Can Allostery Be a Key Strategy for Targeting PTP1B in Drug Discovery? A Lesson from Trodusquemine
Abstract
1. Introduction
2. PTP1B Functions and Involvement in Pathogenic Mechanisms Underlying Human Diseases
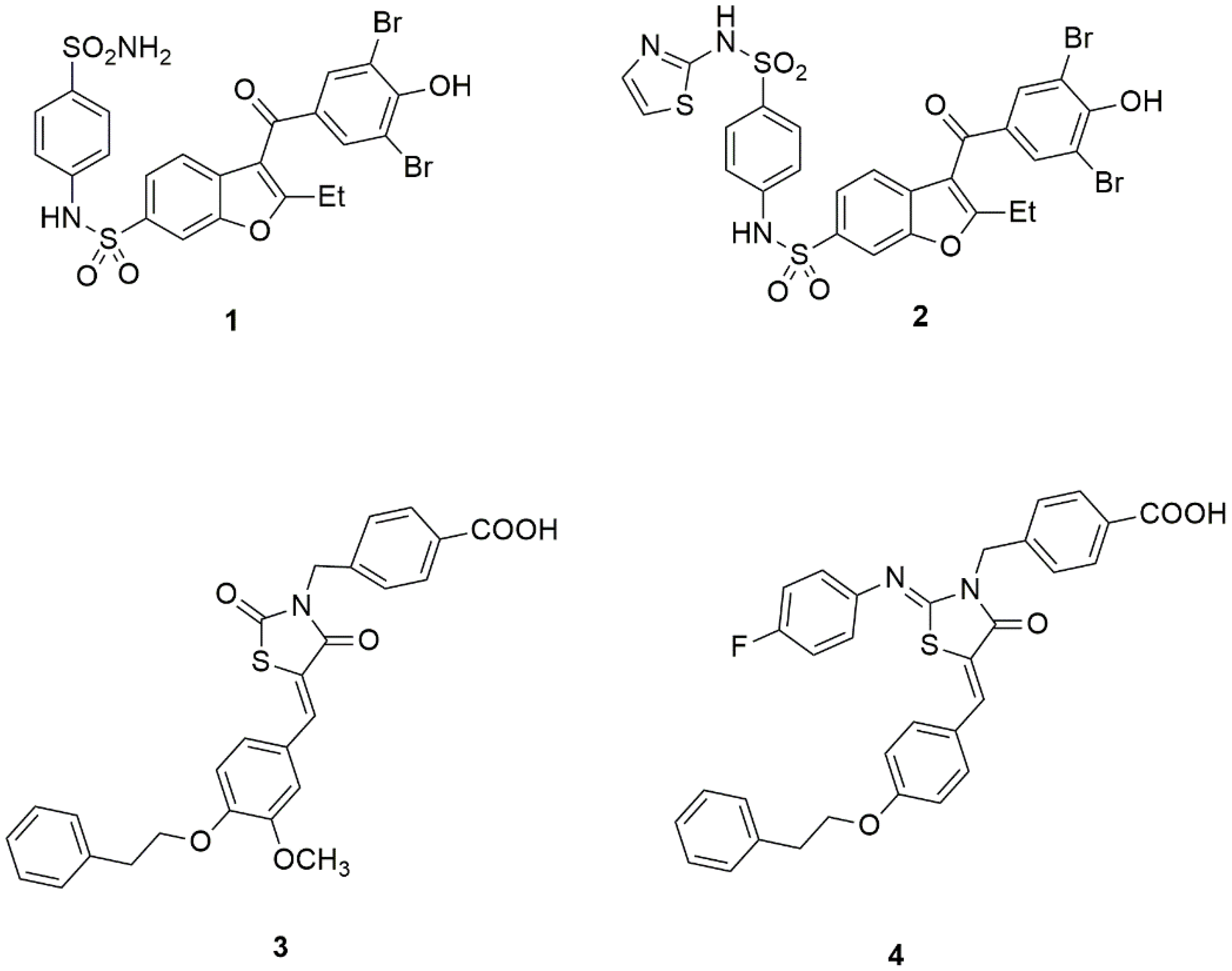
3. PTP1B as a Molecular Target for Designing Novel Drugs
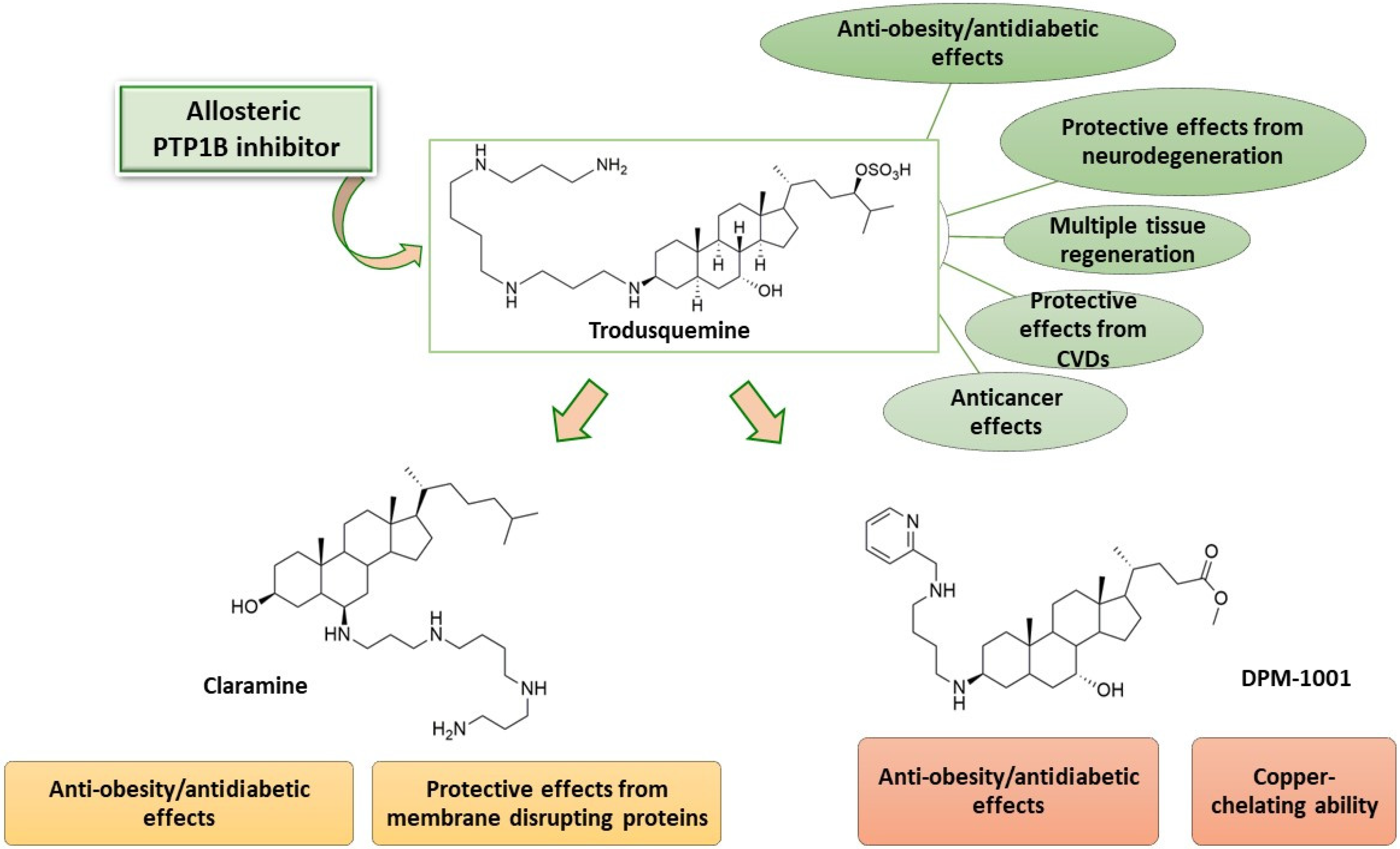
4. Identification of Trodusquemine and Its Mechanism of PTP1B Inhibition
5. Anti-Obesity and Antidiabetic Actions of Trodusquemine
6. Anticancer Activity of Trodusquemine
7. Trodusquemine-Induced Regenerative Effects in Multiple Tissues
8. The Potential of Trodusquemine for the Treatment of Cardiovascular Diseases
9. The Potential of Trodusquemine for the Treatment of Neurodegenerative Disorders
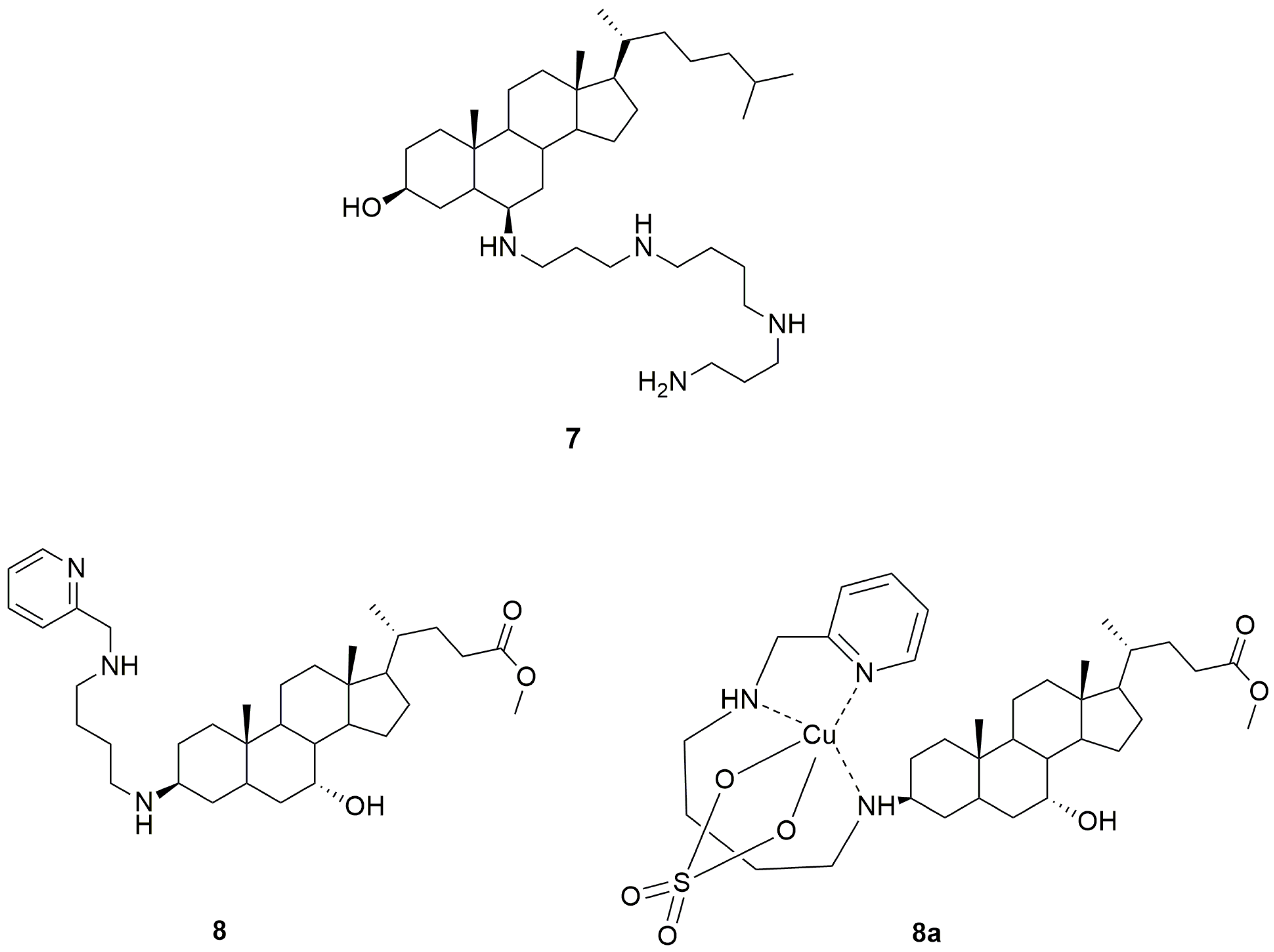
10. Synthetic Analogues of Trodusquemine
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennasroune, A.; Gardin, A.; Aunis, D.; Crémel, G.; Hubert, P. Tyrosine Kinase Receptors as Attractive Targets of Cancer Therapy. Crit. Rev. Oncol. Hemat. 2004, 50, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Bialy, L.; Waldmann, H. Inhibitors of Protein Tyrosine Phosphatases: Next-Generation Drugs? Angew. Chem. Int. 2005, 44, 3814–3839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Z.Y. PTP1B as a Drug Target: Recent Developments in PTP1B Inhibitor Discovery. Drug Discov. Today 2007, 12, 373–381. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T. Drug Discovery and Protein Tyrosine Phosphatases. Curr. Med. Chem. 2009, 16, 2095–2176. [Google Scholar] [CrossRef]
- Vintonyak, V.V.; Waldmann, H.; Rauh, D. Using Small Molecules to Target Protein Phosphatases. Bioorg. Med. Chem. 2011, 19, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Ottanà, R. Low Molecular Weight Phosphotyrosine Protein Phosphatases as Emerging Targets for the Design of Novel Therapeutic Agents. J. Med. Chem. 2012, 55, 2–22. [Google Scholar] [CrossRef]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein Tyrosine Phosphatases in the Human Genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef]
- Abdelsalam, S.S.; Korashy, H.M.; Zeidan, A.; Agouni, A. The Role of Protein Tyrosine Phosphatase (PTP)-1B in Cardiovascular Disease and its Interplay with Insulin Resistance. Biomolecules 2019, 9, 286. [Google Scholar] [CrossRef]
- Beddows, C.A.; Dodd, G.T. Insulin on the Brain: The Role of Central Insulin Signalling in Energy and Glucose Homeostasis. J. Neuroendocrinol. 2021, 33, e12947. [Google Scholar] [CrossRef]
- Villamar-Cruz, O.; Loza-Mejia, M.A.; Arias-Romero, L.E.; Camacho-Arroyo, I. Recent Advances in PTP1B Signaling in Metabolism and Cancer. Bioscience Rep. 2021, 41, BSR20211994. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Bence-Hanulec, K.K.; Stricker-Krongrad, A.; Haj, F.; Wang, Y.; Minokoshi, Y.; Kim, Y.B.; Elmquist, J.K.; Tartaglia, L.A.; Kahn, B.B.; et al. PTP1B Regulates Leptin Signal Transduction In Vivo. Dev. Cell 2002, 2, 489–495. [Google Scholar] [CrossRef]
- Cheng, A.; Uetani, N.; Simoncic, P.D.; Chaubey, V.P.; Lee-Loy, A.; McGlade, C.J.; Kennedy, B.P.; Tremblay, M.L. Attenuation of Leptin Action and Regulation of Obesity by Protein Tyrosine Phosphatase 1B. Dev. Cell 2002, 2, 497–503. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Dodd, G.T.; Tiganis, T. Protein Tyrosine Phosphatases in Hypothalamic Insulin and Leptin Signaling. Trends Pharmacol. Sci. 2015, 36, 661–674. [Google Scholar] [CrossRef]
- Bakke, J.; Haj, F.G. Protein-Tyrosine Phosphatase 1B Substrates and Metabolic Regulation. Semin. Cell Dev. Biol. 2015, 37, 58–65. [Google Scholar] [CrossRef]
- Teimouri, M.; Hosseini, H.; ArabSadeghabadi, Z.; Babaei-Khorzoughi, R.; Gorgani-Firuzjaee, S.; Meshkani, R. The Role of Protein Tyrosine Phosphatase 1B (PTP1B) in the Pathogenesis of Type 2 Diabetes Mellitus and its Complications. J. Physiol. Biochem. 2022, 78, 307–322. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Salmeen, A.; Andersen, J.N.; Myers, M.P.; Tonks, N.K.; Barford, D. Molecular Basis for the Dephosphorylation of the Activation Segment of the Insulin Receptor by Protein Tyrosine Phosphatase 1B. Mol. Cell. 2000, 6, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Delibegovic, M.; Bence, K.; Mody, N.; Hong, E.-G.; Ko, H.J.; Kim, J.K.; Kahn, B.B.; Neel, B.G. Improved Glucose Homeostasis in Mice with Muscle-Specific Deletion of Protein-Tyrosine Phosphatase 1B. Mol. Cell. Biol. 2007, 27, 7727–7734. [Google Scholar] [CrossRef] [PubMed]
- Delibegovic, M.; Zimmer, D.; Kauffman, C.; Rak, K.; Hong, E.; Cho, Y.; Kim, J.K.; Kahn, B.B.; Neel, B.G.; Bence, K.K. Liver-Specific Deletion of Protein-Tyrosine Phosphatase 1B (PTP1B) Improves Metabolic Syndrome and Attenuates Diet-Induced Endoplasmic Reticulum Stress. Diabetes 2009, 58, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.T.; Tiganis, T. Insulin Action in the Brain: Roles in Energy and Glucose Homeostasis. J. Neuroendocrinol. 2017, 29, e12513. [Google Scholar] [CrossRef]
- Sainz, N.; Barrenetxe, J.; Moreno-Aliaga, M.J.; Martinez, J.A. Leptin Resistance and Diet-Induced Obesity: Central and Peripheral Actions of Leptin. Metabolism 2015, 64, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Thon, M.; Hosoi, T.; Ozawa, K. Possible Integrative Actions of Leptin and Insulin Signaling in the Hypothalamus Targeting Energy Homeostasis. Front. Endocrinol. 2016, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin Resistance and Alzheimer’s Disease. BMB Rep. 2009, 42, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Frittitta, L.; Miscio, G.; Bozzali, M.; Baratta, R.; Centra, M.; Spampinato, D.; Santagati, M.G.; Ercolino, T.; Cisternino, C.; et al. A Variation in 3’ UTR of hPTP1B Increases Specific Gene Expression and Associates with Insulin Resistance. Am. J. Hum. Genet. 2002, 70, 806–812. [Google Scholar] [CrossRef]
- Miranda, S.; Gonzalez-Rodriguez, A.; Revuelta-Cervantes, J.; Rondinone, C.M.; Valverde, A.M. Beneficial Effects of PTP1B Deficiency on Brown Adipocyte Differentiation and Protection Against Apoptosis Induced by Pro- and Anti-Inflammatory Stimuli. Cell. Sign. 2010, 22, 645–659. [Google Scholar] [CrossRef]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.-C.; et al. Increased Insulin Sensitivity and Obesity Resistance in Mice Lacking the Protein Tyrosine Phosphatase-1B Gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased Energy Expenditure, Decreased Adiposity, and Tissue-Specific Insulin Sensitivity in Protein-Tyrosine Phosphatase 1B-Deficient Mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Bence, K.K.; Delibegovic, M.; Xue, B.; Gorgun, C.Z.; Hotamisligil, G.S.; Neel, B.G.; Kahn, B.B. Neuronal PTP1B Regulates Body Weight, Adiposity and Leptin Action. Nat. Med. 2006, 12, 917–924. [Google Scholar] [CrossRef]
- Nieto-Vazquez, I.; Fernandez-Veledo, S.; De Alvaro, C.; Rondinone, C.M.; Valverde, A.M.; Lorenzo, M. Protein-Tyrosine Phosphatase 1B-Deficient Myocytes Show Increased Insulin Sensitivity and Protection against Tumor Necrosis Factor-α-Induced Insulin Resistance. Diabetes 2007, 56, 404–413. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Kim, Y.B.; Welsh, L.A.; Kershaw, E.E.; Neel, B.G.; Kahn, B.B. Protein-Tyrosine Phosphatase 1B Expression Is Induced by Inflammation In Vivo. J. Biol. Chem. 2008, 283, 14230–14241. [Google Scholar] [CrossRef] [PubMed]
- Gum, R.J.; Gaede, L.L.; Koterski, S.L.; Heindel, M.; Clampit, J.E.; Zinker, B.A.; Trevillyan, J.M.; Ulrich, R.G.; Jirousek, M.R.; Rondinone, C.M. Reduction of Protein Tyrosine Phosphatase 1B Increases Insulin-Dependent Signalling in ob/ob Mice. Diabetes 2003, 52, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Digenio, A.; Pham, N.C.; Watts, L.M.; Morgan, E.S.; Jung, S.W.; Baker, B.F.; Geary, R.S.; Bhanot, S. Antisense inhibition of protein tyrosine phosphatase 1B with IONIS-PTP-1BRx improves insulin sensitivity and reduces weight in overweight patients with type 2 diabetes. Diabetes Care 2018, 41, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Grewal, A.S.; Grover, R.; Sharma, N.; Chopra, B.; Dhingra, A.K.; Arora, S.; Redhu, S.; Lather, V. Recent Updates on Development of Protein-Tyrosine Phosphatase 1B Inhibitors for Treatment of Diabetes, Obesity and Related Disorders. Bioorg. Chem. 2022, 121, 105626. [Google Scholar] [CrossRef]
- McNay, E.C.; Ong, C.T.; McCrimmon, R.J.; Cresswell, J.; Bogan, J.S.; Sherwin, R.S. Hippocampal Memory Processes Are Modulated by Insulin and High-Fat-Induced Insulin Resistance. Neurobiol. Learn. Mem. 2010, 93, 546–553. [Google Scholar] [CrossRef]
- Vieira, M.N.N.; Lyra e Silva, N.M.; Ferreira, S.T.; De Felice, F.G. Protein Tyrosine Phosphatase 1B (PTP1B): A Potential Target for Alzheimer’s Therapy? Front. Aging Neurosci. 2017, 9, 7. [Google Scholar] [CrossRef]
- Bonda, D.J.; Stone, J.G.; Torres, S.L.; Siedlak, S.L.; Perry, G.; Kryscio, R.; Jicha, G.; Casadesus, G.; Smith, M.A.; Zhu, X.; et al. Dysregulation of Leptin Signaling in Alzheimer Disease: Evidence for Neuronal Leptin Resistance. J. Neurochem. 2014, 128, 162–172. [Google Scholar] [CrossRef]
- Maioli, S.; Lodeiro, M.; Merino-Serrais, P.; Falahati, F.; Khan, W.; Puerta, E.; Codita, A.; Rimondini, R.; Ramirez, M.J.; Simmons, A.; et al. Alterations in Brain Leptin Signalling in Spite of Unchanged CSF Leptin Levels in Alzheimer’s Disease. Aging Cell. 2015, 14, 122–129. [Google Scholar] [CrossRef]
- Ozek, C.; Kanoski, S.E.; Zhang, Z.Y.; Grill, H.J.; Bence, K.K. Protein-Tyrosine Phosphatase 1B (PTP1B) Is a Novel Regulator of Central Brain-Derived Neurotrophic Factor and Tropomyosin Receptor Kinase B (Trkb) Signaling. J. Biol. Chem. 2014, 289, 31682–31692. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, S.; Kim, S.; Kwon, Y.; Kim, K.; Chung, C.G.; Lee, S.; Lee, S.B.; Kim, H. Neuroprotective Effects of Protein Tyrosine Phosphatase 1B Inhibition against ER Stress-Induced Toxicity. Mol. Cells 2017, 40, 280–290. [Google Scholar] [CrossRef]
- Song, G.J.; Jung, M.; Kim, J.H.; Park, H.; Rahman, M.H.; Zhang, S.; Zhang, Z.Y.; Park, D.H.; Kook, H.; Lee, I.K.; et al. A Novel Role for Protein Tyrosine Phosphatase 1B as a Positive Regulator of Neuroinflammation. J. Neuroinflamm. 2016, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; McQueeney, K.E.; Burnett, J.C.; Wipf, P.; Sharlow, E.R. Small Molecule Targeting of PTPs in Cancer. Int. J. Biochem. Cell B 2018, 96, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.K.; Elferink, L.A. Checks and Balances: Interplay of RTKS and PTPs in Cancer Progression. Biochem. Pharmacol. 2011, 82, 435–440. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Z.; Liu, Y.; Zhou, X.; Sun, F.; Liu, Y.; Li, L.; Hua, S.; Zhao, Y.; Gao, H.; et al. PTP1B Markedly Promotes Breast Cancer Progression and is Regulated by MiR-193a-3p. FEBS J. 2019, 286, 1136–1153. [Google Scholar] [CrossRef] [PubMed]
- Lessard, L.; Stuible, M.; Tremblay, M.L. The Two Faces of PTP1B in Cancer. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 613–619. [Google Scholar] [CrossRef]
- Julien, S.G.; Dubè, N.; Read, M.; Penney, J.; Paquet, M.; Han, Y.; Kennedy, B.P.; Muller, W.J.; Tremblay, M.L. Protein Tyrosine Phosphatase 1B Deficiency or Inhibition Delays ErbB2-Induced Mammary Tumorigenesis and Protect from Lung Metastasis. Nat. Gen. 2007, 39, 338–346. [Google Scholar] [CrossRef]
- Bentires-Alj, M.; Neel, B.G. Protein-Tyrosine Phosphatase 1B Is Required for HER2/Neu-Induced Breast Cancer. Cancer Res. 2007, 67, 2420–2424. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.-; Liu, B.; Zhu, Z. Suppression of PTP1B in Gastric Cancer Cells in Vitro Induces a Change in the Genome-Wide Expression Profile and Inhibits Gastric Cancer Cell Growth. Cell Biol. Int. 2010, 34, 747–753. [Google Scholar] [CrossRef]
- Lessard, L.; Labbé, D.P.; Deblois, G.; Beǵin, L.R.; Hardy, S.; Mes-Masson, A.M.; Saad, F.; Trotman, L.C.; Giquère, V.; Tremblay, M.L. PTP1B Is an Androgen Receptor-Regulated Phosphatase that Promotes the Progression of Prostate Cancer. Cancer Res. 2012, 72, 1529–1537. [Google Scholar] [CrossRef]
- Hoekstra, E.; Das, A.M.; Swets, M.; Cao, W.; van der Woude, C.J.; Bruno, M.J.; Peppelenbosch, M.P.; Kuppen, P.J.K.; Ten Hagen, T.L.; Fuhler, G.M. Increased PTP1B Expression and Phosphatase Activity in Colorectal Cancer Results in a More Invasive Phenotype and Worse Patient Outcome. Oncotarget 2016, 7, 21922–21938. [Google Scholar] [CrossRef]
- Dubé, N.; Bourdeau, A.; Heinonen, K.M.; Cheng, A.; Loy, A.L.; Tremblay, M.L. Genetic Ablation of Protein Tyrosine Phosphatase 1B Accelerates Lymphomagenesis of p53-Null Mice through the Regulation of B-cell Development. Cancer Res. 2005, 65, 10088–10095. [Google Scholar] [CrossRef] [PubMed]
- Tamrakar, A.K.; Maurya, C.K.; Ray, A.K. PTP1B Inhibitors for Type 2 Diabetes Treatment: A Patent Review (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, P.J.; Huth, J.R.; Tse, C. Predicting Protein Druggability. Drug Discov. Today 2005, 10, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Coleman, R.G.; Smyth, K.T.; Cao, Q.; Soulard, P.; Caffrey, D.R.; Salzberg, A.C.; Huang, E.S. Structure-Based Maximal Affinity Model Predicts Small-Molecule Druggability. Nat. Biotechnol. 2007, 25, 71–75. [Google Scholar] [CrossRef]
- Ghattas, M.A.; Raslan, N.; Sadeq, A.; Sorkhy, M.A.; Atatreh, N. Druggability Analysis and Classification of Protein Tyrosine Phosphatase Active Sites. Drug Des. Devel. Ther. 2016, 10, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Popov, D. Novel Protein Tyrosine Phosphatase 1B Inhibitors: Interaction Requirements for Improved Intracellular Efficacy in Type 2 Diabetes Mellitus and Obesity Control. Biochem. Biophys. Res. Commun. 2011, 410, 377–381. [Google Scholar] [CrossRef]
- Stanford, S.M.; Bottini, N. Targeting Tyrosine Phosphatases: Time to End the Stigma. Trends Pharmacol. Sci. 2017, 38, 524–540. [Google Scholar] [CrossRef]
- Köhn, M. Turn and Face the Strange: A New View on Phosphatases. ACS Cent. Sci. 2020, 6, 467–477. [Google Scholar] [CrossRef]
- Liu, R.; Mathieu, C.; Berthelet, J.; Zhang, W.; Dupret, J.-M.; Rodrigues Lima, F. Human Protein Tyrosine Phosphatase 1B (PTP1B): From Structure to Clinical Inhibitor Perspectives. Int. J. Mol. Sci. 2022, 23, 7027. [Google Scholar] [CrossRef]
- Nichols, A.J.; Mashal, R.D.; Balkan, B. Toward the Discovery of Small Molecule PTP1B Inhibitors for the Treatment of Metabolic Diseases. Drug. Dev. Res. 2006, 67, 559–566. [Google Scholar] [CrossRef]
- Koren, S.; Fantus, I.G. Inhibition of the Protein Tyrosine Phosphatase PTP1B: Potential Therapy for Obesity, Insulin Resistance and Type-2 Diabetes Mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Scapin, G.; Patel, S.B.; Becker, J.W.; Wang, Q.; Desponts, C.; Waddleton, D.; Skorey, K.; Cromlish, W.; Bayly, C.; Therien, M.; et al. The Structural Basis for the Selectivity of Benzotriazole Inhibitors of PTP1B. Biochemistry 2003, 42, 11451–11459. [Google Scholar] [CrossRef] [PubMed]
- Puius, Y.A.; Zhao, Y.; Sullivan, M.; Lawrence, D.S.; Almo, S.C.; Zhang, Z.Y. Identification of a Second Aryl Phosphate-Binding Site in Protein-Tyrosine Phosphatase 1B: A Paradigm for Inhibitor Design. Proc. Natl. Acad. Sci. USA 1997, 94, 13420–13425. [Google Scholar] [CrossRef]
- Combs, A.P. Recent Advances in the Discovery of Competitive Protein Tyrosine Phosphatase 1B Inhibitors for the Treatment of Diabetes, Obesity and Cancer. J. Med. Chem. 2010, 53, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Ottanà, R.; Ciurleo, R.; Paoli, P.; Manao, G.; Camici, G.; Laggner, C.; Langer, T. Structure-Based Optimization of Benzoic Acids as Inhibitors of Protein Tyrosine Phosphatase 1B and Low Molecular Weight Protein Tyrosine Phosphatase. ChemMedChem 2009, 4, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Ottanà, R.; Maccari, R.; Amuso, S.; Wolber, G.; Schuster, D.; Herdlinger, S.; Manao, G.; Camici, G.; Paoli, P. New 4-[(5-arylidene-2-arylimino-4-oxo-3-thiazolidinyl)methyl]Benzoic Acids Active as Protein Tyrosine Phosphatase Inhibitors Endowed with Insulinomimetic Effect on Mouse C2C12 Skeletal Muscle Cells. Eur. J. Med. Chem. 2012, 50, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Bonham, C.A.; Rus, I.A.; Shrestha, O.K.; Gauss, C.M.; Haque, A.; Tocilj, A.; Joshua-Tor, L.; Tonks, N.K. Harnessing Insulin- And Leptin-Induced Oxidation of PTP1B for Therapeutic Development. Nat. Commun. 2018, 9, 283. [Google Scholar] [CrossRef]
- Wiesmann, C.; Barr, K.J.; Kung, J.; Zhu, J.; Erlanson, D.A.; Shen, W.; Fahr, B.J.; Zhong, M.; Taylor, L.; Randal, M.; et al. Allosteric Inhibition of Protein Tyrosine Phosphatase 1B. Nat. Struct. Mol. Biol. 2004, 11, 730–737. [Google Scholar] [CrossRef]
- Morishita, K.; Shoji, Y.; Tanaka, S.; Fukui, M.; Ito, Y.; Kitao, T.; Ozawa, S.; Hirono, S.; Shirahase, H. Novel Non-Carboxylate Benzoylsulfonamide-Based Protein Tyrosine Phosphatase 1B Inhibitors with Non-Competitive Actions. Chem. Pharm. Bull. 2017, 65, 1144–1160. [Google Scholar] [CrossRef]
- Elhassan, R.M.; Hou, X.; Fang, H. Recent Advances in the Development of Allosteric Protein Tyrosine Phosphatase Inhibitors for Drug Discovery. Med. Res. Rev. 2022, 42, 1064–1110. [Google Scholar] [CrossRef]
- Ottanà, R.; Maccari, R.; Mortier, J.; Caselli, A.; Amuso, S.; Camici, G.; Rotondo, A.; Wolber, G.; Paoli, P. Synthesis, Biological Activity and Structure-Activity Relationships of New Benzoic Acid-Based Protein Tyrosine Phosphatase Inhibitors Endowed with Insulinomimetic Effects in Mouse C2C12 Skeletal Muscle Cells. Eur. J. Med. Chem. 2014, 71, 112–127. [Google Scholar] [CrossRef]
- Ottanà, R.; Paoli, P.; Naß, A.; Lori, G.; Cardile, V.; Adornato, I.; Rotondo, A.; Graziano, A.C.E.; Wolber, G.; Maccari, R. Discovery of 4-[(5-arylidene-4-oxothiazolidin-3-yl)methyl]benzoic Acid Derivatives Active as Novel Potent Allosteric Inhibitors of Protein Tyrosine Phosphatase 1B: In Silico Studies and in Vitro Evaluation as Insulinomimetic and Anti-Inflammatory Agents. Eur. J. Med. Chem. 2017, 127, 840–858. [Google Scholar] [CrossRef] [PubMed]
- Olmez, E.O.; Alakent, B. Alpha7 Helix Plays an Important Role in the Conformational Stability of PTP1B. J. Biomol. Struct. Dyn. 2011, 28, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Beumer, C.; Simard, J.R.; Grütter, C.; Rauh, D. Selective Detection of Allosteric Phosphatase Inhibitors. J. Am. Chem. Soc. 2013, 135, 6838–6841. [Google Scholar] [CrossRef]
- Maccari, R.; Del Corso, A.; Paoli, P.; Adornato, I.; Lori, G.; Balestri, F.; Cappiello, M.; Naß, A.; Wolber, G.; Ottanà, R. An Investigation on 4-Thiazolidinone Derivatives as Dual Inhibitors of Aldose Reductase and Protein Tyrosine Phosphatase 1B, in the Search for Potential Agents for the Treatment of Type 2 Diabetes Mellitus and Its Complications. Bioorg. Med. Chem. Lett. 2018, 28, 3712–3720. [Google Scholar] [CrossRef] [PubMed]
- Ottanà, R.; Paoli, P.; Cappiello, M.; Nguyen, T.N.; Adornato, I.; Del Corso, A.; Genovese, M.; Nesi, I.; Moschini, R.; Naß, A.; et al. In Search for Multi-Target Ligands as Potential Agents for Diabetes Mellitus and its Complications—A Structure-Activity Relationship Study on Inhibitors of Aldose Reductase and Protein Tyrosine Phosphatase 1B. Molecules 2021, 26, 330. [Google Scholar] [CrossRef]
- Maccari, R.; Wolber, G.; Genovese, M.; Sardelli, G.; Talagayev, V.; Balestri, F.; Luti, S.; Santi, A.; Moschini, R.; Del Corso, A.; et al. Designed Multiple Ligands for the Treatment of Type 2 Diabetes Mellitus and its Complications: Discovery of (5-Arylidene-4-Oxo-2-Thioxothiazolidin-3-Yl)alkanoic Acids Active as Novel Dual-Targeted PTP1B/AKR1B1 Inhibitors. Eur. J. Med. Chem. 2023, 252, 115270. [Google Scholar] [CrossRef]
- Moore, K.S.; Wehrli, S.; Rodert, H.; Rogers, M.; Forrest, J.N.; McCrimmon, D.; Zasloff, M. Squalamine: An Aminosterol Antibiotic from the Shark. Proc. Nat. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar] [CrossRef]
- Sills, A.K., Jr.; Williams, J.I.; Tyler, B.M.; Epstein, D.S.; Sipos, E.P.; Davis, J.D.; McLane, M.P.; Pitchford, S.; Cheshire, K.; Gannon, F.H.; et al. Squalamine Inhibits Angiogenesis and Solid Tumor Growth in Vivo and Perturbs Embryonic Vasculature. Cancer Res. 1998, 58, 2784–2792. [Google Scholar]
- Rao, M.N.; Shinnar, A.E.; Noecker, L.A.; Chao, T.L.; Feibush, B.; Snyder, B.; Sharkansky, I.; Sarkahian, A.; Zhang, X.; Jones, S.R.; et al. Aminosterols from the Dogfish Shark Squalus Acanthias. J. Nat. Prod. 2000, 63, 631–635. [Google Scholar] [CrossRef]
- Shu, Y.; Jones, S.R.; Kinney, W.A.; Selinsky, B.S. The Synthesis of Spermine Analogs of the Shark Aminosterol Squalamine. Steroids 2002, 67, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Salmi, C.; Loncle, C.; Vidal, N.; Laget, M.; Letourneux, Y.; Brunel, J.M. Antimicrobial Activities of 3-Amino- and Polyaminosterol Analogues of Squalamine and Trodusquemine. J. Enzym. Inhib. Med. Chem. 2008, 23, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Salmi, C.; Loncle, C.; Vidal, N.; Letourneux, Y.; Brune, J.M. New Stereoselective Titanium Reductive Amination Synthesis of 3-Amino and Polyaminosterol Derivatives Possessing Antimicrobial Activities. Eur. J. Med. Chem. 2008, 43, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Koveal, D.; Miller, D.H.; Xue, B.; Akshinthala, S.D.; Kragelj, J.; Ringkjøbing Jensen, M.; Gauss, C.M.; Page, R.; Blackledge, M.; et al. Targeting the Disordered C-Terminus of PTP1B with an Allosteric Inhibitor. Nat. Chem. Biol. 2014, 10, 558–566. [Google Scholar] [CrossRef]
- Zasloff, M.; Williams, J.I.; Chen, Q.; Anderson, M.; Maeder, T.; Holroyd, K.; Jones, S.; Kinney, W.; Cheshire, K.; McLane, M. A Spermine-Coupled Cholesterol Metabolite from the Shark with Potent Appetite Suppressant and Antidiabetic Properties. Int. J. Obes. 2001, 25, 689–697. [Google Scholar] [CrossRef]
- Ahima, R.S.; Patel, H.R.; Takahashi, N.; Qi, Y.; Hileman, S.M.; Zasloff, M.A. Appetite Suppression and Weight Reduction by a Centrally Active Aminosterol. Diabetes 2002, 51, 2099–2104. [Google Scholar] [CrossRef]
- Takahashi, N.; Qi, Y.; Patel, H.R.; Ahima, R.S. A Novel Aminosterol Reverses Diabetes and Fatty Liver Disease in Obese Mice. J. Hepatol. 2004, 41, 391–398. [Google Scholar] [CrossRef]
- Lantz, K.A.; Emeigh Hart, S.G.; Planey, S.L.; Roitman, M.F.; Ruiz-White, I.A.; Wolfe, H.R.; McLane, M.P. Inhibition of PTP1B by Trodusquemine (MSI-1436) Causes Fat-specific Weight Loss in Diet-induced Obese Mice. Obesity 2010, 18, 1516–1523. [Google Scholar] [CrossRef]
- Roitman, M.F.; Wescott, S.; Cone, J.J.; McLane, M.P.; Wolfe, H.R. MSI-1436 Reduces Acute Food Intake without Affecting Dopamine Transporter Activity. Pharmacol. Biochem. Behav. 2010, 97, 138–143. [Google Scholar] [CrossRef]
- Pandey, N.R.; Zhou, X.; Qin, Z.; Zaman, T.; Gomez-Smith, M.; Keyhanian, K.; Anisman, H.; Brunel, J.M.; Stewart, A.F.R.; Chen, H.H. The LIM Domain only 4 Protein Is a Metabolic Responsive Inhibitor of Protein Tyrosine Phosphatase 1B that Controls Hypothalamic Leptin Signaling. J. Neurosci. 2013, 33, 12647–12655. [Google Scholar] [CrossRef]
- Pandey, N.R.; Zhou, X.; Zaman, T.; Cruz, S.A.; Qin, Z.; Lu, M.; Keyhanian, K.; Brunel, J.M.; Stewart, A.F.R.; Chen, H.H. LMO4 Is Required to Maintain Hypothalamic Insulin Signaling. Biochem. Bioph. Res. Commun. 2014, 450, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K. From NASH to Diabetes and from Diabetes to NASH: Mechanisms and Treatment Options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, L.; Łyczko, J.; Alicka, M.; Bourebaba, N.; Szumny, A.; Fal, A.M.; Marycz, K. Inhibition of Protein-Tyrosine Phosphatase PTP1B and LMPTP Promotes Palmitate/Oleate-Challenged HepG2 Cell Survival by Reducing Lipoapoptosis, Improving Mitochondrial Dynamics and Mitigating Oxidative and Endoplasmic Reticulum Stress. J. Clin. Med. 2020, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Mody, N.; Owen, C.; Czopek, A.; Zimmer, D.; Bentires-Alj, M.; Bence, K.K.; Delibegović, M. Liver-specific Deletion of Protein Tyrosine Phosphatase (PTP) 1B Improves Obesity- and Pharmacologically Induced Endoplasmic Reticulum Stress. Biochem. J. 2011, 438, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, L.; Komakula, S.S.B.; Weiss, C.; Adrar, N.; Marycz, K. The PTP1B Selective Inhibitor MSI-1436 Mitigates Tunicamycin-Induced ER Stress in Human Hepatocarcinoma Cell Line through XBP1 Splicing Modulation. PLoS ONE 2023, 18, e0278566. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Chambers, J.E.; Marciniak, S.J. Cellular Mechanisms of Endoplasmic Reticulum Stress Signaling in Health and Disease. 2. Protein Misfolding and ER Stress. Am. J. Physiol. Cell Physiol. 2014, 307, C657–C670. [Google Scholar] [CrossRef]
- Ariyasu, D.; Yoshida, H.; Hasegawa, Y. Endoplasmic Reticulum (ER) Stress and Endocrine Disorders. Int. J. Mol. Sci. 2017, 18, 382. [Google Scholar] [CrossRef]
- Bourebaba, L.; Kornicka-Garbowska, K.; Al Naem, M.; Röcken, M.; Łyczko, J.; Marycz, K. MSI-1436 Improves EMS Adipose Derived Progenitor Stem Cells in the Course of Adipogenic Differentiation through Modulation of ER Stress, Apoptosis, and Oxidative Stress. Stem. Cell Res. Ther. 2021, 12, 97. [Google Scholar] [CrossRef]
- Kornicka-Garbowska, K.; Bourebaba, L.; Röcken, M.; Marycz, K. Inhibition of Protein Tyrosine Phosphatase Improves Mitochondrial Bioenergetics and Dynamics, Reduces Oxidative Stress, and Enhances Adipogenic Differentiation Potential in Metabolically Impaired Progenitorstem Cells. Cell Commun. Signal 2021, 19, 106. [Google Scholar] [CrossRef]
- Fiedler, L.R. Inhibiting the Inhibitors, PTP1B as a Therapeutic Target in Myocardial Infarction. Heart Res. Open J. 2018, 5, 8–11. [Google Scholar] [CrossRef]
- Limbocker, R.; Errico, S.; Barbut, D.; Knowles, T.P.J.; Vendruscolo, M.; Chiti, F.; Zasloff, M. Squalamine and Trodusquemine: Two Natural Products for Neurodegenerative Diseases, from Physical Chemistry to the Clinic. Nat. Prod. Rep. 2022, 39, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Maguire-Nguyen, K.K.; Rando, T.A.; Zasloff, M.A.; Strange, K.B.; Yin, V.P. The Protein Tyrosine Phosphatase 1B Inhibitor MSI-1436 Stimulates Regeneration of Heart and Multiple Other Tissues. NPJ Regen. Med. 2017, 2, 4. [Google Scholar] [CrossRef]
- Figueiredo, A.; Leal, E.C.; Carvalho, E. Protein Tyrosine Phosphatase 1B Inhibition as a Potential Therapeutic Target for Chronic Wounds in Diabetes. Pharmacol. Res. 2020, 159, 104977. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, M.; Remy, E.; Devaux, C.; Dautreaux, B.; Henry, J.P.; Bauer, F.; Mulder, P.; Hooft van Huijsduijnen, R.; Bombrun, A.; Thuillez, C.; et al. Improvement of Peripheral Endothelial Dysfunction by Protein Tyrosine Phosphatase Inhibitors in Heart Failure. Circulation 2006, 114, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Lanahan, A.A.; Lech, D.; Dubrac, A.; Zhang, J.; Zhuang, Z.W.; Eichmann, A.; Simons, M. PTP1b Is a Physiologic Regulator of Vascular Endothelial Growth Factor Signaling in Endothelial Cells. Circulation 2014, 130, 902–909. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Li, J.; Liu, Y.; Zhang, C.Y.; Zhang, Y.; Zen, K. Protein Tyrosine Phosphatase 1B Impairs Diabetic Wound Healing through Vascular Endothelial Growth Factor Receptor 2 Dephosphorylation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 163–174. [Google Scholar] [CrossRef]
- Schürmann, C.; Goren, I.; Linke, A.; Pfeilschifter, J.; Frank, S. Deregulated Unfolded Protein Response in Chronic Wounds of Diabetic Ob/Ob Mice: A Potential Connection to Inflammatory and Angiogenic Disorders in Diabetes-Impaired Wound Healing. Biochem. Biophys. Res. Commun. 2014, 446, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, P.A.; Delile, E.; Coquerel, D.; Brunel, J.M.; Renet, S.; Tamion, F.; Richard, V. Protein Tyrosine Phosphatase 1B Regulates Endothelial Endoplasmic Reticulum Stress; Role in Endothelial Dysfunction. Vascul. Pharmacol. 2018, 109, 36–44. [Google Scholar] [CrossRef]
- Krenz, M. Friend or foe? Unraveling the Complex Roles of Protein Tyrosine Phosphatases in Cardiac Disease and Development. Cell. Signal. 2022, 93, 110297. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Saad, M.J.A.; Duncan, B.B. Subclinical Inflammation and Obesity, Diabetes and Related Disorders. Drug Discov. Today Dis. Mech. 2005, 2, 307–312. [Google Scholar] [CrossRef]
- Thompson, D.; Morrice, N.; Grant, L.; Le Sommer, S.; Lees, E.K.; Mody, N.; Wilson, H.M.; Delibegovic, M. Pharmacological Inhibition of Protein Tyrosine Phosphatase 1B Protects Against Atherosclerotic Plaque Formation in the LDLR−/− Mouse Model of Atherosclerosis. Clin. Sci. 2017, 131, 2489–2501. [Google Scholar] [CrossRef]
- Thompson, D.; Morrice, N.; Grant, L.; Le Sommer, S.; Ziegler, K.; Whitfield, P.; Mody, N.; Wilson, H.M.; Delibegovic, M. Myeloid Protein Tyrosine Phosphatase 1B (PTP1B) Deficiency Protects Against Atherosclerotic Plaque Formation in the Apoe-/- Mouse Model of Atherosclerosis with Alterations in IL10/AMPKα Pathway. Mol. Metab. 2017, 6, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Vercauteren, M.; Kurtz, B.; Ouvrard-Pascaud, A.; Mulder, P.; Henry, J.P.; Besnier, M.; Waget, A.; Hooft Van Huijsduijnen, R.; Tremblay, M.L.; et al. Reduction of Heart Failure by Pharmacological Inhibition or Gene Deletion of Protein Tyrosine Phosphatase 1B. J. Mol. Cell. Cardiol. 2012, 52, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Schwarzer, M.; Schrepper, A.; Amorim, P.A.; Blum, D.; Hain, C.; Faerber, G.; Haendeler, J.; Altschmied, J.; Doenst, T. Increased Protein Tyrosine Phosphatase 1B (PTP1B) Activity and Cardiac Insulin Resistance Precede Mitochondrial and Contractile Dysfunction in Pressure-Overloaded Hearts. J Am Heart Assoc. 2018, 7, e008865. [Google Scholar] [CrossRef] [PubMed]
- Perni, M.; Flagmeier, P.; Limbocker, R.; Cascella, R.; Aprile, F.A.; Galvagnion, C.; Heller, G.T.; Meisl, G.; Chen, S.W.; Kumita, J.R.; et al. Multistep Inhibition of α-Synuclein Aggregation and Toxicity in Vitro and in Vivo by Trodusquemine. ACS Chem. Biol. 2018, 13, 2308–2319. [Google Scholar] [CrossRef] [PubMed]
- Perni, M.; Galvagnion, C.; Maltsev, A.; Meisl, G.; Müller, M.B.D.; Challa, P.K.; Kirkegaard, J.B.; Flagmeier, P.; Cohen, S.I.A.; Cascella, R.; et al. A Natural Product Inhibits the Initiation of a-Synuclein Aggregation and Suppresses Its Toxicity. Proc. Natl. Acad. Sci. USA 2017, 114, E1009–E1017. [Google Scholar] [CrossRef]
- Perni, M.; van der Goot, A.; Limbocker, R.; van Ham, T.J.; Aprile, F.A.; Xu, C.K.; Flagmeier, P.; Thijssen, K.; Sormanni, P.; Fusco, G.; et al. Comparative Studies in the A30P and A53T a-Synuclein C. Elegans Strains to Investigate the Molecular Origins of Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 552549. [Google Scholar] [CrossRef]
- Limbocker, R.; Staats, R.; Chia, S.; Ruggeri, F.S.; Mannini, B.; Xu, C.K.; Perni, M.; Cascella, R.; Bigi, A.; Sasser, L.R.; et al. Squalamine and its Derivatives Modulate the Aggregation of Amyloid-b and a-Synuclein and Suppress the Toxicity of Their Oligomers. Front. Neurosci. 2021, 15, 680026. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Sutherland, D.; Madrid, J.A.; Rol, M.A.; Frucht, S.; Isaacson, S.; Pagan, F.; Maddux, B.N.; Li, G.; Tse, W.; et al. Targeting Neurons in the Gastrointestinal Tract to Treat Parkinson’s Disease. Clin. Park. Relat. Disord. 2019, 1, 2–7. [Google Scholar] [CrossRef]
- Limbocker, R.; Mannini, B.; Ruggeri, F.S.; Cascella, R.; Xu, C.K.; Perni, M.; Chia, S.; Chen, S.W.; Habchi, J.; Bigi, A.; et al. Trodusquemine Displaces Protein Misfolded Oligomers from Cell Membranes and Abrogates Their Cytotoxicity through a Generic Mechanism. Commun. Biol. 2020, 3, 435. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Kreiser, R.P.; Wright, A.K.; Block, N.R.; Hollows, J.E.; Nguyen, L.T.; LeForte, K.; Mannini, B.; Vendruscolo, M.; Limbocker, R. Therapeutic Strategies to Reduce the Toxicity of Misfolded Protein Oligomers. Int. J. Mol. Sci. 2020, 21, 8651. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-β Protein Dimers Isolated Directly from Alzheimer’s Brains Impair Synaptic Plasticity and Memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Limbocker, R.; Chia, S.; Ruggeri, F.S.; Perni, M.; Cascella, R.; Heller, G.T.; Meisl, G.; Mannini, B.; Habchi, J.; Michaels, T.C.T.; et al. Trodusquemine Enhances Aβ42 Aggregation but Suppresses its Toxicity by Displacing Oligomers from Cell Membranes. Nat. Commun. 2019, 10, 225. [Google Scholar] [CrossRef]
- Evangelisti, E.; Cascella, R.; Becatti, M.; Marrazza, G.; Dobson, C.M.; Chiti, F.; Stefani, M.; Cecchi, C. Binding affinity of amyloid oligomers to cellular membranes is a generic indicator of cellular dysfunction in protein misfolding diseases. Sci. Rep. 2016, 6, 32721. [Google Scholar] [CrossRef]
- Errico, S.; Lucchesi, G.; Odino, D.; Muscat, S.; Capitini, C.; Bugelli, C.; Canale, C.; Ferrando, R.; Grasso, G.; Barbut, D.; et al. Making Biological Membrane Resistant to the Toxicity of Misfolded Protein Oligomers: A Lesson from Trodusquemine. Nanoscale 2020, 12, 22596. [Google Scholar] [CrossRef] [PubMed]
- Errico, S.; Ramshini, H.; Capitini, C.; Canale, C.; Spaziano, M.; Barbut, D.; Calamai, M.; Zasloff, M.; Oropesa-Nuñez, R.; Vendruscolo, M.; et al. Quantitative Measurement of the Affinity of Toxic and Nontoxic Misfolded Protein Oligomers for Lipid Bilayers and of its Modulation by Lipid Composition and Trodusquemine. ACS Chem. Neurosci. 2021, 12, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Capitini, C.; Pesce, L.; Fani, G.; Mazzamuto, G.; Genovese, M.; Franceschini, A.; Paoli, P.; Pieraccini, G.; Zasloff, M.; Chiti, F.; et al. Studying the Trafficking of Labelled Trodusquemine and its Application as Nerve Marker for Light-Sheet and Expansion Microscopy. FASEB J. 2022, 36, e22655. [Google Scholar] [CrossRef]
- Barletti, B.; Lucchesi, G.; Muscat, S.; Errico, S.; Barbut, D.; Danani, A.; Zasloff, M.; Grasso, G.; Chiti, F.; Caminati, G. Reorganization of the Outer Layer of a Model of the Plasma Membrane Induced by a Neuroprotective Aminosterol. Colloids Surf. B 2023, 222, 113115. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Fava, A.; Plastino, M.; Montalcini, T.; Pujia, A. Possible Implications of Insulin Resistance and Glucose Metabolism in Alzheimer’s Disease Pathogenesis. J. Cell. Mol. Med. 2011, 15, 1807–1821. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Jurado, J.; Hernandez, F.; Avila, J. GSK-3β, a Pivotal Kinase in Alzheimer Disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Ricke, K.M.; Cruz, S.A.; Qin, Z.; Farrokhi, K.; Sharmin, F.; Zhang, L.; Zasloff, M.A.; Stewart, A.F.R.; Chen, H.H. Neuronal Protein Tyrosine Phosphatase 1B Hastens Amyloid β-Associated Alzheimer’s Disease in Mice. J. Neurosci. 2020, 40, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cano, A.; Sanchez-Lopez, E.; Carrasco, M.; Verdaguer, E.; Fortuna, A.; Folch, J.; Bulló, M.; Auladell, C.; Camins, A.; et al. Protein Tyrosine Phosphatase 1B (PTP1B) as a Potential Therapeutic Target for Neurological Disorders. Biomed. Pharmacother. 2022, 55, 113709. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Tonks, N.K. Anxious Moments for the Protein Tyrosine Phosphatase PTP1B. Trends Neuros. 2015, 38, 462–465. [Google Scholar] [CrossRef]
- Qin, Z.; Zhou, X.; Pandey, N.R.; Vecchiarelli, H.A.; Stewart, C.A.; Zhang, X.; Lagace, D.C.; Brunel, J.M.; Be´ı¨que, J.C.; Stewart, A.F.R.; et al. Chronic Stress Induces Anxiety via an Amygdalar Intracellular Cascade that Impairs Endocannabinoid Signaling. Neuron 2015, 85, 1319–1331. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, L.; Cruz, S.A.; Stewart, A.F.R.; Chen, H.H. Activation of Tyrosine Phosphatase PTP1B in Pyramidal Neurons Impairs Endocannabinoid Signaling by Tyrosine Receptor Kinase Trkb and Causes Schizophrenia-Like Behaviors in Mice. Neuropsychopharmacology 2020, 45, 1884–1895. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, L.; Zasloff, M.A.; Stewart, A.F.R.; Chen, H.H. Ketamine’s schizophrenia-like effects are prevented by targeting PTP1B. Neurobiol. Dis. 2021, 155, 105397. [Google Scholar] [CrossRef]
- Qin, Z.; Pandey, N.R.; Zhou, X.; Stewart, C.A.; Hari, A.; Huang, H.; Stewart, A.F.R.; Brunel, J.M.; Chen, H.H. Functional Properties of Claramine: A Novel PTP1B Inhibitor and Insulin-Mimetic Compound. Biochem. Biophys. Res. Commun. 2015, 458, 21–27. [Google Scholar] [CrossRef]
- Blanchet, M.; Borselli, D.; Rodallec, A.; Peiretti, F.; Vidal, N.; Bolla, J.M.; Digiorgio, C.; Morrison, K.R.; Wuest, W.M.; Brunel, J.M. Claramines: A New Class of Broad-Spectrum Antimicrobial Agents with Bimodal Activity. ChemMedChem 2018, 13, 1018–1027. [Google Scholar] [CrossRef]
- Dodd, G.T.; Xirouchaki, C.E.; Eramo, M.; Mitchell, C.A.; Andrews, Z.B.; Henry, B.A.; Cowley, M.A.; Tiganis, T. Intranasal Targeting of Hypothalamic PTP1B and TCPTP Reinstates Leptin and Insulin Sensitivity and Promotes Weight Loss in Obesity. Cell Rep. 2019, 28, 2905–2922. [Google Scholar] [CrossRef] [PubMed]
- Kreiser, R.P.; Wright, A.K.; Sasser, L.R.; Rinauro, D.J.; Gabriel, J.M.; Hsu, C.M.; Hurtado, J.A.; McKenzie, T.L.; Errico, S.; Albright, J.A.; et al. A Brain-Permeable Aminosterol Regulates Cell Membranes to Mitigate the Toxicity of Diverse Pore-Forming Agents. ACS Chem. Neurosci. 2022, 13, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Konidaris, K.F.; Gasser, G.; Tonks, N.K. A Potent, Selective, and Orally Bioavailable Inhibitor of the Protein-Tyrosine Phosphatase PTP1B Improves Insulin and Leptin Signaling in Animal Models. J. Biol. Chem. 2018, 293, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Felice, C.; Rivera, K.; Pappin, D.J.; Tonks, N.K. DPM-1001 Decreased Copper Levels and Ameliorated Deficits in a Mouse Model of Wilson’s Disease. Genes Dev. 2018, 32, 944–952. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maccari, R.; Ottanà, R. Can Allostery Be a Key Strategy for Targeting PTP1B in Drug Discovery? A Lesson from Trodusquemine. Int. J. Mol. Sci. 2023, 24, 9621. https://doi.org/10.3390/ijms24119621
Maccari R, Ottanà R. Can Allostery Be a Key Strategy for Targeting PTP1B in Drug Discovery? A Lesson from Trodusquemine. International Journal of Molecular Sciences. 2023; 24(11):9621. https://doi.org/10.3390/ijms24119621
Chicago/Turabian StyleMaccari, Rosanna, and Rosaria Ottanà. 2023. "Can Allostery Be a Key Strategy for Targeting PTP1B in Drug Discovery? A Lesson from Trodusquemine" International Journal of Molecular Sciences 24, no. 11: 9621. https://doi.org/10.3390/ijms24119621
APA StyleMaccari, R., & Ottanà, R. (2023). Can Allostery Be a Key Strategy for Targeting PTP1B in Drug Discovery? A Lesson from Trodusquemine. International Journal of Molecular Sciences, 24(11), 9621. https://doi.org/10.3390/ijms24119621