S. Seizures in the developing brain result in a long-lasting decrease in GABA(B) inhibitory postsynaptic currents in the rat hippocampus. Neurobiol. Dis. 2010, 37, 704–710. [Google Scholar] [CrossRef]
- Cavus, I.; Widi, G.A.; Duckrow, R.B.; Zaveri, H.; Kennard, J.T.; Krystal, J.; Spencer, D.D. 50 Hz hippocampal stimulation in refractory epilepsy: Higher level of basal glutamate predicts greater release of glutamate. Epilepsia 2016, 57, 288–297. [Google Scholar] [CrossRef]
- Naylor, D.E. Glutamate and GABA in the balance: Convergent pathways sustain seizures during status epilepticus. Epilepsia 2010, 51 (Suppl. 3), 106–109. [Google Scholar] [CrossRef] [PubMed]
- Sessolo, M.; Marcon, I.; Bovetti, S.; Losi, G.; Cammarota, M.; Ratto, G.M.; Fellin, T.; Carmignoto, G. Parvalbumin-Positive Inhibitory Interneurons Oppose Propagation But Favor Generation of Focal Epileptiform Activity. J. Neurosci. 2015, 35, 9544–9557. [Google Scholar] [CrossRef] [PubMed]
- Bonansco, C.; Fuenzalida, M. Plasticity of Hippocampal Excitatory-Inhibitory Balance: Missing the Synaptic Control in the Epileptic Brain. Neural Plast. 2016, 2016, 8607038. [Google Scholar] [CrossRef]
- Cavus, I.; Pan, J.W.; Hetherington, H.P.; Abi-Saab, W.; Zaveri, H.P.; Vives, K.P.; Krystal, J.H.; Spencer, S.S.; Spencer, D.D. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia 2008, 49, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Cavus, I.; Kim, J.; Hetherington, H.P.; Spencer, D.D. Hippocampal extracellular GABA correlates with metabolism in human epilepsy. Metab. Brain Dis. 2008, 23, 457–468. [Google Scholar] [CrossRef]
- Soukupova, M.; Binaschi, A.; Falcicchia, C.; Palma, E.; Roncon, P.; Zucchini, S.; Simonato, M. Increased extracellular levels of glutamate in the hippocampus of chronically epileptic rats. Neuroscience 2015, 301, 246–253. [Google Scholar] [CrossRef]
- Gambardella, A.; Labate, A.; Cifelli, P.; Ruffolo, G.; Mumoli, L.; Aronica, E.; Palma, E. Pharmacological modulation in mesial temporal lobe epilepsy: Current status and future perspectives. Pharmacol. Res. 2016, 113 Pt A, 421–425. [Google Scholar] [CrossRef]
- Riquelme, J.; Wellmann, M.; Sotomayor-Zárate, R.; Bonansco, C. Gliotransmission: A Novel Target for the Development of Antiseizure Drugs. Neuroscientist 2020, 26, 293–309. [Google Scholar] [CrossRef]
- Vezzani, A.; Aronica, E.; Mazarati, A.; Pittman, Q.J. Epilepsy and brain inflammation. Exp. Neurol. 2013, 244, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Ravizza, T.; Balosso, S.; Aronica, E. Glia as a source of cytokines: Implications for neuronal excitability and survival. Epilepsia 2008, 49 (Suppl. 2), 24–32. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.I.; Elisevich, K.V. Brain region and epilepsy-associated differences in inflammatory mediator levels in medically refractory mesial temporal lobe epilepsy. J. Neuroinflammation 2016, 13, 270. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, D.; Stefan, H. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: Uncontrolled inflammation drives disease progression? J. Neurol. Sci. 2010, 296, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hiragi, T.; Ikegaya, Y.; Koyama, R. Microglia after Seizures and in Epilepsy. Cells 2018, 7, 26. [Google Scholar] [CrossRef]
- Ravizza, T.; Gagliardi, B.; Noé, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Beamer, E.; Fischer, W.; Engel, T. The ATP-Gated P2X7 Receptor As a Target for the Treatment of Drug-Resistant Epilepsy. Front. Neurosci. 2017, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, T.; Vezzani, A. Pharmacological targeting of brain inflammation in epilepsy: Therapeutic perspectives from experimental and clinical studies. Epilepsia Open. 2018, 3 (Suppl. 2), 133–142. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Pacheco, A.; Diaz-Hernandez, M.; Arribas-Blázquez, M.; Sanz-Rodriguez, A.; Olivos-Oré, L.A.; Artalejo, A.R.; Alves, M.; Letavic, M.; Miras-Portugal, M.T.; Conroy, R.M.; et al. Transient P2X7 Receptor Antagonism Produces Lasting Reductions in Spontaneous Seizures and Gliosis in Experimental Temporal Lobe Epilepsy. J. Neurosci. 2016, 36, 5920–5932. [Google Scholar] [CrossRef] [PubMed]
- Boison, D. Adenosine dysfunction in epilepsy. Glia 2012, 60, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Thomas, M.J.; Spencer, D.D.; Rundén-Pran, E.; Lai, J.C.; Malthankar, G.V.; Kim, J.H.; Danbolt, N.C.; Ottersen, O.P.; de Lanerolle, N.C. Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 2004, 363, 28–37. [Google Scholar] [CrossRef]
- Steinhäuser, C.; Seifert, G. Glial membrane channels and receptors in epilepsy: Impact for generation and spread of seizure activity. Eur. J. Pharmacol. 2002, 447, 227–237. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef]
- Bonansco, C.; Couve, A.; Perea, G.; Ferradas, C.; Roncagliolo, M.; Fuenzalida, M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur. J. Neurosci. 2011, 33, 1483–1492. [Google Scholar] [CrossRef]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2019, 405, 137–147. [Google Scholar] [CrossRef]
- Dossi, E.; Blauwblomme, T.; Moulard, J.; Chever, O.; Vasile, F.; Guinard, E.; Le Bert, M.; Couillin, I.; Pallud, J.; Capelle, L.; et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci. Transl. Med. 2018, 10, eaar3796. [Google Scholar] [CrossRef]
- Nikolic, L.; Shen, W.; Nobili, P.; Virenque, A.; Ulmann, L.; Audinat, E. Blocking TNFα-driven astrocyte purinergic signaling restores normal synaptic activity during epileptogenesis. Glia 2018, 66, 2673–2683. [Google Scholar] [CrossRef]
- Shigetomi, E.; Hirayama, Y.J.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Role of Purinergic Receptor P2Y1 in Spatiotemporal Ca. J. Neurosci. 2018, 38, 1383–1395. [Google Scholar] [CrossRef]
- Álvarez-Ferradas, C.; Morales, J.C.; Wellmann, M.; Nualart, F.; Roncagliolo, M.; Fuenzalida, M.; Bonansco, C. Enhanced astroglial Ca2+ signaling increases excitatory synaptic strength in the epileptic brain. Glia 2015, 63, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; Ben Achour, S.; Rostaing, P.; Triller, A.; Bessis, A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2012, 109, E197–E205. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, M.; Álvarez-Ferradas, C.; Maturana, C.J.; Sáez, J.C.; Bonansco, C. Astroglial Ca. Front. Cell. Neurosci. 2018, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Heuser, K.; Nome, C.G.; Pettersen, K.H.; Åbjørsbråten, K.S.; Jensen, V.; Tang, W.; Sprengel, R.; Taubøll, E.; Nagelhus, E.A.; Enger, R. Ca2+ Signals in Astrocytes Facilitate Spread of Epileptiform Activity. Cereb. Cortex 2018, 28, 4036–4048. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Alves, M.; Gomez-Villafuertes, R.; Delanty, N.; Farrell, M.A.; O’Brien, D.F.; Miras-Portugal, M.T.; Hernandez, M.D.; Henshall, D.C.; Engel, T. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia 2017, 58, 1603–1614. [Google Scholar] [CrossRef]
- Engel, T.; Brennan, G.P.; Sanz-Rodriguez, A.; Alves, M.; Beamer, E.; Watters, O.; Henshall, D.C.; Jimenez-Mateos, E.M. A calcium-sensitive feed-forward loop regulating the expression of the ATP-gated purinergic P2X7 receptor via specificity protein 1 and microRNA-22. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 255–266. [Google Scholar] [CrossRef]
- Panatier, A.; Vallée, J.; Haber, M.; Murai, K.K.; Lacaille, J.C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Bahrami, F.; Janahmadi, M. Functional contributions of astrocytes in synchronization of a neuronal network model. J. Theor. Biol. 2012, 292, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ashhad, S.; Narayanan, R. Active dendrites regulate the impact of gliotransmission on rat hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E3280–E3289. [Google Scholar] [CrossRef] [PubMed]
- Carmignoto, G.; Haydon, P.G. Astrocyte calcium signaling and epilepsy. Glia 2012, 60, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gonzalo, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Cammarota, M.; Brondi, M.; Vetri, F.; Uva, L.; Pozzan, T.; de Curtis, M.; et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010, 8, e1000352. [Google Scholar] [CrossRef]
- American Psychiatric, A. Anxiety disorders: DSM-5 Selections; American Psychiatric Association Publishing: Arlington, VA, USA, 2016. [Google Scholar]
- Yang, X.; Fang, Y.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; Yang, L.; Lu, M. Global, regional and national burden of anxiety disorders from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Epidemiol. Psychiatr. Sci. 2021, 30, e36. [Google Scholar] [CrossRef]
- Kalin, N.H. The Critical Relationship Between Anxiety and Depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef]
- Swendsen, J.; Conway, K.P.; Degenhardt, L.; Glantz, M.; Jin, R.; Merikangas, K.R.; Sampson, N.; Kessler, R.C. Mental disorders as risk factors for substance use, abuse and dependence: Results from the 10-year follow-up of the National Comorbidity Survey. Addiction 2010, 105, 1117–1128. [Google Scholar] [CrossRef]
- Mendez, M.F. The Relationship Between Anxiety and Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2021, 5, 171–177. [Google Scholar] [CrossRef]
- Gandy, M.; Sharpe, L.; Perry, K.N.; Miller, L.; Thayer, Z.; Boserio, J.; Mohamed, A. Anxiety in epilepsy: A neglected disorder. J. Psychosom. Res. 2015, 78, 149–155. [Google Scholar] [CrossRef]
- Roy-Byrne, P.P.; Davidson, K.W.; Kessler, R.C.; Asmundson, G.J.; Goodwin, R.D.; Kubzansky, L.; Lydiard, R.B.; Massie, M.J.; Katon, W.; Laden, S.K.; et al. Anxiety disorders and comorbid medical illness. Gen. Hosp. Psychiatry 2008, 30, 208–225. [Google Scholar] [CrossRef]
- Costello, H.; Gould, R.L.; Abrol, E.; Howard, R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open. 2019, 9, e027925. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Tu, J.L.; Li, X.H.; Hua, Q.; Liu, W.Z.; Liu, Y.; Pan, B.X.; Hu, P.; Zhang, W.H. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav. Immun. 2021, 91, 505–518. [Google Scholar] [CrossRef]
- Pascual, M.; Baliño, P.; Aragón, C.M.; Guerri, C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of TLR4 and TLR2. Neuropharmacology 2015, 89, 352–359. [Google Scholar] [CrossRef]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 2016, 41, 1874–1887. [Google Scholar] [CrossRef]
- Fourrier, C.; Bosch-Bouju, C.; Boursereau, R.; Sauvant, J.; Aubert, A.; Capuron, L.; Ferreira, G.; Layé, S.; Castanon, N. Brain tumor necrosis factor-α mediates anxiety-like behavior in a mouse model of severe obesity. Brain Behav. Immun. 2019, 77, 25–36. [Google Scholar] [CrossRef]
- Gomes, J.A.S.; Silva, J.F.; Marcal, A.P.; Silva, G.C.; Gomes, G.F.; de Oliveira, A.C.P.; Soares, V.L.; Oliveira, M.C.; Ferreira, A.V.M.; Aguiar, D.C. High-refined carbohydrate diet consumption induces neuroinflammation and anxiety-like behavior in mice. J. Nutr. Biochem. 2020, 77, 108317. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2020.
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Raleigh, J.M.; Abbate, A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr. Heart Fail. Rep. 2015, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Querio, G.; Antoniotti, S.; Geddo, F.; Tullio, F.; Penna, C.; Pagliaro, P.; Gallo, M.P. Ischemic heart disease and cardioprotection: Focus on estrogenic hormonal setting and microvascular health. Vascul Pharmacol. 2021, 141, 106921. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, Z.; Cai, L.; Lin, L.; Liu, J.; Cheng, J. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4293206. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Wang, F.; Li, B.; Dong, Z.; Liu, X.; Zhang, C.; An, F. Association of nucleotide-binding oligomerization domain-like receptor 3 inflammasome and adverse clinical outcomes in patients with idiopathic dilated cardiomyopathy. Clin. Chem. Lab. Med. 2013, 51, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Martine, P.; Chevriaux, A.; Derangere, V.; Apetoh, L.; Garrido, C.; Ghiringhelli, F.; Rebe, C. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell. Death Dis. 2019, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroma, E.; Abbate, A.; Toldo, S. NLRP3 Inflammasome Inhibitors in Cardiovascular Diseases. Molecules 2021, 26, 976. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Duan, F.; Hu, J.; Luo, B.; Huang, B.; Lou, X.; Sun, X.; Li, H.; Zhang, X.; Yin, S.; et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020, 34, 101523. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Korzick, D.H. Estrogen and the female heart. Mol. Cell. Endocrinol. 2014, 389, 31–39. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Gupta, S.; Knowlton, A.A. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: Cross-talk with NF kappa B signaling. J. Mol. Cell. Cardiol. 2004, 36, 577–584. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Sun, L. Heat-shock factor-1, steroid hormones, and regulation of heat-shock protein expression in the heart. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H455–H464. [Google Scholar] [CrossRef]
- Lancaster, T.S.; Jefferson, S.J.; Hunter, J.C.; Lopez, V.; Van Eyk, J.E.; Lakatta, E.G.; Korzick, D.H. Quantitative proteomic analysis reveals novel mitochondrial targets of estrogen deficiency in the aged female rat heart. Physiol. Genom. 2012, 44, 957–969. [Google Scholar] [CrossRef]
- Farruggio, S.; Cocomazzi, G.; Marotta, P.; Romito, R.; Surico, D.; Calamita, G.; Bellan, M.; Pirisi, M.; Grossini, E. Genistein and 17beta-Estradiol Protect Hepatocytes from Fatty Degeneration by Mechanisms Involving Mitochondria, Inflammasome and Kinases Activation. Cell. Physiol. Biochem. 2020, 54, 401–416. [Google Scholar] [CrossRef]
- Wei, A.; Liu, J.; Li, D.; Lu, Y.; Yang, L.; Zhuo, Y.; Tian, W.; Cong, H. Syringaresinol attenuates sepsis-induced cardiac dysfunction by inhibiting inflammation and pyroptosis in mice. Eur. J. Pharmacol. 2021, 913, 174644. [Google Scholar] [CrossRef] [PubMed]
- Margina, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int. J. Environ. Res. Public. Health 2020, 17, 4135. [Google Scholar] [CrossRef] [PubMed]
- Mallah, K.; Couch, C.; Borucki, D.M.; Toutonji, A.; Alshareef, M.; Tomlinson, S. Anti-inflammatory and Neuroprotective Agents in Clinical Trials for CNS Disease and Injury: Where Do We Go From Here? Front. Immunol. 2020, 11, 2021. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chi, X.S.; Li, R.; Hu, X.; Xu, H.X.; Li, J.M.; Zhou, D. Inhibition of P2X7 Receptor Ameliorates Nuclear Factor-Kappa B Mediated Neuroinflammation Induced by Status Epilepticus in Rat Hippocampus. J. Mol. Neurosci. 2017, 63, 173–184. [Google Scholar] [CrossRef]
- Jimenez-Pacheco, A.; Mesuret, G.; Sanz-Rodriguez, A.; Tanaka, K.; Mooney, C.; Conroy, R.; Miras-Portugal, M.T.; Diaz-Hernandez, M.; Henshall, D.C.; Engel, T. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia 2013, 54, 1551–1561. [Google Scholar] [CrossRef]
| Drug | Anti-Inflammatory Mechanism | Preliminary Findings | Therapeutic Potential | References |
|---|---|---|---|---|
| Nalmefene | Inhibition of ethanol-induced TLR4 activation | Reduction in ethanol consumption and the up-regulation of cytokines in PFC | Treatment for alcoholism | [139] |
| Ampicillin + sulbactam | Modulation of NMDA receptor NR2B subunits and HMGB1-associated pathways | Up-regulation of GLT-1 and mGluR5 in NAc shell, and reduction in HMGB1, RAGE and TNF-α | Treatment for alcoholism | [140] |
| Minocycline | Inhibition of microglial activation | Reduction in mRNA expression of IL-1β and IFN-γ microglial activation in the NAc; reduction in NAc protein levels of IBA-1, TNF-α, and IL-6 induced by METH | Treatment for obesity or drug addiction | [129,147] |
| SCH-23390 | Antagonist of D1 receptors | Reduction in NAc protein levels of IBA-1, TNF-α and IL-6 induced by METH | Treatment for drug addiction | [147] |
| Box A | Antagonist of HMGB1 | Reduction in protein levels of IL-1β in VTA, NAc and PFC in rats with acute METH administration | Treatment for drug addiction | [148] |
| Ginger extract | Not fully clear | Reduction in NAc morphine-induced inflammatory markers, p38 MAPK and GFAP | Treatment for drug addiction | [152] |
| Ceftriaxone | Not fully clear | Reduction in mRNA levels of NF-κB and TNF-α in PFC, NAc and VTA | Treatment for tobacco addiction | [155] |
| TREM 2 binding antibody | Monoclonal antibody binding the extracellular domain of human and murine TREM2 | Improvement in cognitive function in experimental amyloidopathy models | Treatment for AD | [220] |
| A-438079 P2X7 receptor antagonist | Inhibition of microglial activation and purinergic-dependent inflammation. | Reduction in neuronal damage, and astroglial and microglial activation | Treatment for seizures and epilepsy | [242,297,298] |
| LPS-RS | TLR4 antagonist (VTA administration) | Reduction in cocaine-primed reinstatement | Treatment for drug addiction | [150] |
| Etanercept | TNF blocker | Decrease in anxiety behavior | Treatment for anxiety disorders | [278] |
| Aminoguanidine | iNOS blocker | Decrease in anxiety behavior | Treatment for anxiety caused by refined carbohydrates diet | [279] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, A.P.; Bonansco, C.; Cruz, G.; Dagnino-Subiabre, A.; Fuenzalida, M.; Negrón, I.; Sotomayor-Zárate, R.; Martínez-Pinto, J.; Jorquera, G. Central and Peripheral Inflammation: A Common Factor Causing Addictive and Neurological Disorders and Aging-Related Pathologies. Int. J. Mol. Sci. 2023, 24, 10083. https://doi.org/10.3390/ijms241210083
Escobar AP, Bonansco C, Cruz G, Dagnino-Subiabre A, Fuenzalida M, Negrón I, Sotomayor-Zárate R, Martínez-Pinto J, Jorquera G. Central and Peripheral Inflammation: A Common Factor Causing Addictive and Neurological Disorders and Aging-Related Pathologies. International Journal of Molecular Sciences. 2023; 24(12):10083. https://doi.org/10.3390/ijms241210083
Chicago/Turabian StyleEscobar, Angélica P., Christian Bonansco, Gonzalo Cruz, Alexies Dagnino-Subiabre, Marco Fuenzalida, Ignacio Negrón, Ramón Sotomayor-Zárate, Jonathan Martínez-Pinto, and Gonzalo Jorquera. 2023. "Central and Peripheral Inflammation: A Common Factor Causing Addictive and Neurological Disorders and Aging-Related Pathologies" International Journal of Molecular Sciences 24, no. 12: 10083. https://doi.org/10.3390/ijms241210083
APA StyleEscobar, A. P., Bonansco, C., Cruz, G., Dagnino-Subiabre, A., Fuenzalida, M., Negrón, I., Sotomayor-Zárate, R., Martínez-Pinto, J., & Jorquera, G. (2023). Central and Peripheral Inflammation: A Common Factor Causing Addictive and Neurological Disorders and Aging-Related Pathologies. International Journal of Molecular Sciences, 24(12), 10083. https://doi.org/10.3390/ijms241210083







