Genome-Wide Identification and Characterization of the Msr Gene Family in Alfalfa under Abiotic Stress
Abstract
1. Introduction
2. Results
2.1. Identification of MsMsr Family Members
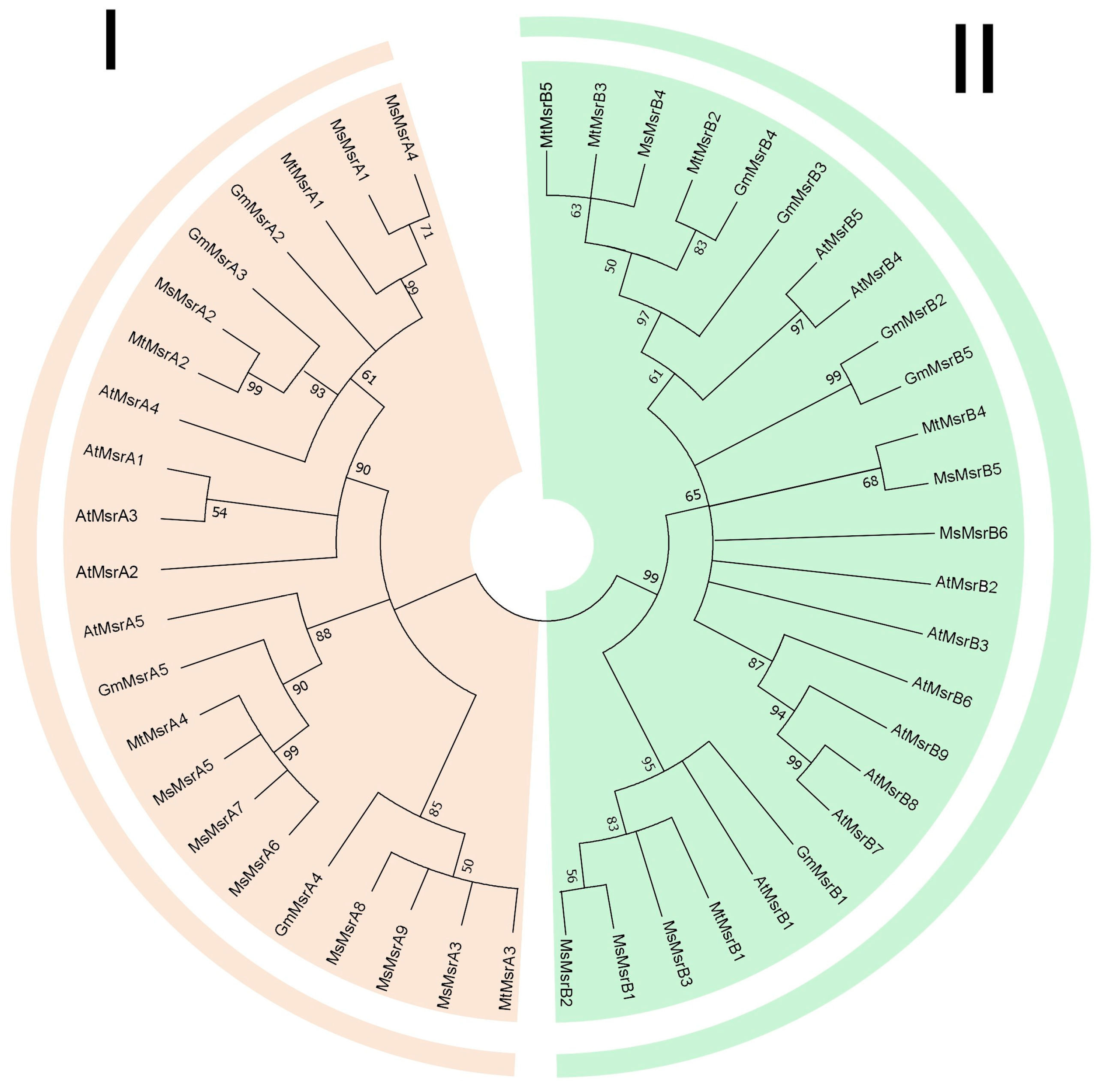
2.2. Phylogenetic Analysis of Msrs
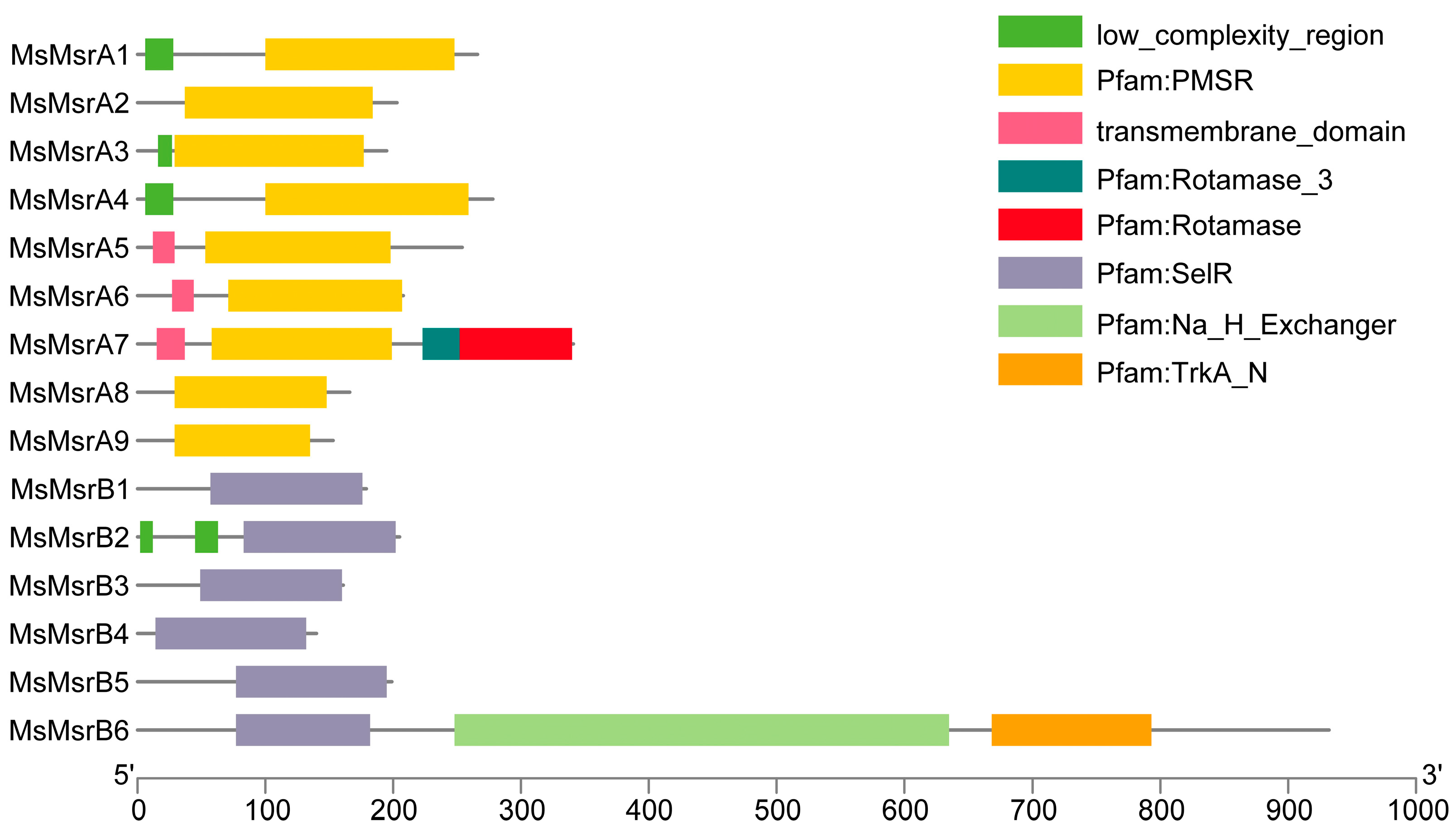
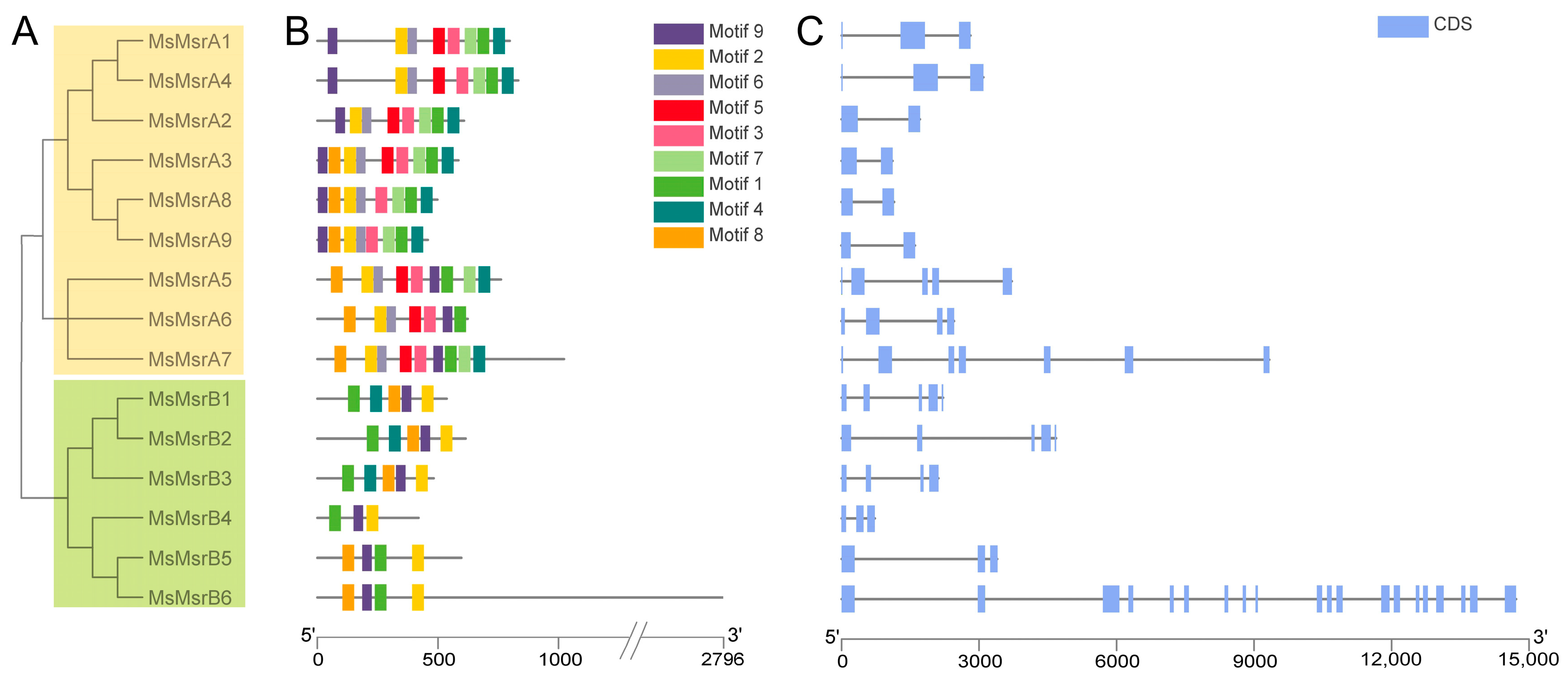
2.3. Sequence and Structure Analysis of MsMsrs
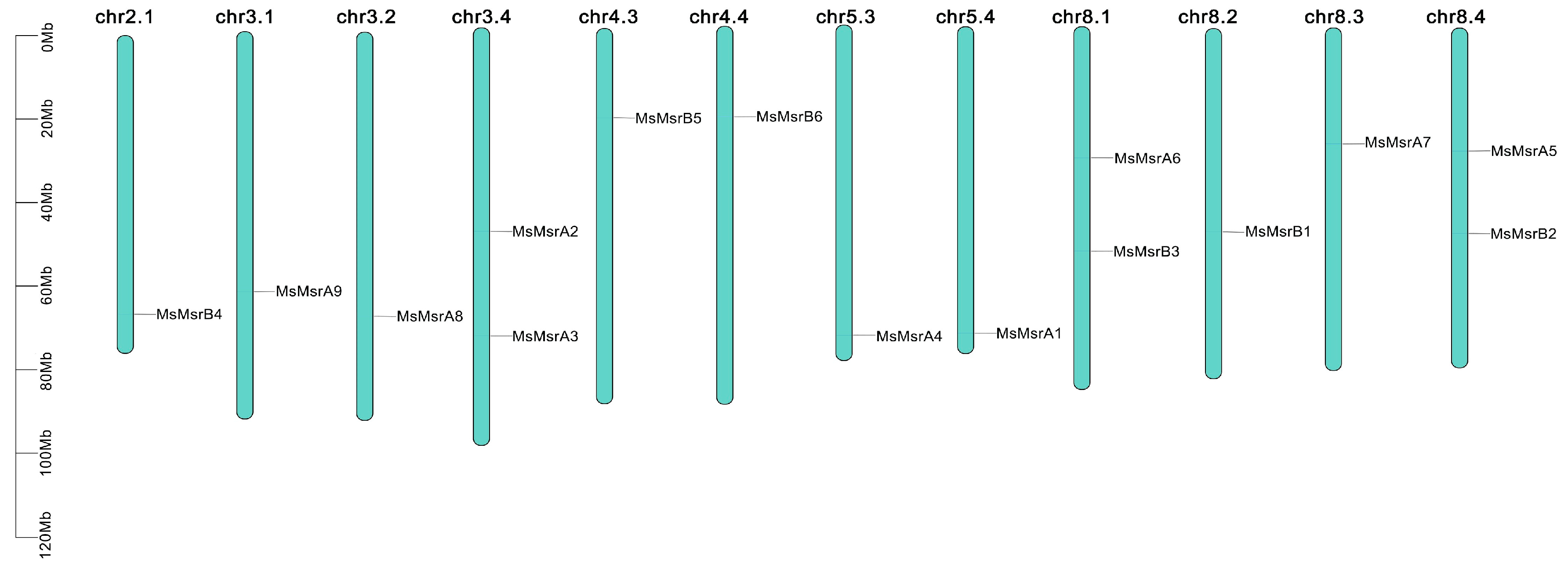
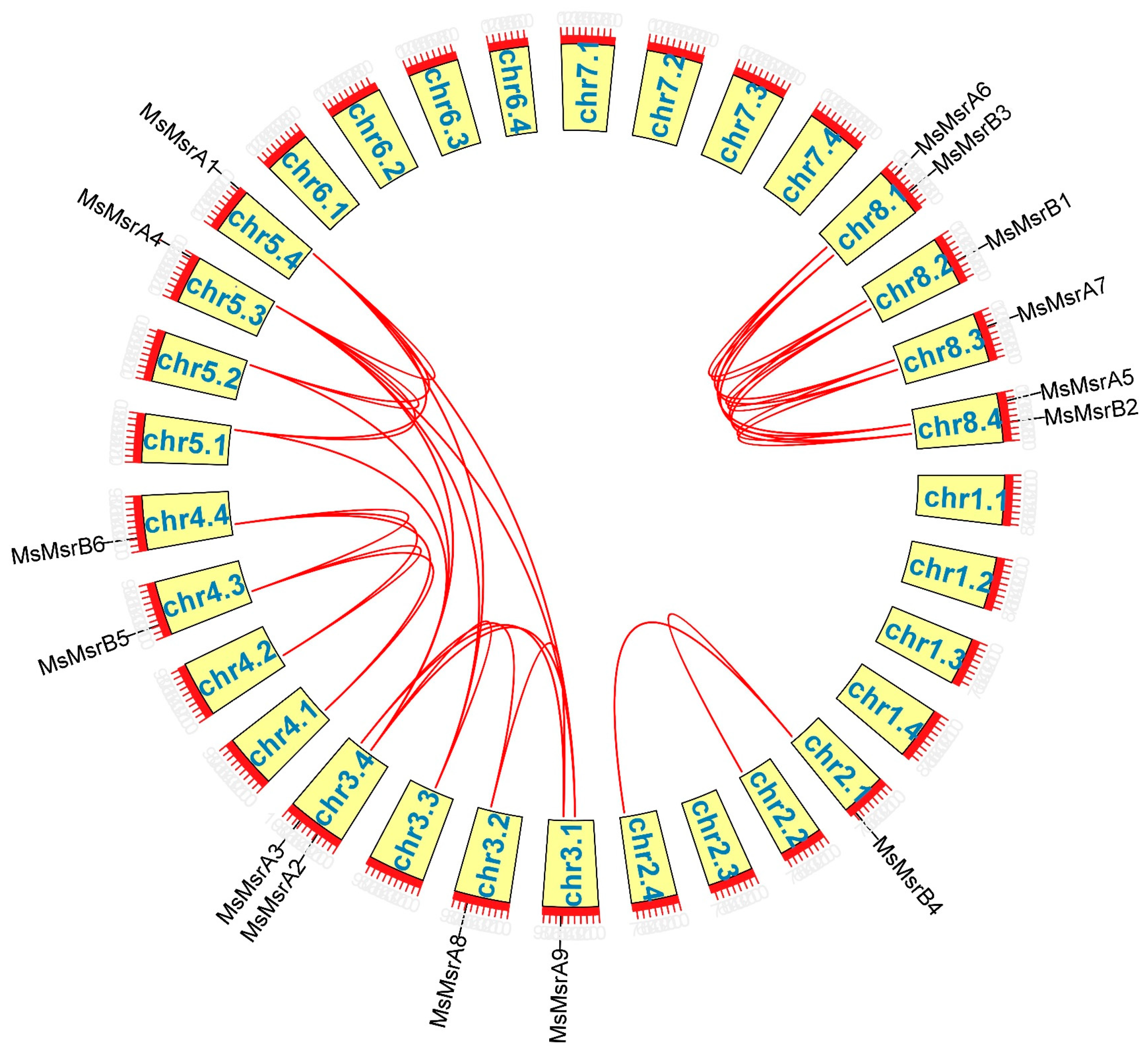
2.4. Chromosomal Mapping and Collinearity Analysis of MsMsr Genes
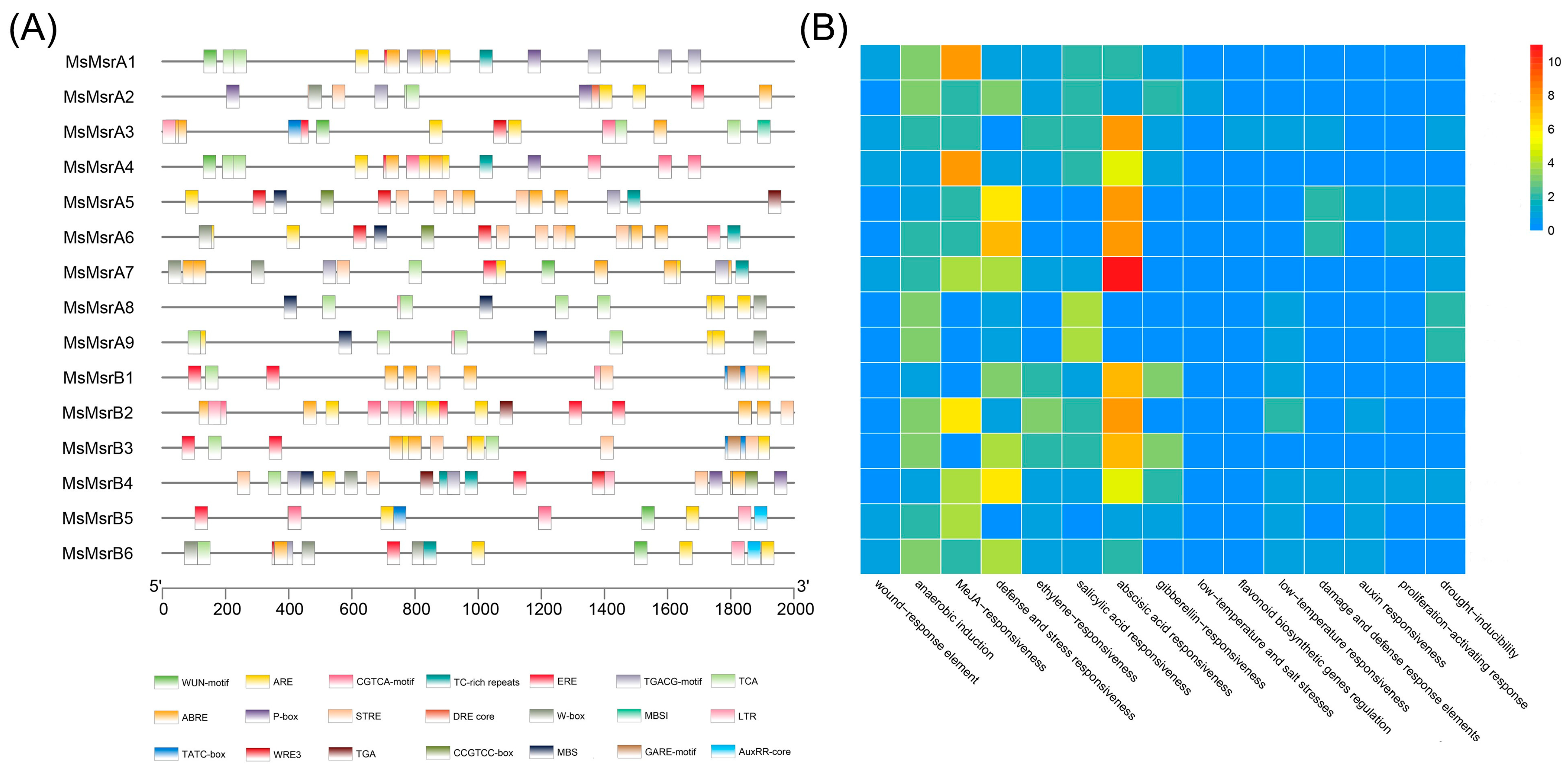
2.5. Cis-Regulatory Elements in MsMsr Gene Promoters
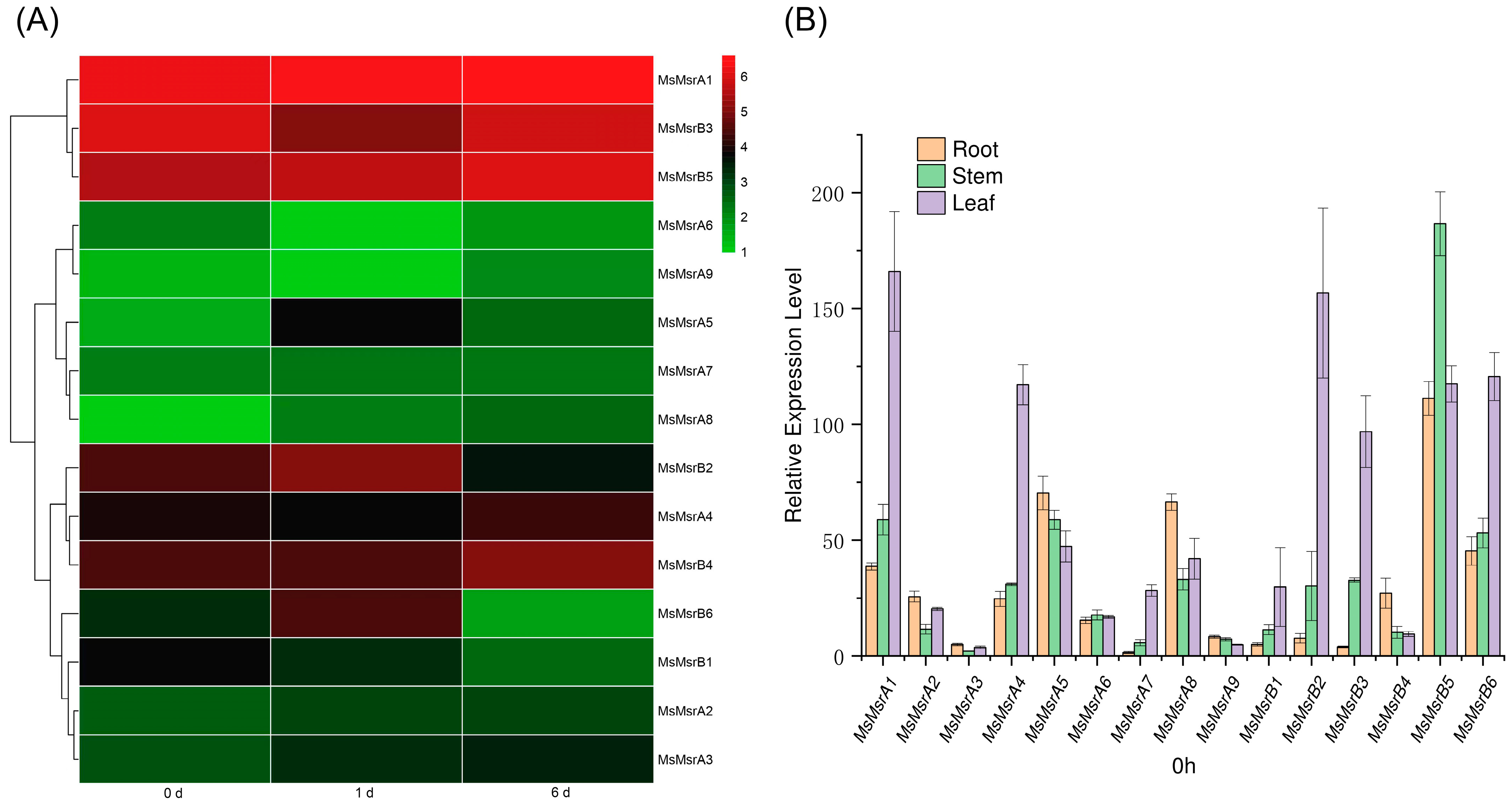
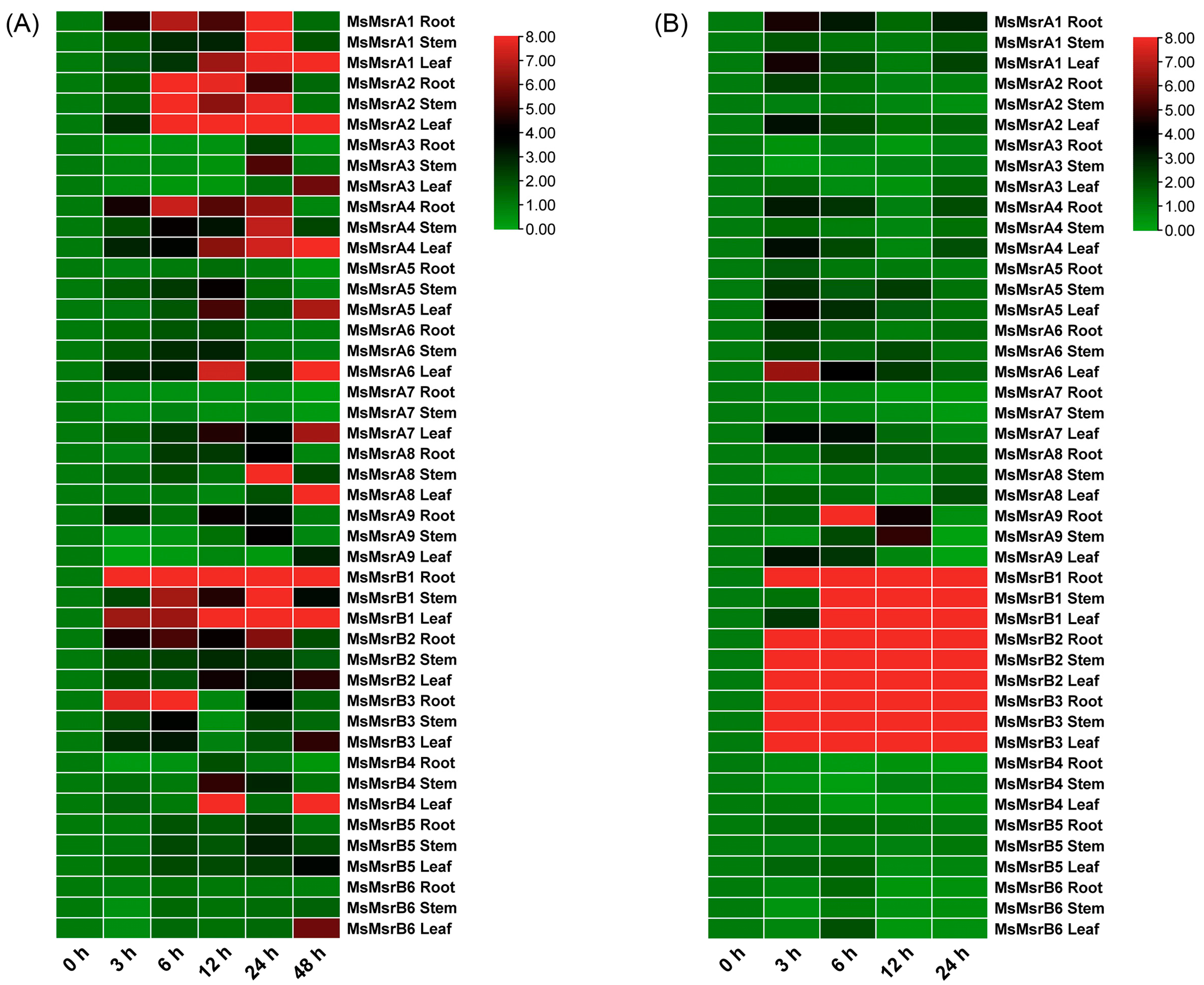
2.6. Expression Analysis of Homologous Genes of 15 MsMsr Genes under Abiotic Stress
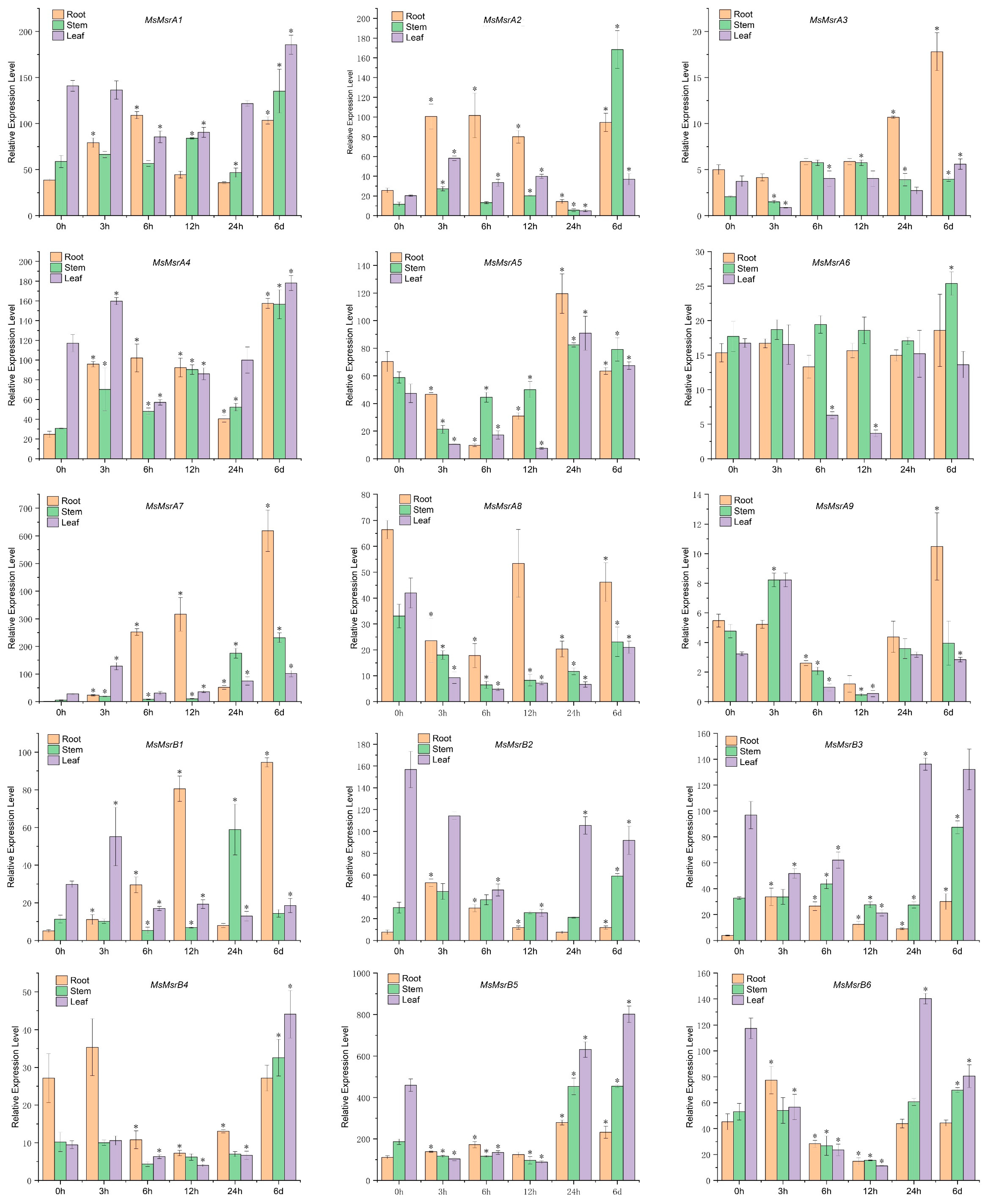
2.7. qRT-PCR Analysis of 15 Homologs of MsMsr Genes
3. Discussion
4. Materials and Methods
4.1. Identification of MsMsr Genes in Alfalfa
4.2. Phylogenetic Analysis, Gene Structure, and Motif Composition of MsMsr Genes
4.3. Chromosomal Mapping, Gene Duplication, and Collinearity Analysis
4.4. Analysis of Cis-acting Elements in Promoter Regions
4.5. Transcriptome and Functional Enrichment Analyses of MsMsr Genes under Salt Treatment
4.6. Plant Materials and Growth Conditions
4.7. Quantitative Real-Time PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radović, J.; Sokolović, D.; Marković, J. Alfalfa-Most Important Perennial Forage Legume in Animal Husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Acharya, J.P.; Lopez, Y.; Gouveia, B.T.; de Bem Oliveira, I.; Resende, M.F.R.; Muñoz, P.R.; Rios, E.F. Breeding Alfalfa (Medicago sativa L.) Adapted to Subtropical Agroecosystems. Agronomy 2020, 10, 742. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.; Liu, Y.; et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Lavine, T.F. The formation, resolution, and optical properties of the diastereoisomeric sulfoxides derived from L-methionine. J. Biol. Chem. 1947, 169, 477–491. [Google Scholar] [CrossRef]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Brot, N.; Weissbach, L.; Werth, J.; Weissbach, H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc. Natl. Acad. Sci. USA 1981, 78, 2155–2158. [Google Scholar] [CrossRef]
- Grimaud, R.; Ezraty, B.; Mitchell, J.K.; Lafitte, D.; Briand, C.; Derrick, P.J.; Barras, F. Repair of oxidized proteins: Identification of a new methionine sulfoxide reductase. J. Biol. Chem. 2001, 276, 48915–48920. [Google Scholar] [CrossRef]
- Zhang, X.H.; Weissbach, H. Origin and evolution of the protein-repairing enzymes methionine sulphoxide reductases. Biol. Rev. Camb. Philos. Soc. 2008, 83, 249–257. [Google Scholar] [CrossRef]
- Delaye, L.; Becerra, A.; Orgel, L.; Lazcano, A. Molecular evolution of peptide methionine sulfoxide reductases (MsrA and MsrB): On the early development of a mechanism that protects against oxidative damage. J. Mol. Evol. 2007, 64, 15–32. [Google Scholar] [CrossRef]
- Moskovitz, J.; Flescher, E.; Berlett, B.S.; Stadtman, E.R. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc. Natl. Acad. Sci. USA 1998, 95, 14071–14075. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Poston, J.M.; Berlett, B.S.; Nosworthy, N.J.; Szczepanowski, R.; Stadtman, E.R. Identification and Characterization of a Putative Active Site for Peptide Methionine Sulfoxide Reductase (MsrA) and Its Substrate Stereospecificity. J. Biol. Chem. 2000, 275, 14167–14172. [Google Scholar] [CrossRef] [PubMed]
- Gabbita, S.P.; Aksenov, M.Y.; Lovell, M.A.; Markesbery, W.R. Decrease in Peptide Methionine Sulfoxide Reductase in Alzheimer’s Disease Brain. J. Neurochem. 1999, 73, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.; Mary, J.; Perichon, M.; Friguet, B. Rat peptide methionine sulphoxide reductase: Cloning of the cDNA, and down-regulation of gene expression and enzyme activity during aging. Biochem. J. 2001, 355, 819. [Google Scholar] [CrossRef]
- Brot, N.; Fliss, H.; Coleman, T.; Weissbach, H. Enzymatic Reduction of Methionine Sulfoxide Residues in Proteins and Peptides. Methods Enzymol. 1984, 107, 352–360. [Google Scholar] [CrossRef]
- Rouhier, N.; Vieira Dos Santos, C.; Tarrago, L.; Rey, P. Plant methionine sulfoxide reductase A and B multigenic families. Photosynth. Res. 2006, 89, 247–262. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Wang, Y.; Chen, Y.; Chu, C. OsMSRA4.1 and OsMSRB1.1, two rice plastidial methionine sulfoxide reductases, are involved in abiotic stress responses. Planta 2009, 230, 227–238. [Google Scholar] [CrossRef]
- Le, D.T.; Tarrago, L.; Watanabe, Y.; Kaya, A.; Lee, B.C.; Tran, U.; Nishiyama, R.; Fomenko, D.E.; Gladyshev, V.N.; Tran, L.-S.P. Diversity of plant methionine sulfoxide reductases B and evolution of a form specific for free methionine sulfoxide. PLoS ONE 2013, 8, e65637. [Google Scholar] [CrossRef]
- Zhu, J.; Ding, P.; Li, Q.; Gao, Y.; Chen, F.; Xia, G. Molecular characterization and expression profile of methionine sulfoxide reductase gene family in maize (Zea mays) under abiotic stresses. Gene 2015, 562, 159–168. [Google Scholar] [CrossRef]
- Ding, P.; Fang, L.; Wang, G.; Li, X.; Huang, S.; Gao, Y.; Zhu, J.; Xiao, L.; Tong, J.; Chen, F.; et al. Wheat methionine sulfoxide reductase A4.1 interacts with heme oxygenase 1 to enhance seedling tolerance to salinity or drought stress. Plant Mol. Biol. 2019, 101, 203–220. [Google Scholar] [CrossRef]
- Ding, P.; Fang, L.; Huang, S.; Zhu, J.; Wang, G.; Xia, G.; Chen, F. Wheat plastidial methionine sulfoxide reductase MSRB3.1 interacts with haem oxygenase 1 to improve osmotic stress tolerance in wheat seedlings. Environ. Exp. Bot. 2021, 188, 104528. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, P.; Guo, Q.; Hu, D.; Fu, X.; Chen, F.; Xia, G. Interaction of wheat methionine sulfoxide reductase TaMSRB5.2 with glutathione S-transferase TaGSTF3-A contributes to seedling osmotic stress resistance. Environ. Exp. Bot. 2022, 194, 104731. [Google Scholar] [CrossRef]
- Romero, H.M.; Berlett, B.S.; Jensen, P.J.; Pell, E.J.; Tien, M. Investigations into the role of the plastidial peptide methionine sulfoxide reductase in response to oxidative stress in Arabidopsis. Plant Physiol. 2004, 136, 3784–3794. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, U.; Murphy, D.J.; Mullineaux, P.M. Arabidopsis peptide methionine sulfoxide reductase2 prevents cellular oxidative damage in long nights. Plant Cell 2004, 16, 908–919. [Google Scholar] [CrossRef]
- Laugier, E.; Tarrago, L.; Vieira Dos Santos, C.; Eymery, F.; Havaux, M.; Rey, P. Arabidopsis thaliana plastidial methionine sulfoxide reductases B, MSRBs, account for most leaf peptide MSR activity and are essential for growth under environmental constraints through a role in the preservation of photosystem antennae. Plant J. 2010, 61, 271–282. [Google Scholar] [CrossRef]
- Vieira Dos Santos, C.; Cuiné, S.; Rouhier, N.; Rey, P. The Arabidopsis plastidic methionine sulfoxide reductase B proteins. Sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress. Plant Physiol. 2005, 138, 909–922. [Google Scholar] [CrossRef]
- Li, C.-W.; Lee, S.-H.; Chieh, P.-S.; Lin, C.-S.; Wang, Y.-C.; Chan, M.-T. Arabidopsis Root-Abundant Cytosolic Methionine Sulfoxide Reductase B Genes MsrB7 and MsrB8 are Involved in Tolerance to Oxidative Stress. Plant Cell Physiol. 2012, 53, 1707–1719. [Google Scholar] [CrossRef]
- Chatelain, E.; Satour, P.; Laugier, E.; Ly Vu, B.; Payet, N.; Rey, P.; Montrichard, F. Evidence for participation of the methionine sulfoxide reductase repair system in plant seed longevity. Proc. Natl. Acad. Sci. USA 2013, 110, 3633–3638. [Google Scholar] [CrossRef]
- Sun, X.; Sun, M.; Jia, B.; Qin, Z.; Yang, K.; Chen, C.; Yu, Q.; Zhu, Y. A Glycine soja methionine sulfoxide reductase B5a interacts with the Ca2+/CAM-binding kinase Gs CBRLK and activates ROS signaling under carbonate alkaline stress. Plant J. 2016, 86, 514–529. [Google Scholar] [CrossRef]
- Hazra, A.; Varshney, V.; Verma, P.; Kamble, N.U.; Ghosh, S.; Achary, R.K.; Gautam, S.; Majee, M. Methionine sulfoxide reductase B5 plays a key role in preserving seed vigor and longevity in rice (Oryza sativa). N. Phytol. 2022, 236, 1042–1060. [Google Scholar] [CrossRef]
- Yan, H.; Jiang, G.; Wu, F.; Li, Z.; Xiao, L.; Jiang, Y.; Duan, X. Sulfoxidation regulation of transcription factor NAC42 influences its functions in relation to stress-induced fruit ripening in banana. J. Exp. Bot. 2021, 72, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L.; Wu, C.; Shan, W.; Cai, D.; Lin, Z.; Wei, W.; Chen, J.; Lu, W.; Kuang, J. Methionine oxidation and reduction of the ethylene signaling component MaEIL9 are involved in banana fruit ripening. J. Integr. Plant Biol. 2023, 65, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zheng, F.; Zhang, D.; Li, C.; Li, M.; Ye, J.; Zhang, Y.; Wang, T.; Ouyang, B.; Hong, Z. Tomato methionine sulfoxide reductase B2 functions in drought tolerance by promoting ROS scavenging and chlorophyll accumulation through interaction with Catalase 2 and RBCS3B. Plant Sci. 2022, 318, 111206. [Google Scholar] [CrossRef]
- Fu, B.L.; Wang, W.Q.; Liu, X.F.; Duan, X.W.; Allan, A.C.; Grierson, D.; Yin, X.R. An ethylene-hypersensitive methionine sulfoxide reductase regulated by NAC transcription factors increases methionine pool size and ethylene production during kiwifruit ripening. N. Phytol. 2021, 232, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, J.; Ding, P.; Chen, F.; Xia, G. The Brachypodium distachyon methionine sulfoxide reductase gene family. Genes Genom. 2017, 39, 975–985. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Long, M. A new function evolved from gene fusion. Genome Res. 2000, 10, 1655–1657. [Google Scholar] [CrossRef]
- Long, M.; VanKuren, N.W.; Chen, S.; Vibranovski, M.D. New gene evolution: Little did we know. Annu. Rev. Genet. 2013, 47, 307–333. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Zhang, L.; Ye, Q.; Liu, N.; Wang, M.; Long, G.; Fan, W.; Long, M.; Wing, R. Gene fusion as an important mechanism to generate new genes in the genus Oryza. Genome Biol. 2022, 23, 130. [Google Scholar] [CrossRef]
- Meiri, D.; Breiman, A. Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90. 1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. 2009, 59, 387–399. [Google Scholar] [CrossRef]
- Bissoli, G.; Niñoles, R.; Fresquet, S.; Palombieri, S.; Bueso, E.; Rubio, L.; García-Sánchez, M.J.; Fernández, J.A.; Mulet, J.M.; Serrano, R.J.T.P.J. Peptidyl-prolyl cis-trans isomerase ROF2 modulates intracellular pH homeostasis in Arabidopsis. Plant J. 2012, 70, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Barragan, V.; Leidi, E.O.; Andres, Z.; Rubio, L.; De Luca, A.; Fernandez, J.A.; Cubero, B.; Pardo, J. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, G.-D.; Li, H.; Zheng, C.-C.; Wu, C.-A. Overexpression of NHX1 s in transgenic Arabidopsis enhances photoprotection capacity in high salinity and drought conditions. Acta Physiol. Plant 2010, 32, 81–90. [Google Scholar] [CrossRef]
- Hu, D.; Guo, Q.; Zhang, Y.; Chen, F. Maize Methionine Sulfoxide Reductase Genes ZmMSRA2 and ZmMSRA5.1 Involved in the Tolerance to Osmotic or Salinity Stress in Arabidopsis and Maize. Plant Mol. Biol. Rep. 2022, 41, 118–133. [Google Scholar] [CrossRef]
- Han, Y.; Du, Y.; Wang, J.; Wu, T. Overexpression of Chinese flowering cabbage BpPMSR3 enhances the tolerance of Arabidopsis thaliana to cadmium. J. Plant Nutr. Soil Sci. 2018, 181, 787–794. [Google Scholar] [CrossRef]
- Sham, A.; Al-Ashram, H.; Whitley, K.; Iratni, R.; El-Tarabily, K.A.; AbuQamar, S.F. Metatranscriptomic analysis of multiple environmental stresses identifies RAP2. 4 gene associated with Arabidopsis immunity to Botrytis cinerea. Sci. Rep. 2019, 9, 17010. [Google Scholar] [CrossRef]
- Tartaglia, M.; Sciarrillo, R.; Zuzolo, D.; Postiglione, A.; Prigioniero, A.; Scarano, P.; Ruggieri, V.; Guarino, C. Exploring an enhanced rhizospheric phenomenon for pluricontaminated soil remediation: Insights from tripartite metatranscriptome analyses. J. Hazard. Mater. 2022, 428, 128246. [Google Scholar] [CrossRef]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. bioRxiv 2018, 289660. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.e.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Li, Q.; He, C.; Pang, Y. Identification and Characterization of Abiotic Stress–Responsive NF-YB Family Genes in Medicago. Int. J. Mol. Sci. 2022, 23, 6906. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, L.; Du, W.; Yan, S.; Wang, Z.; Pang, Y. Identification and Characterization of Salt-and Drought-Responsive AQP Family Genes in Medicago sativa L. Int. J. Mol. Sci. 2022, 23, 3342. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Z.; Chen, S.; Ma, L.; Wang, H.; Dong, R.; Wang, Y.; Liu, Z. Global transcriptome profiling analysis reveals insight into saliva-responsive genes in alfalfa. Plant Cell Rep. 2016, 35, 561–571. [Google Scholar] [CrossRef]
- Mao, P.; Jin, X.; Bao, Q.; Mei, C.; Zhou, Q.; Min, X.; Liu, Z. WRKY transcription factors in Medicago sativa L.: Genome-wide identification and expression analysis under abiotic stress. DNA Cell Biol. 2020, 39, 2212–2225. [Google Scholar] [CrossRef]
| Name | Gene ID | CDS (bp) | Length (aa) | pI | MW (Da) | Predicted Location (s) | Predicted Signal (s) |
|---|---|---|---|---|---|---|---|
| MsMsrA1 | MS.gene038415 | 798 | 265 | 8.75 | 29,419.08 | Plastid | Chloroplast transit peptide |
| MsMsrA2 | MS.gene027087 | 609 | 202 | 8.20 | 23,205.85 | Cytoplasm | None |
| MsMsrA3 | MS.gene29083 | 585 | 194 | 5.48 | 21,737.02 | Cytoplasm | None |
| MsMsrA4 | MS.gene017180 | 834 | 277 | 7.53 | 30,789.65 | Plastid | Chloroplast transit peptide |
| MsMsrA5 | MS.gene012096 | 762 | 253 | 5.79 | 28,908.68 | Endoplasmic reticulum | Signal peptide |
| MsMsrA6 | MS.gene44259 | 624 | 207 | 6.42 | 23,033.94 | Endoplasmic reticulum | Signal peptide |
| MsMsrA7 | MS.gene89646 | 1023 | 340 | 6.05 | 38,010.76 | Endoplasmic reticulum | Signal peptide |
| MsMsrA8 | MS.gene022411 | 498 | 165 | 5.34 | 18,248.1 | Cytoplasm | None |
| MsMsrA9 | MS.gene78714 | 459 | 152 | 5.2 | 16,851.51 | Cytoplasm | None |
| MsMsrB1 | MS.gene032356 | 537 | 178 | 8.48 | 20,193.22 | Cytoplasm, mitochondrion | Mitochondrial transit peptide |
| MsMsrB2 | MS.gene38969 | 615 | 204 | 9.09 | 22,729.57 | Plastid | Chloroplast transit peptide |
| MsMsrB3 | MS.gene007821 | 483 | 160 | 6.29 | 18,119.74 | Cytoplasm, mitochondrion | Mitochondrial transit peptide |
| MsMsrB4 | MS.gene002820 | 420 | 139 | 6.08 | 15,101.88 | Cytoplasm, peroxisome | Peroxisomal targeting signal |
| MsMsrB5 | MS.gene40100 | 597 | 198 | 8.87 | 21,424.38 | Plastid | Chloroplast transit peptide |
| MsMsrB6 | MS.gene053626 | 2796 | 931 | 5.7 | 101,351.14 | Plastid | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Han, X.; Lu, X.; Yang, H.; Wang, Z.-Y.; Chai, M. Genome-Wide Identification and Characterization of the Msr Gene Family in Alfalfa under Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 9638. https://doi.org/10.3390/ijms24119638
Zhao X, Han X, Lu X, Yang H, Wang Z-Y, Chai M. Genome-Wide Identification and Characterization of the Msr Gene Family in Alfalfa under Abiotic Stress. International Journal of Molecular Sciences. 2023; 24(11):9638. https://doi.org/10.3390/ijms24119638
Chicago/Turabian StyleZhao, Xianglong, Xiao Han, Xuran Lu, Haoyue Yang, Zeng-Yu Wang, and Maofeng Chai. 2023. "Genome-Wide Identification and Characterization of the Msr Gene Family in Alfalfa under Abiotic Stress" International Journal of Molecular Sciences 24, no. 11: 9638. https://doi.org/10.3390/ijms24119638
APA StyleZhao, X., Han, X., Lu, X., Yang, H., Wang, Z.-Y., & Chai, M. (2023). Genome-Wide Identification and Characterization of the Msr Gene Family in Alfalfa under Abiotic Stress. International Journal of Molecular Sciences, 24(11), 9638. https://doi.org/10.3390/ijms24119638






