Abstract
Epidemiological studies have associated plasma galectin-4 (Gal-4) levels with prevalent and incident diabetes, and with an increased risk of coronary artery disease. To date, data regarding possible associations between plasma Gal-4 and stroke are lacking. Using linear and logistic regression analyses, we tested Gal-4 association with prevalent stroke in a population-based cohort. Additionally, in mice fed a high-fat diet (HFD), we investigated whether plasma Gal-4 increases in response to ischemic stroke. Plasma Gal-4 was higher in subjects with prevalent ischemic stroke, and was associated with prevalent ischemic stroke (odds ratio 1.52; 95% confidence interval 1.01–2.30; p = 0.048) adjusted for age, sex, and covariates of cardiometabolic health. Plasma Gal-4 increased after experimental stroke in both controls and HFD-fed mice. HFD exposure was devoid of impact on Gal-4 levels. This study demonstrates higher plasma Gal-4 levels in both experimental stroke and in humans that experienced ischemic stroke.
1. Introduction
In previous epidemiological studies, we have shown that increased levels of galectin-4 (Gal-4) are linked with prevalent and incident diabetes [1], as well as increased risk of future myocardial infarction, heart failure, cardiovascular, and all-cause mortality [2]. Whether plasma Gal-4 is also associated with ischemic stroke, which is a major complication in diabetic patients, has not yet been investigated.
Gal-4 is a gastrointestinal tract protein involved in the apical trafficking of proteins, including dipeptidyl peptidase-4 (DPP-4), which accumulates intracellularly in Gal-4-depleted mice [3]. Soluble and membrane-bound DPP-4 inactivate gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1), which are largely responsible for the incretin effect [4]. The latter is defective in individuals with diabetes [5], leading to cardio-metabolically adverse effects [6]. Recent evidence suggests that incretin-based antihyperglycemic agents, including DPP-4 and GLP-1 receptor agonists, exert beneficial effects in patients with diabetes who suffer ischemic stroke by reducing infarct size and promoting recovery [7,8,9,10].
Here, we tested whether plasma Gal-4 concentrations increase after stroke and whether this may be dependent on metabolic syndrome by using a mouse model of ischemic stroke. The clinical relevance of potential stroke-associated Gal-4 alterations was assessed by testing association with prevalent stroke in a population-based cohort study.
2. Results
2.1. Gal-4 Plasma Levels Increase after Experimental Ischemic Stroke
To induce metabolic syndrome, mice were fed a high-fat diet (HFD) for 8 weeks before stroke induction (Figure 1a). Compared to the control diet (CD; 10% fat kcal), HFD (60% fat kcal) over the course of 8 weeks led to increased body weight (in g: 29.8 ± 0.9 in CD vs. 40.5 ± 1.2 in HFD, p < 0.001), higher plasma glucose (in mmol/L: 4.4 ± 0.3 in CD vs. 6.2 ± 0.5 in HFD, p = 0.007), and elevated plasma insulin (in µg/L: 3.2 ± 1.8 in CD vs. 15.8 ± 5.7 in HFD, p = 0.035).
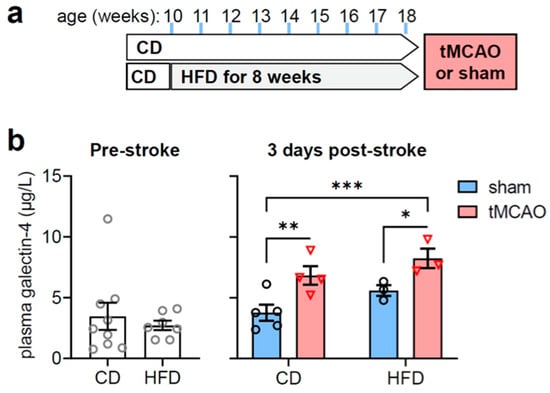
Figure 1.
Stroke-associated increases in galectin-4 plasma concentrations are independent of metabolic syndrome. (a) Study design: mice were fed HFD or CD for 8 consecutive weeks. (b) Plasma galectin-4 levels after 8 weeks of HFD or CD feeding (left panel), and 3 days after stroke or sham surgery in mice fed the HFD and CD (right panel). Data are shown as mean ± SEM. Symbols (open grey circles = pre-stroke, open black circles = post-sham surgery, open red triangles = post-tMCAo) represent individual mice. * p < 0.05, ** p < 0.01, *** p < 0.001 based on Fischer’s LSD post-hoc comparison after significant effect of diet and stroke in ANOVA. CD = control diet, HFD = high-fat diet, tMCAo = transient middle cerebral artery occlusion.
Stroke induced by transient middle cerebral artery occlusion (tMCAo) was confirmed by lower neurological function (i.e., higher neuro-scores after induction of stroke compared to sham surgery: 0 in CD and HFD for sham vs. 8.8 ± 5.2 in CD and 6.7 ± 3.2 in HFD for tMCAo) and apparent ischemic lesions (41 ± 13% in CD, 37 ± 15% in HFD). Mice on CD and HFD had a poorer survival rate after tMCAo compared to mice on conventional rodent chow (survival at 1 day after tMCAo in %: 100 for conventional rodent chow vs. 83 for CD and 50 for HFD).
While HFD had negligible impact on plasma Gal-4, tMCAO resulted in increased Gal-4 plasma levels, as measured 3 days after stroke (Figure 1b; ANOVA effects: diet F(1,11) = 5.00, p = 0.047, stroke F(1,11) = 15.50, p = 0.002, interaction F(1,11) = 0.081, p = 0.781).
An independent mouse cohort confirmed the stroke-associated increase of plasma Gal-4 concentrations (Figure 2). Plasma Gal-4 concentration was elevated in mice fed conventional rodent chow (5.3% fat kcal) at 1 day post-stroke compared to sham-operated controls (p = 0.0129). Moreover, plasma Gal-4 levels did not differ between sham-operated mice on the conventional chow diet, CD or HFD (Gal-4 concentration in µg/µL: 3.4 ± 0.6 for rodent chow, 3.8 ± 0.7 for CD, 5.6 ± 0.4 for HFD; ANOVA: p = 0.1283).
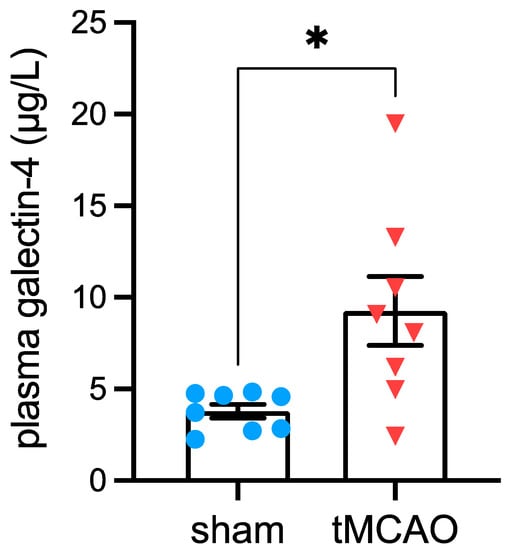
Figure 2.
Stroke-associated increases in galectin-4 plasma concentrations. Plasma galectin-4 levels 1 day after tMCAo or sham surgery in mice fed conventional rodent chow. Data are shown as mean ± SEM. Symbols (full blue circles = post-sham surgery, full red triangles = post-tMCAo) represent individual mice. * p < 0.05 based unpaired t-test. tMCAo = transient middle cerebral artery occlusion.
2.2. Gal-4 Associates with Prevalent Stroke
Subjects with prevalent ischemic stroke (n = 59) were older, more often men, had diabetes mellitus to higher extent, and were more often treated for hypertension. Gal-4 was significantly higher in subjects with prevalent ischemic stroke than in subjects without (Table 1).

Table 1.
Characteristics of the human study population. Values are means (±standard deviation) and numbers (percent).
In logistic regressions, Gal-4 was associated with prevalent ischemic stroke in an unadjusted model and following adjustments for age and sex as well as the main covariates related to weight, diabetes, and cardiovascular health (Table 2). In univariate regression analyses, each doubling of Gal-4 concentration was associated with higher BMI (β 0.87; p = 8.3 × 10−8) and a higher probability of antihypertensive treatment (OR 2.24 [1.90–2.63]; p = 2.2 × 10−22). Gal-4 had no significant association with systolic blood pressure (p = 0.678).

Table 2.
Associations between plasma galectin-4 and prevalent stroke.
3. Discussion
This is the first study to show higher plasma Gal-4 levels in patients with prevalent stroke. Verification in a murine model of experimental stroke supports a direct interaction between Gal-4 plasma levels and ischemic stroke. The association of increases in plasma Gal-4 with higher BMI in the tested human cohort necessitated the investigation of whether HFD feeding has an effect on Gal-4 levels in a controlled experimental setting. Testing whether HFD feeding increases Gal-4 plasma levels through the assessment of Gal-4 levels after 8 weeks of HFD prior to stroke revealed that HFD has a negligible impact on plasma Gal-4 levels in mice. From these data, it can be concluded that impaired fasting glucose and diabetes status (both apparent in mice after 8 weeks of HDF exposure) are not directly linked to alterations in Gal-4 levels. Although this is in contrast to results from previously published epidemiological studies that report associations between high Gal-4 levels and prevalent as well as incident diabetes [1], our findings showing a direct effect of stroke on plasma Gal-4 elevation are supported by studies reporting similar relationships between plasma Gal-4 and the incidence of myocardial infarction [2], heart failure [2,11], and cardiovascular and all-cause mortality [2]. Together, these data rather suggest that changes in Gal-4 levels are generally associated with the occurrence of cardiovascular events, including stroke. This notion is underpinned by the results of the current investigation, showing that stroke-induced plasma Gal-4 elevation is independent of metabolic syndrome.
The mechanisms by which Gal-4 concentration increase post-stroke are not yet known. Nonetheless, several pathogenic processes post-stroke may be affected by altered Gal-4 homeostasis. Gal-4 has been reported to be involved in immunoregulatory functions through the activation and differentiation of monocytes [12], which have been proposed as central players in the detrimental innate proinflammatory response post-stroke [13]. In addition to inflammation, the formation of new vessels through angiogenesis is thought to participate in functional stroke recovery [14]. Gal-4 has been linked to the augmented secretion of circulating cytokines responsible for endothelial activation related to angiogenesis and thus cancer metastasis [15]. Earlier studies supported its role in the proliferation and migration of different cell types [16], emphasizing a potential involvement in angiogenic processes that may also occur post-stroke. Similarly, arteriogenesis, as a major process to improve collateral flow, has been associated with Gal-4 [17]. As patients with good collateral flow experience more favorable stroke outcomes and metabolic risk factors such as diabetes are associated with poor leptomeningeal collateral status [18], investigating the role of Gal-4 in this respect may be promising.
Taken together, the relatively sparse knowledge base available on the role of Gal-4 in stroke pathology and related processes, as well as its relationship to risk factors for stroke, such as metabolic syndrome, and diabetes, necessitate mechanistic and clinical studies to investigate the relative potential of Gal-4 as a prognostic marker for stroke outcome or even its potential as therapeutic target.
Study Limitations
The assessed population cohort (i.e., the Malmö Preventive Project Re-Examination cohort) consists of mainly elderly, white European men. Thus, our findings might not be generalizable to the general population. As is common to all observational studies, no conclusions about causality can be drawn. Further, this study did not aim at identifying molecular mechanisms involved in Gal-4 alterations post-stroke. With a fast dissemination of the findings that Gal-4 plasma levels increase with stroke, we instead want to stimulate further research on this important topic and pave the way for additional mechanistic studies that may include the testing of the relative potential of Gal-4 as a prognostic marker for stroke outcome, its potential as a therapeutic target, or the investigation of plasma Gal-4 as prognostic parameter for stroke incidence in patients with diabetes or metabolic syndrome.
4. Materials and Methods
4.1. Mouse Study
Male C57BL/6J mice (8 weeks old; Taconic, Skensved, Denmark) were group-housed under a 12 h light–dark cycle in enriched cages with access to food and water ad libitum. After acclimatization, at 9 weeks of age, mice were started on high-fat (HFD, 60% fat kcal; N = 12) or control (CD, 10% fat kcal; N = 12) diets (Research Diets, New Brunswick, NJ-USA) for 8 consecutive weeks as previously described [19]. Where possible, experimenters were blinded to group allocation during sample processing and data analysis.
4.1.1. Mouse Model of Stroke
Transient middle cerebral artery occlusion (tMCAo) was performed as previously described [20]. Briefly, the middle cerebral artery was transiently occluded using a monofilament (9–10 mm coating length, 0.19 ± 0.01 mm tip diameter; Doccol, Sharon, MA, USA) in anesthetized mice (isoflurane in 70% N2O, 30% O2). Reperfusion was initiated after 60 min. Cerebral blood flow was monitored using laser Doppler flowmetry (Moor Instruments, Axminster, UK). The same protocol without occlusion was used for sham surgery. Neurological function was evaluated using the sum of focal and general scores ranging between 0 (no deficits) and 56 (the poorest performance) [20]. Coronal brain slices (1 mm thick) were stained with 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, #93140), and infarct area was determined and presented as percentage of contralateral hemisphere [20]. Group sizes were determined based on prior experiments, considering estimated group specific attrition. Mice from CD (n = 12) and HFD (n = 12) groups were randomly assigned to tMCAo or sham surgery. In the CD group, 6 mice were subjected to tMCAo and 6 mice to sham surgery, while 8 of the HFD mice were subjected to tMCAo and 4 to sham surgery. A separate cohort of mice fed on conventional rodent chow (SAFE A30; Safe Diets, Augy, France) was utilized to assess direct effects of ischemic stroke on plasma Gal-4 levels (n = 8 for sham and tMCAo, respectively).
4.1.2. Plasma Analyses
Pre-surgery blood from the saphenous vein and post-stroke blood withdrawn from vena cava prior to transcardial perfusion was collected into EDTA-coated tubes (#41.1395.105, Sarstedt, Nürnbrecht, Germany), and separated by centrifugation at 1000× g for 10 min at room temperature. Plasma glucose was measured using the glucose oxidase method coupled to a peroxidase reaction oxidizing the AmplexRed reagent (#A12222, Invitrogen, Fisher Scientific, Göteborg, Sweden), as detailed before [21]. Commercially available ELISA kits were used to determine plasma insulin (#10-1247-10, Mercodia, Uppsala, Sweden) and Gal-4 (#NBP2-76725, Novus Biologicals, Bio-Techne, Abingdon, UK).
4.1.3. Statistics
Data were analyzed by Prism 9.3.0 (GraphPad, San Diego, CA, USA). After normality testing (Kolmogorov–Smirnov and Shapiro–Wilk tests), data were analyzed by either Student’s t-test (HFD effect pre-stroke) or Mann–Whitney (stroke effect) or 2-way ANOVAs with diet and stroke as factors. Significant diet, stroke, or interaction effects were followed by Fisher’s least significant difference (LSD) tests for independent comparisons. Normally distributed data are presented as mean ± standard error of the mean (SEM). Data that are not normally distributed are presented as median ± interquartile range (IQR).
4.2. Human Study
Within the Malmö Preventive Project [22] Re-Examination cohort, a sub-sample of participants was randomly selected based on glucometabolic status [23], i.e., 1/3 normoglycemic; 1/3 with impaired fasting glucose (IFG), and 1/3 with diabetes (ntotal = 1792). Blood samples provided by 1737 individuals were analyzed with proximity extension assay technology (Proseek Multiplex CVD III from Olink Bioscience, Uppsala, Sweden). The CVD III panel consists of 92 proteins, among them galectin-4, with either established or proposed associations with CVD, inflammation, and metabolism. Complete data for all co-variates were available in 1688 subjects.
4.2.1. Examinations
Standardized methods were used to measure anthropometrics and blood pressure, as described elsewhere [23]. Fasting plasma glucose and high-density lipoprotein (HDL) cholesterol were analyzed using a Beckman Coulter LX20 (Beckman Coulter, Brea, CA, USA) at Department of Chemistry, Skåne University Hospital, Malmö. Fasting blood samples for proteomic analyses were stored at −80 °C until time of analysis.
4.2.2. Definitions
Prevalent stroke was defined as prior ischemic stroke, and data were collected through regional and national registers, defined as ICD9 codes 433–434 or ICD10 codes I63.0–I63.9. IFG was defined as fasting plasma glucose ≥5.6 mmol/L. Prevalent diabetes was defined as either previously known diabetes or new-onset diabetes (two separate measurements of fasting plasma glucose ≥7.0 mmol/L or one measurement ≥11.1 mmol/L) [23]. Smoking was self-reported and defined as present smoking. Antihypertensive treatment was defined as use of any blood pressure-lowering medicine and retrieved through the National Prescribed Drug Register (starting in 2005).
4.2.3. Statistics
Associations between Gal-4 and prevalent ischemic stroke were explored using logistic regression models in three steps: (1) unadjusted; (2) adjusted for age and sex (Model (1), and (3) adjusted for body mass index, systolic blood pressure, prevalent IFG/diabetes, HDL-cholesterol, antihypertensive treatment, and smoking (Model 2). Groups were compared using Student’s t-tests or χ2 tests, where appropriate. Associations between Gal-4 and continuous variables were analyzed using univariate linear regressions, and associations between Gal-4 and binary variables using univariate logistic regressions.
Author Contributions
J.P.P.V. and H.M. performed mouse experiments and analyzed data. A.M., J.M.N.D., A.J. and M.M. designed the study, analyzed data, and wrote the manuscript. J.P.P.V., H.M., A.J., P.M.N., U.L., M.H.O., J.M.N.D., M.M. and A.M. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The MPP was funded by the Heart and Lung Foundation of Sweden (2004045806), Region Skåne (PMN), Merck Sharp & Dohme, Hulda and E Conrad Mossfelts Foundation and Ernhold Lundströms Foundation. Further funding was provided from the Swedish Research Council (2019-01130, JMND and 2022-00973, MM), Diabetesfonden (Dia2019-440, JMND), Direktör Albert Påhlssons Foundation (JMND, AM), Hjärnfonden (FO2021-0112, AM), Crafoord Foundation (20220654, AM, 20220566, MM) the Heart and Lung Foundation (20210354, MM), Stroke Riksförbundet (AM), German Academic Exchange Service (HM), and Sparbanken Stiftelse (V2021/1851, AM). The authors acknowledge generous financial support from The Knut and Alice Wallenberg foundation, the Faculty of Medicine at Lund University and Region Skåne. JMND acknowledges support from the Lund University Diabetes Centre, which is funded by the Swedish Research Council (Strategic Research Area EXODIAB, grant 2009-1039) and the Swedish Foundation for Strategic Research (grant IRC15-0067).
Institutional Review Board Statement
Animal experiments were performed according to EU Directive 2010/63/EU and approved by the Malmö/Lund Committee for Animal Experiment Ethics (Dnr 5.8.18-08160/2021). The study was conducted in accordance with the Declaration of Helsinki and approved by the ethical review board of Lund University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available upon request from Steering Committee of the Malmö Preventive Project study by contacting data manager Anders Dahlin (anders.dahlin@med.lu.se), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available due to ethical and legal restrictions related to the Swedish Biobanks in Medical Care Act (2002:297) and the Personal Data Act (1998:204).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
AU—arbitrary unit, AHT—antihypertensive treatment, BMI—body mass index, CD—control diet, DPP-4—dipeptidyl peptidase-4, Gal-4—galectin, GIP—gastric inhibitory polypeptide, GLP-1—glucagon-like peptide 1, HFD—high-fat diet, HDL—high density lipoprotein, IFG—impaired fasting glucose, IQR—interquartile range, MMP-Res—Malmö Preventive Project [14] Re-Examination cohort, OR—odds ratio, SBP—systolic blood pressure, SEM—standard error of the mean, tMCAo—transient middle cerebral artery occlusion.
References
- Molvin, J.; Pareek, M.; Jujic, A.; Melander, O.; Råstam, L.; Lindblad, U.; Daka, B.; Leósdóttir, M.; Nilsson, P.M.; Olsen, M.H.; et al. Using a Targeted Proteomics Chip to Explore Pathophysiological Pathways for Incident Diabetes- The Malmö Preventive Project. Sci. Rep. 2019, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Molvin, J.; Jujić, A.; Melander, O.; Pareek, M.; Råstam, L.; Lindblad, U.; Daka, B.; Leósdóttir, M.; Nilsson, P.M.; Olsen, M.H.; et al. Proteomic exploration of common pathophysiological pathways in diabetes and cardiovascular disease. ESC Heart Fail. 2020, 7, 4151–4158. [Google Scholar] [CrossRef] [PubMed]
- Delacour, D.; Gouyer, V.; Zanetta, J.P.; Drobecq, H.; Leteurtre, E.; Grard, G.; Moreau-Hannedouche, O.; Maes, E.; Pons, A.; André, S.; et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J. Cell Biol. 2005, 169, 491–501. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef]
- Zhong, J.; Maiseyeu, A.; Davis, S.N.; Rajagopalan, S. DPP4 in cardiometabolic disease: Recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 2015, 116, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, J.; Jung, H.S.; Kim, B.; Kim, Y.E.; Lim, D.S.; Kim, S.D.; Song, Y.S. Anti-inflammatory Effect of Glucagon Like Peptide-1 Receptor Agonist, Exendin-4, through Modulation of IB1/JIP1 Expression and JNK Signaling in Stroke. Exp. Neurobiol. 2017, 26, 227–239. [Google Scholar] [CrossRef]
- Filchenko, I.; Simanenkova, A.; Chefu, S.; Kolpakova, M.; Vlasov, T. Neuroprotective effect of glucagon-like peptide-1 receptor agonist is independent of glycaemia normalization in type two diabetic rats. Diab. Vasc. Dis. Res. 2018, 15, 567–570. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Rullman, E.; Melin, M.; Mandić, M.; Gonon, A.; Fernandez-Gonzalo, R.; Gustafsson, T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin. Res. Cardiol. 2020, 109, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Shin, J.S.; Chung, H.; Park, C.G. Galectin-4 Interaction with CD14 Triggers the Differentiation of Monocytes into Macrophage-like Cells via the MAPK Signaling Pathway. Immune Netw. 2019, 19, e17. [Google Scholar] [CrossRef] [PubMed]
- Urra, X.; Cervera, A.; Obach, V.; Climent, N.; Planas, A.M.; Chamorro, A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke 2009, 40, 1262–1268. [Google Scholar] [CrossRef]
- Arai, K.; Jin, G.; Navaratna, D.; Lo, E.H. Brain angiogenesis in developmental and pathological processes: Neurovascular injury and angiogenic recovery after stroke. Febs J. 2009, 276, 4644–4652. [Google Scholar] [CrossRef]
- Chen, C.; Duckworth, C.A.; Fu, B.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.G. Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis. Br. J. Cancer 2014, 110, 741–752. [Google Scholar] [CrossRef]
- Etulain, J.; Negrotto, S.; Tribulatti, M.V.; Croci, D.O.; Carabelli, J.; Campetella, O.; Rabinovich, G.A.; Schattner, M. Control of Angiogenesis by Galectins Involves the Release of Platelet-Derived Proangiogenic Factors. PLoS ONE 2014, 9, e96402. [Google Scholar] [CrossRef]
- van der Hoeven, N.W.; Hollander, M.R.; Yıldırım, C.; Jansen, M.F.; Teunissen, P.F.; Horrevoets, A.J.; van der Pouw Kraan, T.C.; van Royen, N. The emerging role of galectins in cardiovascular disease. Vascul Pharmacol. 2016, 81, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.K.; Smith, E.E.; Coutts, S.B.; Welsh, D.G.; Faber, J.E.; Goyal, M.; Hill, M.D.; Demchuk, A.M.; Damani, Z.; Cho, K.H.; et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann. Neurol. 2013, 74, 241–248. [Google Scholar] [CrossRef]
- Garcia-Serrano, A.M.; Vieira, J.P.P.; Fleischhart, V.; Duarte, J.M.N. Taurine and N-acetylcysteine treatments prevent memory impairment and metabolite profile alterations in the hippocampus of high-fat diet-fed female mice. Nutr. Neurosci. 2022, 1–13. [Google Scholar] [CrossRef]
- Battistella, R.; Kritsilis, M.; Matuskova, H.; Haswell, D.; Cheng, A.X.; Meissner, A.; Nedergaard, M.; Lundgaard, I. Not All Lectins Are Equally Suitable for Labeling Rodent Vasculature. Int. J. Mol. Sci. 2021, 22, 11554. [Google Scholar] [CrossRef]
- Duarte, J.M.N.; Morgenthaler, F.D.; Gruetter, R. Glycogen Supercompensation in the Rat Brain After Acute Hypoglycemia is Independent of Glucose Levels During Recovery. Neurochem. Res. 2017, 42, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Berglund, G.; Nilsson, P.; Eriksson, K.F.; Nilsson, J.A.; Hedblad, B.; Kristenson, H.; Lindgärde, F. Long-term outcome of the Malmö preventive project: Mortality and cardiovascular morbidity. J. Intern. Med. 2000, 247, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Leosdottir, M.; Willenheimer, R.; Plehn, J.; Borgquist, R.; Gudmundsson, P.; Harris, T.B.; Launer, L.J.; Bjornsdottir, H.; Nilsson, P.M.; Gudnason, V. Myocardial structure and function by echocardiography in relation to glucometabolic status in elderly subjects from 2 population-based cohorts: A cross-sectional study. Am. Heart J. 2010, 159, 414–420.e4. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).