Molecular Farming of Pembrolizumab and Nivolumab
Abstract
1. Introduction
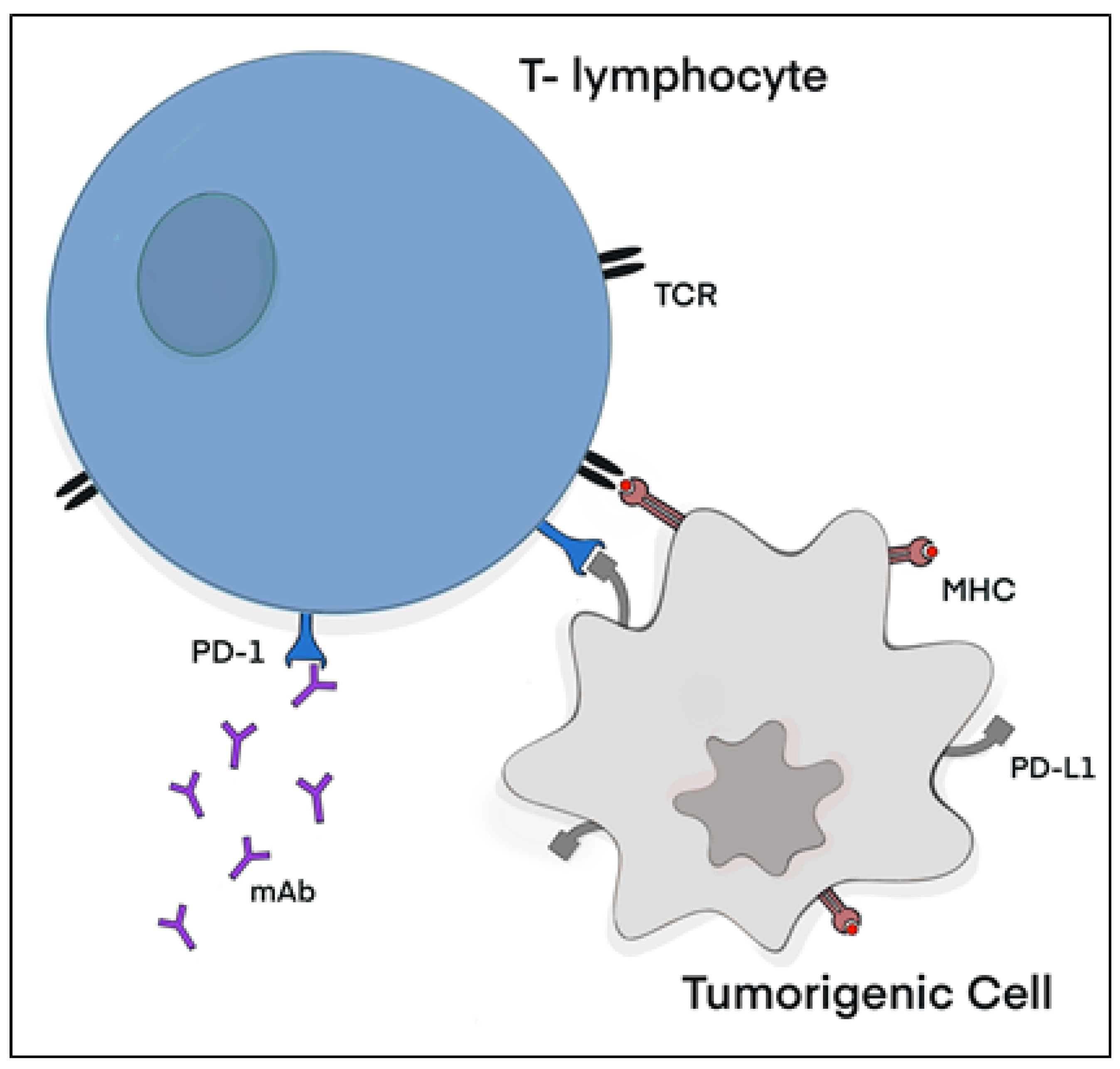
2. PD-1/PD-L1 Axis in Cancer
3. Monoclonal Antibodies
3.1. Immune Checkpoint Inhibitors
Pembrolizumab and Nivolumab
4. Traditional Manufacturing Methods
5. Molecular Farming
5.1. Vector Construction
5.2. Agroinfiltration and Plant Growth
5.3. Purification
5.4. Structural and Functional Assays
6. Advantages and Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 19 February 2023).
- Mattila, P.O.; Babar, Z.-U.-D.; Suleman, F. Assessing the Prices and Affordability of Oncology Medicines for Three Common Cancers within the Private Sector of South Africa. BMC Health Serv. Res. 2021, 21, 661. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S.; et al. Integrative Oncology: Addressing the Global Challenges of Cancer Prevention and Treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.; Longo, V.D. Starvation, Stress Resistance, and Cancer. Trends Endocrinol. Metab. 2018, 29, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Hervas-Stubbs, S.; Glennie, M.; Pardoll, D.M.; Chen, L. Immunostimulatory Monoclonal Antibodies for Cancer Therapy. Nat. Rev. Cancer 2007, 7, 95–106. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging New Therapeutic Antibody Derivatives for Cancer Treatment. Signal Transduct. Target Ther. 2022, 7, 39. [Google Scholar] [CrossRef]
- Moussavou, G.; Ko, K.; Lee, J.-H.; Choo, Y.-K. Production of Monoclonal Antibodies in Plants for Cancer Immunotherapy. BioMed Res. Int. 2015, 2015, 306164. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer Immunotherapy: Pros, Cons and Beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Franzin, R.; Netti, G.S.; Spadaccino, F.; Porta, C.; Gesualdo, L.; Stallone, G.; Castellano, G.; Ranieri, E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front. Immunol. 2020, 11, 574271. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Li, J.; Adhikari, R.; Fu, L. PD-1/PD-L1 Based Combinational Cancer Therapy: Icing on the Cake. Front. Pharmacol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Zhang, C.; Wang, Y.; Cheng, T.; Duan, L.; Tong, Z.; Tan, S.; Zhang, H.; Saw, P.E.; et al. Tumor Cell-Intrinsic PD-1 Receptor Is a Tumor Suppressor and Mediates Resistance to PD-1 Blockade Therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 6640–6650. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol 2017, 8, 561. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci 2020, 27, 1. [Google Scholar] [CrossRef]
- Phakham, T.; Bulaon, C.J.I.; Khorattanakulchai, N.; Shanmugaraj, B.; Buranapraditkun, S.; Boonkrai, C.; Sooksai, S.; Hirankarn, N.; Abe, Y.; Strasser, R.; et al. Functional Characterization of Pembrolizumab Produced in Nicotiana Benthamiana Using a Rapid Transient Expression System. Front. Plant Sci. 2021, 12, 1956. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Phakham, T.; Buranapraditkun, S.; Siriwattananon, K.; Boonkrai, C.; Pisitkun, T.; Hirankarn, N.; Strasser, R.; Abe, Y.; Phoolcharoen, W. Structural and In Vitro Functional Analyses of Novel Plant-Produced Anti-Human PD1 Antibody. Sci. Rep. 2019, 9, 15205. [Google Scholar] [CrossRef]
- Yokoyama, W.M.; Christensen, M.; Dos Santos, G.; Miller, D.; Ho, J.; Wu, T.; Dziegelewski, M.; Neethling, F.A. Production of Monoclonal Antibodies. Curr. Protoc. Immunol. 2013, 102, 2.5.1–2.5.29. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of Recombinant Antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yao, H.; Li, C.; Fang, J.-Y.; Xu, J. Regulation of PD-L1: Emerging Routes for Targeting Tumor Immune Evasion. Front. Pharmacol. 2018, 9, 536. [Google Scholar] [CrossRef]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 2362. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhan, P.; Song, Y. PD-L1 over-Expression and Survival in Patients with Non-Small Cell Lung Cancer: A Me-ta-Analysis. Transl. Lung Cancer Res. 2015, 4, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Zhang, H.; Zhou, Z.; Wang, Q. Regulation of PD-L1 Expression in Cancer and Clinical Implications in Immuno-therapy. Am. J. Cancer Res. 2020, 10, 1–11. [Google Scholar] [PubMed]
- Antonangeli, F.; Natalini, A.; Garassino, M.C.; Sica, A.; Santoni, A.; Di Rosa, F. Regulation of PD-L1 Expression by NF-ΚB in Cancer. Front. Immunol. 2020, 11, 584626. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feng, Y.; Lu, L.; Wang, H.; Dai, L.; Li, Y.; Zhang, P. Interferon-γ-Induced PD-L1 Surface Expression on Human Oral Squamous Carcinoma via PKD2 Signal Pathway. Immunobiology 2012, 217, 385–393. [Google Scholar] [CrossRef]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-Induced Activation of JAK1 and JAK2 Suppresses Tumor Cell Susceptibility to NK Cells through Upregulation of PD-L1 Expression. Oncoimmunology 2015, 4, e1008824. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Zeng, F.; Li, S.; Guo, W.; Chen, L.; Li, G.; Zhang, Z.; Wang, Q.J.; Deng, F. Protein Kinase Ds Promote Tumor Angiogenesis through Mast Cell Recruitment and Expression of Angiogenic Factors in Prostate Cancer Microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 114. [Google Scholar] [CrossRef]
- Guo, R.; Li, Y.; Wang, Z.; Bai, H.; Duan, J.; Wang, S.; Wang, L.; Wang, J. Hypoxia-Inducible Factor-1α and Nuclear Factor-ΚB Play Important Roles in Regulating Programmed Cell Death Ligand 1 Expression by Epidermal Growth Factor Receptor Mutants in Non-Small-Cell Lung Cancer Cells. Cancer Sci. 2019, 110, 1665–1675. [Google Scholar] [CrossRef]
- Lim, S.-O.; Li, C.-W.; Xia, W.; Cha, J.-H.; Chan, L.-C.; Wu, Y.; Chang, S.-S.; Lin, W.-C.; Hsu, J.-M.; Hsu, Y.-H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef]
- Green, M.R.; Monti, S.; Rodig, S.J.; Juszczynski, P.; Currie, T.; O’Donnell, E.; Chapuy, B.; Takeyama, K.; Neuberg, D.; Golub, T.R.; et al. Integrative Analysis Reveals Selective 9p24.1 Amplification, Increased PD-1 Ligand Expression, and Further Induction via JAK2 in Nodular Sclerosing Hodgkin Lymphoma and Primary Mediastinal Large B-Cell Lymphoma. Blood 2010, 116, 3268–3277. [Google Scholar] [CrossRef]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.M.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 Blockade with Nivolumab in Relapsed/Refractory Primary Central Nervous System and Testicular Lymphoma. Blood 2017, 129, 3071–3073. [Google Scholar] [CrossRef]
- Barret, M.T.; Anderson, K.S.; Lenkiewicz, E.; Andreozzi, M.; Cunliffe, H.E.; Klassen, C.L.; Dueck, A.C.; McCullough, A.E.; Reddy, S.K.; Ramanathan, R.K.; et al. Genomic Amplification of 9p24.1 Targeting JAK2, PD-L1, and PD-L2 Is Enriched in High-Risk Triple Negative Breast Cancer. Oncotarget 2015, 6, 26483–26493. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC Regulates the Antitumor Immune Response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef]
- Chang, F.; Steelman, L.S.; Lee, J.T.; Shelton, J.G.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Signal Transduction Mediated by the Ras/Raf/MEK/ERK Pathway from Cytokine Receptors to Transcription Factors: Potential Targeting for Therapeutic Intervention. Leukemia 2003, 17, 1263–1293. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.; He, Q.; Yang, B.; Cao, J. Small Molecule Inhibitors Targeting the PD-1/PD-L1 Signaling Pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Zak, K.M.; Grudnik, P.; Magiera, K.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Structure 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ Cytotoxic T Lymphocytes in Cancer Immunotherapy: A Review. J. Cell Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Trapani, J.A. Target Cell Apoptosis Induced by Cytotoxic T Cells and Natural Killer Cells Involves Synergy between the Pore-Forming Protein, Perforin, and the Serine Protease, Granzyme B. Aust. N. Z. J. Med. 1995, 25, 793–799. [Google Scholar] [CrossRef]
- Lin, D.Y.; Tanaka, Y.; Iwasaki, M.; Gittis, A.G.; Su, H.-P.; Mikami, B.; Okazaki, T.; Honjo, T.; Minato, N.; Garboczi, D.N. The PD-1/PD-L1 Complex Resembles the Antigen-Binding Fv Domains of Antibodies and T Cell Receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 3011–3016. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 Pathway in the Immune Response. Am. J. Transplant. 2012, 12, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- De Carné Trécesson, S.; Souazé, F.; Basseville, A.; Bernard, A.-C.; Pécot, J.; Lopez, J.; Bessou, M.; Sarosiek, K.A.; Letai, A.; Barillé-Nion, S.; et al. BCL-XL Directly Modulates RAS Signalling to Favour Cancer Cell Stemness. Nat. Commun. 2017, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, Q.; Zhang, R. PD-1/PD-L1 Pathway Blockade Works as an Effective and Practical Therapy for Cancer Immunotherapy. Cancer Biol. Med. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Pandolfi, P.P. The PTEN–PI3K Pathway: Of Feedbacks and Cross-Talks. Oncogene 2008, 27, 5527–5541. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.H. The History of Monoclonal Antibody Development—Progress, Remaining Challenges and Future Innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef]
- Wang, S.S.; Yan, Y.S.; Ho, K. US FDA-Approved Therapeutic Antibodies with High-Concentration Formulation: Summaries and Perspectives. Antib. Ther. 2021, 4, 262–272. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef]
- Janda, A.; Bowen, A.; Greenspan, N.S.; Casadevall, A. Ig Constant Region Effects on Variable Region Structure and Function. Front. Microbiol. 2016, 7, 22. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.; Tian, W. How to Select IgG Subclasses in Developing Anti-Tumor Therapeutic Antibodies. J. Hematol. Oncol. 2020, 13, 45. [Google Scholar] [CrossRef]
- van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef]
- Kellner, C.; Otte, A.; Cappuzzello, E.; Klausz, K.; Peipp, M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemotherapy 2017, 44, 327–336. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef]
- Tans, R.; van Rijswijck, D.M.H.; Davidson, A.; Hannam, R.; Ricketts, B.; Tack, C.J.; Wessels, H.J.C.T.; Gloerich, J.; van Gool, A.J. Affimers as an Alternative to Antibodies for Protein Biomarker Enrichment. Protein Expr. Purif. 2020, 174, 105677. [Google Scholar] [CrossRef]
- Urquhart, L. Top Companies and Drugs by Sales in 2021. Nat. Rev. Drug Discov. 2022, 21, 251. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.; Yang, X.; Long, J.; Bai, Y.; Yang, X.; Mao, Y.; Sang, X.; Seery, S.; Zhao, H. Combination Regimens with PD-1/PD-L1 Immune Checkpoint Inhibitors for Gastrointestinal Malignancies. J. Hematol. Oncol. 2019, 12, 42. [Google Scholar] [CrossRef]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Brunner-Weinzierl, M.C.; Rudd, C.E. CTLA-4 and PD-1 Control of T-Cell Motility and Migration: Implications for Tumor Immunotherapy. Front. Immunol. 2018, 9, 2737. [Google Scholar] [CrossRef]
- Lipson, E.J.; Drake, C.G. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clin. Cancer Res. 2011, 17, 6958–6962. [Google Scholar] [CrossRef]
- KEYTRUDA® (Pembrolizumab). Available online: https://www.keytruda.com/ (accessed on 2 April 2023).
- Raedler, L.A. Keytruda (Pembrolizumab): First PD-1 Inhibitor Approved for Previously Treated Unresectable or Metastatic Melanoma. Am. Health Drug Benefits 2015, 8, 96–100. [Google Scholar] [PubMed]
- Freshwater, T.; Kondic, A.; Ahamadi, M.; Li, C.H.; de Greef, R.; de Alwis, D.; Stone, J.A. Evaluation of Dosing Strategy for Pembrolizumab for Oncology Indications. J. Immunother. Cancer 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Dosing Schedule for KEYTRUDA® (Pembrolizumab)|HCP. Available online: https://www.keytrudahcp.com/dosing/options/ (accessed on 2 April 2023).
- OPDIVO® (Nivolumab). Available online: https://www.opdivo.com/ (accessed on 2 April 2023).
- Kooshkaki, O.; Derakhshani, A.; Hosseinkhani, N.; Torabi, M.; Safaei, S.; Brunetti, O.; Racanelli, V.; Silvestris, N.; Baradaran, B. Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 4427. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, Z.-J. Turning Cold Tumors into Hot Tumors by Improving T-Cell Infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Sevenich, L. Turning “Cold” Into “Hot” Tumors—Opportunities and Challenges for Radio-Immunotherapy Against Primary and Metastatic Brain Cancers. Front. Oncol. 2019, 9, 163. [Google Scholar] [CrossRef]
- Komiya, T.; Huang, C.H. Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Front. Oncol. 2018, 8, 423. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.-H.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Carrera, P.M.; Kantarjian, H.M.; Blinder, V.S. The Financial Burden and Distress of Patients with Cancer: Understanding and Stepping-up Action on the Financial Toxicity of Cancer Treatment. CA Cancer J. Clin. 2018, 68, 153–165. [Google Scholar] [CrossRef]
- Osei Afriyie, D.; Krasniq, B.; Hooley, B.; Tediosi, F.; Fink, G. Equity in Health Insurance Schemes Enrollment in Low and Middle-Income Countries: A Systematic Review and Meta-Analysis. Int. J. Equity Health 2022, 21, 21. [Google Scholar] [CrossRef]
- Karki, U.; Fang, H.; Guo, W.; Unnold-Cofre, C.; Xu, J. Cellular Engineering of Plant Cells for Improved Therapeutic Protein Production. Plant Cell Rep. 2021, 40, 1087–1099. [Google Scholar] [CrossRef]
- Hammers, C.M.; Stanley, J.R. Antibody Phage Display: Technique and Applications. J. Investig. Dermatol. 2014, 134, 1–5. [Google Scholar] [CrossRef]
- Moraes, J.Z.; Hamaguchi, B.; Braggion, C.; Speciale, E.R.; Cesar, F.B.V.; Soares, G. de F. da S.; Osaki, J.H.; Pereira, T.M.; Aguiar, R.B. Hybridoma Technology: Is It Still Useful? Curr. Res. Immunol. 2021, 2, 32–40. [Google Scholar] [CrossRef]
- Zheng, K.; Yarmarkovich, M.; Bantog, C.; Bayer, R.; Patapoff, T.W. Influence of Glycosylation Pattern on the Molecular Properties of Monoclonal Antibodies. mAbs 2014, 6, 649–658. [Google Scholar] [CrossRef]
- Aebi, M. N-Linked Protein Glycosylation in the ER. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2013, 1833, 2430–2437. [Google Scholar] [CrossRef]
- Mitra, S.; Tomar, P.C. Hybridoma Technology; Advancements, Clinical Significance, and Future Aspects. J. Genet. Eng. Biotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Parray, H.A.; Shukla, S.; Samal, S.; Shrivastava, T.; Ahmed, S.; Sharma, C.; Kumar, R. Hybridoma Technology a Versatile Method for Isolation of Monoclonal Antibodies, Its Applicability across Species, Limitations, Advancement and Future Perspectives. Int. Immunopharmacol. 2020, 85, 106639. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Shan, L.-L.; Liang, F.; Du, C.-Y.; Li, J.-J. Strategies and Considerations for Improving Recombinant Antibody Production and Quality in Chinese Hamster Ovary Cells. Front. Bioeng. Biotechnol. 2022, 10, 856049. [Google Scholar] [CrossRef]
- Mallbris, L.; Davies, J.; Glasebrook, A.; Tang, Y.; Glaesner, W.; Nickoloff, B.J. Molecular Insights into Fully Human and Humanized Monoclonal Antibodies. J. Clin. Aesthet. Dematol. 2016, 9, 13–15. [Google Scholar]
- Brüggemann, M.; Osborn, M.J.; Ma, B.; Hayre, J.; Avis, S.; Lundstrom, B.; Buelow, R. Human Antibody Production in Transgenic Animals. Arch. Immunol. Ther. Exp. 2015, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R. The Immunogenicity of Humanized and Fully Human Antibodies. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.T.; Dear, P.H.; Foote, J.; Neuberger, M.S.; Winter, G. Replacing the Complementarity-Determining Regions in a Human Antibody with Those from a Mouse. Nature 1986, 321, 522–525. [Google Scholar] [CrossRef]
- Diamos, A.G.; Hunter, J.G.L.; Pardhe, M.D.; Rosenthal, S.H.; Sun, H.; Foster, B.C.; DiPalma, M.P.; Chen, Q.; Mason, H.S. High Level Production of Monoclonal Antibodies Using an Optimized Plant Expression System. Front. Bioeng. Biotechnol. 2020, 7, 472. [Google Scholar] [CrossRef]
- Buyel, J.F. Plant Molecular Farming—Integration and Exploitation of Side Streams to Achieve Sustainable Biomanufacturing. Front. Plant Sci. 2019, 9, 1893. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; de La Vega, M.-A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola Virus Disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Bulaon, C.J.I.; Phoolcharoen, W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 2020, 9, 842. [Google Scholar] [CrossRef]
- Nessa, M.U.; Rahman, M.A.; Kabir, Y. Plant-Produced Monoclonal Antibody as Immunotherapy for Cancer. BioMed Res. Int. 2020, 2020, 3038564. [Google Scholar] [CrossRef]
- Fus-Kujawa, A.; Prus, P.; Bajdak-Rusinek, K.; Teper, P.; Gawron, K.; Kowalczuk, A.; Sieron, A.L. An Overview of Methods and Tools for Transfection of Eukaryotic Cells in Vitro. Front. Bioeng. Biotechnol. 2021, 9, 701031. [Google Scholar] [CrossRef]
- Schiavinato, M.; Marcet-Houben, M.; Dohm, J.C.; Gabaldón, T.; Himmelbauer, H. Parental Origin of the Allotetraploid Tobacco Nicotiana Benthamiana. Plant J. 2020, 102, 541–554. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel PEff Vector Based on the Genome of Potato Virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef]
- Bundock, P.; Hooykaas, P.J.J. Integration of Agrobacterium Tumefaciens T-DNA in the Saccharomyces Cerevisiae Genome by Illegitimate Recombination. Proc. Natl. Acad. Sci. USA 1996, 93, 15272–15275. [Google Scholar] [CrossRef]
- Voinnet, O.; Rivas, S.; Mestre, P.; Baulcombe, D. Retracted: An Enhanced Transient Expression System in Plants Based on Suppression of Gene Silencing by the P19 Protein of Tomato Bushy Stunt Virus. Plant J. 2003, 33, 949–956. [Google Scholar] [CrossRef]
- Horn, M.E.; Woodard, S.L.; Howard, J.A. Plant Molecular Farming: Systems and Products. Plant Cell Rep. 2004, 22, 711–720. [Google Scholar] [CrossRef]
- Young, J.L.; Dean, D.A. Chapter Three—Electroporation-Mediated Gene Delivery. In Advances in Genetics; Huang, L., Liu, D., Wagner, E., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 89, pp. 49–88. ISBN 0065-2660. [Google Scholar]
- Prudhomme, N.; Pastora, R.; Muselius, B.; McLean, M.D.; Cossar, D.; Geddes-McAlister, J. Exposure of Agrobacterium Tumefaciens to Agroinfiltration Medium Demonstrates Cellular Remodelling and May Promote Enhanced Adaptability for Molecular Pharming. Can. J. Microbiol. 2020, 67, 85–97. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust Estimation of Bacterial Cell Count from Optical Density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving Accuracy of Cell and Chromophore Concentration Measurements Using Optical Density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Chen, L.; Kuss, S.; Compton, R.G. Detection of Escherichia Coli Bacteria by Impact Electrochemistry. Analyst 2018, 143, 4840–4843. [Google Scholar] [CrossRef]
- Leuzinger, K.; Dent, M.; Hurtado, J.; Stahnke, J.; Lai, H.; Zhou, X.; Chen, Q. Efficient Agroinfiltration of Plants for High-Level Transient Expression of Recombinant Proteins. JoVE 2013, e50521. [Google Scholar] [CrossRef]
- Fujiuchi, N.; Matoba, N.; Matsuda, R. Environment Control to Improve Recombinant Protein Yields in Plants Based on Agrobacterium-Mediated Transient Gene Expression. Front. Bioeng. Biotechnol. 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Gkotsi, D.S.; Smith, D.R.M.; Goss, R.J.M.; Caputi, L.; O’Connor, S.E. Nicotiana Benthamiana as a Transient Expression Host to Produce Auxin Analogs. Front. Plant Sci. 2020, 11, 581675. [Google Scholar] [CrossRef] [PubMed]
- Goulet, M.-C.; Gaudreau, L.; Gagné, M.; Maltais, A.-M.; Laliberté, A.-C.; Éthier, G.; Bechtold, N.; Martel, M.; D’Aoust, M.-A.; Gosselin, A.; et al. Production of Biopharmaceuticals in Nicotiana benthamiana—Axillary Stem Growth as a Key Determinant of Total Protein Yield. Front. Plant Sci. 2019, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Mathur, J.; Griffiths, S.; Barton, K.; Schattat, M.H. Chapter Eight—Green-to-Red Photoconvertible MEosFP-Aided Live Imaging in Plants. In Methods in Enzymology; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 504, pp. 163–181. ISBN 0076-6879. [Google Scholar]
- Grom, M.; Kozorog, M.; Caserman, S.; Pohar, A.; Likozar, B. Protein a Affinity Chromatography of Chinese Hamster Ovary (CHO) Cell Culture Broths Containing Biopharmaceutical Monoclonal Antibody (MAb): Experiments and Mechanistic Transport, Binding and Equilibrium Modeling. J. Chromatogr. B 2018, 1083, 44–56. [Google Scholar] [CrossRef]
- Liu, H.F.; Ma, J.; Winter, C.; Bayer, R. Recovery and Purification Process Development for Monoclonal Antibody Production. mAbs 2010, 2, 480–499. [Google Scholar] [CrossRef]
- Rogers, D.M.; Jasim, S.B.; Dyer, N.T.; Auvray, F.; Réfrégiers, M.; Hirst, J.D. Electronic Circular Dichroism Spectroscopy of Proteins. Chem 2019, 5, 2751–2774. [Google Scholar] [CrossRef]
- Jang, A.; Cheon, D.; Hwang, E.; Kim, Y. Structural Stability of Cutibacterium Acnes Acyl Carrier Protein Studied Using CD and NMR Spectroscopy. J. Anal. Sci. Technol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Castilho, A.; Gattinger, P.; Grass, J.; Jez, J.; Pabst, M.; Altmann, F.; Gorfer, M.; Strasser, R.; Steinkellner, H. N-Glycosylation Engineering of Plants for the Biosynthesis of Glycoproteins with Bisected and Branched Complex N-Glycans. Glycobiology 2011, 21, 813–823. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Oh, Y.J.; Kajiura, H.; Hamorsky, K.T.; Fujiyama, K.; Matoba, N. Hydroponic Treatment of Nicotiana Benthamiana with Kifunensine Modifies the N-Glycans of Recombinant Glycoprotein Antigens to Predominantly Man9 High-Mannose Type upon Transient Overexpression. Front. Plant Sci. 2018, 9, 62. [Google Scholar] [CrossRef]
- Klimyuk, V.; Pogue, G.; Herz, S.; Butler, J.; Haydon, H. Production of Recombinant Antigens and Antibodies in Nicotiana Benthamiana Using ‘Magnifection’ Technology: GMP-Compliant Facilities for Small- and Large-Scale Manufacturing. In Plant Viral Vectors; Palmer, K., Gleba, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 127–154. ISBN 978-3-642-40829-8. [Google Scholar]
- Basaran, P.; Rodríguez-Cerezo, E. Plant Molecular Farming: Opportunities and Challenges. Crit. Rev. Biotechnol. 2008, 28, 153–172. [Google Scholar] [CrossRef]
- Chen, Q.; Lai, H. Gene Delivery into Plant Cells for Recombinant Protein Production. BioMed Res. Int. 2015, 2015, 932161. [Google Scholar] [CrossRef]
- Ma, J.K.-C.; Drossard, J.; Lewis, D.; Altmann, F.; Boyle, J.; Christou, P.; Cole, T.; Dale, P.; van Dolleweerd, C.J.; Isitt, V.; et al. Regulatory Approval and a First-in-Human Phase I Clinical Trial of a Monoclonal Antibody Produced in Transgenic Tobacco Plants. Plant Biotechnol. J. 2015, 13, 1106–1120. [Google Scholar] [CrossRef]
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical Analysis of the Commercial Potential of Plants for the Production of Recombinant Proteins. Front. Plant Sci. 2019, 10, 720. [Google Scholar] [CrossRef]
- Reshetnikova, M.; Pugacheva, I.; Lukina, Y. Trends of the German Biotech Market. E3S Web Conf. 2021, 295, 01037. [Google Scholar] [CrossRef]

| mAb | Target | FDA Approval Year | Main Indications | Mechanism of Action |
|---|---|---|---|---|
| Pembrolizumab (Keytruda®, Merck, NJ, USA) | PD-1 | 2014 | Melanoma, head and neck cancer, NSCLC, lymphoma, kidney, breast, esophageal, colorectal, endometrial, urothelial and cervical cancer. | Inhibition of PD-1/PD-L1 immune checkpoint |
| Nivolumab (OPDVIO®, Bristol-Myers Squibb, NY, USA) | PD-1 | 2014 | Melanoma, head and neck cancer, NSCLC, pleural mesothelioma, lymphoma, kidney, liver, colorectal, stomach, esophageal and urothelial cancer. | Inhibition of PD-1/PD-L1 immune checkpoint |
| Bevacizumab (Avastin®, San Francisco, CA, USA) | VEGF-A | 2004 | Colorectal cancer, NSCLC, renal cell carcinoma, glioblastoma, breast, ovarian and cervical cancer | Inhibition of angiogenesis |
| Trastuzumab (Herceptin®, San Francisco, CA, USA) | HER2 | 1998 | Breast cancer, esophageal cancer and gastric cancer | Inhibition of HER2 mediated cell signaling pathways |
| Rituximab (Rituxan®, San Francisco, CA, USA) | CD20 | 1997 | Non-Hodgkin’s lymphoma and chronic lymphocytic leukemia | Activation of Fc-effector functions (ADCC, ADCP and CDC) |
| mAb | Structure | Expression | Price (2022–2023) | Average Duration of Course |
|---|---|---|---|---|
| Pembrolizumab | Humanized IgG4 | Recombinant Chinese hamster ovary (CHO) cells | USD 10,683 per 200 mg infusion every 3 weeks | 2 years |
| Nivolumab | Human IgG4 | Recombinant CHO cells | USD 7194 per 240 mg infusion every 2 weeks | 2 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stark, M.C.; Joubert, A.M.; Visagie, M.H. Molecular Farming of Pembrolizumab and Nivolumab. Int. J. Mol. Sci. 2023, 24, 10045. https://doi.org/10.3390/ijms241210045
Stark MC, Joubert AM, Visagie MH. Molecular Farming of Pembrolizumab and Nivolumab. International Journal of Molecular Sciences. 2023; 24(12):10045. https://doi.org/10.3390/ijms241210045
Chicago/Turabian StyleStark, Michael C., Anna M. Joubert, and Michelle H. Visagie. 2023. "Molecular Farming of Pembrolizumab and Nivolumab" International Journal of Molecular Sciences 24, no. 12: 10045. https://doi.org/10.3390/ijms241210045
APA StyleStark, M. C., Joubert, A. M., & Visagie, M. H. (2023). Molecular Farming of Pembrolizumab and Nivolumab. International Journal of Molecular Sciences, 24(12), 10045. https://doi.org/10.3390/ijms241210045






