High Plasma Angiopoietin-2 Levels Predict the Need to Initiate Dialysis within Two Years in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Results
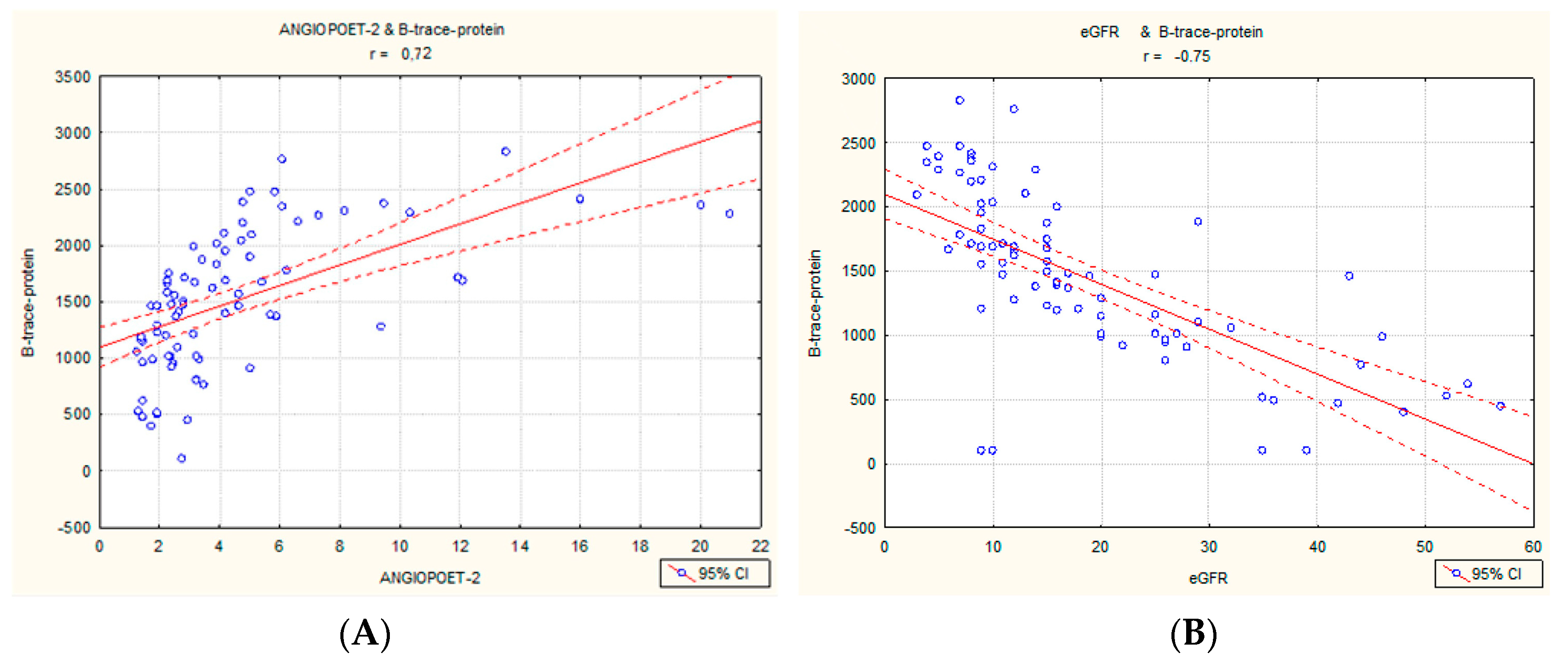
2.1. Correlations in the Entire Cohort
2.2. Univariate and Multivariate Regression
2.3. Ang-2, VCAM, and B-Trace Protein in Progressors vs. Nonprogressors
2.4. Impact of Angiopoetin-2 on Renal Survival (RRT Initiation)
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Study Design
4.2.1. Lung Ultrasonography (B-Lines)
4.2.2. Echocardiography with GLS
4.2.3. Bioimpedance
4.2.4. Six-Minute Walk Test
4.2.5. Quantification of Circulating Biomarkers
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, J.-C.; Zhang, L.-X. Prevalence and Disease Burden of Chronic Kidney Disease; Springer: Singapore, 2019; Volume 1165. [Google Scholar] [CrossRef]
- Gentile, G.; Remuzzi, G. Novel Biomarkers for Renal Diseases ? None for the Moment (but One). SLAS Discov. Adv. Sci. Drug Discov. 2016, 21, 655–670. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin—Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.A.; Tzvetkova, D.; Nikolov, D.B. Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure 2005, 13, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, J.C.F.; Liu, Y.; Etheridge, A.S.; Yazdani, A.; Kindler, H.L.; Kelly, W.K.; Nixon, A.B.; Innocenti, F. Plasma levels of angiopoietin-2, VEGF-A, and VCAM-1 as markers of bevacizumab-induced hypertension: CALGB 80303 and 90401 (Alliance). Angiogenesis 2022, 25, 47–55. [Google Scholar] [CrossRef]
- Yuan, H.T.; Khankin, E.V.; Karumanchi, S.A.; Parikh, S.M. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol. Cell Biol. 2009, 29, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef]
- Daly, C.; Pasnikowski, E.; Burova, E.; Wong, V.; Aldrich, T.H.; Griffiths, J.; Ioffe, E.; Daly, T.J.; Fandl, J.P.; Papadopoulos, N.; et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15491–15496. [Google Scholar] [CrossRef]
- Ong, T.; McClintock, D.E.; Kallet, R.H.; Ware, L.B.; Matthay, M.A.; Liu, K.D. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit. Care Med. 2010, 38, 1845–1851. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Richard-Greenblatt, M.; Wright, J.; Crowley, V.M.; Kain, K.C. Endothelial Activation: The Ang/Tie Axis in Sepsis. Front. Immunol. 2018, 9, 838. [Google Scholar] [CrossRef]
- Eklund, L.; Kangas, J.; Saharinen, P. Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clin. Sci. 2017, 131, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Pfister, F.; Wang, Y.; Schreiter, K.; Hagen, F.V.; Altvater, K.; Hoffmann, S.; Deutsch, U.; Hammes, H.-P.; Feng, Y. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol. 2010, 47, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, E.; Lorentz, P.; Kopfstein, L.; Christofori, G. Angiopoietin-1 and -2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 2011, 71, 5717–5727. [Google Scholar] [CrossRef]
- Chong, A.Y.; Caine, G.J.; Freestone, B.; Blann, A.D.; Lip, G.Y. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J. Am. Coll. Cardiol. 2004, 43, 423–428. [Google Scholar] [CrossRef]
- Lim, H.S.; Lip, G.Y.; Blann, A.D. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: Relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 2005, 180, 113–118. [Google Scholar] [CrossRef]
- Hobohm, L.; Kölmel, S.; Niemann, C.; Kümpers, P.; Krieg, V.J.; Bochenek, M.L.; Lukasz, A.H.; Reiss, Y.; Plate, K.-H.; Liebetrau, C.; et al. Role of angiopoietin-2 in venous thrombus resolution and chronic thromboembolic disease. Eur. Respir. J. 2021, 58, 2004196. [Google Scholar] [CrossRef]
- Desideri, L.F.; Traverso, C.E.; Nicolò, M. The emerging role of the Angiopoietin-Tie pathway as therapeutic target for treating retinal diseases. Expert Opin. Ther. Targets 2022, 26, 145–154. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Chiu, Y.-W.; Kuo, H.-T.; Lee, J.-J.; Lee, S.-C.; Chen, T.-H.; Lin, M.-Y.; Hwang, S.-J.; Kuo, M.-C.; Hsu, Y.-L.; et al. The interaction between fluid status and angiopoietin-2 in adverse renal outcomes of chronic kidney disease. PLoS ONE 2017, 12, e0173906. [Google Scholar] [CrossRef]
- Hennings, A.; Hannemann, A.; Rettig, R.; Dörr, M.; Nauck, M.; Völzke, H.; Lerch, M.M.; Lieb, W.; Friedrich, N. Circulating Angiopoietin-2 and Its Soluble Receptor Tie-2 Concentrations Are Related to Renal Function in Two Population-Based Cohorts. PLoS ONE 2016, 11, e0166492. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Chiu, Y.-W.; Tsai, J.-C.; Kuo, H.-T.; Lee, S.-C.; Hung, C.-C.; Lin, M.-Y.; Hwang, S.-J.; Kuo, M.-C.; Chen, H.-C. Association of angiopoietin-2 with renal outcome in chronic kidney disease. PLoS ONE 2014, 9, e108862. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Lee, C.-S.; Chiu, Y.-W.; Lee, J.-J.; Lee, S.-C.; Hsu, Y.-L.; Kuo, M.-C. Angiopoietin-2, Renal Deterioration, Major Adverse Cardiovascular Events and All- Cause Mortality in Patients with Diabetic Nephropathy. Kidney Blood Press. Res. 2018, 43, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef]
- Lee, K.W.; Lip, G.Y.H.; Blann, A.D. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation 2004, 110, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Lee, C.-S.; Chiu, Y.-W.; Kuo, H.-T.; Lee, S.-C.; Hwang, S.-J.; Kuo, M.-C.; Chen, H.-C. Angiopoietin-2, Angiopoietin-1 and subclinical cardiovascular disease in Chronic Kidney Disease. Sci. Rep. 2016, 6, 39400. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Lee, C.-S.; Chiu, Y.-W.; Kuo, H.-T.; Lee, S.-C.; Hwang, S.-J.; Kuo, M.-C.; Chen, H.-C. Angiopoietin-2 as a Prognostic Biomarker of Major Adverse Cardiovascular Events and All- Cause Mortality in Chronic Kidney Disease. PLoS ONE 2015, 10, e0135181. [Google Scholar] [CrossRef]
- Chang, F.-C.; Liu, C.-H.; Luo, A.-J.; Huang, T.T.-M.; Tsai, M.-H.; Chen, Y.-J.; Lai, C.-F.; Chiang, C.-K.; Lin, T.-H.; Chiang, W.-C.; et al. Angiopoietin-2 inhibition attenuates kidney fibrosis by hindering chemokine C-C motif ligand 2 expression and apoptosis of endothelial cells. Kidney Int. 2022, 102, 780–797. [Google Scholar] [CrossRef]
- Bontekoe, J.; Lee, J.; Bansal, V.; Syed, M.; Hoppensteadt, D.; Maia, P.; Walborn, A.; Liles, J.; Brailovsky, E.; Fareed, J. Biomarker Profiling in Stage 5 Chronic Kidney Disease Identifies the Relationship between Angiopoietin-2 and Atrial Fibrillation. Clin. Appl. Thromb. 2018, 24, 269S–276S. [Google Scholar] [CrossRef]
- Park, J.-H.; Marwick, T.H. Use and Limitations of E/e’ to Assess Left Ventricular Filling Pressure by Echocardiography. J. Cardiovasc. Ultrasound 2011, 19, 169–173. [Google Scholar] [CrossRef]
- Baber, U.; Gutierrez, O.M.; Levitan, E.B.; Warnock, D.G.; Farkouh, M.E.; Tonelli, M.; Safford, M.M.; Muntner, P. Risk for recurrent coronary heart disease and all-cause mortality among individuals with chronic kidney disease compared with diabetes mellitus, metabolic syndrome, and cigarette smokers. Am. Heart J. 2013, 166, 373–380.e2. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yang, J.-W.; Yoo, J.S.; Choi, S.O.; Han, B.-G. Association between E/e’ ratio and fluid overload in patients with predialysis chronic kidney disease. PLoS ONE 2017, 12, e0184764. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Q.; Zhi, G.; Zhang, L.; Huang, D.; Dangsheng, H.; Zhang, M. The application of lung ultrasound in acute decompensated heart failure in heart failure with preserved and reduced ejection fraction. Echocardiography 2017, 34, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Lousa, I.; Reis, F.; Beirão, I.; Alves, R.; Belo, L.; Santos-Silva, A. New Potential Biomarkers for Chronic Kidney Disease Management-A Review of the Literature. Int. J. Mol. Sci. 2020, 22, 43. [Google Scholar] [CrossRef]
- Mansour, S.G.; Bhatraju, P.K.; Coca, S.G.; Obeid, W.; Wilson, F.P.; Stanaway, I.B.; Jia, Y.; Thiessen-Philbrook, H.; Go, A.S.; Ikizler, T.A.; et al. Angiopoietins as Prognostic Markers for Future Kidney Disease and Heart Failure Events after Acute Kidney Injury. J. Am. Soc. Nephrol. 2022, 33, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Frassi, F.; Agricola, E.; Gligorova, S.; Gargani, L.; Mottola, G. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J. Am. Soc. Echocardiogr. 2006, 19, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Benedetto, F.A.; Tripepi, R.; Rastelli, S.; Castellino, P.; Tripepi, G.; Picano, E.; Zoccali, C. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc. Imaging 2010, 3, 586–594. [Google Scholar] [CrossRef]
- Picano, E.; Gargani, L.; Gheorghiade, M. Why, when, and how to assess pulmonary congestion in heart failure: Pathophysiological, clinical, and methodological implications. Heart Fail. Rev. 2010, 15, 63–72. [Google Scholar] [CrossRef]
- Torino, C.; Gargani, L.; Sicari, R.; Letachowicz, K.; Ekart, R.; Fliser, D.; Covic, A.; Siamopoulos, K.; Stavroulopoulos, A.; Massy, Z.A.; et al. The agreement between auscultation and lung ultrasound in hemodialysis patients: The LUST study. Clin. J. Am. Soc. Nephrol. 2016, 11, 2005–2011. [Google Scholar] [CrossRef]
- Enright, P.L. The Six-Minute Walk Test. Respir. Care 2003, 48, 783–785. Available online: http://rc.rcjournal.com/content/respcare/48/8/783.full.pdf (accessed on 1 May 2023).

| Parameter | Mean | Median | 25–75% [IQR] |
|---|---|---|---|
| Age (years) | 52.6 | 52.0 | 42–66 |
| Body mass index (kg/m2) | 25.9 | 25.7 | 22.2–27.9 |
| 6-MWT (m) | 407.6 | 420.0 | 360–480 |
| Sys BP (mmHg) | 144.3 | 144.0 | 127–160 |
| Dia BP (mmHg) | 87.4 | 85.0 | 80–100 |
| Heart rate (1/min) | 71.6 | 70.0 | 62–81 |
| Biochemistry | |||
| Serum creatinine (mg/dL) | 4.3 | 3.8 | 2.3–5.8 |
| eGFR (mL/min/1.73 m2) | 21.9 | 16.0 | 10–28 |
| Urea (mg/dL) | 105.2 | 97.0 | 64–142 |
| BNP (ng/mL) | 376.7 | 103.3 | 37.6–287.8 |
| cTnI (ng/dL) | 17.4 | 8.9 | 4.3–18.8 |
| Hemoglobin (mmol/L) | 11.2 | 11.0 | 9.6–12.4 |
| Sodium (mmol/L) | 140.3 | 140.0 | 139–142 |
| Urine osmolality (mOsm/kg H2O) | 338.3 | 335.5 | 259–402 |
| Serum osmolality (mOsm/kg H2O) | 303.2 | 303.5 | 295–313 |
| Uric acid (mg/dL) | 7.3 | 7.3 | 6.3–8.3 |
| Serum albumin (g/dL) | 3.6 | 3.6 | 3.2–4.1 |
| Total protein (g/dL) | 6.2 | 6.2 | 5.5–6.9 |
| C-reactive protein (mg/L) | 7.7 | 2.4 | 1.1–6 |
| Procalcitonin (ng/mL) | 0.1 | 0.1 | 0.0–0.1 |
| Ultrasound examination | |||
| B-lines (Lung US) | 10.8 | 7.0 | 3–13 |
| LVEF (%) | 61.4 | 65.0 | 60–65 |
| GLS | −17.6 | −18.0 | −19.8–−14.9 |
| Mitral E/e′ | 8.9 | 8.3 | 6.7–10.2 |
| LVM index | 130.7 | 123.0 | 99–156 |
| Bioimpedance | |||
| ECW (L) | 22.5 | 21.7 | 17.1–26.1 |
| ICW (L) | 23.8 | 23.2 | 18–27.4 |
| ECW/ICW | 1.0 | 0.9 | 0.8–1.1 |
| Biomarkers of congestion or endothelial injury/activation | |||
| VCAM-1 (ng/mL) | 1476.1 | 1358.0 | 1081.5–1759.0 |
| ANGIOPOETIN-2 (ng/mL) | 4.3 | 3.1 | 2.3–5.0 |
| VEGF-C (ng/mL) | 4.9 | 4.7 | 3.7–5.8 |
| Copeptin (pg/mL) | 893.8 | 574.0 | 342.5–1432.5 |
| B-trace-protein (ng/mL) | 1537.4 | 1497.0 | 1095–1993 |
| BMI | ECW/ ICW | 6 MWT | B-Lines | e/e′ | EF | GLS | VCAM-1 | Ang-2 | VEGF-C | CPP | B-TP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.14 | 0.35 * | −0.47 | 0.13 | 0.28 | 0.02 | 0.10 | −0.10 | −0.06 | −0.19 | −0.33 | −0.12 |
| BNP | −0.11 | 0.46 | −0.38 | 0.40 | 0.42 | −0.10 | 0.28 | 0.50 | 0.58 | −0.12 | 0.00 | 0.46 |
| cTnI | −0.16 | 0.31 | −0.35 | 0.32 | 0.45 | −0.13 | 0.41 | 0.44 | 0.42 | −0.02 | −0.13 | 0.15 |
| Hemoglobin | 0.19 | −0.48 | 0.33 | −0.33 | −0.01 | 0.01 | −0.01 | −0.21 | −0.48 | 0.20 | −0.01 | −0.39 |
| Na | −0.12 | 0.28 | −0.11 | 0.05 | −0.09 | 0.02 | −0.18 | 0.18 | 0.17 | −0.11 | −0.07 | −0.01 |
| U osmolality | 0.18 | 0.06 | 0.20 | −0.02 | −0.03 | 0.21 | 0.06 | −0.40 | −0.30 | −0.03 | −0.12 | −0.45 |
| S osmolality | 0.08 | 0.06 | −0.11 | 0.11 | −0.11 | 0.06 | −0.11 | 0.09 | 0.23 | −0.02 | −0.02 | 0.22 |
| Uric acid | 0.26 | 0.01 | 0.10 | −0.10 | −0.28 | 0.13 | −0.35 | −0.17 | −0.18 | −0.21 | −0.03 | −0.42 |
| S creatinine | −0.10 | 0.08 | −0.09 | 0.26 | −0.04 | −0.11 | 0.03 | 0.48 | 0.57 | 0.03 | −0.01 | 0.74 |
| eGFR | 0.20 | −0.20 | 0.19 | −0.29 | −0.02 | 0.11 | −0.04 | −0.49 | −0.61 | 0.12 | −0.12 | −0.75 |
| urea | −0.13 | 0.23 | −0.18 | 0.26 | −0.09 | −0.02 | −0.05 | 0.25 | 0.30 | −0.09 | 0.10 | 0.23 |
| S albumin | 0.13 | −0.35 | 0.15 | −0.08 | −0.12 | −0.15 | −0.03 | −0.09 | 0.02 | −0.20 | −0.12 | 0.19 |
| CRP | −0.12 | 0.26 | −0.31 | 0.24 | 0.17 | −0.01 | 0.01 | 0.24 | 0.34 | −0.14 | −0.15 | 0.19 |
| procalcitonin | −0.11 | 0.24 | −0.37 | 0.37 | 0.36 | −0.16 | 0.14 | 0.58 | 0.56 | 0.08 | −0.18 | 0.61 |
| Univariate Regression | ||||
|---|---|---|---|---|
| Risk Factor | HR | 2.5% | 97.5% | p-Value |
| VCAM-1 | 1.0010 | 1.0000 | 1.0010 | 0.0017 |
| Ang-2 | 1.1770 | 1.0380 | 1.3350 | 0.0113 |
| B-trace-protein | 1.0010 | 1.0010 | 1.0020 | 0.0001 |
| VEGF-C | 1.0000 | 0.9968 | 1.0001 | 0.4520 |
| eGFR | 0.8867 | 0.8477 | 0.9276 | 0.0000 |
| BNP | 1.0000 | 0.9998 | 1.0010 | 0.2520 |
| Copeptin | 0.9998 | 0.9994 | 1.0000 | 0.5280 |
| Regression | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Risk Factor | HR | 2.5% | 97.5% | p-Value | HR | 2.5% | 97.5% | p-Value |
| 1 | VCAM-1 | 1.0010 | 1.0000 | 1.0010 | 0.0017 | 1.0007 | 1.0001 | 1.0010 | 0.0144 |
| Ang-2 | 1.1770 | 1.0380 | 1.3350 | 0.0113 | 1.1179 | 0.9801 | 1.2750 | 0.0968 | |
| Copeptin | 0.9998 | 0.9994 | 1.0000 | 0.5280 | 0.9999 | 0.9994 | 1.0000 | 0.7679 | |
| 2 | Ang-2 | 1.1770 | 1.0380 | 1.3350 | 0.0113 | 1.1790 | 1.0157 | 1.3690 | 0.0304 |
| BNP | 1.0000 | 0.9998 | 1.0010 | 0.2520 | 1.0000 | 0.9994 | 1.0010 | 0.9582 | |
| Parameter | Group I, n = 31 (Nonprogressors) | Group II, n = 47 (Progressors) | Dialysis Group (CKD5-HD), n = 22 | p < 0.05 | |||
|---|---|---|---|---|---|---|---|
| Median | 25–75% [IQR] | Median | 25–75% [IQR] | Median | 25–75% [IQR] | ||
| Age (years) | 51.0 | 42–64 | 59.0 | 44–68 | 52.5 | 38.5–65.5 | Ns |
| Body mass index (kg/m2) | 24.6 | 22.3–27 | 25.7 | 22.3–28 | 25.4 | 21.5–30 | Ns |
| 6-MWT (m) | 440.0 | 395–500 | 400.0 | 360–475 | 390.0 | 320–460 | I vs. II, I vs. HD |
| Sys BP (mmHg) | 144.0 | 125–150 | 140.0 | 127–150 | 149.5 | 127.5–170 | Ns |
| Dia BP (mmHg) | 85.0 | 80–94 | 83.0 | 80–93 | 97.0 | 80–100 | I vs. HD, II vs. HD |
| Heart rate (1/min) | 71.5 | 63–75 | 70.0 | 62–80 | 69.0 | 58–90 | Ns |
| Serum creatinine (mg/dL) | 2.3 | 1.5–3.2 | 3.8 | 2.4–5.2 | 6.7 | 5.4–7.6 | I vs. II, I vs. HD, II vs. HD |
| eGFR (mL/min/1.73 m2) | 28.0 | 22–46 | 17.0 | 11–27 | 9.0 | 7.5–11 | I vs. II, I vs. HD, II vs. HD |
| 24 M eGFR (mL/min/1.73 m2) | 31.0 | 23–50 | 6.0 | 5–13 | 6.0 | 30–54 | I vs. II, I vs. HD |
| Urea (mg/dL) | 76.0 | 58–93 | 112.0 | 66–142 | 131.0 | 70–158 | I vs. II, I vs. HD |
| BNP (ng/mL) | 56.6 | 23.2–208 | 105.2 | 48–188.6 | 274.5 | 54.8–856 | I vs. II, I vs. HD, II vs. HD |
| cTnI (ng/dL) | 8.3 | 3.6–16.8 | 8.2 | 4.1–13.7 | 15.9 | 4.4–30 | Ns |
| Hemoglobin (mmol/L) | 11.7 | 10.3–13 | 11.0 | 9.6–12.4 | 10.1 | 9.2–11.9 | I vs. HD |
| Sodium (mmol/L) | 140.0 | 138–143 | 140.0 | 139–142 | 140.0 | 139–141 | Ns |
| Urine osmolality (mOsm/kg H2O) | 402.0 | 339–450 | 319.0 | 248–399 | 293.0 | 239–326 | I vs. II, I vs. HD, II vs. HD |
| Serum osmolality (mOsm/kg H2O) | 297.0 | 291–306 | 304.5 | 299–317 | 309.0 | 296–313 | I vs. II, I vs. HD, II vs. HD |
| Uric acid (mg/dL) | 7.5 | 6.6–8.3 | 7.4 | 6.4–8.4 | 6.7 | 4.2–8.1 | Ns |
| Serum albumin (g/dL) | 3.5 | 2.8–4 | 3.5 | 3.2–4 | 3.9 | 3.3–4.2 | Ns |
| C-reactive protein (mg/L) | 2.1 | 1.1–6.4 | 1.8 | 0.8–5 | 5.0 | 2–9.2 | Ns |
| Procalcitonin (ng/mL) | 0.05 | 0.0–0.1 | 0.07 | 0.0–0.1 | 0.18 | 0.1–0.3 | I vs. HD, II vs. HD |
| B-lines (LUS) | 5.0 | 3–12 | 7.0 | 4–13 | 8.0 | 5.5–22 | I vs. II, I vs. HD, II vs. HD |
| ECW (L) | 21.1 | 17.9–24.4 | 23.3 | 17.7–26.4 | 20.7 | 15.9–28 | Ns |
| ICW (L) | 24.9 | 18–28.3 | 23.8 | 20–27.1 | 22.7 | 18–26.4 | Ns |
| ECW/ICW | 0.9 | 0.8–1 | 1.0 | 0.8–1.1 | 0.9 | 0.9–1.1 | Ns |
| Ejection fraction (%) | 65.0 | 60–65 | 65.0 | 60–65 | 60.0 | 55–65 | Ns |
| GLS | −18.9 | −20–−16.7 | −18.2 | −21.5–−15 | −15.9 | −19.1–−13.4 | Ns |
| Mitral E/e′ | 7.0 | 6.2 | 8.2 | 6.4–10 | 10.0 | 7.4–11 | Ns |
| LVM index | 110.0 | 100–134 | 125.0 | 94–153 | 139.0 | 103–187 | I vs. HD |
| VCAM-1 (ng/mL) | 1134.5 | 1005–1415 | 1300.0 | 1076–1636 | 1685.0 | 1386.5–2240 | I vs. II, I vs. HD, II vs. HD |
| Ang-2 (ng/mL) | 2.4 | 1.9–3.5 | 2.9 | 2.2–4.2 | 5.1 | 4.2–8.1 | I vs. II, I vs. HD, II vs. HD |
| VEGF-C (ng/mL) | 4.7 | 3.7–6 | 5.0 | 4.3–5.9 | 4.6 | 3.0–5.6 | Ns |
| Copeptin (pg/mL) | 490.0 | 271–739 | 572.0 | 357–960 | 362.5 | 322–576 | II vs. HD |
| B-trace protein (ng/mL) | 963.0 | 562.5–1364 | 1406.0 | 1014–1711 | 2088.0 | 1683–2374 | I vs. II, I vs. HD, II vs. HD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, A.; Kusztal, M.; Gołębiowski, T.; Letachowicz, K.; Goździk, A.; Kościelska-Kasprzak, K.; Tukiendorf, A.; Krajewska, M. High Plasma Angiopoietin-2 Levels Predict the Need to Initiate Dialysis within Two Years in Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 10036. https://doi.org/10.3390/ijms241210036
Szymczak A, Kusztal M, Gołębiowski T, Letachowicz K, Goździk A, Kościelska-Kasprzak K, Tukiendorf A, Krajewska M. High Plasma Angiopoietin-2 Levels Predict the Need to Initiate Dialysis within Two Years in Patients with Chronic Kidney Disease. International Journal of Molecular Sciences. 2023; 24(12):10036. https://doi.org/10.3390/ijms241210036
Chicago/Turabian StyleSzymczak, Anna, Mariusz Kusztal, Tomasz Gołębiowski, Krzysztof Letachowicz, Anna Goździk, Katarzyna Kościelska-Kasprzak, Andrzej Tukiendorf, and Magdalena Krajewska. 2023. "High Plasma Angiopoietin-2 Levels Predict the Need to Initiate Dialysis within Two Years in Patients with Chronic Kidney Disease" International Journal of Molecular Sciences 24, no. 12: 10036. https://doi.org/10.3390/ijms241210036
APA StyleSzymczak, A., Kusztal, M., Gołębiowski, T., Letachowicz, K., Goździk, A., Kościelska-Kasprzak, K., Tukiendorf, A., & Krajewska, M. (2023). High Plasma Angiopoietin-2 Levels Predict the Need to Initiate Dialysis within Two Years in Patients with Chronic Kidney Disease. International Journal of Molecular Sciences, 24(12), 10036. https://doi.org/10.3390/ijms241210036






