Secreted Cytokines within the Urine of AKI Patients Modulate TP53 and SIRT1 Levels in a Human Podocyte Cell Model
Abstract
1. Introduction
2. Results
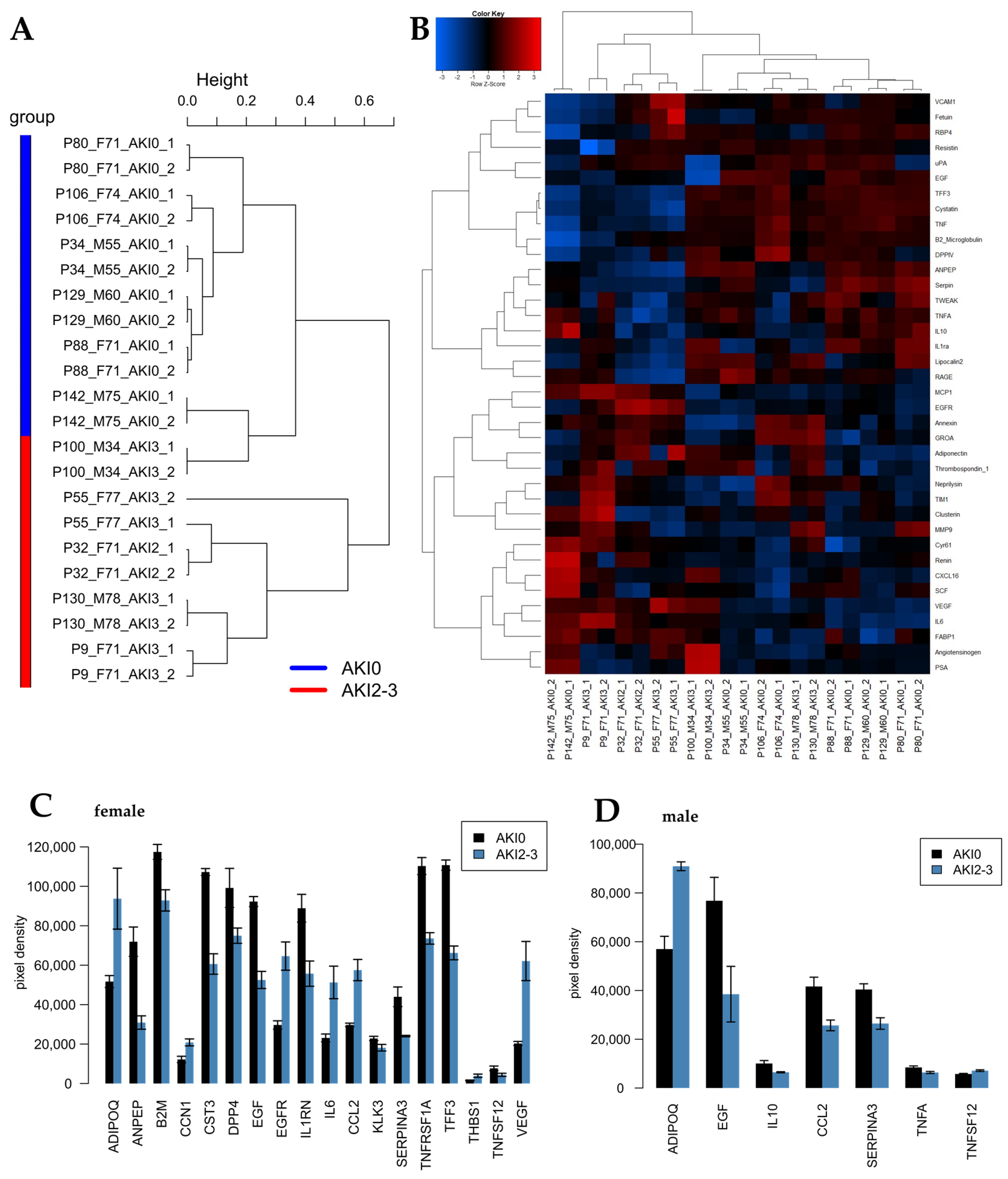
2.1. Identification of Cytokines Upregulated in Urine Derived from AKI Patients 24 h Post-Operation
2.2. Identification of Cytokines Upregulated in Urine Derived from AKI Patients 72 h Post-Operation
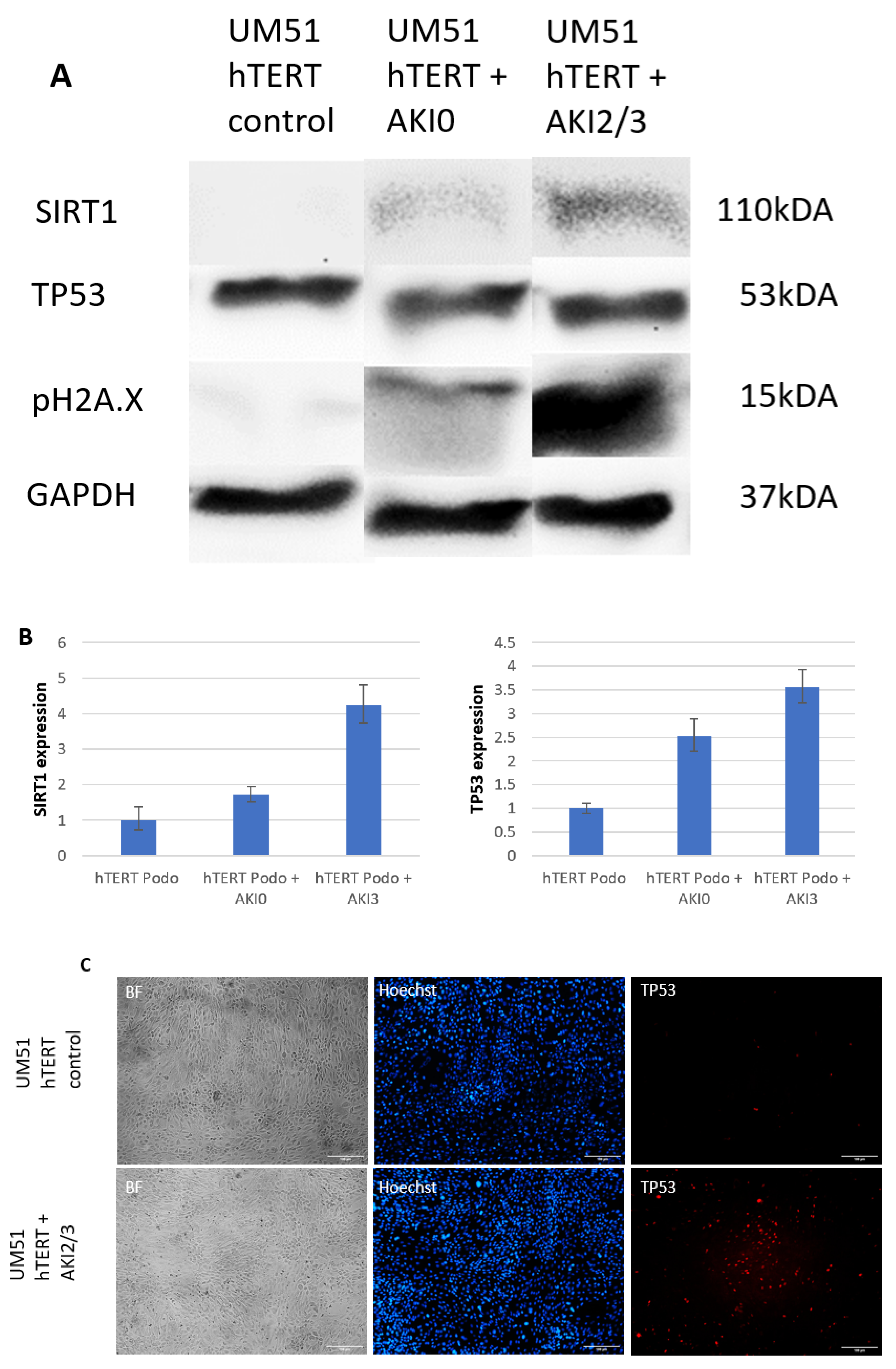
2.3. Effect of AKI Stage 2/3 Urine on Podocytes
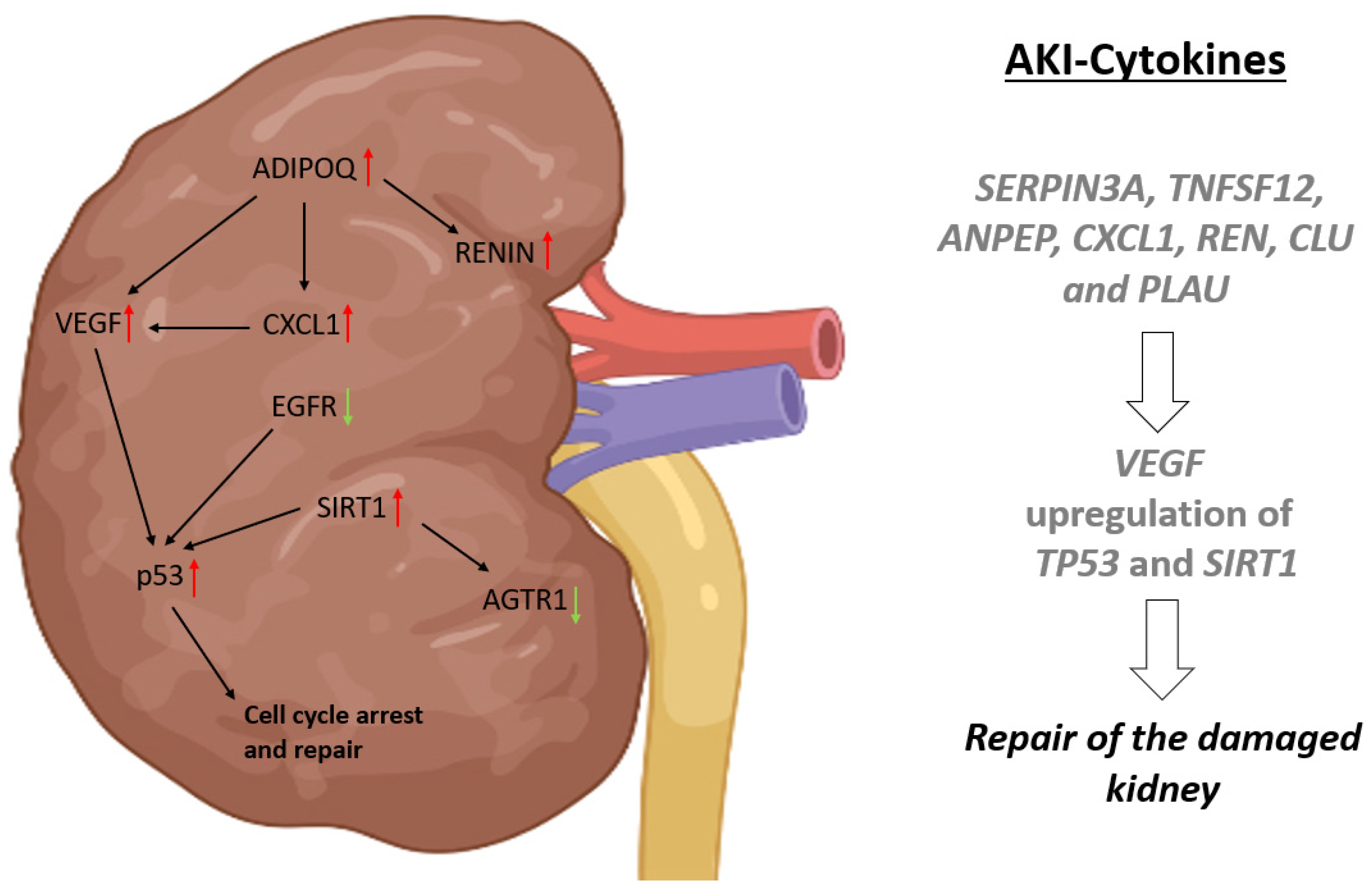
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Secretome Analyses
4.3. Cell Culture Conditions
4.4. Relative Quantification of Podocyte-Associated Gene Expression by Real-Time PCR
4.5. Immunofluorescence-Based Detection of Protein Expression
4.6. Western Blotting
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eknoyan, G. Emergence of the concept of acute renal failure. Am. J. Nephrol. 2002, 22, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Ronco, C.; Bellomo, R. Conceptual advances and evolving terminology in acute kidney disease. Nat. Rev. Nephrol. 2021, 17, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury, Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Thakar, C.V. Perioperative acute kidney injury. Adv. Chronic Kidney Dis. 2013, 20, 67–75. [Google Scholar] [CrossRef]
- Antunes, P.E.; Prieto, D.; Ferrão de Oliveira, J.; Antunes, M.J. Renal dysfunction after myocardial revascularization. Eur. J. Cardio Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2004, 25, 597–604. [Google Scholar] [CrossRef]
- Chertow, G.M.; Lazarus, J.M.; Christiansen, C.L.; Cook, E.F.; Hammermeister, K.E.; Grover, F.; Daley, J. Preoperative renal risk stratification. Circulation 1997, 95, 878–884. [Google Scholar] [CrossRef]
- Chertow, G.M.; Levy, E.M.; Hammermeister, K.E.; Grover, F.; Daley, J. Independent association between acute renal failure and mortality following cardiac surgery. Am. J. Med. 1998, 104, 343–348. [Google Scholar] [CrossRef]
- Ostermann, M.E.; Taube, D.; Morgan, C.J.; Evans, T.W. Acute renal failure following cardiopulmonary bypass: A changing picture. Intensive Care Med. 2000, 26, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Nadasdy, T.; Laszik, Z.; Blick, K.E.; Johnson, L.D.; Silva, F.G. Proliferative activity of intrinsic cell populations in the normal human kidney. J. Am. Soc. Nephrol. 1994, 4, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, R.; Brown, D.; Schwarz, C.; Bonventre, J.V. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Investig. 1994, 93, 2175–2188. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Moran, A.; Igarashi, P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J. Clin. Investig. 2005, 115, 1756–1764. [Google Scholar] [CrossRef]
- Canaud, G.; Bonventre, J.V. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2015, 30, 575–583. [Google Scholar] [CrossRef]
- Clements, M.E.; Chaber, C.J.; Ledbetter, S.R.; Zuk, A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE 2013, 8, e70464. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Dagher, P.C.; Sandoval, R.M.; Campos, S.B.; Ashush, H.; Fridman, E.; Brafman, A.; Faerman, A.; Atkinson, S.J.; Thompson, J.D.; et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 1754–1764. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, G.; Yang, T.; Megyesi, J.; Price, P.M.; Dong, Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2007, 293, F1282–F1291. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Lai, K.N.; Lan, H.Y. Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J. Am. Soc. Nephrol. 2010, 21, 31–41. [Google Scholar] [CrossRef]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef]
- Deng, C.-X. SIRT1, is it a tumor promoter or tumor suppressor? Int. J. Biol. Sci. 2009, 5, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-K.; Song, E.-K.; Guo, Y.; Ou, X.; Mantel, C.; Broxmeyer, H.E. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2008, 2, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Raji-amirhasani, A.; Khaksari, M.; Darvishzadeh Mahani, F.; Hajializadeh, Z. Activators of SIRT1 in the kidney and protective effects of SIRT1 during acute kidney injury (AKI) (effect of SIRT1 activators on acute kidney injury). Clin. Exp. Nephrol. 2021, 25, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Jo, J.; Kim, K.; An, H.-J.; Gwon, M.-G.; Gu, H.; Kim, H.-J.; Yang, A.Y.; Kim, S.-W.; Jeon, E.J.; et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants 2019, 8, 322. [Google Scholar] [CrossRef]
- Mahani, F.D.; Khaksari, M.; Raji-Amirhasani, A. Renoprotective effects of estrogen on acute kidney injury: The role of SIRT1. Int. Urol. Nephrol. 2021, 53, 2299–2310. [Google Scholar] [CrossRef]
- Erichsen, L.; Kloss, L.D.F.; Thimm, C.; Bohndorf, M.; Schichel, K.; Wruck, W.; Adjaye, J. Derivation of the Immortalized Cell Line UM51-PrePodo-hTERT and Its Responsiveness to Angiotensin II and Activation of the RAAS Pathway. Cells 2023, 12, 342. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef]
- Cai, L.; Xu, S.; Piao, C.; Qiu, S.; Li, H.; Du, J. Adiponectin induces CXCL1 secretion from cancer cells and promotes tumor angiogenesis by inducing stromal fibroblast senescence. Mol. Carcinog. 2016, 55, 1796–1806. [Google Scholar] [CrossRef]
- Montero, E.; Abreu, C.; Tonino, P. Relationship between VEGF and p53 expression and tumor cell proliferation in human gastrointestinal carcinomas. J. Cancer Res. Clin. Oncol. 2008, 134, 193–201. [Google Scholar] [CrossRef]
- Pierzchalski, P.; Reiss, K.; Cheng, W.; Cirielli, C.; Kajstura, J.; Nitahara, J.A.; Rizk, M.; Capogrossi, M.C.; Anversa, P. p53 Induces myocyte apoptosis via the activation of the renin-angiotensin system. Exp. Cell Res. 1997, 234, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Marudamuthu, A.S.; Tsukasaki, Y.; Ikebe, M.; Fu, J.; Shetty, S. p53- and PAI-1-mediated induction of C-X-C chemokines and CXCR2: Importance in pulmonary inflammation due to cigarette smoke exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L496–L506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, S.; Spee, C.; Ishikawa, K.; Hinton, D.R. SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by Resveratrol and its relevance to choroidal neovascularization. Cytokine 2015, 76, 549–552. [Google Scholar] [CrossRef]
- Chandel, N.; Ayasolla, K.; Wen, H.; Lan, X.; Haque, S.; Saleem, M.A.; Malhotra, A.; Singhal, P.C. Vitamin D receptor deficit induces activation of renin angiotensin system via SIRT1 modulation in podocytes. Exp. Mol. Pathol. 2017, 102, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, A.; Vorgias, C.E.; Chrousos, G.P.; Rogakou, E.P. Genome Instability and γH2AX. Int. J. Mol. Sci. 2017, 18, 1979. [Google Scholar] [CrossRef] [PubMed]
- Bendale, D.S.; Karpe, P.A.; Chhabra, R.; Shete, S.P.; Shah, H.; Tikoo, K. 17-β Oestradiol prevents cardiovascular dysfunction in post-menopausal metabolic syndrome by affecting SIRT1/AMPK/H3 acetylation. Br. J. Pharmacol. 2013, 170, 779–795. [Google Scholar] [CrossRef]
- Ota, H.; Akishita, M.; Akiyoshi, T.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Eto, M.; Ouchi, Y. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: Protective role of eNOS and SIRT1. PLoS ONE 2012, 7, e29598. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, J.; Cai, T.; Wang, Z.; Zhu, G. Testosterone antagonizes paraquat-induced cardiomyocyte senescence via the mIGF-1/SIRT1 signaling pathway. Braz. J. Med. Biol. Res. 2020, 53, e9849. [Google Scholar] [CrossRef]
- Ucero, A.C.; Benito-Martin, A.; Fuentes-Calvo, I.; Santamaria, B.; Blanco, J.; Lopez-Novoa, J.M.; Ruiz-Ortega, M.; Egido, J.; Burkly, L.C.; Martinez-Salgado, C.; et al. TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim. Biophys. Acta 2013, 1832, 1744–1755. [Google Scholar] [CrossRef]
- Tögel, F.; Zhang, P.; Hu, Z.; Westenfelder, C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J. Cell. Mol. Med. 2009, 13, 2109–2114. [Google Scholar] [CrossRef]
- Sharma, N.; Anders, H.-J.; Gaikwad, A.B. Fiend and friend in the renin angiotensin system: An insight on acute kidney injury. Biomed. Pharmacother. 2018, 110, 764–774. [Google Scholar] [CrossRef]
- Ma, C.; Liu, G.; Liu, W.; Xu, W.; Li, H.; Piao, S.; Sui, Y.; Feng, W. CXCL1 stimulates decidual angiogenesis via the VEGF-A pathway during the first trimester of pregnancy. Mol. Cell. Biochem. 2021, 476, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-W.; Xia, G.-K.; Wu, Y.; Chen, W.; Xiang, Z.; Schwarz, R.E.; Brekken, R.A.; Awasthi, N.; He, Y.-L.; Zhang, C.-H. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett. 2015, 359, 335–343. [Google Scholar] [CrossRef]
- Divella, R.; Daniele, A.; DE Luca, R.; Simone, M.; Naglieri, E.; Savino, E.; Abbate, I.; Gadaleta, C.D.; Ranieri, G. Circulating Levels of VEGF and CXCL1 Are Predictive of Metastatic Organotropismin in Patients with Colorectal Cancer. Anticancer Res. 2017, 37, 4867–4871. [Google Scholar] [PubMed]
- Nakagawa, N.; Yao, N.; Hirayama, T.; Ishida, M.; Ishida, H.; Wada, A.; Fujino, T.; Saijo, Y.; Kikuchi, K.; Hasebe, N. Potential impact of renin-angiotensin system inhibitors and calcium channel blockers on plasma high-molecular-weight adiponectin levels in hemodialysis patients. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2011, 34, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.B.; Massaro, A.N.; Soler-García, A.A.; Perazzo, S.; Ray, P.E. A novel urinary biomarker profile to identify acute kidney injury (AKI) in critically ill neonates: A pilot study. Pediatr. Nephrol. 2013, 28, 2179–2188. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Koralkar, R.; Hundley, H.E.; Montesanti, A.; Parwar, P.; Sonjara, S.; Ambalavanan, N. Urine biomarkers predict acute kidney injury in newborns. J. Pediatr. 2012, 161, 270–275.e1. [Google Scholar] [CrossRef]
- Staun-Ram, E.; Goldman, S.; Shalev, E. p53 Mediates epidermal growth factor (EGF) induction of MMP-2 transcription and trophoblast invasion. Placenta 2009, 30, 1029–1036. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Li, Y.; Xu, Y.; He, Q.; Harris, R.C. EGF Receptor-Dependent YAP Activation Is Important for Renal Recovery from AKI. J. Am. Soc. Nephrol. 2018, 29, 2372–2385. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Carrier, F.; Johnson, A.C.; Ogdon, S.E.; Fornace, A.J. Identification of an additional p53-responsive site in the human epidermal growth factor receptor gene promotor. Oncogene 1997, 15, 1095–1101. [Google Scholar] [CrossRef]
- Samarakoon, R.; Dobberfuhl, A.D.; Cooley, C.; Overstreet, J.M.; Patel, S.; Goldschmeding, R.; Meldrum, K.K.; Higgins, P.J. Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cell. Signal. 2013, 25, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Faubel, S.; Askenazi, D.J.; Cerda, J.; Fissell, W.H.; Heung, M.; Humphreys, B.D.; Koyner, J.L.; Liu, K.D.; Mour, G.; et al. Clinical Use of the Urine Biomarker TIMP-2 × IGFBP7 for Acute Kidney Injury Risk Assessment. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 68, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.M.; Heung, M. The use of cell cycle arrest biomarkers in the early detection of acute kidney injury. Is this the new renal troponin? Nefrologia 2018, 38, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Saad, A.; Herrmann, S.M.; Eirin Massat, A.; McKusick, M.A.; Misra, S.; Lerman, L.O.; Textor, S.C. Changes in inflammatory biomarkers after renal revascularization in atherosclerotic renal artery stenosis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2016, 31, 1437–1443. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, L.; Thimm, C.; Bohndorf, M.; Rahman, M.S.; Wruck, W.; Adjaye, J. Activation of the Renin-Angiotensin System Disrupts the Cytoskeletal Architecture of Human Urine-Derived Podocytes. Cells 2022, 11, 1095. [Google Scholar] [CrossRef]
- Wruck, W.; Boima, V.; Erichsen, L.; Thimm, C.; Koranteng, T.; Kwakyi, E.; Antwi, S.; Adu, D.; Adjaye, J. Urine-based detection of biomarkers indicative of chronic kidney disease in a patient cohort from Ghana. J. Pers. Med. 2022, 13, 38. [Google Scholar] [CrossRef]
- Engel, J.E.; Williams, E.; Williams, M.L.; Bidwell, G.L.; Chade, A.R. Targeted VEGF (Vascular Endothelial Growth Factor) Therapy Induces Long-Term Renal Recovery in Chronic Kidney Disease via Macrophage Polarization. Hypertension 2019, 74, 1113–1123. [Google Scholar] [CrossRef]
- Basile, D.P.; Friedrich, J.L.; Spahic, J.; Knipe, N.; Mang, H.; Leonard, E.C.; Changizi-Ashtiyani, S.; Bacallao, R.L.; Molitoris, B.A.; Sutton, T.A. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Ren. Physiol. 2011, 300, F721–F733. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056. [Google Scholar] [CrossRef]
- Lee, J.T.; Gu, W. SIRT1: Regulator of p53 Deacetylation. Genes Cancer 2013, 4, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Niederer, F.; Ospelt, C.; Brentano, F.; Hottiger, M.O.; Gay, R.E.; Gay, S.; Detmar, M.; Kyburz, D. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann. Rheum. Dis. 2011, 70, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Washida, N.; Tokuyama, H.; Hayashi, K.; Itoh, H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun. 2008, 372, 51–56. [Google Scholar] [CrossRef]
- Gao, P.; Xu, T.-T.; Lu, J.; Li, L.; Xu, J.; Hao, D.-L.; Chen, H.-Z.; Liu, D.-P. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J. Mol. Med. 2013, 92, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Erichsen, L.; Adjaye, J. Crosstalk between age accumulated DNA-damage and the SIRT1-AKT-GSK3ß axis in urine derived renal progenitor cells. Aging 2022, 14, 8179–8204. [Google Scholar] [CrossRef]
- Andrade, L.; Rodrigues, C.E.; Gomes, S.A.; Noronha, I.L. Acute Kidney Injury as a Condition of Renal Senescence. Cell Transplant. 2018, 27, 739–753. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Kibbe, W.A.; Lin, S.M. lumi: A pipeline for processing Illumina microarray. Bioinformatics 2008, 24, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Graffmann, N.; Ring, S.; Kawala, M.-A.; Wruck, W.; Ncube, A.; Trompeter, H.-I.; Adjaye, J. Modeling Nonalcoholic Fatty Liver Disease with Human Pluripotent Stem Cell-Derived Immature Hepatocyte-Like Cells Reveals Activation of PLIN2 and Confirms Regulatory Functions of Peroxisome Proliferator-Activated Receptor Alpha. Stem Cells Dev. 2016, 25, 1119–1133. [Google Scholar] [CrossRef] [PubMed]

| Parameter | No AKI (n = 6) | AKI 2 and 3 (n = 6) | p-Value |
|---|---|---|---|
| Age [years] | 68 ± 8 | 69 ± 18 | 0.37 |
| Gender | |||
| Female [%] | 3 (50) | 3 (50) | 1.0 |
| Male [%] | 3 (50) | 3 (50) | 1.0 |
| Comorbidities | |||
| Obesity [%] | 2 (33) | 3 (50) | 1.0 |
| Hypertension [%] | 5 (93) | 4 (67) | 1.0 |
| Diabetes Mellitus [%] | 2 (33) | 2 (33) | 1.0 |
| CKD [%] | 0 (0) | 2 (33) | 0.455 |
| Preoperative creatinine [mg/dL] | 1.18 ± 0.44 | 1.85 ± 0.90 | 0.126 |
| Operation time [minutes] | 282 ± 66 | 287 ± 86 | 0.699 |
| CPB time [minutes] | 191 ± 65 | 170 ± 51 | 0.589 |
| X-clamp [minutes] | 109 ± 33 | 103 ± 52 | 1.0 |
| Creatinine postop. [mg/dL] | 1.2 ± 0.34 | 1.43 ± 0.42 | 0.268 |
| Creatinine on day 3 [mg/dL] | 1.08 ± 0.50 | 2.03 ± 1.29 | 0.097 |
| Lactate postop. [mmol/L] | 2.5 ± 0.9 | 7.5 ± 3.2 | 0.004 |
| Lactate on day 3 [mmol/L] | 2.2 ± 2.5 | 1.7 ± 0.5 | 0.296 |
| APACHE-II postop. | 26 ± 2 | 32 ± 3 | 0.008 |
| Ventilation [hours] | 20 ± 8 | 186 ± 253 | 0.020 |
| LOICUS [days] | 2 ± 2 | 13 ± 11 | 0.014 |
| LOHS [days] | 18 ± 12 | 42 ± 20 | 0.030 |
| Last creatinine [mg/dL] | 1.03 ± 0.33 | 2.07 ± 0.72 | 0.016 |
| Mortality in hospital | 0 (0) | 1 (17) | 1.0 |
| Cytokine | Female 24 h Post-Surgery | Male 24 h Post-Surgery | Female 72 h Post-Surgery | Male 72 h Post-Surgery |
|---|---|---|---|---|
| ADIPOQ | Up | Up | ||
| CCN1 | Up | Down | ||
| EGFR | Up | Up | ||
| IL6 | Up | |||
| CCL2 | Up | Down | Down | Up |
| THBS1 | Up | |||
| VEGF | Up | Up | Up | |
| ANPEP | Down | |||
| B2M | Down | Down | ||
| CST3 | Down | |||
| DDP4 | Down | |||
| EGF | Down | Down | Down | |
| IL1RN | Down | Up | Down | |
| KLK3 | Down | |||
| SERPIN3A | Down | Down | Down | Down |
| TNFRSF1A | Down | |||
| TTF3 | Down | |||
| TNFSF12 | Down | Up | Down | Down |
| IL10 | Down | Down | ||
| TNFA | Down | |||
| ANPEP | Up | Up | ||
| CXCL1 | Up | Up | ||
| REN | Up | Up | ||
| CLU | Down | Down | ||
| MMP9 | Down | |||
| MME | Down | |||
| KLK3 | Down | |||
| AGER | Down | |||
| PLAU | Down | Down | ||
| AHSG | Up | |||
| ANXA5 | Down | |||
| IL6 | Down | |||
| SKP1 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erichsen, L.; Thimm, C.; Wruck, W.; Kaierle, D.; Schless, M.; Huthmann, L.; Dimski, T.; Kindgen-Milles, D.; Brandenburger, T.; Adjaye, J. Secreted Cytokines within the Urine of AKI Patients Modulate TP53 and SIRT1 Levels in a Human Podocyte Cell Model. Int. J. Mol. Sci. 2023, 24, 8228. https://doi.org/10.3390/ijms24098228
Erichsen L, Thimm C, Wruck W, Kaierle D, Schless M, Huthmann L, Dimski T, Kindgen-Milles D, Brandenburger T, Adjaye J. Secreted Cytokines within the Urine of AKI Patients Modulate TP53 and SIRT1 Levels in a Human Podocyte Cell Model. International Journal of Molecular Sciences. 2023; 24(9):8228. https://doi.org/10.3390/ijms24098228
Chicago/Turabian StyleErichsen, Lars, Chantelle Thimm, Wasco Wruck, Daniela Kaierle, Manon Schless, Laura Huthmann, Thomas Dimski, Detlef Kindgen-Milles, Timo Brandenburger, and James Adjaye. 2023. "Secreted Cytokines within the Urine of AKI Patients Modulate TP53 and SIRT1 Levels in a Human Podocyte Cell Model" International Journal of Molecular Sciences 24, no. 9: 8228. https://doi.org/10.3390/ijms24098228
APA StyleErichsen, L., Thimm, C., Wruck, W., Kaierle, D., Schless, M., Huthmann, L., Dimski, T., Kindgen-Milles, D., Brandenburger, T., & Adjaye, J. (2023). Secreted Cytokines within the Urine of AKI Patients Modulate TP53 and SIRT1 Levels in a Human Podocyte Cell Model. International Journal of Molecular Sciences, 24(9), 8228. https://doi.org/10.3390/ijms24098228






