Comprehensive Insight into Colorectal Cancer Metabolites and Lipids for Human Serum: A Proof-of-Concept Study
Abstract
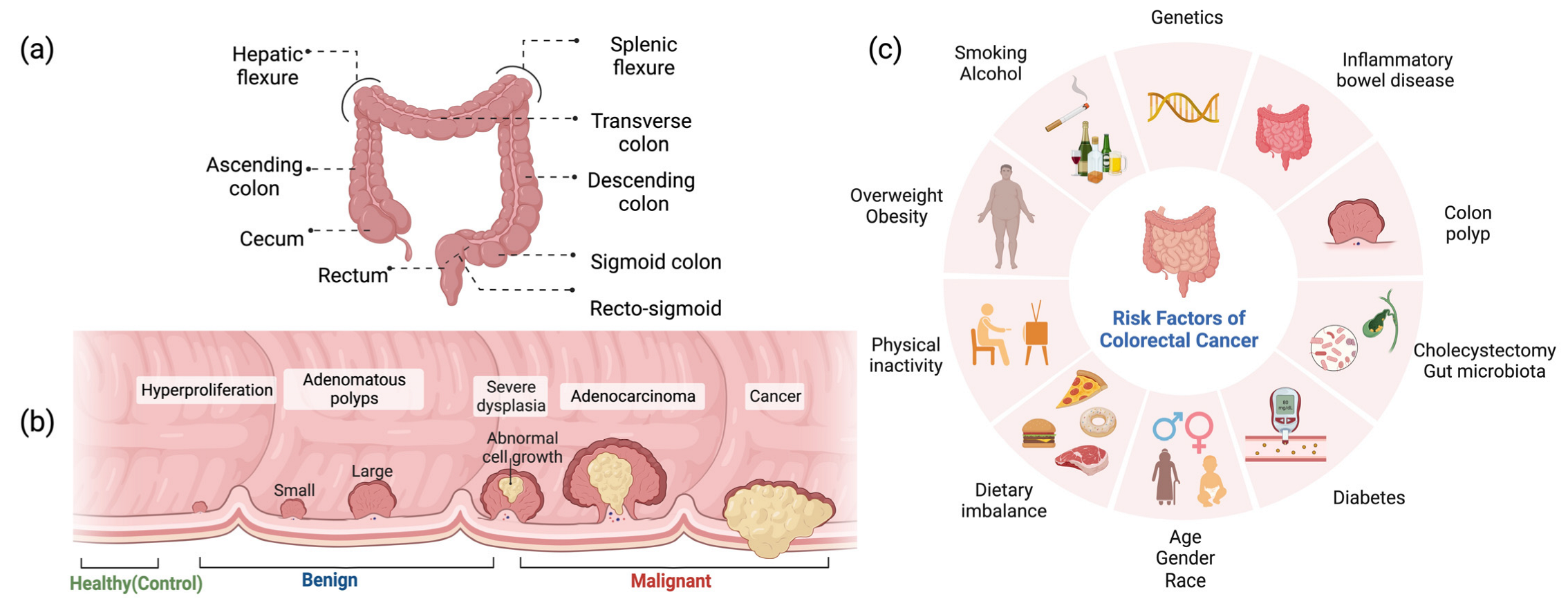
1. Introduction
2. Results
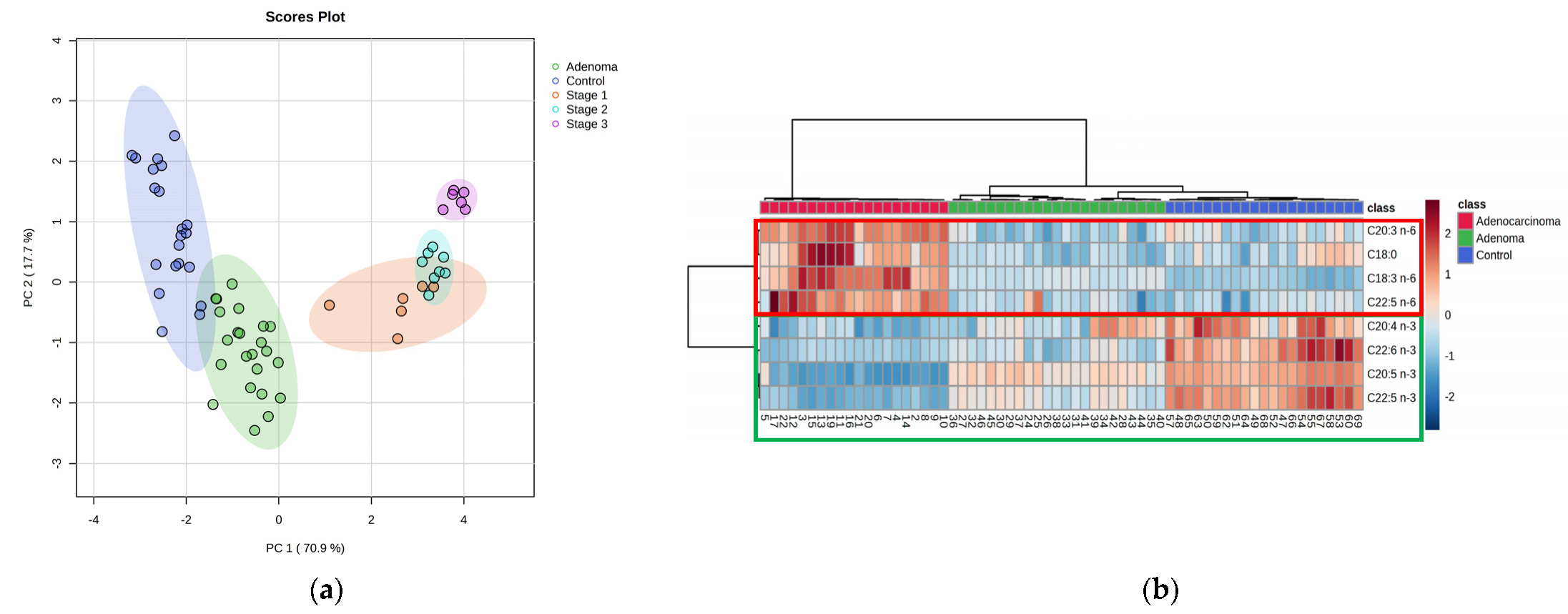
2.1. Lipidomic Profiling of Colorectal Cancer Serum by GC×GC-LR/HR-TOFMS
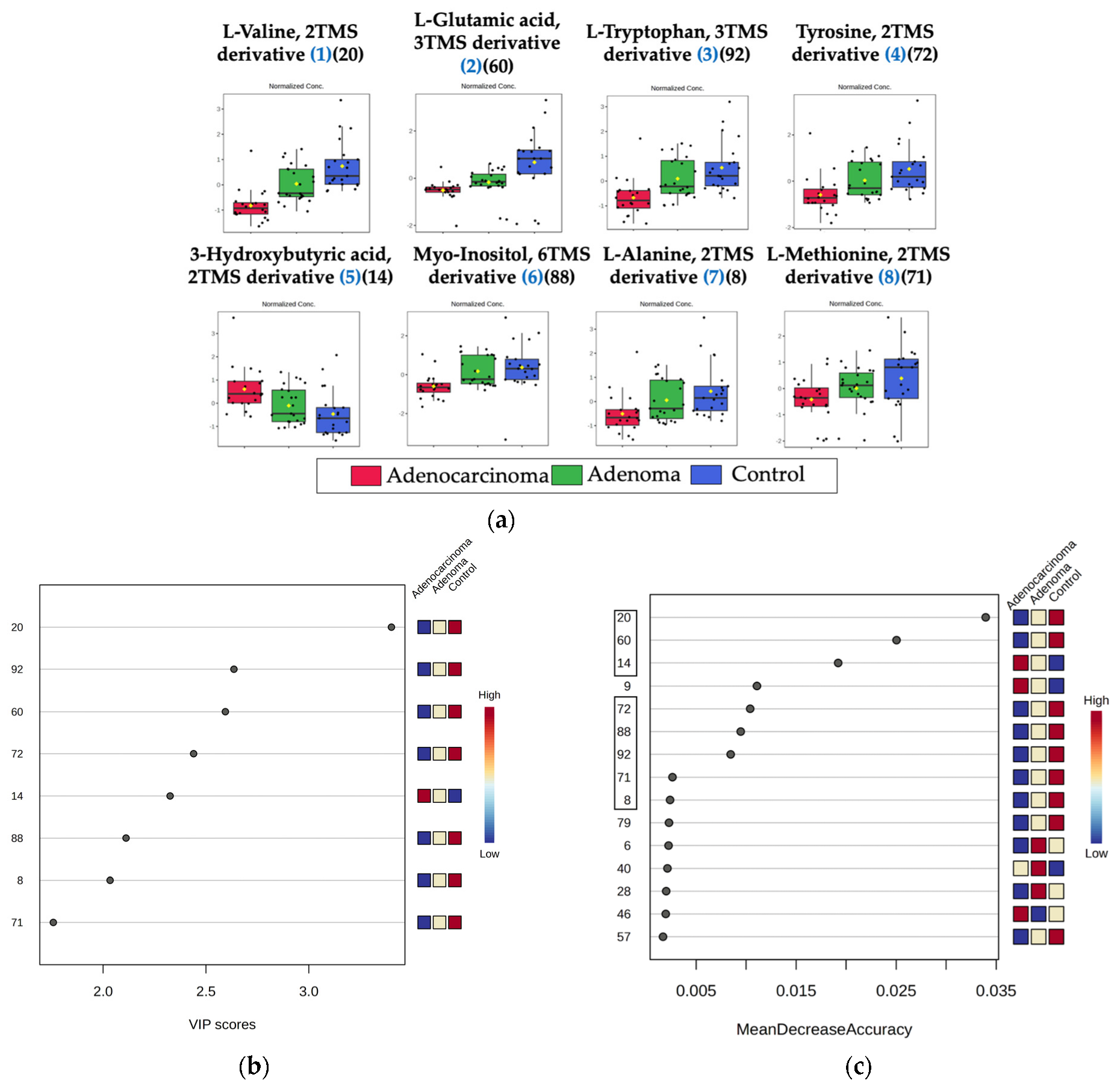
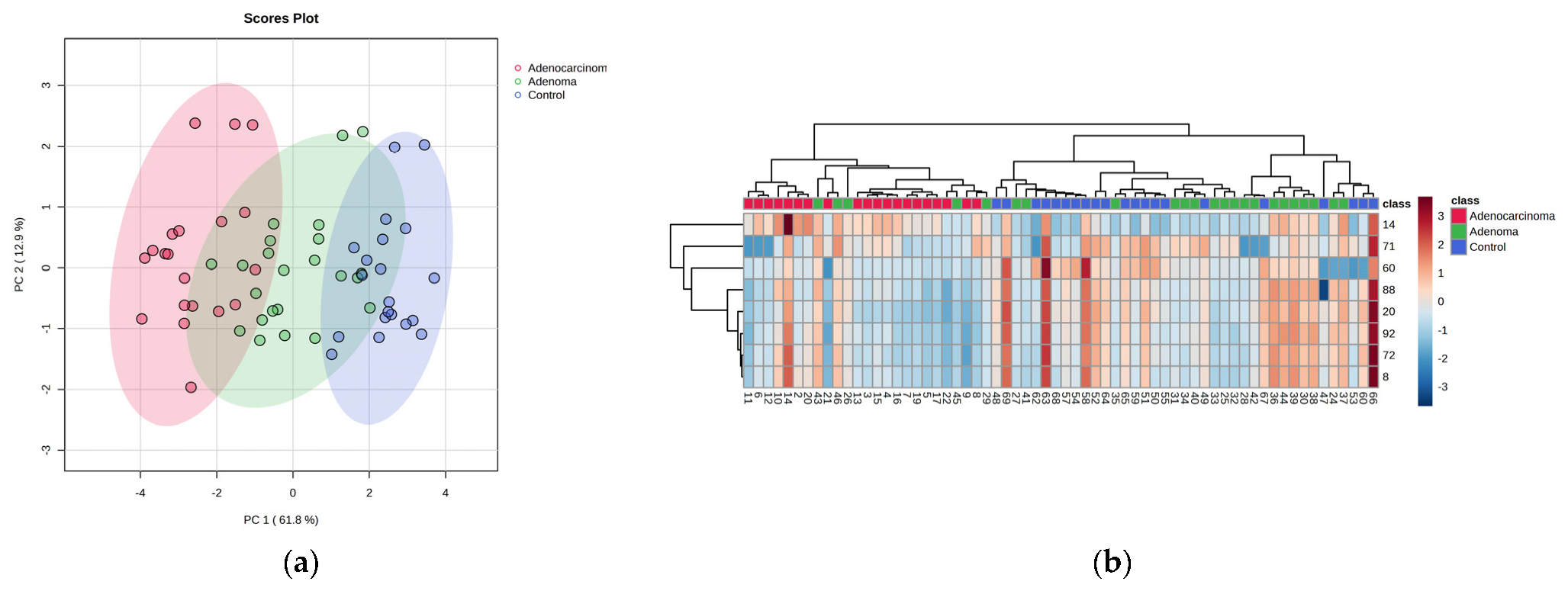
2.2. Metabolomic Profiling of Colorectal Cancer Serum by GC×GC-LR/HR-TOFMS
3. Discussion
4. Materials and Methods
4.1. Chemicals, Standards, and Samples
4.2. The Instrumental Method
4.3. Sample Preparation
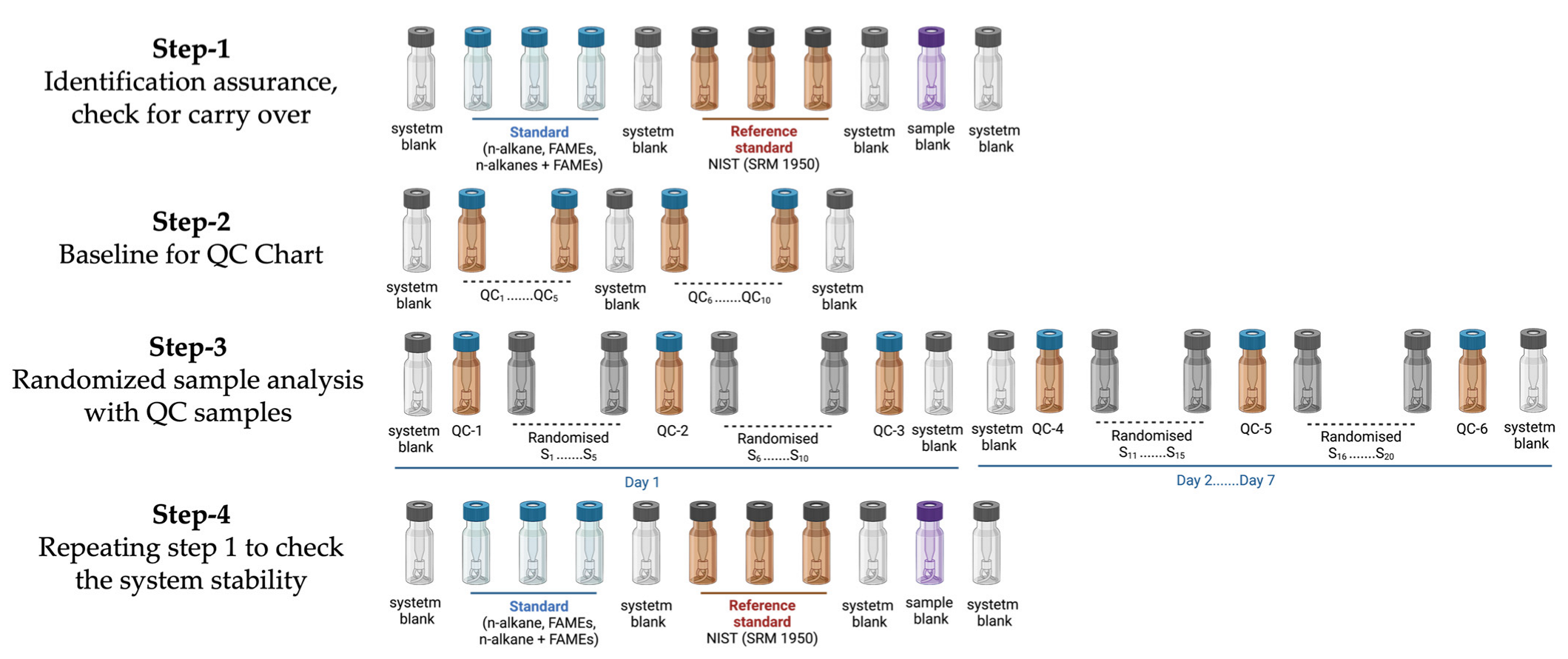
4.4. QAQC: The Injection Sequence
4.5. Data Processing and Chemometrics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Colorectal Cancer Awareness Month 2022. Available online: https://www.iarc.who.int/featured-news/colorectal-cancer-awareness-month-2022/ (accessed on 22 April 2023).
- Harber, I.; Zeidan, D.; Aslam, M.N. Colorectal Cancer Screening: Impact of COVID-19 Pandemic and Possible Consequences. Life 2021, 11, 1297. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Ferrari, A.; Neefs, I.; Hoeck, S.; Peeters, M.; Van Hal, G. Towards Novel Non-Invasive Colorectal Cancer Screening Methods: A Comprehensive Review. Cancers 2021, 13, 1820. [Google Scholar] [CrossRef]
- Karsa, L.; Patnick, J.; Segnan, N. European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis; Publications Office of the European Union: Luxembourg, 2010; ISBN 978-92-79-16435-4. Available online: https://data.europa.eu/doi/10.2772/1458 (accessed on 20 April 2023).
- Ullah, I.; Yang, L.; Yin, F.-T.; Sun, Y.; Li, X.-H.; Li, J.; Wang, X.-J. Multi-Omics Approaches in Colorectal Cancer Screening and Diagnosis, Recent Updates and Future Perspectives. Cancers 2022, 14, 5545. [Google Scholar] [CrossRef]
- Ivanisevic, J.; Want, E.J. From Samples to Insights into Metabolism: Uncovering Biologically Relevant Information in LC-HRMS Metabolomics Data. Metabolites 2019, 9, 308. [Google Scholar] [CrossRef]
- Bedia, C. Experimental Approaches in Omic Sciences, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 82, ISBN 9780444640444. [Google Scholar]
- Di Giovanni, N.; Meuwis, M.A.; Louis, E.; Focant, J.F. Untargeted Serum Metabolic Profiling by Comprehensive Two-Dimensional Gas Chromatography-High-Resolution Time-of-Flight Mass Spectrometry. J. Proteome Res. 2020, 19, 1013–1028. [Google Scholar] [CrossRef]
- Jing, Y.; Wu, X.; Gao, P.; Fang, Z.; Wu, J.; Wang, Q.; Li, C.; Zhu, Z.; Cao, Y. Rapid Differentiating Colorectal Cancer and Colorectal Polyp Using Dried Blood Spot Mass Spectrometry Metabolomic Approach. IUBMB Life 2017, 69, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Geijsen, A.J.M.R.; van Roekel, E.H.; van Duijnhoven, F.J.B.; Achaintre, D.; Bachleitner-Hofmann, T.; Baierl, A.; Bergmann, M.M.; Boehm, J.; Bours, M.J.L.; Brenner, H.; et al. Plasma Metabolites Associated with Colorectal Cancer Stage: Findings from an International Consortium. Int. J. Cancer 2020, 146, 3256–3266. [Google Scholar] [CrossRef] [PubMed]
- Holowatyj, A.N.; Gigic, B.; Herpel, E.; Scalbert, A.; Schneider, M.; Ulrich, C.M.; Achaintre, D.; Brezina, S.; van Duijnhoven, F.J.B.; Gsur, A.; et al. Distinct Molecular Phenotype of Sporadic Colorectal Cancers Among Young Patients Based on Multiomics Analysis. Gastroenterology 2020, 158, 1155–1158.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, B.; Zhang, F.; Yang, Y.; Shen, X.; Li, Z.; Zhao, W.; Zhang, Y.; Deng, K.; Rong, Z.; et al. Development of a Correlative Strategy to Discover Colorectal Tumor Tissue Derived Metabolite Biomarkers in Plasma Using Untargeted Metabolomics. Anal. Chem. 2019, 91, 2401–2408. [Google Scholar] [CrossRef]
- Purcaro, G.; Cordero, C.; Liberto, E.; Bicchi, C.; Conte, L.S. Toward a Definition of Blueprint of Virgin Olive Oil by Comprehensive Two-Dimensional Gas Chromatography. J. Chromatogr. A 2014, 1334, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; Agnoletto, E.; Cancemi, G.; Di Marco, V.; Traldi, P.; Pucciarelli, S.; Nitti, D.; Agostini, M. Altered Plasma Levels of Decanoic Acid in Colorectal Cancer as a New Diagnostic Biomarker. Anal. Bioanal. Chem. 2016, 408, 6321–6328. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Xiang, Y.B.; Rothman, N.; Yu, D.; Li, H.L.; Yang, G.; Cai, H.; Ma, X.; Lan, Q.; Gao, Y.T.; et al. Prospective Study of Blood Metabolites Associated with Colorectal Cancer Risk. Int. J. Cancer 2018, 143, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Moore, S.C.; Boca, S.; Huang, W.Y.; Xiong, X.; Stolzenberg-Solomon, R.; Sinha, R.; Sampson, J.N. A Prospective Study of Serum Metabolites and Colorectal Cancer Risk. Cancer 2014, 120, 3049–3057. [Google Scholar] [CrossRef]
- Farshidfar, F.; Weljie, A.M.; Kopciuk, K.A.; Hilsden, R.; McGregor, S.E.; Buie, W.D.; MacLean, A.; Vogel, H.J.; Bathe, O.F. A Validated Metabolomic Signature for Colorectal Cancer: Exploration of the Clinical Value of Metabolomics. Br. J. Cancer 2016, 115, 848–857. [Google Scholar] [CrossRef]
- Uchiyama, K.; Yagi, N.; Mizushima, K.; Higashimura, Y.; Hirai, Y.; Okayama, T.; Yoshida, N.; Katada, K.; Kamada, K.; Handa, O.; et al. Serum Metabolomics Analysis for Early Detection of Colorectal Cancer. J. Gastroenterol. 2017, 52, 677–694. [Google Scholar] [CrossRef]
- Long, Y.; Sanchez-Espiridion, B.; Lin, M.; White, L.; Mishra, L.; Raju, G.S.; Kopetz, S.; Eng, C.; Hildebrandt, M.A.T.; Chang, D.W.; et al. Global and Targeted Serum Metabolic Profiling of Colorectal Cancer Progression. Cancer 2017, 123, 4066–4074. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, Y.; Shu, D.; Liang, X.; Hu, X.; Xie, Y.; Lin, D.; Li, H. Metabolomics Analysis in Serum from Patients with Colorectal Polyp and Colorectal Cancer by 1H-NMR Spectrometry. Dis. Markers 2019, 2019, 3491852. [Google Scholar] [CrossRef]
- Wu, J.; Wu, M.; Wu, Q. Identification of Potential Metabolite Markers for Colon Cancer and Rectal Cancer Using Serum Metabolomics. J. Clin. Lab. Anal. 2020, 34, e23333. [Google Scholar] [CrossRef]
- Udo, R.; Katsumata, K.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Sunamura, M.; Nagakawa, Y.; Soya, R.; Sugimoto, M.; Tsuchida, A. Urinary Charged Metabolite Profiling of Colorectal Cancer Using Capillary Electrophoresis-Mass Spectrometry. Sci. Rep. 2020, 10, 21057. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Y.; Liang, J.; Huang, Y.; Ma, C.; Liu, X.; Yang, J. NMR-Based Metabolomic Techniques Identify Potential Urinary Biomarkers for Early Colorectal Cancer Detection. Oncotarget 2017, 8, 105819–105831. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and Metabolomic Analyses Reveal Distinct Stage-Specific Phenotypes of the Gut Microbiota in Colorectal Cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Kim, M.; Vogtmann, E.; Ahlquist, D.A.; Devens, M.E.; Kisiel, J.B.; Taylor, W.R.; White, B.A.; Hale, V.L.; Sung, J.; Chia, N.; et al. Fecal Metabolomic Signatures in Colorectal Adenoma Patients Are Associated with Gut Microbiota and Early Events of Colorectal Cancer Pathogenesis. MBio 2020, 11, e03186-19. [Google Scholar] [CrossRef]
- Kim, D.J.; Yang, J.; Seo, H.; Lee, W.H.; Ho Lee, D.; Kym, S.; Park, Y.S.; Kim, J.G.; Jang, I.J.; Kim, Y.K.; et al. Colorectal Cancer Diagnostic Model Utilizing Metagenomic and Metabolomic Data of Stool Microbial Extracellular Vesicles. Sci. Rep. 2020, 10, 2860. [Google Scholar] [CrossRef]
- Spener, F.; Lagarde, M.; Géloën, A.; Record, M. What Is Lipidomics? Eur. J. Lipid Sci. Technol. 2003, 105, 481. [Google Scholar] [CrossRef]
- Han, X.; Gross, R.W. Global Analyses of Cellular Lipidomes Directly from Crude Extracts of Biological Samples by ESI Mass Spectrometry: A Bridge to Lipidomics. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid Classification, Structures and Tools. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Sledzinski, T.; Mika, A. Changes in Lipids Composition and Metabolism in Colorectal Cancer: A Review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Cancer, C.; Zaytseva, Y. Lipid Metabolism as a Targetable Metabolic Vulnerability in Colorectal Cancer. Cancers 2021, 13, 301. [Google Scholar]
- Menendez, J.A.; Lupu, R. Fatty Acid Synthase and the Lipogenic Phenotype in Cancer Pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Holčapek, M.; Liebisch, G.; Ekroos, K. Lipidomic Analysis. Anal. Chem. 2018, 90, 4249–4257. [Google Scholar] [CrossRef]
- Zhang, Y.; He, C.; Qiu, L.; Wang, Y.; Qin, X.; Liu, Y.; Li, Z. Serum Unsaturated Free Fatty Acids: A Potential Biomarker Panel for Early-Stage Detection of Colorectal Cancer. J. Cancer 2016, 7, 477–483. [Google Scholar] [CrossRef]
- Bartolucci, G.; Pallecchi, M.; Menicatti, M.; Moracci, L.; Pucciarelli, S.; Agostini, M.; Crotti, S. A Method for Assessing Plasma Free Fatty Acids from C2 to C18 and Its Application for the Early Detection of Colorectal Cancer. J. Pharm. Biomed. Anal. 2022, 215, 114762. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Nishiumi, S.; Shinohara, M.; Hatano, N.; Ikeda, A.; Yoshie, T.; Kobayashi, T.; Shiomi, Y.; Irino, Y.; Takenawa, T.; et al. Serum Fatty Acid Profiling of Colorectal Cancer by Gas Chromatography/Mass Spectrometry. Biomark. Med. 2011, 5, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Kobiela, J.; Pakiet, A.; Czumaj, A.; Sokołowska, E.; Makarewicz, W.; Chmielewski, M.; Stepnowski, P.; Marino-Gammazza, A.; Sledzinski, T. Preferential Uptake of Polyunsaturated Fatty Acids by Colorectal Cancer Cells. Sci. Rep. 2020, 10, 1954. [Google Scholar] [CrossRef]
- Cottet, V.; Vaysse, C.; Scherrer, M.L.; Ortega-Deballon, P.; Lakkis, Z.; Delhorme, J.B.; Deguelte-Lardière, S.; Combe, N.; Bonithon-Kopp, C. Fatty Acid Composition of Adipose Tissue and Colorectal Cancer: A Case-Control Study. Am. J. Clin. Nutr. 2015, 101, 192–201. [Google Scholar] [CrossRef]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.H.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M.; Paulus, V.; Guissard, F.; Graff, S.; et al. Exhaled Volatile Organic Compounds Are Able to Discriminate between Neutrophilic and Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.H.; Zanella, D.; Vercammen, J.; Henket, M.; Schleich, F.; Louis, R.; Focant, J.F. Multimodal Combination of GC × GC-HRTOFMS and SIFT-MS for Asthma Phenotyping Using Exhaled Breath. Sci. Rep. 2020, 10, 16159. [Google Scholar] [CrossRef] [PubMed]
- Zanella, D.; Guiot, J.; Stefanuto, P.H.; Giltay, L.; Henket, M.; Guissard, F.; André, B.; Malaise, M.; Potjewijd, J.; Schleich, F.; et al. Breathomics to Diagnose Systemic Sclerosis Using Thermal Desorption and Comprehensive Two-Dimensional Gas Chromatography High-Resolution Time-of-Flight Mass Spectrometry. Anal. Bioanal. Chem. 2021, 413, 3813–3822. [Google Scholar] [CrossRef]
- Di Giovanni, N.; Meuwis, M.A.; Louis, E.; Focant, J.F. Specificity of Metabolic Colorectal Cancer Biomarkers in Serum through Effect Size. Metabolomics 2020, 16, 88. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of Fatty Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A. Very Long-Chain Fatty Acids: Elongation, Physiology and Related Disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Tiuca, I.-D. Importance of Fatty Acids in Physiopathology of Human Body. Fat. Acids 2017, 3–22. [Google Scholar] [CrossRef]
- Murff, H.J.; Shu, X.O.; Li, H.; Dai, Q.; Kallianpur, A.; Yang, G.; Cai, H.; Wen, W.; Gao, Y.T.; Zheng, W. A Prospective Study of Dietary Polyunsaturated Fatty Acids and Colorectal Cancer Risk in Chinese Women. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wen, X.; Gu, F.; Zhang, X.; Li, J.; Liu, Y.; Dong, J.; Deng, X.; Zhu, X.; Tian, Y. Role of Serum Polyunsaturated Fatty Acids in the Development of Colorectal Cancer. Int. J. Clin. Exp. Med. 2015, 8, 15900–15909. [Google Scholar]
- Shi, D.D.; Fang, Y.J.; Jiang, Y.L.; Dong, T.; Zhang, Z.L.; Ma, T.; Zhou, R.L.; Ou, Q.J.; Zhang, C.X. Serum Levels of N-3 Polyunsaturated Fatty Acids and Colorectal Cancer Risk in Chinese Population. Br. J. Nutr. 2023, 1–11. [Google Scholar] [CrossRef]
- Linseisen, J.; Grundmann, N.; Zoller, D.; Kuhn, T.; Jansen, E.H.J.M.; Chajes, V.; Fedirko, V.; Weiderpass, E.; Dahm, C.C.; Overvad, K.; et al. Red Blood Cell Fatty Acids and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol. Biomarkers Prev. 2021, 30, 874–885. [Google Scholar] [CrossRef]
- Jagadeesh, G.; Balakumar, P.; Maung-U, K. Pathophysiology and Pharmacotherapy of Cardiovascular Disease; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–1342. ISBN 978-3-319-15961-4. [Google Scholar] [CrossRef]
- Gold, A.; Choueiry, F.; Jin, N.; Mo, X.; Zhu, J. The Application of Metabolomics in Recent Colorectal Cancer Studies: A State-of-the-Art Review. Cancers 2022, 14, 725. [Google Scholar] [CrossRef]
- Fatima, S.; Hu, X.; Huang, C.; Zhang, W.; Cai, J.; Huang, M.; Gong, R.H.; Chen, M.; Ho, A.H.M.; Su, T.; et al. High-Fat Diet Feeding and Palmitic Acid Increase CRC Growth in Β2AR-Dependent Manner. Cell Death Dis. 2019, 10, 711. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Zhao, W.; Deng, K.; Wang, Z.; Yang, C.; Ma, L.; Openkova, M.S.; Hou, Y.; Li, K. Metabolomics for Biomarker Discovery in the Diagnosis, Prognosis, Survival and Recurrence of Colorectal Cancer: A Systematic Review. Oncotarget 2017, 8, 35460–35472. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Brietzke, E. Recent Advances in Lipidomics: Analytical and Clinical Perspectives. Prostaglandins Other Lipid Mediat. 2017, 128–129, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Dejong, T.; Dubois, L.M.; Markey, A.; Gengler, N.; Wavreille, J.; Stefanuto, P.H.; Focant, J.F. Lipid Serum Profiling of Boar-Tainted and Untainted Pigs Using GC×GC–TOFMS: An Exploratory Study. Metabolites 2022, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]


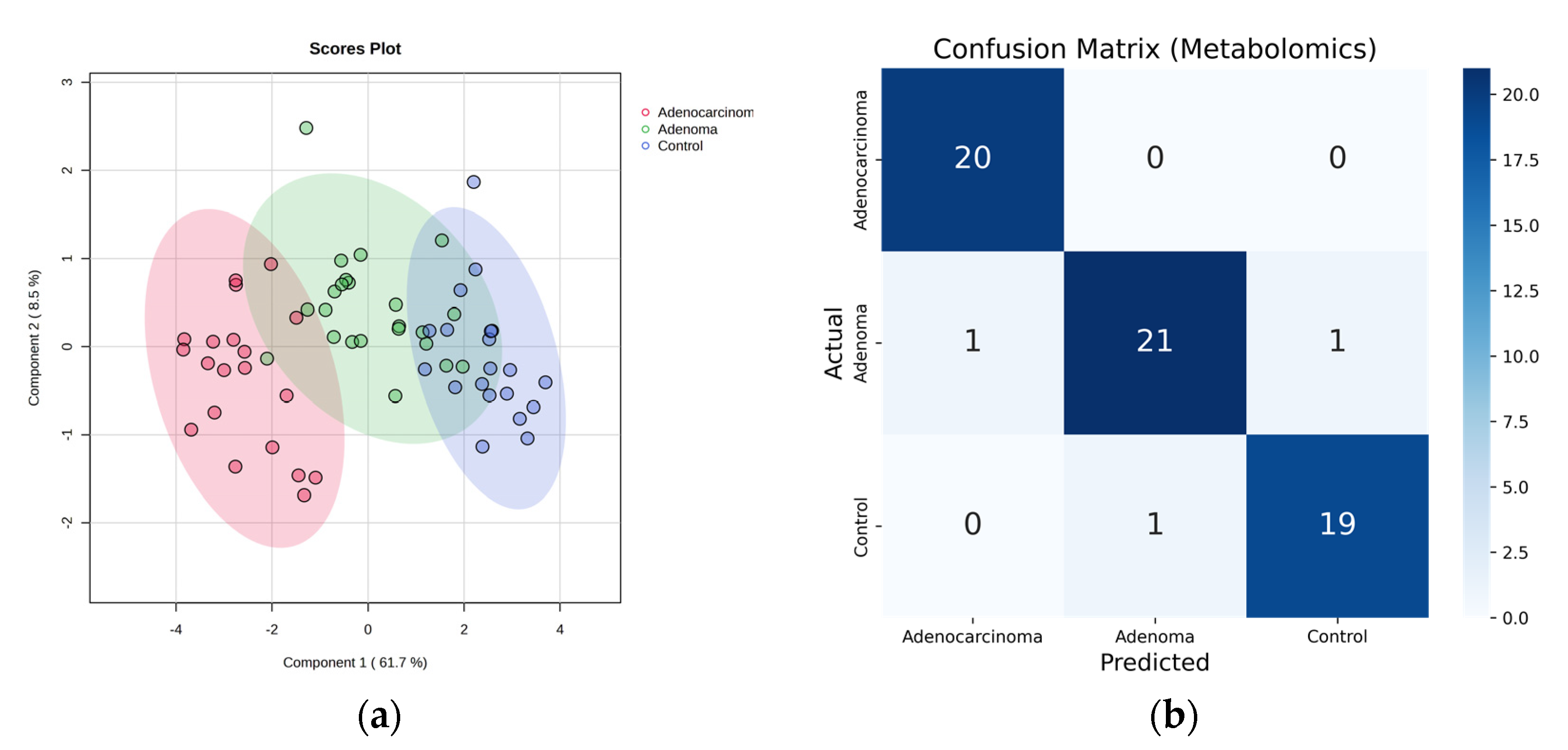
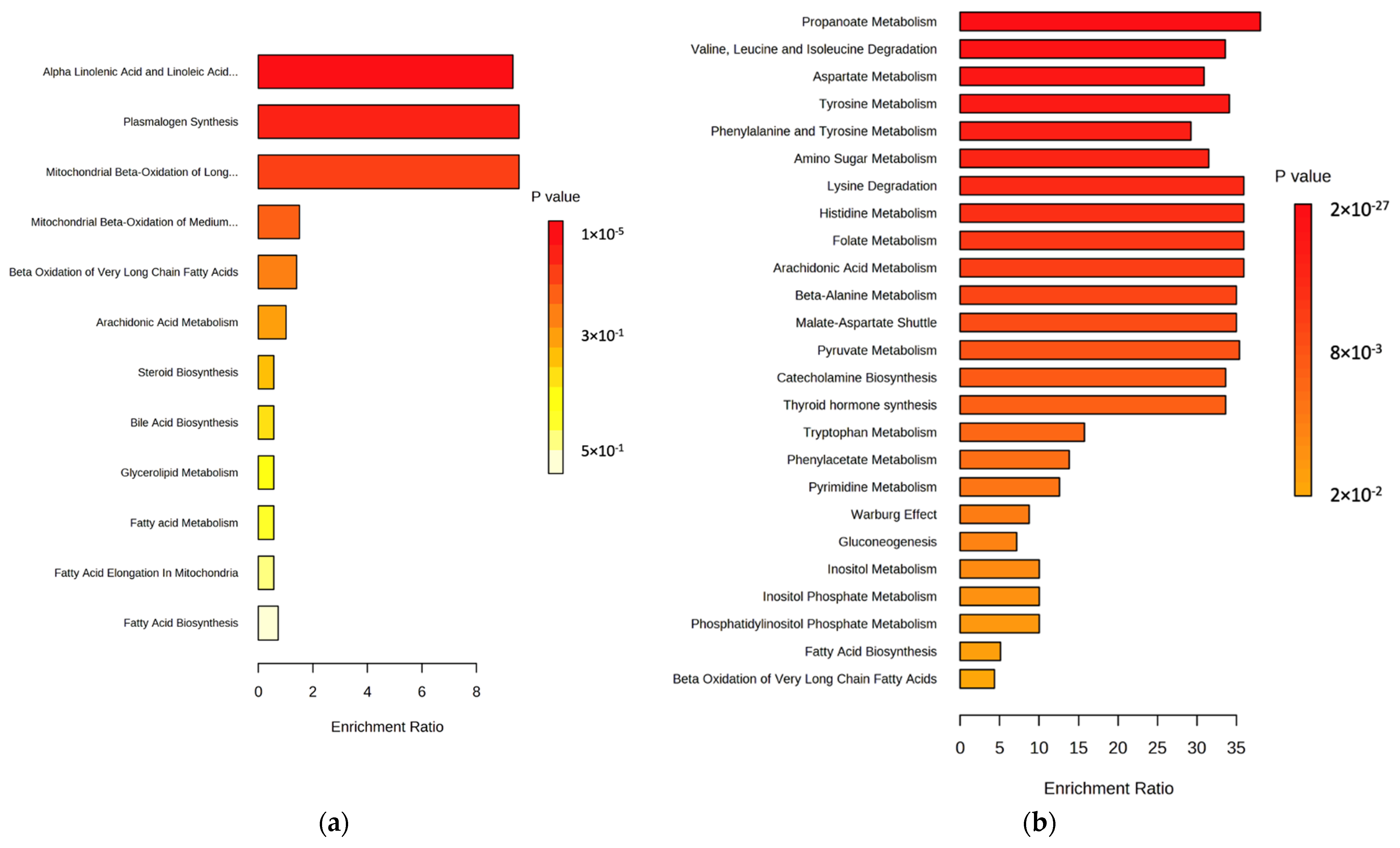
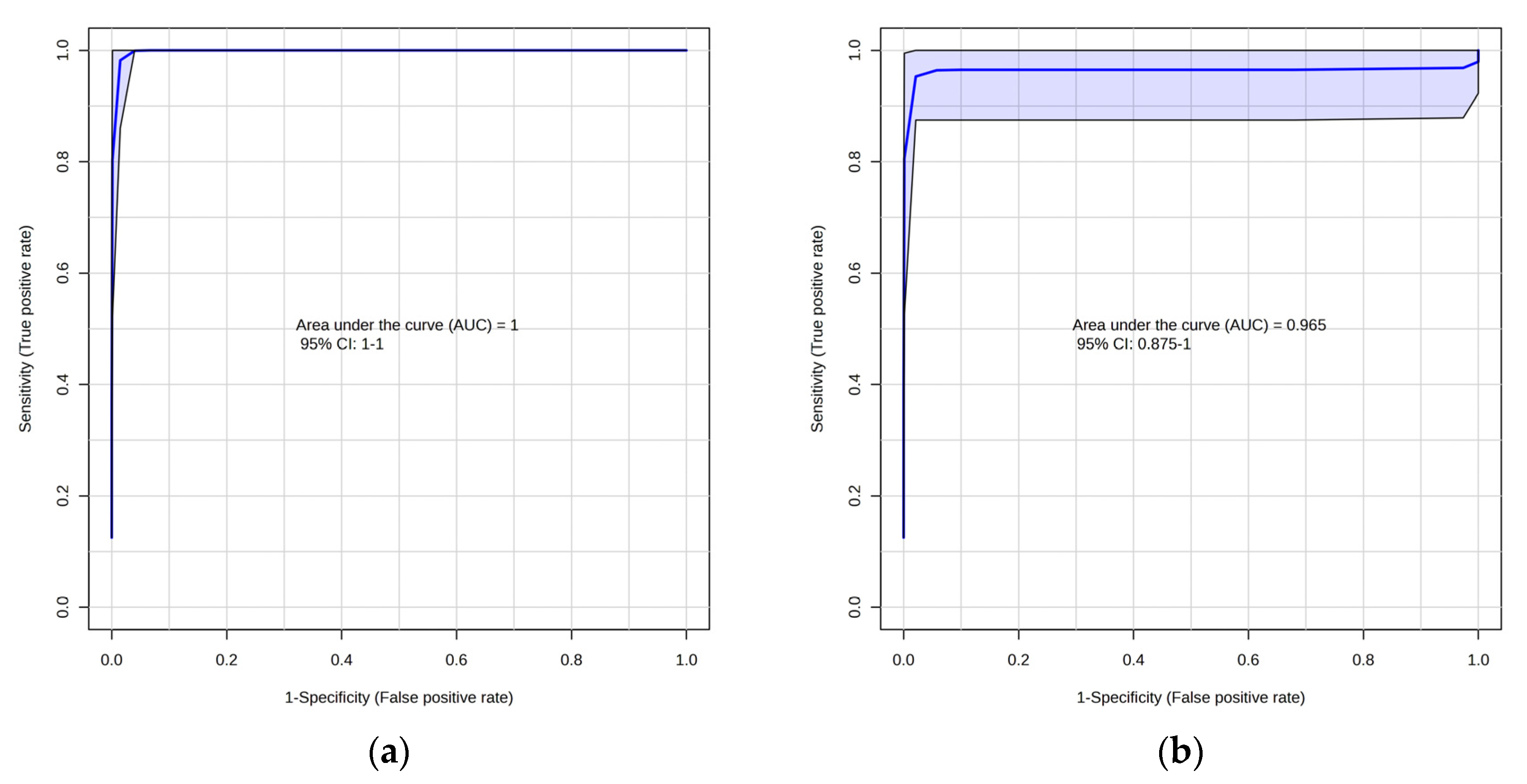
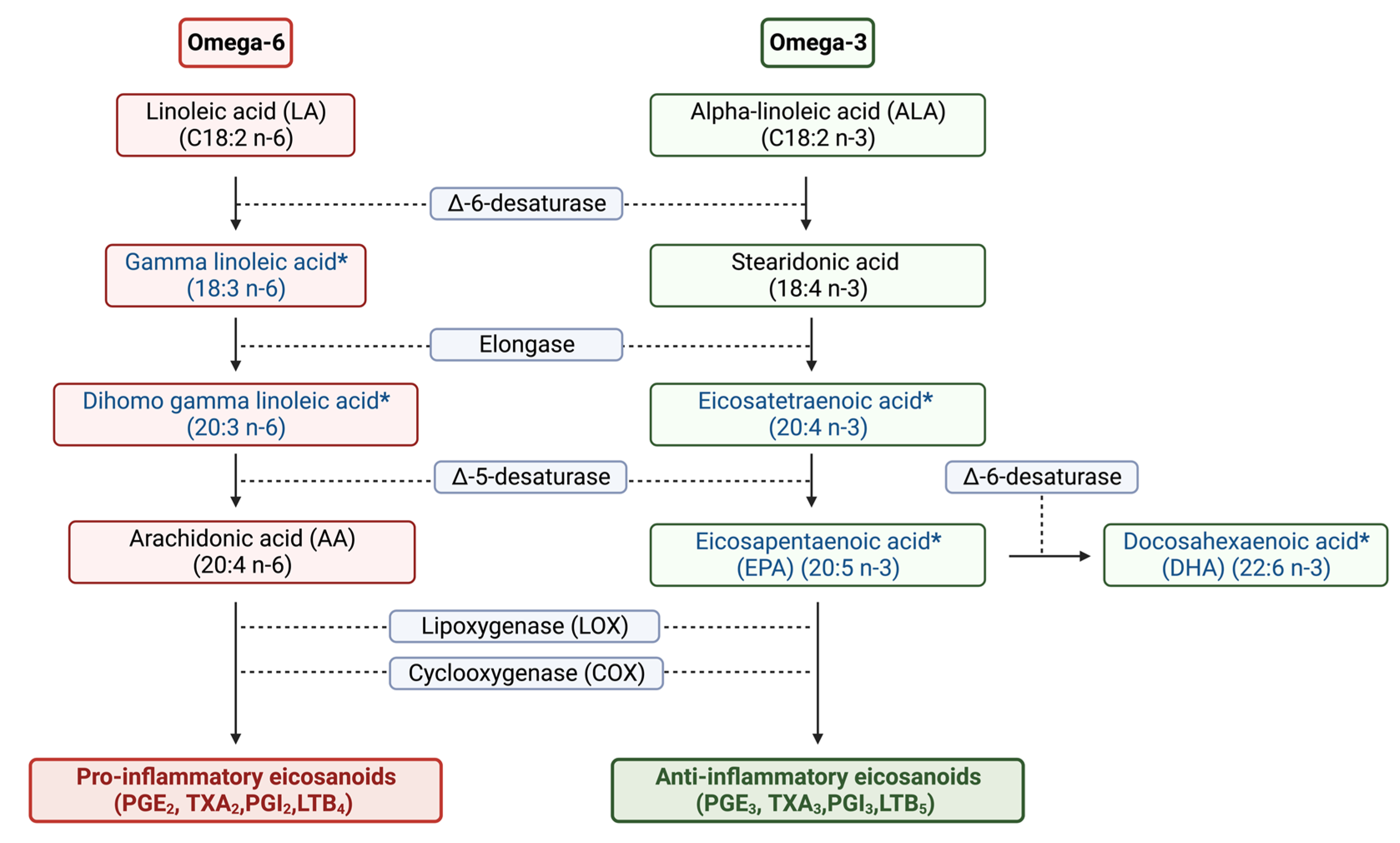

| Adenocarcinoma | Adenoma | Control | |
|---|---|---|---|
| Total no of participants (Female/male) | 20 (9/11) | 23 (12/11) | 21 (9/12) |
| Female age (Mean ± SD) | 66.50 ± 7.99 | 64.70 ± 8.69 | 65.81 ± 8.73 |
| Male age (Mean ± SD) | 69.45 ± 8.54 | 64.70 ± 8.69 | 65.81 ± 8.93 |
| Location 1 (A/T/D/S/R/recto-sigmoid/splenic flexure) | (7/0/3/3/6/0/1) | (7/1/4/3/7/1/0) | - |
| pTNM staging 2 Stage -I (pT1N0M0 or pT1NxMx) Stage -II (pT2N0Mx or pT2NxMx) Stage -III (pT3N0Mx or ypT3N0Mx) | 6 8 6 | - | - |
| Alcohol (Yes/No) | 10/10 | 10/13 | 12/9 |
| Smoking (Yes/No) | 5/15 | 4/19 | 3/18 |
| Potential ID | Class | CAS | Similarity | Reverse | Probability (%) | △ LRI | (FDR) <0.05 | VIP Score (>1) | MDA (>0.008) |
|---|---|---|---|---|---|---|---|---|---|
| C20:4 n-3 ** | PUFA (ω-3) | 132712-70-0 | 882 | 875 | 30.1 | 12 | 2.6 × 10−4 | 1.9847 | 0.0193 |
| C20:5 n-3 * | PUFA (ω-3) | 2734-47-6 | 890 | 892 | 58.5 | 8 | 1.1 × 10−11 | 2.8251 | 0.0790 |
| C22:5 n-3 ** | PUFA (ω-3) | 108698-02-8 | 851 | 851 | 74.1 | 13 | 5.3 × 10−3 | 2.1896 | 0.0324 |
| C22:6 n-3 * | PUFA (ω-3) | 2566-90-7 | 900 | 910 | 72.9 | 16 | 2.0 × 10−3 | 1.6093 | 0.0193 |
| C18:3 n-6 * | PUFA (ω-6) | 16326-32-2 | 875 | 875 | 56.3 | 11 | 9.7 × 10−8 | 2.4366 | 0.0463 |
| C20:3 n-6 * | PUFA (ω-6) | 21061-10-9 | 919 | 907 | 69.7 | 12 | 7.5 × 10−8 | 1.9479 | 0.0369 |
| C22:5 n-6 ** | PUFA (ω-6) | - | 897 | 883 | 28.6 | 18 | 7.5 × 10−8 | 2.2631 | 0.0448 |
| C18:0 * | SFA | 112-61-8 | 925 | 955 | 84.3 | 1 | 7.0 × 10−3 | 1.1598 | 0.0087 |
| Name | CAS | Similarity | Reverse | Probability (%) | △ LRI | p-Value (<0.05) | VIP Score (>1.7) | MDA (>0.0023) |
|---|---|---|---|---|---|---|---|---|
| L-Alanine, 2TMS derivative | 27844-07-1 | 861 | 862 | 91.4 | 14 | 8.85 × 10−3 | 2.0337 | 0.0024 |
| 3-Hydroxybutyric acid, 2TMS derivative | 55133-94-3 | 907 | 912 | 65.4 | 12 | 1.49 × 10−3 | 2.3255 | 0.0192 |
| L-Valine, 2TMS derivative | 7364-44-5 | 851 | 936 | 82.4 | 17 | 1.99 × 10−7 | 3.4019 | 0.0340 |
| L-Methionine, 2TMS derivative | 27844-10-6 | 898 | 898 | 98.7 | 8 | 3.16 × 10−3 | 1.7578 | 0.0026 |
| L-Glutamic acid, 3TMS derivative | 15985-07-6 | 878 | 878 | 94.2 | 13 | 1.90 × 10−4 | 2.5943 | 0.0250 |
| L-Tyrosine, 3TMS derivative | 55638-45-4 | 909 | 923 | 97.4 | 1 | 9.33 × 10−4 | 2.4399 | 0.0104 |
| Myo-Inositol, 6TMS derivative | 2582-79-8 | 925 | 925 | 87.7 | 11 | 3.31 × 10−2 | 2.1113 | 0.0095 |
| L-Tryptophan, 3TMS derivative | 55429-28-2 | 897 | 901 | 72.7 | 6 | 2.00 × 10−4 | 2.6359 | 0.0085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, K.; Orlando, T.; Meuwis, M.-A.; Louis, E.; Stefanuto, P.-H.; Focant, J.-F. Comprehensive Insight into Colorectal Cancer Metabolites and Lipids for Human Serum: A Proof-of-Concept Study. Int. J. Mol. Sci. 2023, 24, 9614. https://doi.org/10.3390/ijms24119614
Bhatt K, Orlando T, Meuwis M-A, Louis E, Stefanuto P-H, Focant J-F. Comprehensive Insight into Colorectal Cancer Metabolites and Lipids for Human Serum: A Proof-of-Concept Study. International Journal of Molecular Sciences. 2023; 24(11):9614. https://doi.org/10.3390/ijms24119614
Chicago/Turabian StyleBhatt, Kinjal, Titziana Orlando, Marie-Alice Meuwis, Edouard Louis, Pierre-Hugues Stefanuto, and Jean-François Focant. 2023. "Comprehensive Insight into Colorectal Cancer Metabolites and Lipids for Human Serum: A Proof-of-Concept Study" International Journal of Molecular Sciences 24, no. 11: 9614. https://doi.org/10.3390/ijms24119614
APA StyleBhatt, K., Orlando, T., Meuwis, M.-A., Louis, E., Stefanuto, P.-H., & Focant, J.-F. (2023). Comprehensive Insight into Colorectal Cancer Metabolites and Lipids for Human Serum: A Proof-of-Concept Study. International Journal of Molecular Sciences, 24(11), 9614. https://doi.org/10.3390/ijms24119614






