Energetic Polymer Possessing Furazan, 1,2,3-Triazole, and Nitramine Subunits
Abstract
1. Introduction
2. Results and Discussion
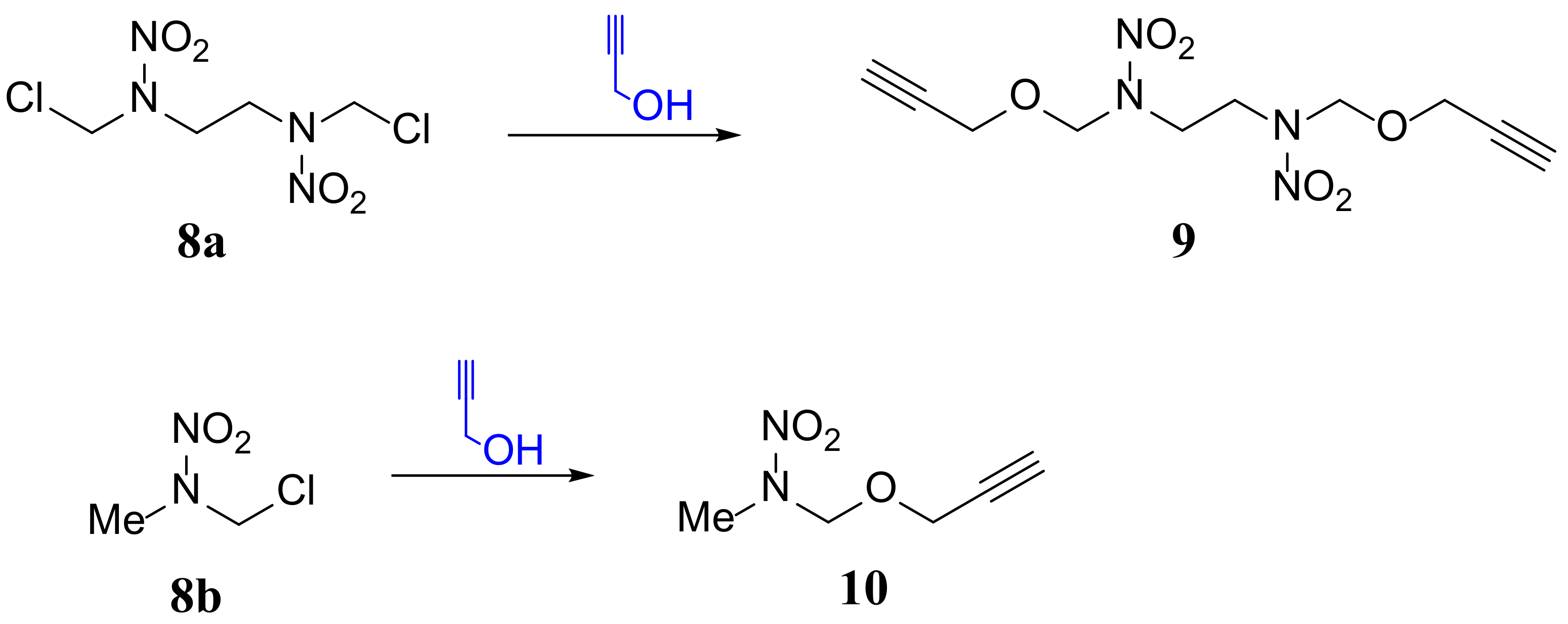
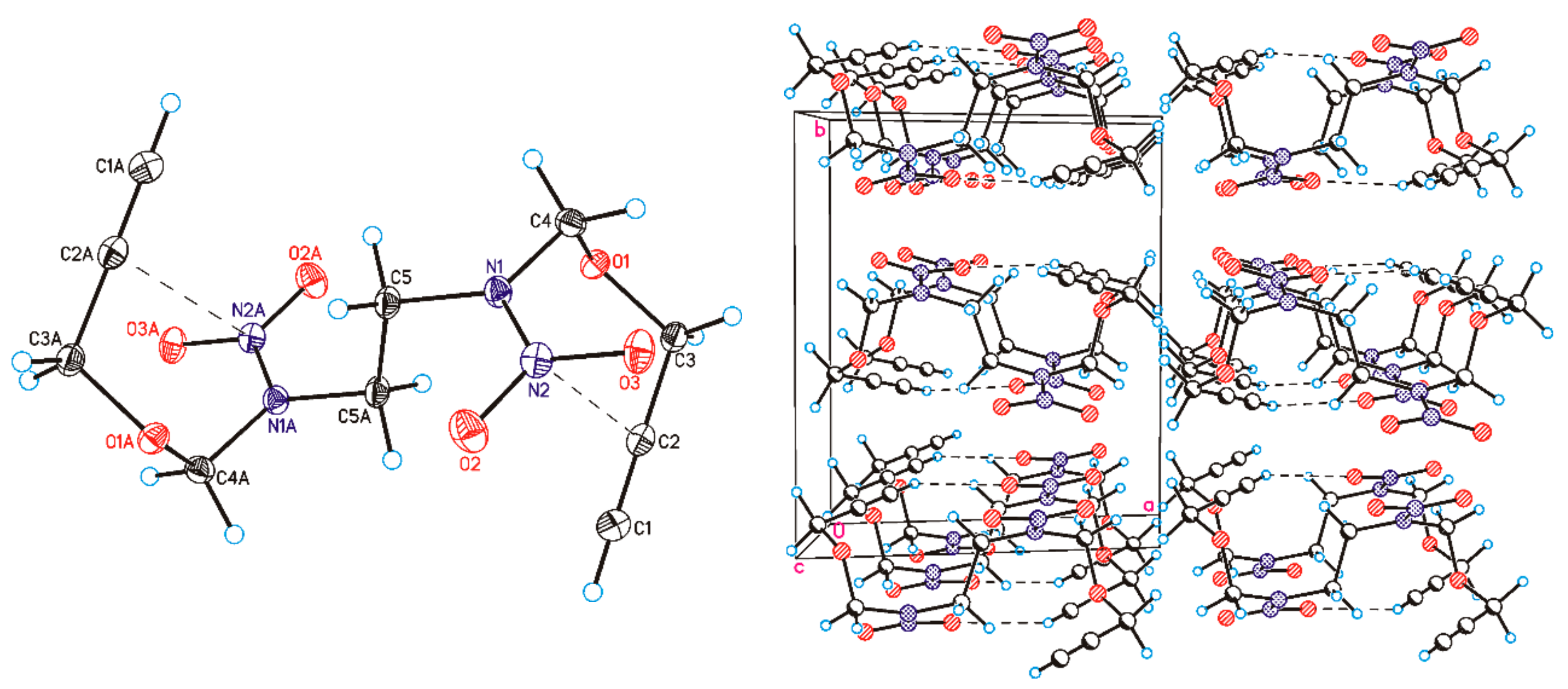
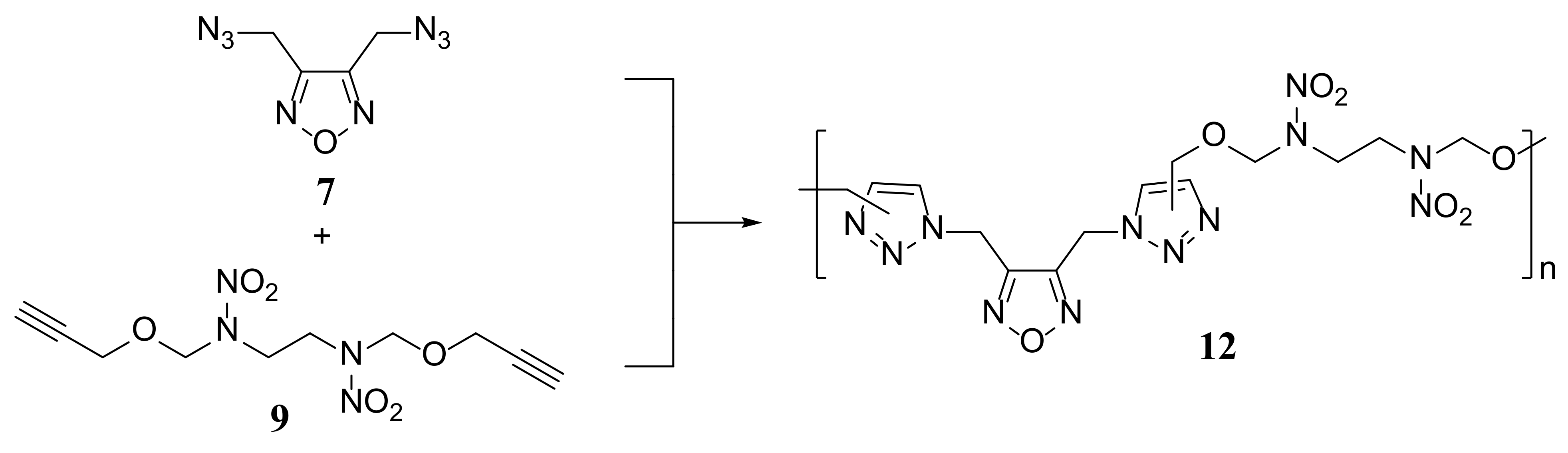
2.1. Synthesis and Characterization of Monomers and Polymer
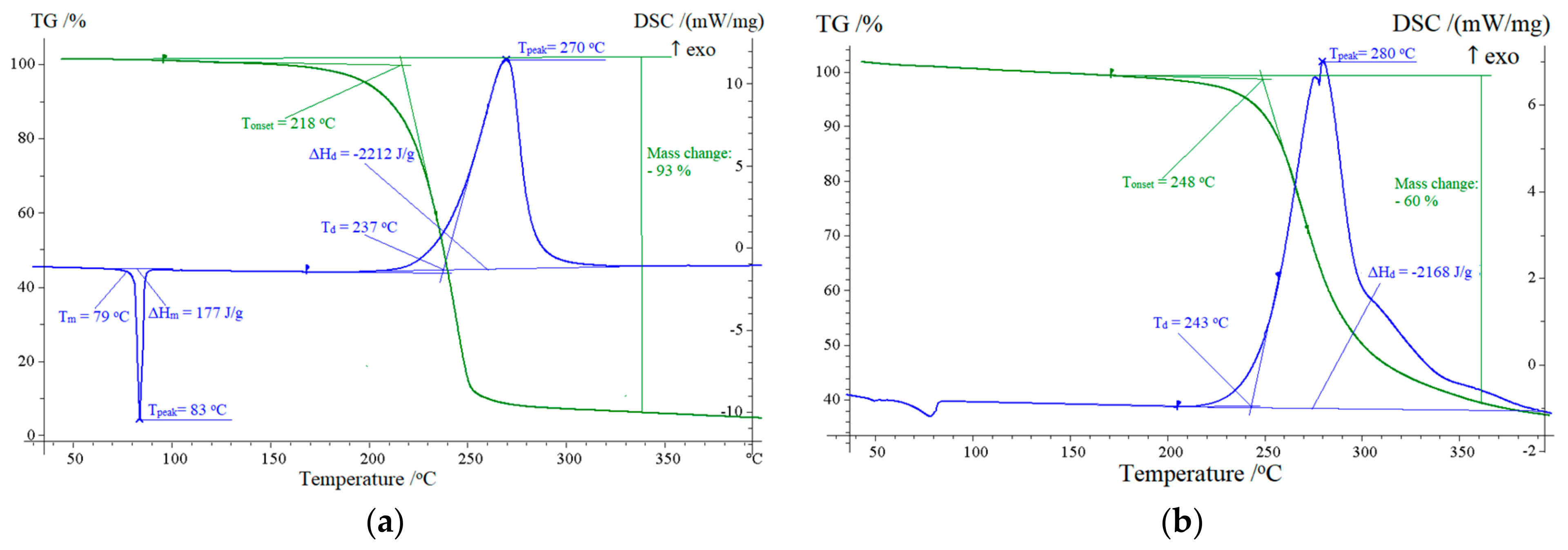
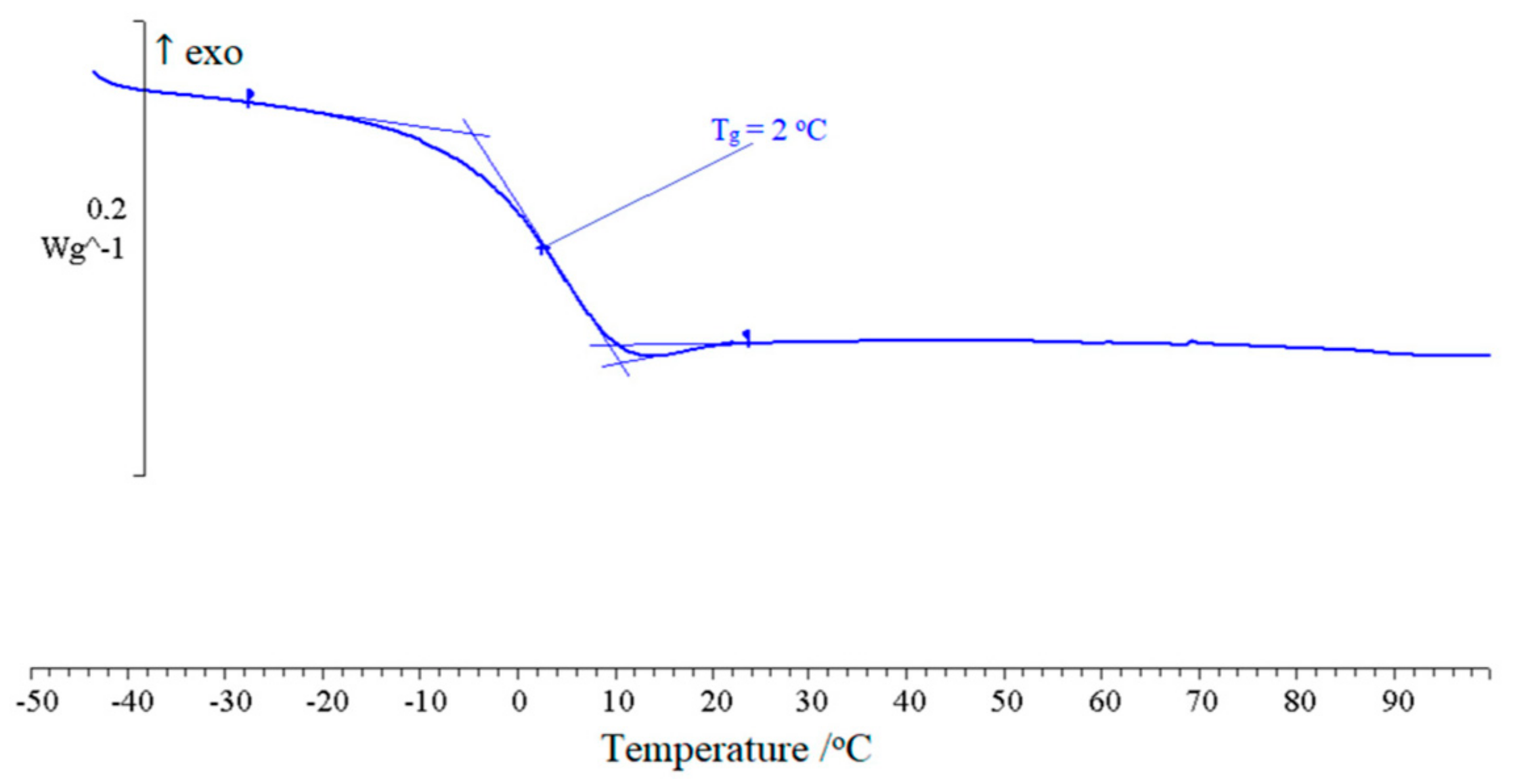
2.2. Thermal Analysis
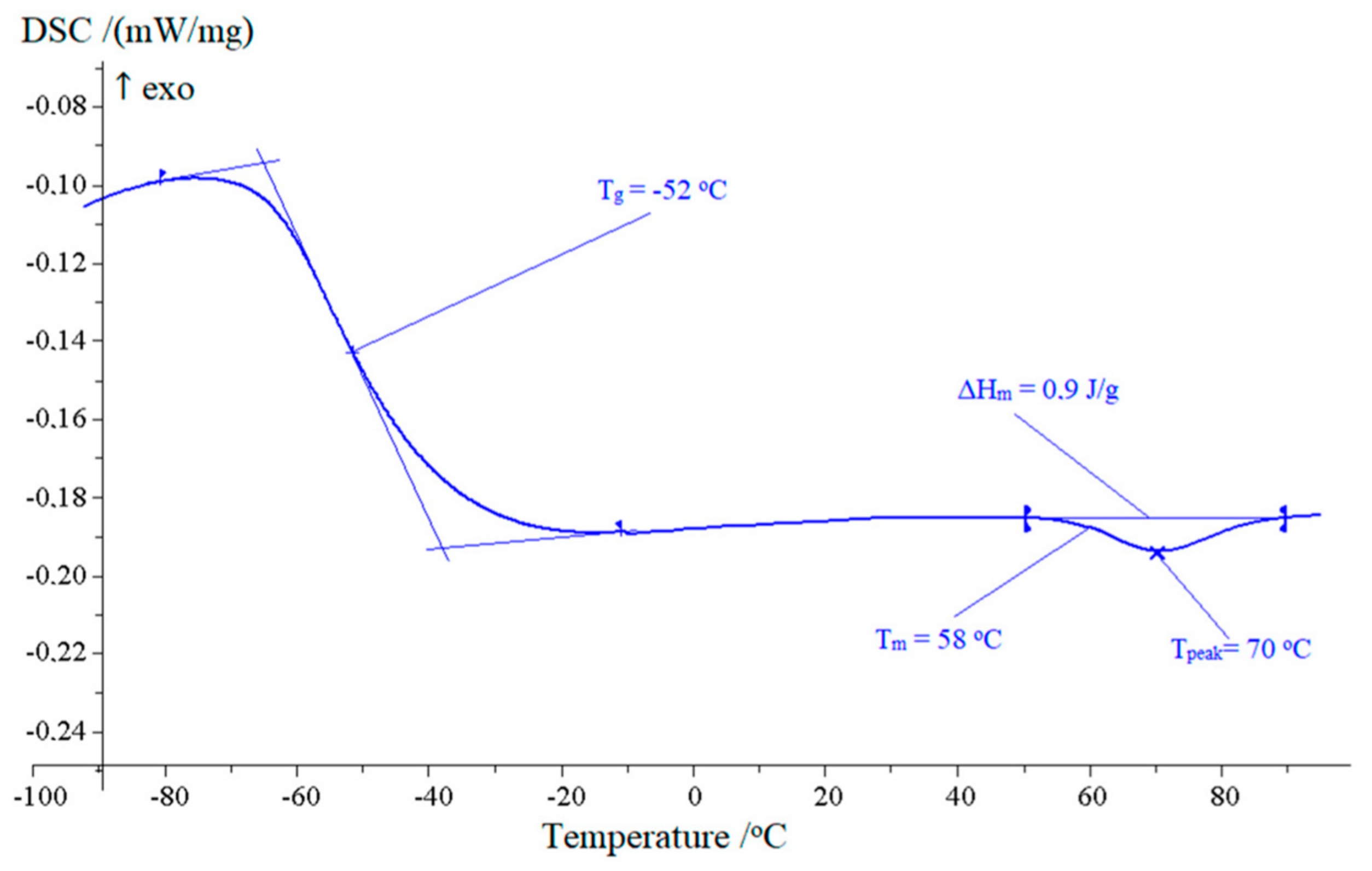
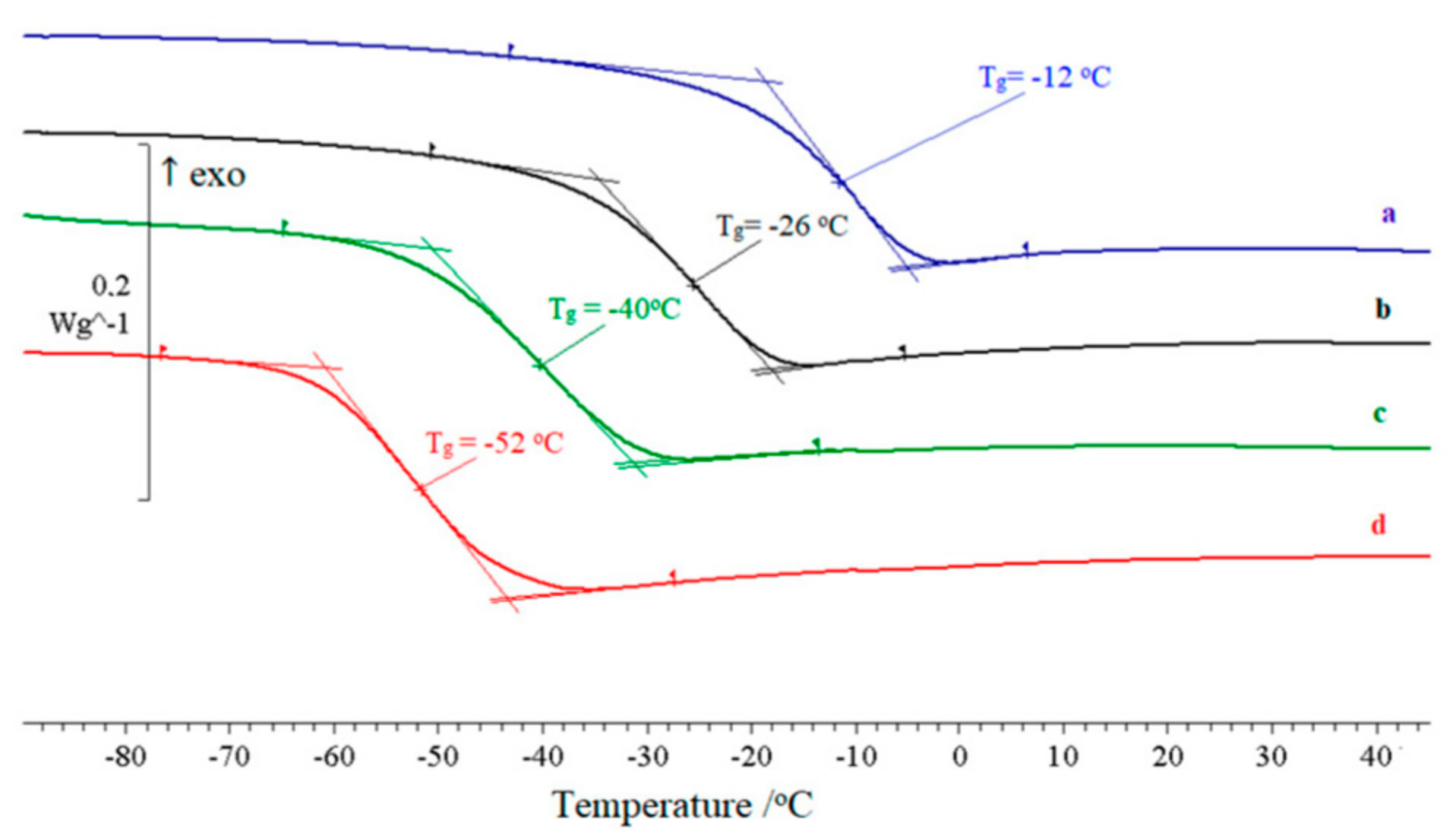
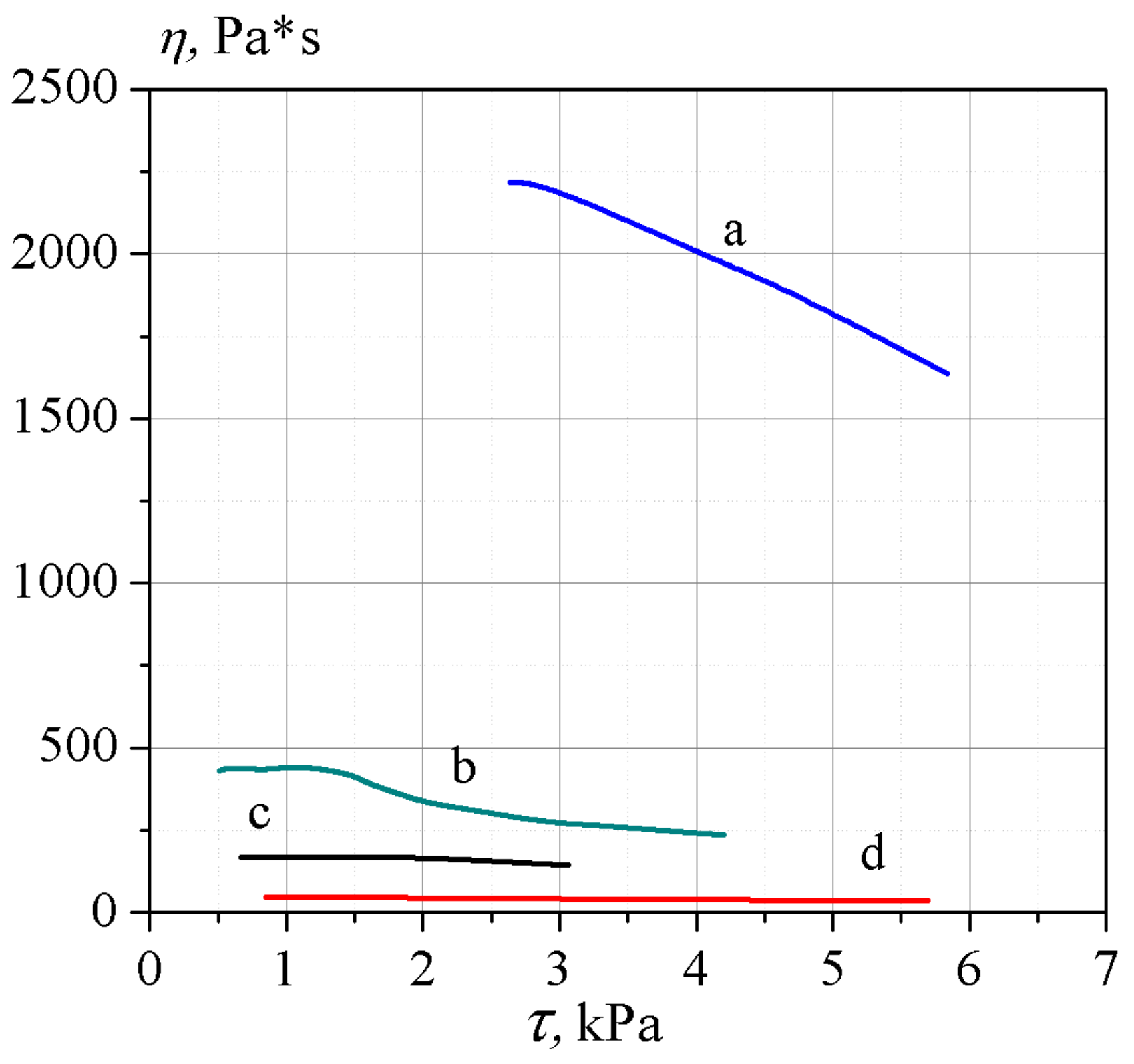
2.3. Plastification
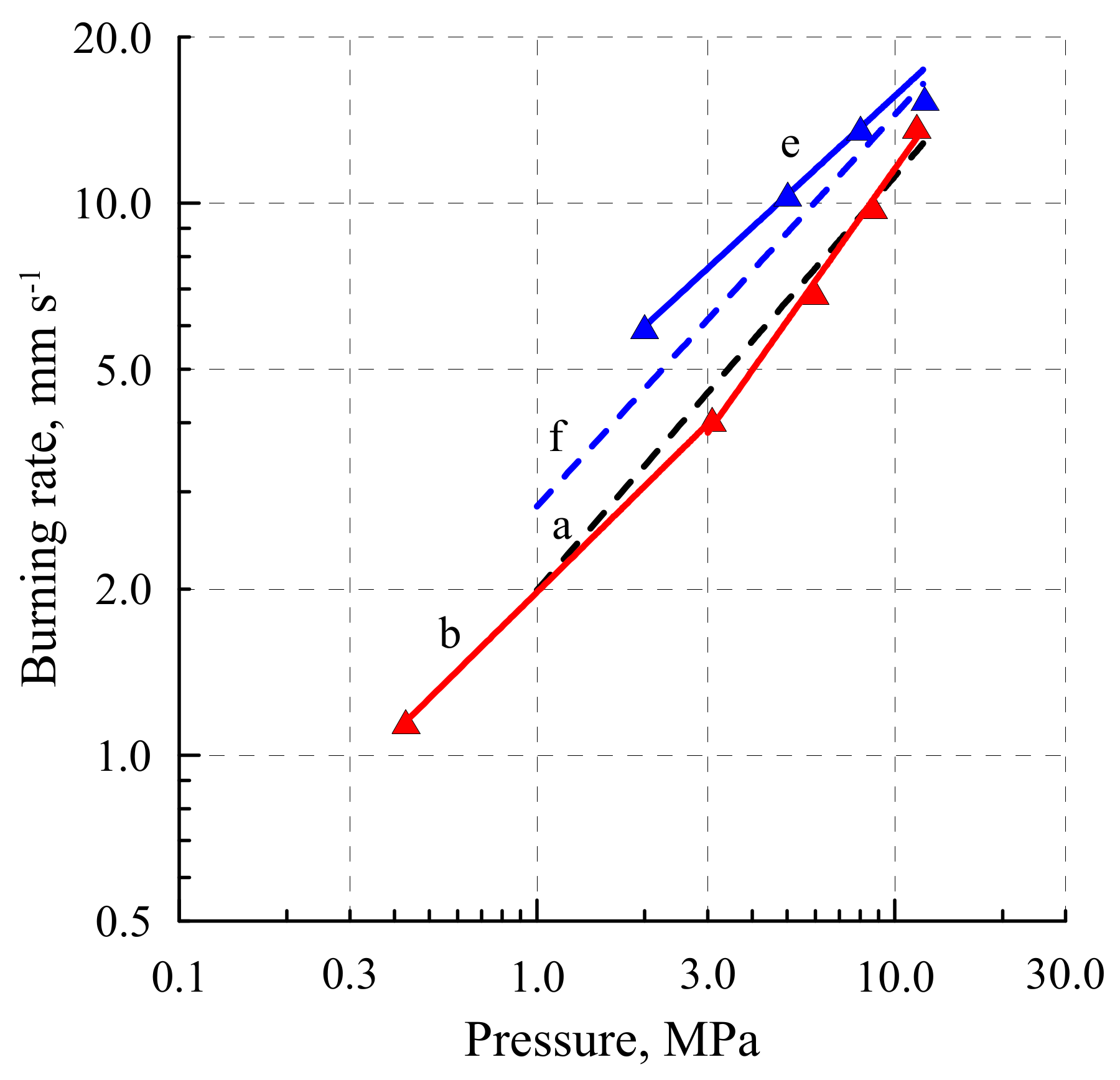
2.4. Combustion
2.5. Gunpowder
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubota, N. Propellants and Explosives; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Davenas, A. Development of modern solid propellants. J. Propuls. Power 2003, 19, 1108–1128. [Google Scholar] [CrossRef]
- Pavlovets, G.A.; Tsutsuran, V.I. Physicochemical Properties of Powders and Propellants; Russian Ministry of Defense Publishing House: Moscow, Russia, 2009. (In Russian)
- DeLuca, L.T.; Shimada, T.; Sinditskii, V.P.; Calabro, M. (Eds.) Chemical Rocket Propulsion: A Comprehensive Survey of Energetic Materials; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Singh, H.; Shekhar, H. Solid Rocket Propellants: Science and Technology Challenges; Royal Society of Chemistry: Cambridge, UK, 2017. [Google Scholar]
- Lysien, K.; Stolarczyk, A.; Jarosz, T. Solid Propellant Formulations: A Review of Recent Progress and Utilized Components. Materials 2021, 14, 6657. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, W.; Zhou, Z.; Liu, T.; Xia, H.; Huang, S.; Zhang, Q. Recent advances in hypergolic ionic liquids with broad potential for propellant applications. FirePhysChem 2022, 2, 236–252. [Google Scholar] [CrossRef]
- Gayathri, S.; Reshmi, S. Nitrato Functionalized Polymers for High Energy Propellants and Explosives: Recent Advances. Polym. Adv. Technol. 2017, 28, 1539–1550. [Google Scholar] [CrossRef]
- Liu, J. Nitrate Esters Chemistry and Technology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Dou, J.; Xu, M.; Tan, B.; Lu, X.; Mo, H.; Wang, B.; Liu, N. Research progress of nitrate ester binders. FirePhysChem 2023, 3, 54–77. [Google Scholar] [CrossRef]
- Ang, H.G.; Pisharath, S. Energetic Polymers: Binders and Plasticizers for Enhancing Performance, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Kumari, D.; Balakshe, R.; Banerjee, S.; Singh, H. Energetic Plasticizers for Gun & Rocket Propellants. Rev. J. Chem. 2012, 2, 240–262. [Google Scholar] [CrossRef]
- Pasquinet, E. Nitrogen-Rich Polymers as Candidates for Energetic Applications. In New Polymers for Special Applications; INTECH: London, UK, 2012; Chapter 10; pp. 313–338. [Google Scholar] [CrossRef]
- Paraskos, A.J. Energetic Polymers: Synthesis and Applications. In Energetic Materials; Shukla, M.K., Boddu, V., Steevens, J., Damavarapu, R., Leszczynski, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Chapter 4; pp. 91–134. [Google Scholar]
- Jarosz, T.; Stolarczyk, A.; Wawrzkiewicz-Jalowiecka, A.; Pawlus, K.; Miszczyszyn, K. Glycidyl Azide Polymer and its Derivatives-Versatile Binders for Explosives and Pyrotechnics: Tutorial Review of Recent Progress. Molecules 2019, 24, 4475. [Google Scholar] [CrossRef]
- Lysien, K.; Stolarczyk, A.; Jarosz, T. Energetic Polyoxetanes as High-Performance Binders for Energetic Composites: A Critical Review. Polymers 2022, N14, 4651. [Google Scholar] [CrossRef]
- Gozin, M.; Fershtat, L.L. Recent Advances in Chemistry of Nitrogen-Rich Energetic Polymers and Plasticizers. In Nitrogen-Rich Energetic Materials; Gozin, M., Fershtat, L.L., Eds.; Wiley-VCH GmbH: Weinheim, Germany, 2023; pp. 189–238. [Google Scholar] [CrossRef]
- Lempert, D.B.; Kazakov, A.I.; Sheremetev, A.B. Comparative ballistic efficiency of solid composite propellants: Which plasticizer/polymer combination is the energetically preferred binder? Mendeleev Commun. 2022, 32, 601–603. [Google Scholar] [CrossRef]
- Lempert, D.B.; Sheremetev, A.B. The energetic potential of azo- and azoxyfurazan nitro derivatives as components of composite rocket propellants. Chem. Heterocycl. Compd. 2016, 52, 1070–1077. [Google Scholar] [CrossRef]
- Lempert, D.B.; Dorofeenko, E.M. Reasons for the anomalous dependence of the specific impulse of rocket propellants on the content of borohydride. Combust. Explos. Shock. Waves 2013, 49, 472–477. [Google Scholar] [CrossRef]
- Dorofeenko, E.M.; Soglasnova, S.I.; Nechiporenko, G.N.; Lempert, D.B. Optimization of the Binder Formulation to Increase the Energetic Performance of Polynitrogen Oxidizers in Metal-Free Compositions. Combust. Explos. Shock. Waves 2018, 54, 698–703. [Google Scholar] [CrossRef]
- Kizhnyaev, V.N.; Vereshchagin, L.I. Vinyltetrazoles: Synthesis and properties. Russ. Chem. Rev. 2003, 72, 143–164. [Google Scholar] [CrossRef]
- Kizhnyaev, V.N.; Pokatilov, F.A.; Vereshchagin, L.I. Carbochain polymers with oxadiazole, triazole, and tetrazole cycles. Polym. Sci. Ser. C 2008, 50, 1–21. [Google Scholar] [CrossRef]
- Kizhnyaev, V.N.; Pokatilov, F.A.; Vereshchagin, L.I.; Verkhozina, O.N.; Petrova, T.L.; Prodaikov, A.G.; Ratovskii, G.V.; Tyukalova, O.V. Synthesis of energetic polynuclear and polymeric nitroazole systems. Russ. J. Appl. Chem. 2009, 82, 1769–1775. [Google Scholar] [CrossRef]
- Kizhnyaev, V.N.; Golobokova, T.V.; Pokatilov, F.A.; Vereshchagin, L.I.; Estrin, Y.I. Synthesis of energetic triazole- and tetrazole-containing oligomers and polymers. Chem. Heterocycl. Compd. 2017, 53, 682–692. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. 1,2,5-Oxadiazole-Based High-Energy-Density Materials: Synthesis and Performance. ChemPlusChem 2020, 85, 13–42. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Bi, F.; Wang, B. Energetic materials based on poly furazan and furoxan structures. Chin. Chem. Lett. 2020, 31, 2375–2394. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Churakov, A.M.; Egorov, M.P.; Fershtat, L.L.; Klenov, M.S.; Kuchurov, I.V.; Makhova, N.N.; Smirnov, G.A.; Tomilov, Y.V.; Tartakovsky, V.A. Advanced energetic materials: Novel strategies and versatile applications. Mendeleev Commun. 2021, 31, 731–749. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, B.; Qiu, L.; Xu, R.; Sheremetev, A.B. Recent synthetic efforts towards high energy density materials: How to design high-performance energetic structures? FirePhysChem 2022, 2, 83–139. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Shatunova, E.V. Furazanyl Ethers of Pentaerythritol Derivates. In Proceedings of the 28th International Annual ICT-Conference, Combustion and Detonation, Karlsruhe, Germany, 24–27 June 1997. FRG, 94/1-8. [Google Scholar]
- Sheremetev, A.B.; Yudin, I.L. Unusual Oxidation of 4-amino-4H,8H-bisfurazano-[3,4-b;3′,4′-e]pyrazines. Mendeleev Commun. 2002, 12, 66–67. [Google Scholar] [CrossRef]
- Willer, R.L. Calculation of the density and detonation properties of C, H, N, O and F compounds: Use in the design and synthesis of new energetic materials. J. Mex. Chem. Soc. 2009, 53, 108–119. [Google Scholar] [CrossRef]
- Lotmentsev, Y.M.; Kondakova, N.N.; Bakeshko, A.V.; Kozeev, A.M.; Sheremetev, A.B. 3-Alkyl-4-nitrofurazans—Plasticizers for polymers. Chem. Heterocycl. Comp. 2017, 53, 740–745. [Google Scholar] [CrossRef]
- Gorman, I.E.; Willer, R.L.; Kemp, L.K.; Storey, R.F. Development of a triazole-cure resin system for composites: Evaluation of alkyne curatives. Polymer 2012, 53, 2548–2558. [Google Scholar] [CrossRef]
- Chavez, D.E.; Tappan, B.C.; Kuehl, V.A.; Schmalzer, A.M.; Leonard, P.W.; Wu, R.; Imler, G.H.; Parrish, D.A. [3+2] Click chemistry approach to tetrazine containing polymers. RSC Adv. 2022, 12, 28490–28493. [Google Scholar] [CrossRef]
- Petrov, A.O.; Karpov, S.V.; Malkov, G.V.; Shastin, A.V.; Badamshina, E.R. New non-symmetric azido-diacetylenic s-triazine monomer for polycycloaddition. Mendeleev Commun. 2022, 32, 464–466. [Google Scholar] [CrossRef]
- Johnson, J.A.; Finn, M.G.; Koberstein, J.T.; Turro, N.J. Construction of Linear Polymers, Dendrimers, Networks, and Other Polymeric Architectures by Copper-Catalyzed Azide-Alkyne Cycloaddition ‘‘Click’’ Chemistry. Macromol. Rapid Commun. 2008, 29, 1052–1072. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Santos, C.S.; Oliveira, R.J.; Oliveira, R.N.; Freitas, J.C.R. 1,2,3-Triazoles: General and key synthetic strategies. Arkivoc 2020, i, 219–271. [Google Scholar] [CrossRef]
- Aflak, N.; Ayouchia, H.B.E.; Bahsis, L.; Anane, H.; Julve, M.; Stiriba, S.-E. Recent Advances in Copper-Based Solid Heterogeneous Catalysts for Azide–Alkyne Cycloaddition Reactions. Int. J. Mol. Sci. 2022, 23, 2383. [Google Scholar] [CrossRef]
- Bakulev, V.A.; Shafran, Y.M.; Beliaev, N.A.; Beryozkina, T.V.; Volkova, N.N.; Joy, M.N.; Fan, Z. Heterocyclic azides: Advances in their chemistry. Russ. Chem. Rev. 2022, 91, RCR5042. [Google Scholar] [CrossRef]
- Androsov, A.S.; Denisyuk, A.P.; Tokarev, N.P.; Fominov, K.G. Role of individual components in the catalysis of the combustion of ballistic powders. Combust. Explos. Shock. Waves 1975, 11, 14–20. [Google Scholar] [CrossRef]
- Androsov, A.S.; Denisyuk, A.P.; Tokarev, N.P. Mechanism of the effect of composite lead-copper catalysts on powder Combustion. Combust. Explos. Shock. Waves 1978, 14, 184–187. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.; Elbeih, A.; Helal, F. The effect of different copper salts on the mechanical and ballistic characteristics of double base rocket propellants. Cent. Eur. J. Energetic Mater. 2016, 13, 469–482. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, R.; Nie, H.; Yan, Q.; Liu, J.; Sun, Y. Insight into the precise catalytic mechanism of CuO on the decomposition and combustion of core–shell Al@AP particles. Fuel 2023, 346, 128294. [Google Scholar] [CrossRef]
- Khakhalev, A.V.; Anisimov, A.A.; Sheremetev, A.B. 3,4-Bis(bromomethyl)furazan and its N-oxide. Chem. Heterocycl. Compd. 2022, 58, 263–266. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Averina, E.B.; Kuznetsova, T.S.; Zefirov, N.S. Synthesis of new 3,4-disubstituted furazans. Chem. Heterocycl. Compd. 2001, 36, 1091–1096. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Mel’nikova, S.F.; Kokareva, E.S.; Nekrutenko, R.E.; Strizhenko, K.V.; Suponitsky, K.Y.; Pham, T.D.; Pivkina, A.N.; Sinditskii, V.P. Nitroxy- and azidomethyl azofurazans as advanced energetic materials. Def. Technol. 2022, 18, 1369–1381. [Google Scholar] [CrossRef]
- Min, B.S.; Kim, S.J.; Kim, W.H.; Moon, H.S.; Hwang, G.W.; Jeon, H.B.; Cho, H.R.; Jung, J.Y. An Energetic Prepolymer for Solid Propellant Binder and Manufacturing Method Thereof. Korean Patent KR2386460, 15 April 2022. [Google Scholar]
- Gribov, P.S.; Suponitsky, K.Y.; Sheremetev, A.B. Efficient Synthesis of N-(Chloromethyl)nitramines via TiCl4-Catalyzed Chlorodeacetoxylation. New J. Chem. 2022, 46, 17548–17553. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. In A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Keith, T.A. AIMAll (Version 15.05.18); TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]
- Gribov, P.S.; Kon’kova, T.S.; Suponitsky, K.Y.; Sheremetev, A.B. Dipropargyl ethers possessing nitramine units. Mendeleev Commun. 2023, 34, 466–468. [Google Scholar]
- Karpov, S.V.; Petrov, A.O.; Malkov, G.V.; Badamshina, E.R. The Gaussian G4 enthalpy of formation of propargylamine and propargyloxy derivatives of triazido-s-triazine. Mendeleev Commun. 2022, 32, 338–340. [Google Scholar] [CrossRef]
- Wamhoff, H. 1,2,3-Triazoles and their Benzo Derivatives. Compr. Heterocycl. Chem. 1984, 5, 669–732. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Binke, N.; Rong, L.; Xianqi, C.; Yuan, W.; Rongzu, H.; Qingsen, Y. Study on the Melting Process of Nitrocellulose by Thermal Analysis Method. J. Therm. Anal. Calorim. 1999, 58, 249–256. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Kubasov, A.A. Chemical Kinetics and Catalysis; Part 1; Moscow State University Publishing House: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Prokudin, V.G.; Nazin, G.M. Gas-phase cyclodecomposition of furazane and furazane N-oxide. Bull. Acad. Sci. USSR Div. Chem. Sci. 1987, 36, 199–201. [Google Scholar] [CrossRef]
- Kumar, A.S.; Ghule, V.D.; Subrahmanyam, S.; Sahoo, A.K. Synthesis of Thermally Stable Energetic 1,2,3-Triazole Derivatives. Chem. A Eur. J. 2013, 19, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xue, Y.; Qian, L.; Yang, H.; Cheng, G. Combination of 1,2,3-triazole and 1,2,4-triazole frameworks for new high-energy and low-sensitivity compounds. Energetic Mater. Front. 2021, 2, 131–138. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hosseini, S.G.; Rahimi-Nasrabadi, M.; Hajimirsadeghi, S.S.; Momenian, H. Effect of nitrate content on thermal decomposition of nitrocellulose. J. Hazard. Mater. 2009, 162, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Zinoviev, V.M.; Kutsenko, G.V.; Ermilov, A.S.; Boldavnin, I.I. High-Energy Plasticizers of Solid Rocket and Gun Propellants; Perm STU Publishing House: Perm, Russia, 2010. (In Russian) [Google Scholar]
- Vinogradov, D.B.; Bulatov, P.V.; Petrov, E.Y.; Gribov, P.S.; Kondakova, N.N.; Il’icheva, N.N.; Stepanova, E.R.; Denesyuk, A.P.; Sizov, V.A.; Sinditskii, V.P.; et al. Promising oxygen- and nitrogen-rich azidonitramino ether plasticizers for energetic materials. Molecules 2022, 27, 7749. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.P.; Biblarz, O. Rocket Propulsion Elements, 9th ed.; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Agrawal, J.P. High Energy Materials: Propellants, Explosives and Pyrotechnics; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Sinditskii, V.P.; Egorshev, V.Y.; Serushkin, V.V.; Levshenkov, A.I.; Berezin, M.V.; Filatov, S.A.; Smirnov, S.P. Evaluation of Decomposition Kinetics of Energetic Materials in the Combustion Wave. Thermochim. Acta 2009, 496, 1–12. [Google Scholar] [CrossRef]
- Denisyuk, A.P.; Shepelev, Y.G.; Yudaev, S.V.; Kalashnikov, I.V. Combustion of systems containing linear nitramines. Combust Explos Shock Waves 2005, 41, 206–214. [Google Scholar] [CrossRef]
- Sarner, S.F. Propellant Chemistry; Reinhold: New York, NY, USA, 1966. [Google Scholar]
- Kostochko, A.V.; Kazban, B.M. Gunpowder, Solid Composite Propellants and Their Properties; INTRA-M: Moscow, Russia, 2015. (In Russian) [Google Scholar]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.N., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Suponitsky, K.Y.; Smol’yakov, A.F.; Ananyev, I.V.; Khakhalev, A.V.; Gidaspov, A.A.; Sheremetev, A.B. 3,4-Dinitrofurazan: Structural Nonequivalence of ortho-Nitro Groups as a Key Feature of the Crystal Structure and Density. ChemistrySelect 2020, 5, 14543–14548. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Fedyanin, I.V.; Karnoukhova, V.A.; Zalomlenkov, V.A.; Gidaspov, A.A.; Bakharev, V.V.; Sheremetev, A.B. Energetic co-crystal of a primary metal-free explosive with BTF. Ideal pair for co-crystallization. Molecules 2021, 26, 7452. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Masunov, A.E.; Antipin, M.Y. Conformational dependence of the first molecular hyperpolarizability in the computational design of nonlinear optical materials for optical switching. Mendeleev. Commun. 2008, 18, 265–267. [Google Scholar] [CrossRef]





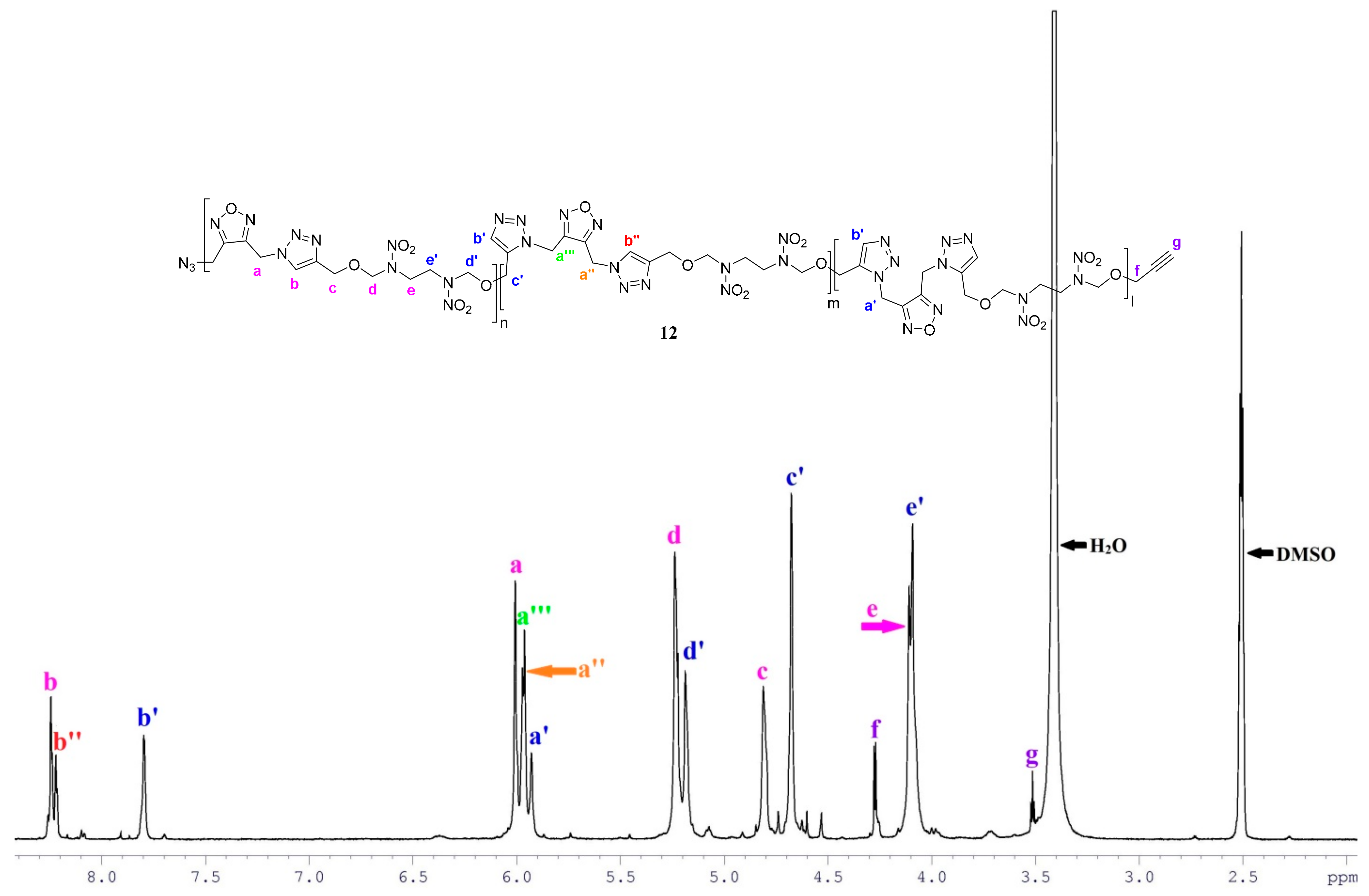






| Entry | Reaction Time (h) | Mn2 (g mol−1) | MW3 (g mol−1) | ĐM4 |
|---|---|---|---|---|
| 1 | 2 | 1210 | 1720 | 1.42 |
| 2 | 10 | 1810 | 3338 | 1.84 |
| 3 | 18 | 2450 | 4539 | 1.85 |
| 4 | 42 | 2532 | 5122 | 2.02 |
| 5 | 66 | 2766 | 6747 | 2.44 |
| 6 5 | 66 | 14,200 | 42,400 | 2.99 |
| Sample | DSC | TGA | |||
|---|---|---|---|---|---|
| Tonset, 1 °C | Tpeak, 2 °C | ∆Hd, 3 J g−1 | Tonset, 4 °C | Δm, 5 % | |
| 7 | 207 | 215 | - 6 | 103 | 98 |
| 9 | 237 | 270 | −2273 | 218 | 93 |
| 12 | 243 | 280 | −2168 | 248 | 60 |
| NC (12.5% N) | 197 | 204 | −1550 | 166 | 98 |
| Heating Rate, °C min−1 | Tpeak, °C | Enthalpy of Decomposition, ∆Hd, J g−1 | k·103, c−1 |
|---|---|---|---|
| 5 | 271 | −2176 | 5.96 |
| 10 | 280 | −2168 | 11.5 |
| 15 | 286 | −2226 | 16.9 |
| 20 | 290 | −2199 | 22.2 |
| Polymer | Plasticizer | C, 1 % wt. | Tg, °C | ∆Cp, 2 J g−1·K |
|---|---|---|---|---|
| 12 | Z1 | 50 | −51 | 0.615 |
| Z3 | 50 | −39 | 0.607 | |
| Z8 | 50 | −43 | 0.766 | |
| NG | 40 | −12 | 0,618 | |
| 50 | −27 | 0.647 | ||
| 60 | −40 | 0.713 | ||
| 70 | −52 | 0.775 | ||
| DEGDN | 20 | 2 | 0.519 | |
| NC | NG | 50 | −52 | 0.591 |
| Percentage NG, % wt | Viscosity, Pa·c |
|---|---|
| 40 * | 2192 |
| 50 | 269 |
| 60 | 142 |
| 70 | 43 |
| Material | Burning Rate rb = Apn. | rb 2,6 mm c−1 | rb 10, 7 mm c−1 | ||
|---|---|---|---|---|---|
| ∆p, 3 MPa | n 4 | A 5 | |||
| 9 | 2–15 | 0.48 | 1.24 | 1.7 | 3.7 |
| 121 | 0.4–3 | 0.64 | 2.0 | 3.3 | 11.5 |
| 122 | 0.1–3 | 0.54 | 1.5 | 2.2 | 9.4 |
| 12/NG (60:40) | 2–12 | 0.60 | 3.96 | 6.0 | 15.8 |
| NC | 1–12 | 0.73 | 2.82 | 3.3 | 10.7 |
| NC/NG (60:40) | 1–12 | 0.71 | A 3 | 4.6 | 14.5 |
| Composition | F, 1 kJ kg−1 | Tc, 2 K |
|---|---|---|
| NC/NG (70:30) | 1155 | 3730 |
| 12/NG (70:30) | 933 | 2380 |
| 12/NG (60:40) | 1038 | 2584 |
| 12/NG (50:50) | 1147 | 2975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribov, P.S.; Kondakova, N.N.; Il’icheva, N.N.; Stepanova, E.R.; Denisyuk, A.P.; Sizov, V.A.; Dotsenko, V.D.; Vinogradov, D.B.; Bulatov, P.V.; Sinditskii, V.P.; et al. Energetic Polymer Possessing Furazan, 1,2,3-Triazole, and Nitramine Subunits. Int. J. Mol. Sci. 2023, 24, 9645. https://doi.org/10.3390/ijms24119645
Gribov PS, Kondakova NN, Il’icheva NN, Stepanova ER, Denisyuk AP, Sizov VA, Dotsenko VD, Vinogradov DB, Bulatov PV, Sinditskii VP, et al. Energetic Polymer Possessing Furazan, 1,2,3-Triazole, and Nitramine Subunits. International Journal of Molecular Sciences. 2023; 24(11):9645. https://doi.org/10.3390/ijms24119645
Chicago/Turabian StyleGribov, Pavel S., Natalia N. Kondakova, Natalia N. Il’icheva, Evgenia R. Stepanova, Anatoly P. Denisyuk, Vladimir A. Sizov, Varvara D. Dotsenko, Dmitry B. Vinogradov, Pavel V. Bulatov, Valery P. Sinditskii, and et al. 2023. "Energetic Polymer Possessing Furazan, 1,2,3-Triazole, and Nitramine Subunits" International Journal of Molecular Sciences 24, no. 11: 9645. https://doi.org/10.3390/ijms24119645
APA StyleGribov, P. S., Kondakova, N. N., Il’icheva, N. N., Stepanova, E. R., Denisyuk, A. P., Sizov, V. A., Dotsenko, V. D., Vinogradov, D. B., Bulatov, P. V., Sinditskii, V. P., Suponitsky, K. Y., Il’in, M. M., Keshtov, M. L., & Sheremetev, A. B. (2023). Energetic Polymer Possessing Furazan, 1,2,3-Triazole, and Nitramine Subunits. International Journal of Molecular Sciences, 24(11), 9645. https://doi.org/10.3390/ijms24119645






