Investigating the Inhibition of FTSJ1, a Tryptophan tRNA-Specific 2′-O-Methyltransferase by NV TRIDs, as a Mechanism of Readthrough in Nonsense Mutated CFTR
Abstract
1. Introduction
2. Results and Discussion
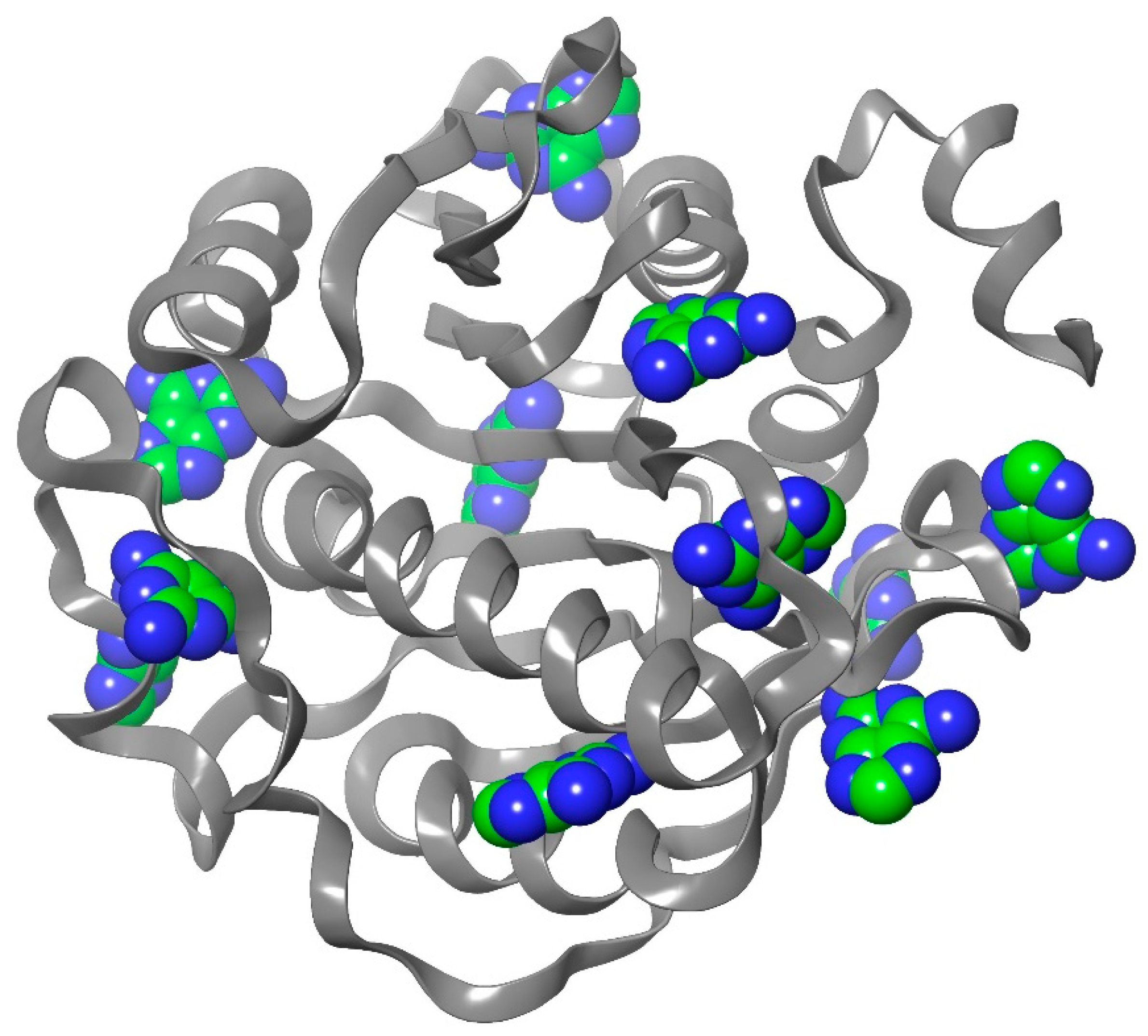
2.1. Homology Modeling
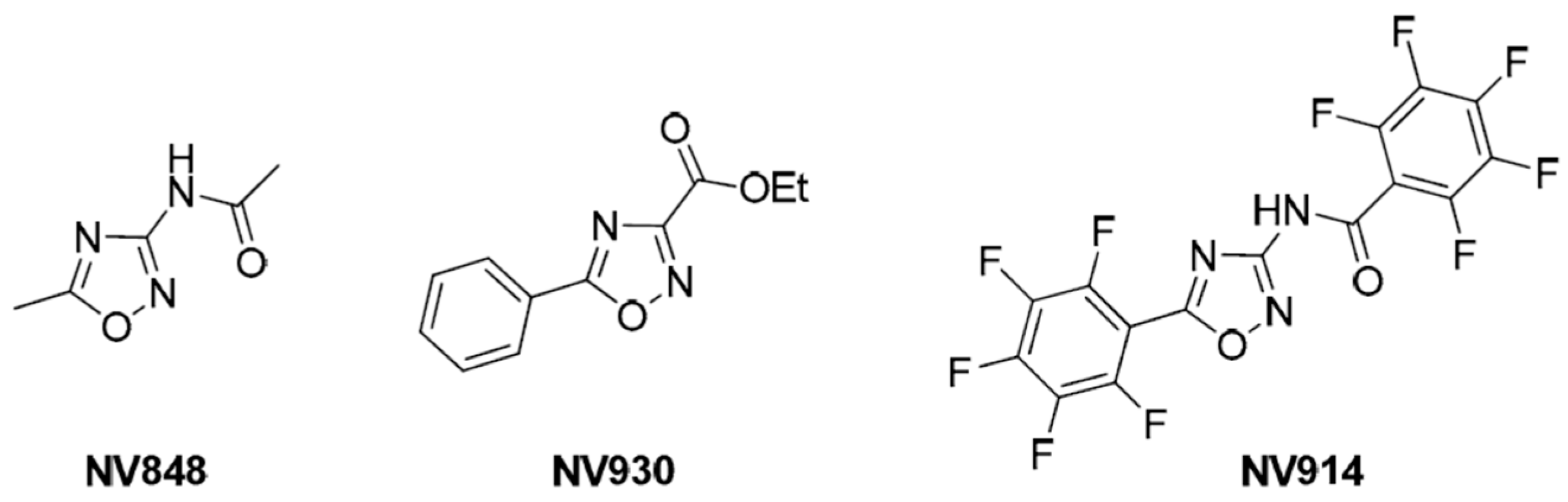
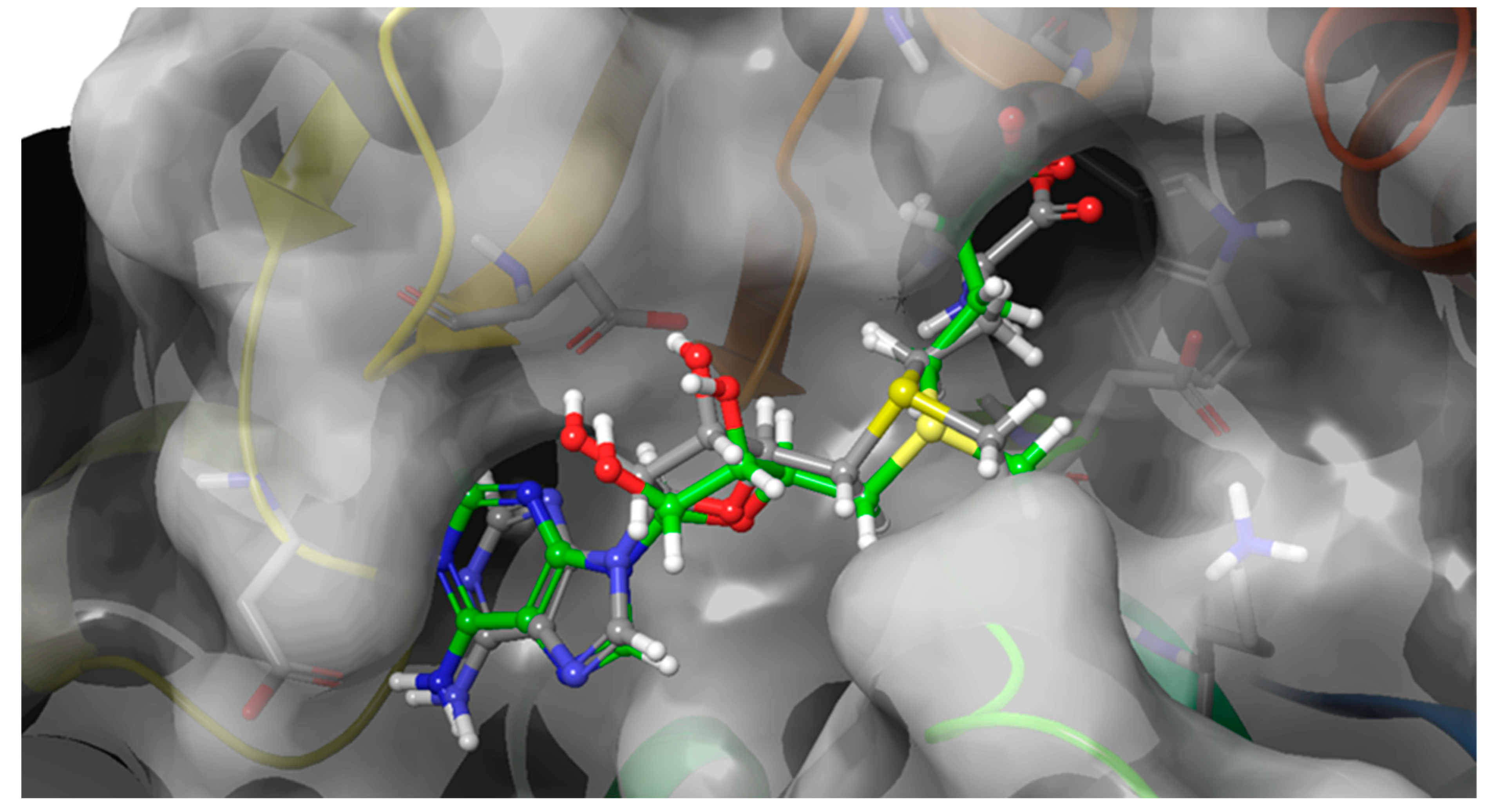
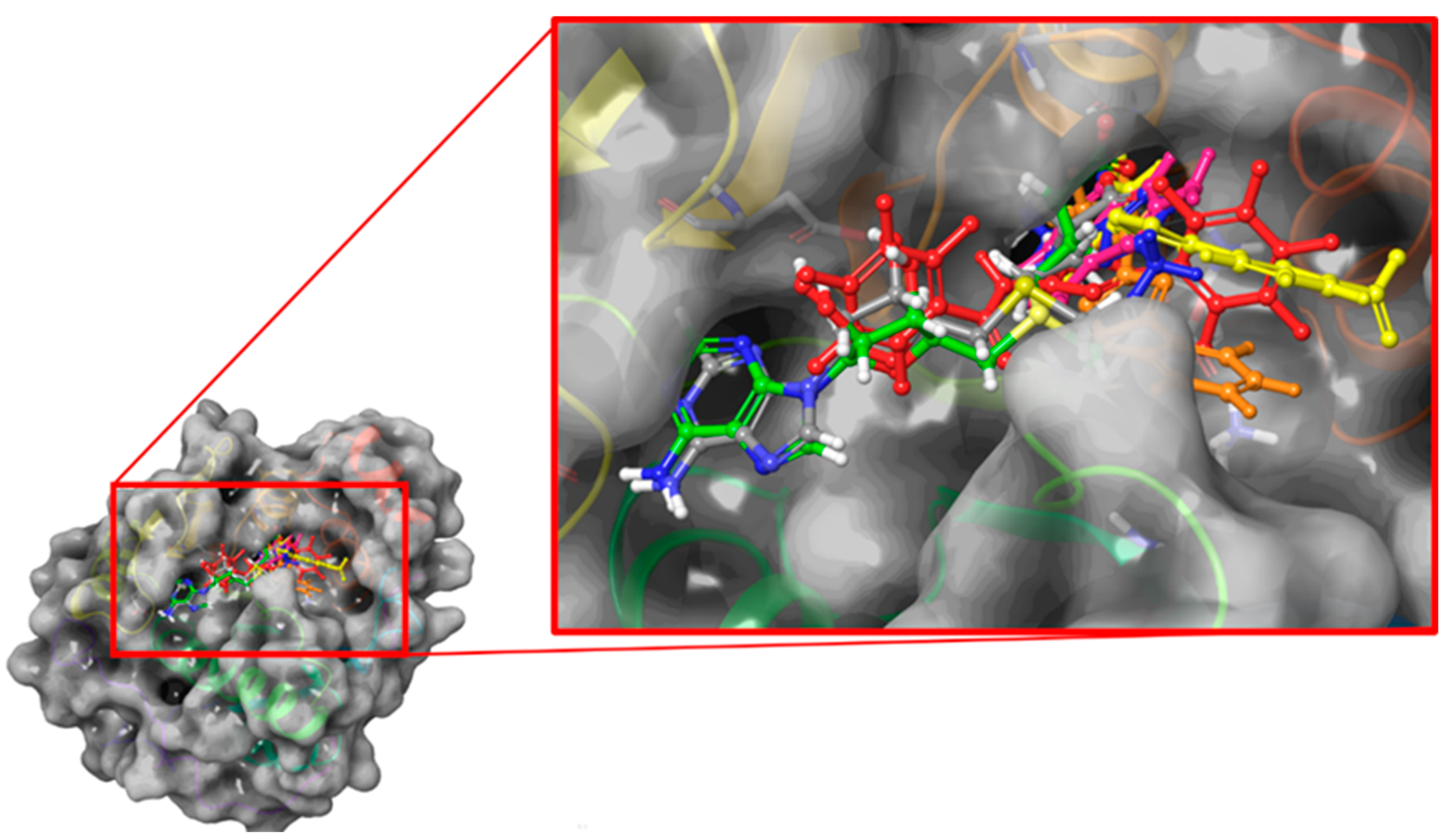
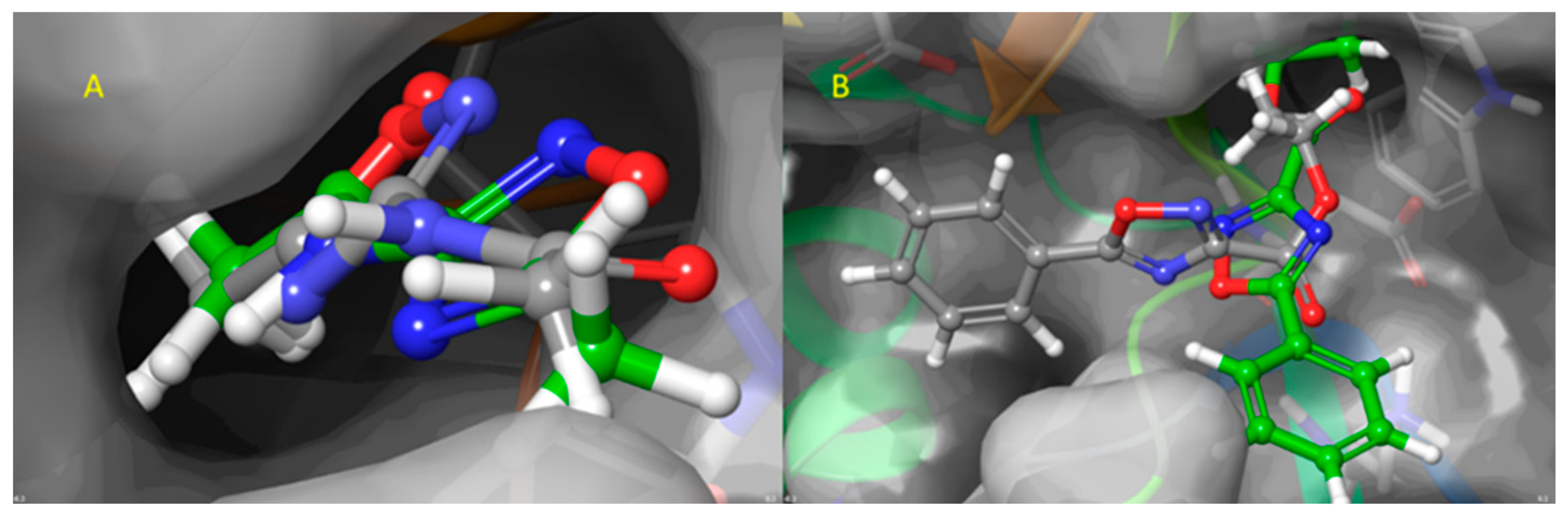
2.2. Blind Docking and Semi-Flexible Docking Analysis
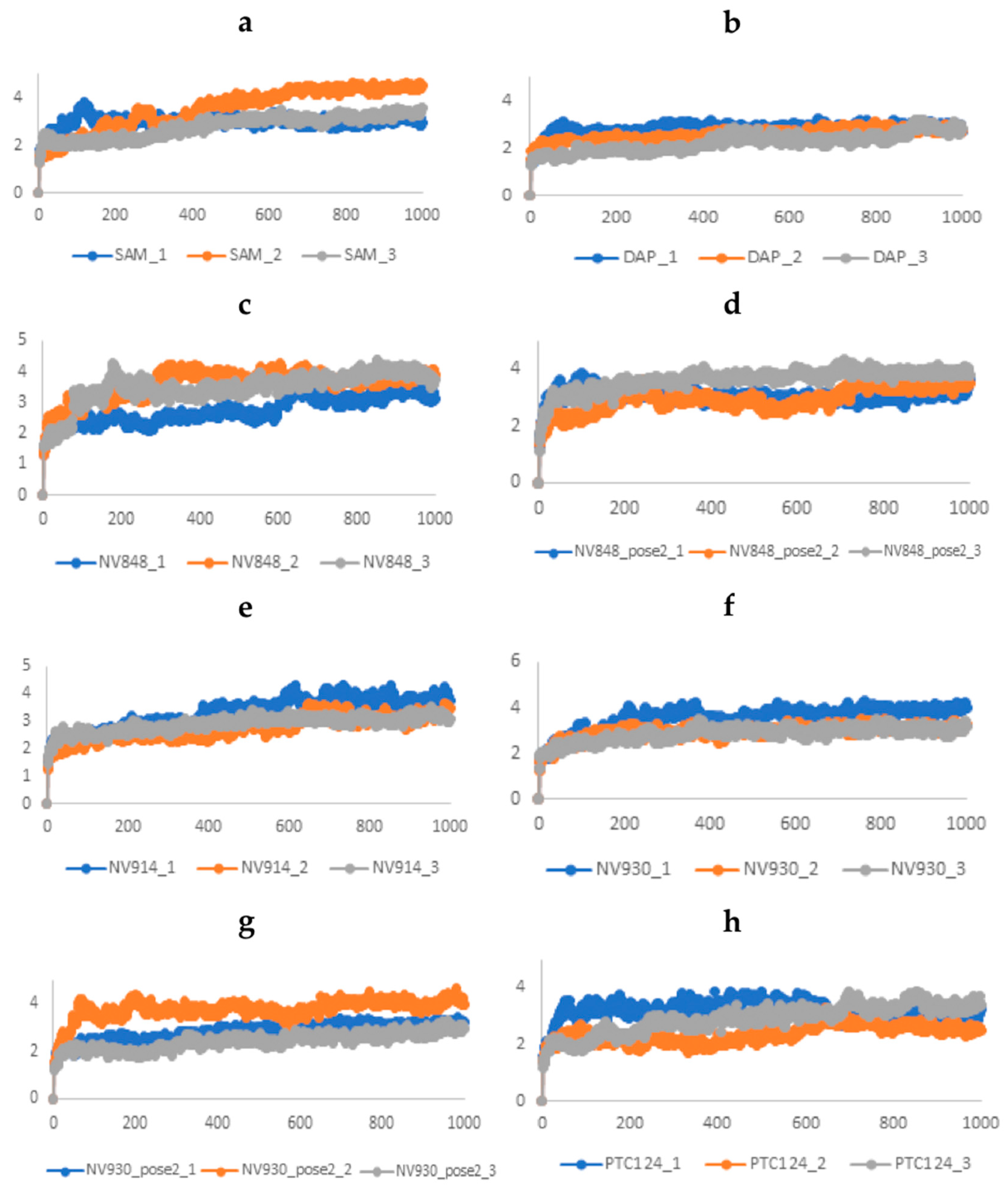
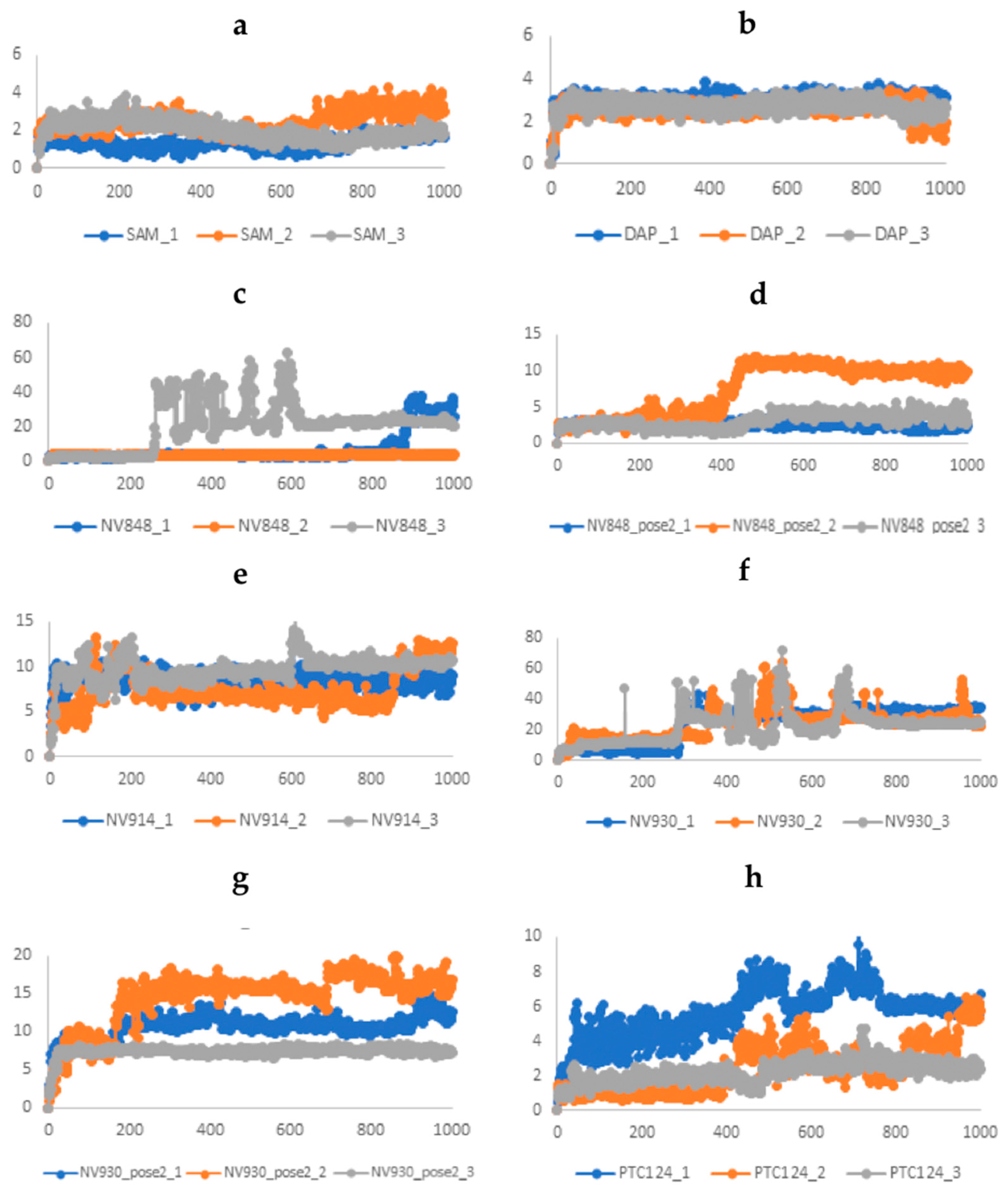
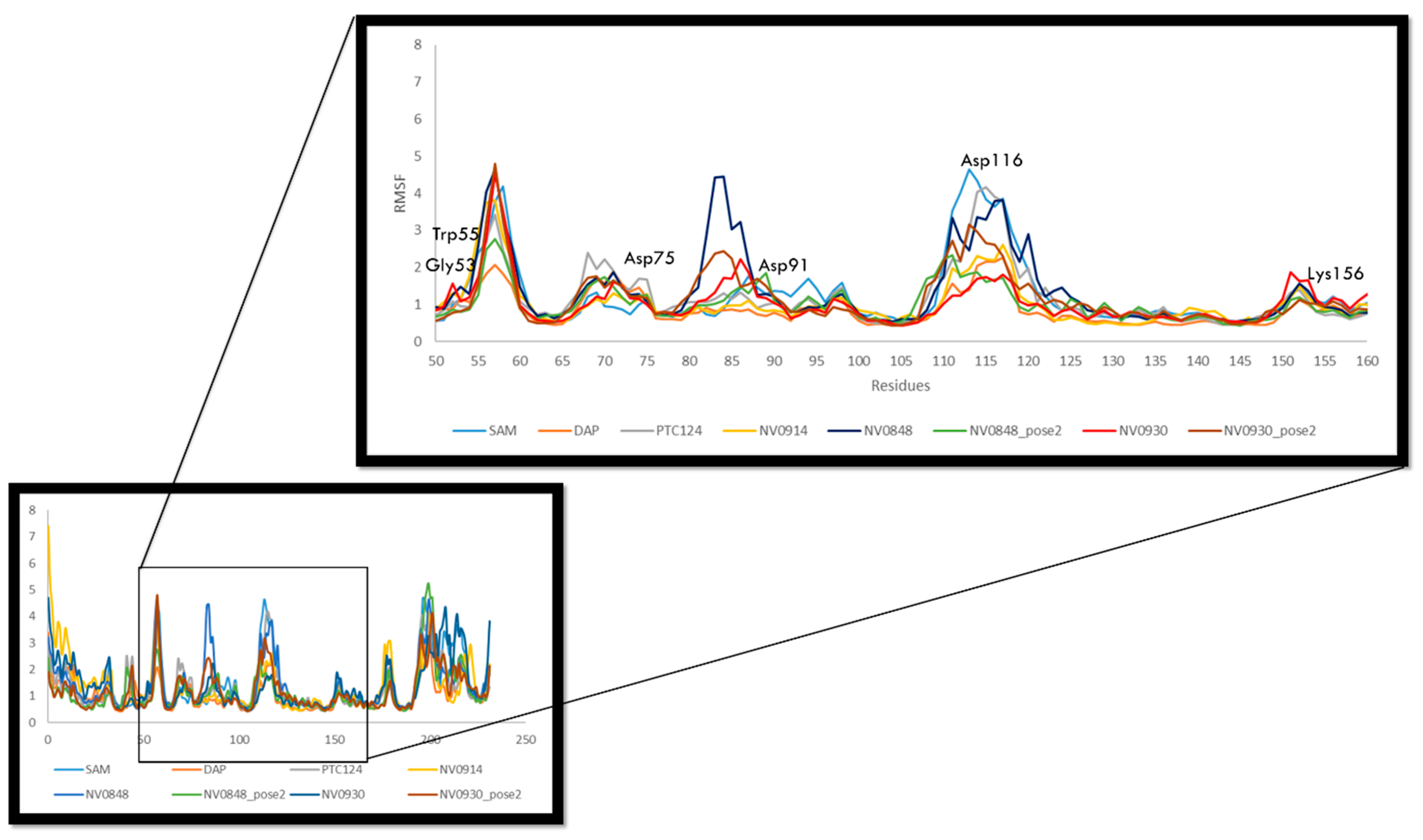
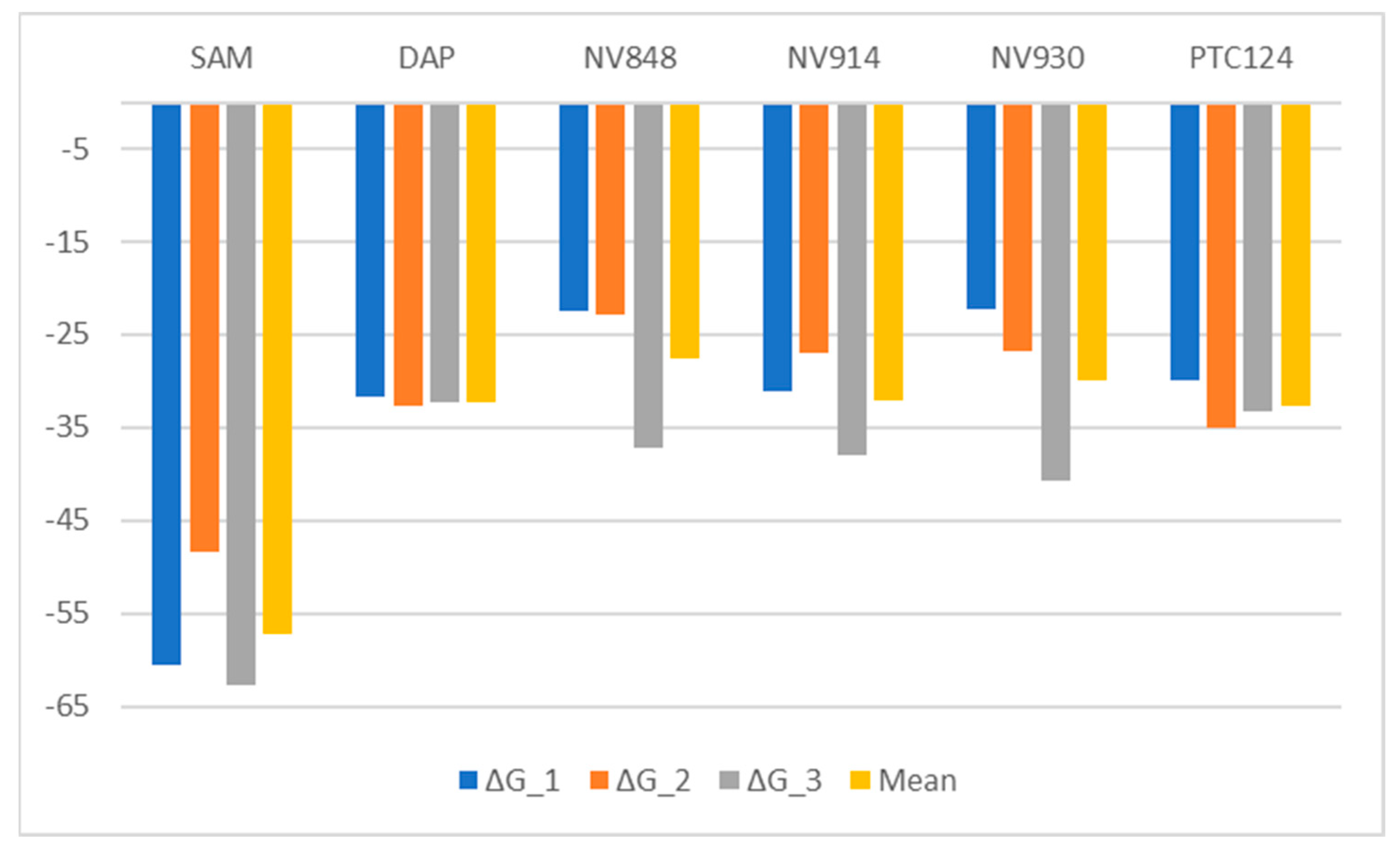
2.3. Molecular Dynamics and Free Energy Analysis
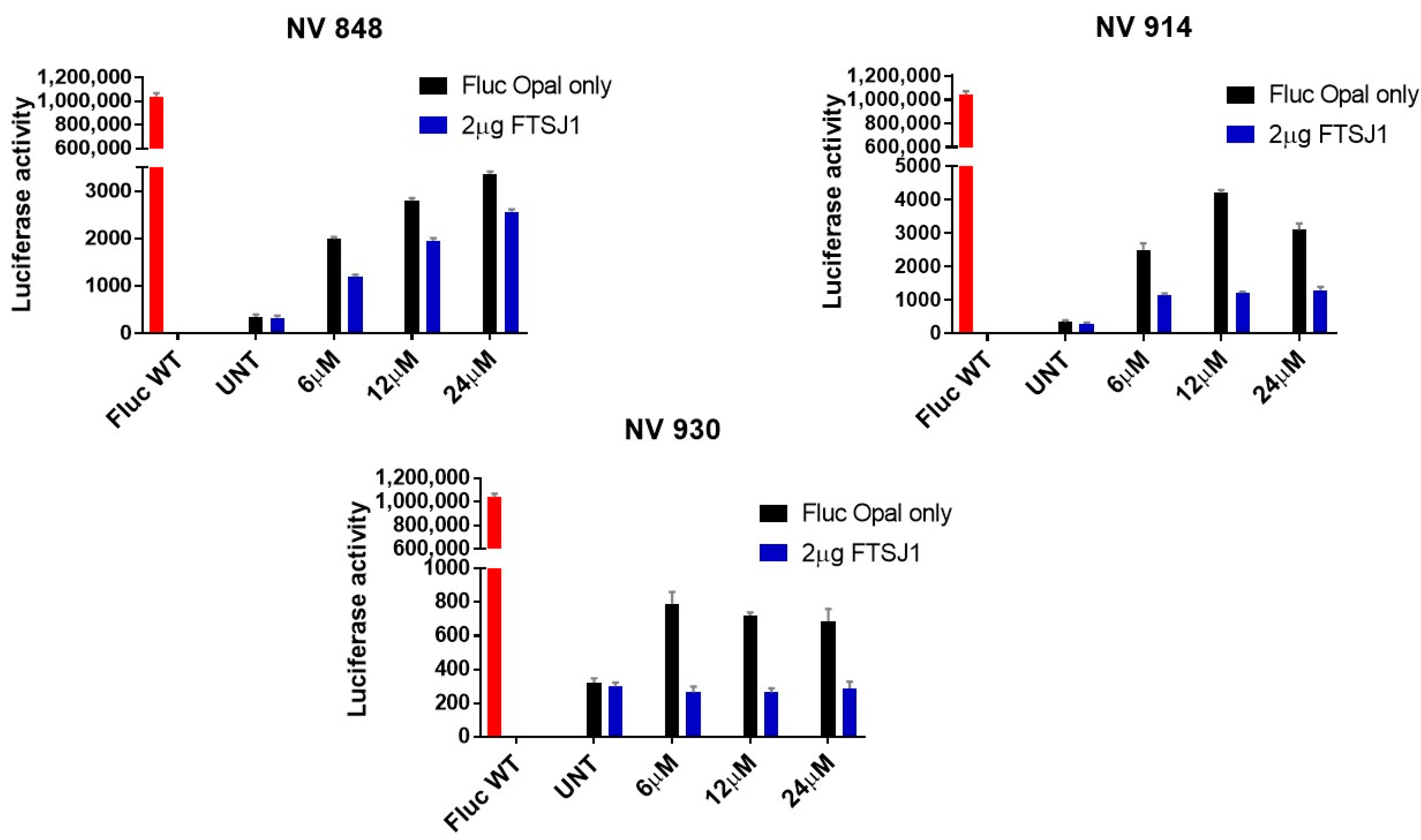
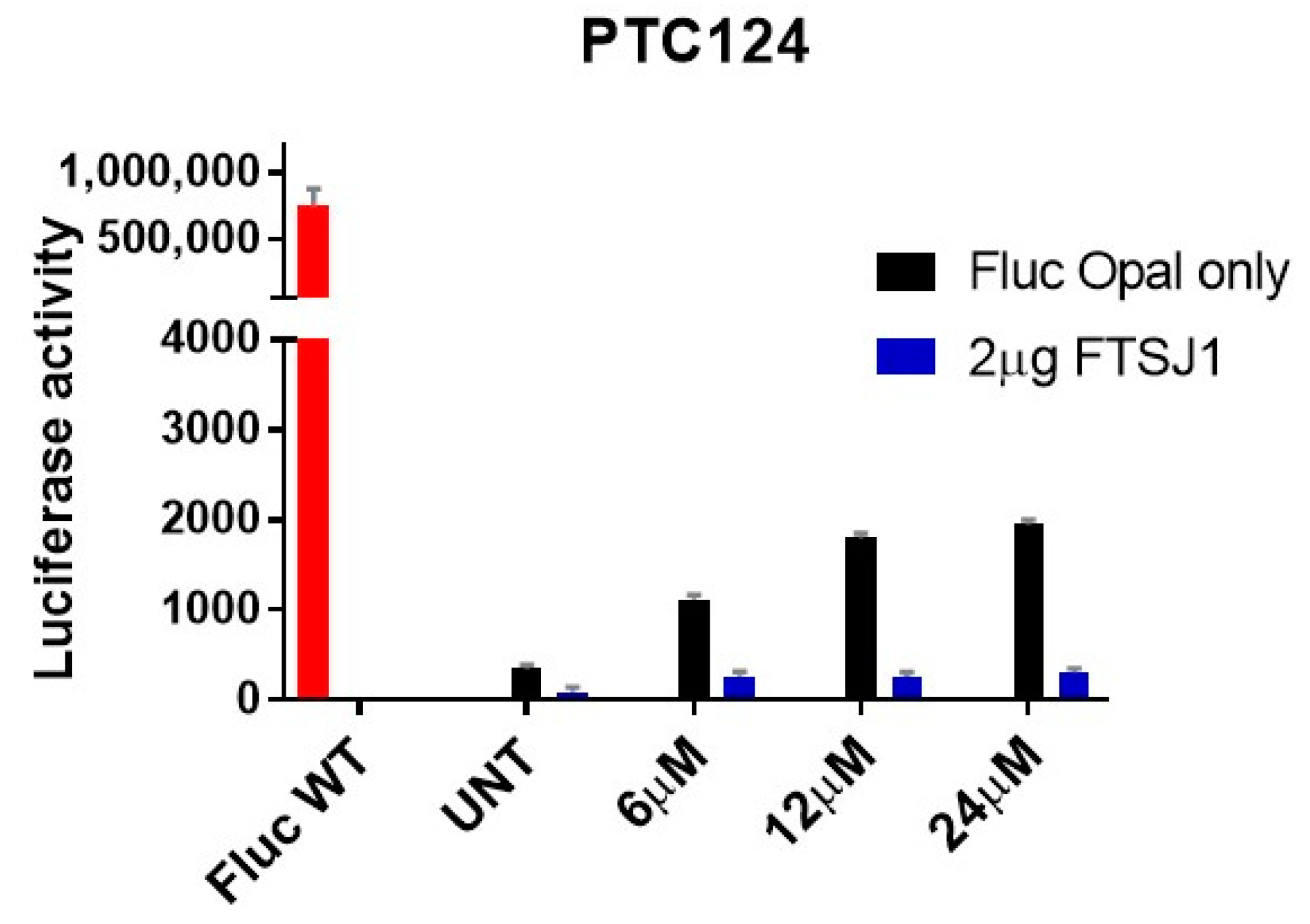
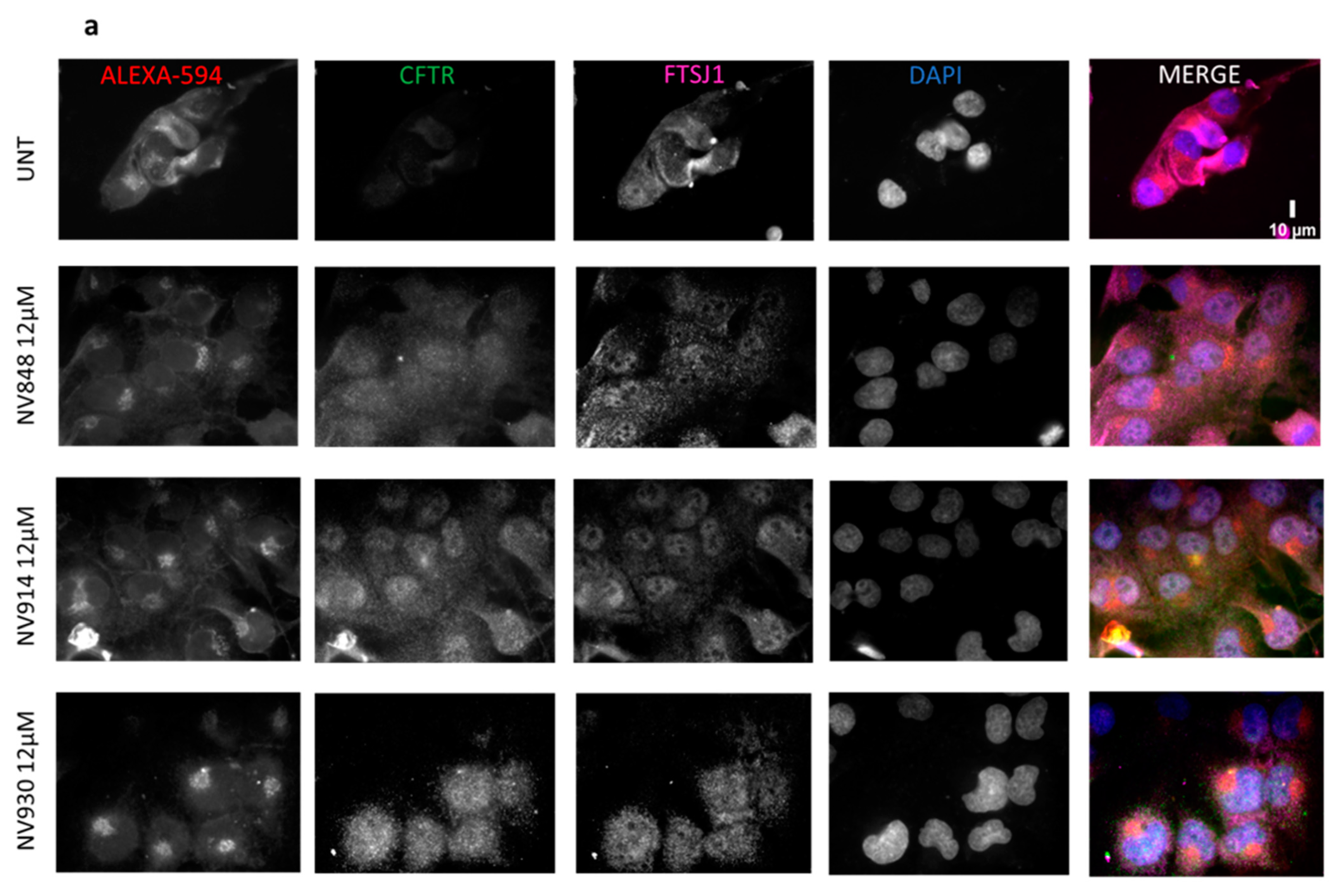
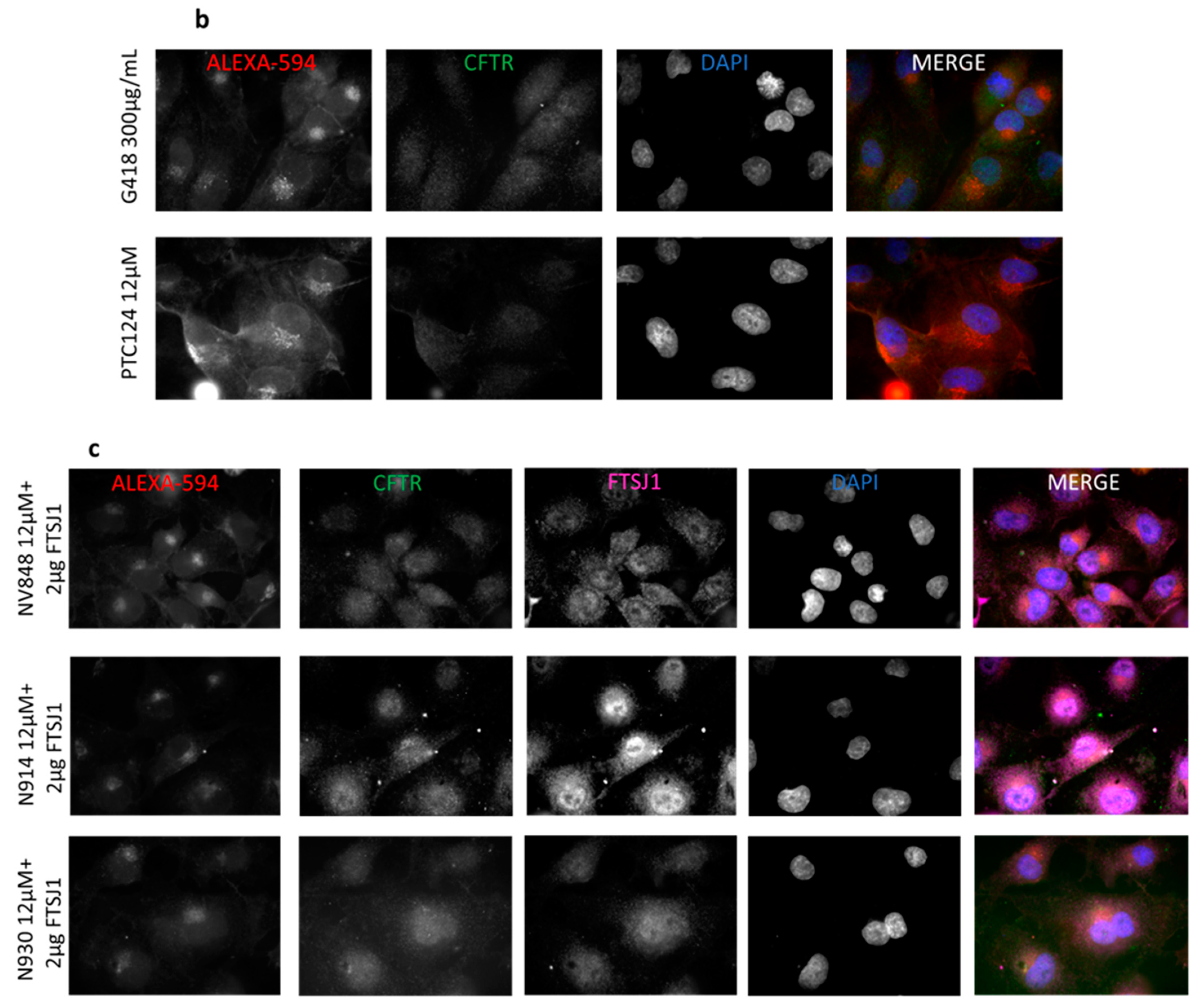
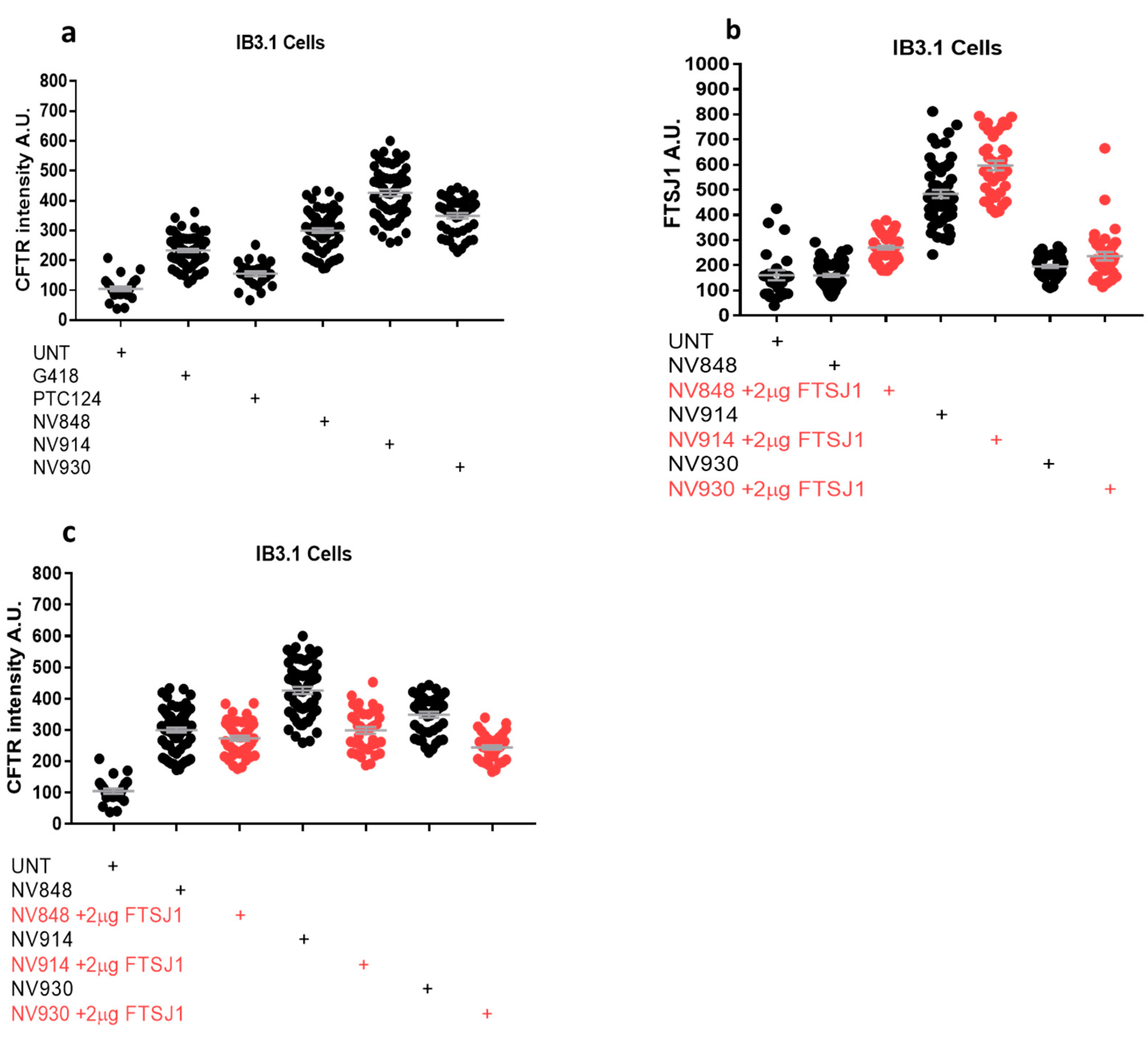
2.4. In Vitro Analysis
3. Materials and Methods
3.1. Homology Modeling and Protein Preparation
3.2. Blind Docking and Semi-Flexible Docking
3.3. Molecular Dynamics and MM-GBSA Calculations
3.4. Cell Culture and NVs Resuspension
3.5. Luminescence Assay
3.6. Immunofluorescence Microscopy
3.7. Image Analysis of Immunofluorescence Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crick, F. Central Dogma of Molecular Biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Lejeune, F. Deciphering the Molecular Mechanism of Stop Codon Readthrough. Biol. Rev. 2021, 96, 310–329. [Google Scholar] [CrossRef]
- Freitag, J.; Ast, J.; Bölker, M. Cryptic Peroxisomal Targeting via Alternative Splicing and Stop Codon Read-through in Fungi. Nature 2012, 485, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.G.; Foo, C.K.; Belletier, N.G.; Gavis, E.R.; Weissman, J.S. Ribosome Profiling Reveals Pervasive and Regulated Stop Codon Readthrough in Drosophila Melanogaster. Elife 2013, 2013, e01179. [Google Scholar] [CrossRef] [PubMed]
- Loughran, G.; Chou, M.Y.; Ivanov, I.P.; Jungreis, I.; Kellis, M.; Kiran, A.M.; Baranov, P.V.; Atkins, J.F. Evidence of Efficient Stop Codon Readthrough in Four Mammalian Genes. Nucleic Acids Res. 2014, 42, 8928–8938. [Google Scholar] [CrossRef]
- Rajon, E.; Masel, J. Evolution of Molecular Error Rates and the Consequences for Evolvability. Proc. Natl. Acad. Sci. USA 2011, 108, 1082–1087. [Google Scholar] [CrossRef]
- Fearon, K.; McClendon, V.; Bonetti, B.; Bedwell, D.M. Premature Translation Termination Mutations Are Efficiently Suppressed in a Highly Conserved Region of Yeast Ste6p, a Member of the ATP-Binding Cassette (ABC) Transporter Family. J. Biol. Chem. 1994, 269, 17802–17808. [Google Scholar] [CrossRef]
- Campofelice, A.; Lentini, L.; Di Leonardo, A.; Melfi, R.; Tutone, M.; Pace, A.; Pibiri, I. Strategies against Nonsense: Oxadiazoles as Translational Readthrough-Inducing Drugs (TRIDs). Int. J. Mol. Sci. 2019, 20, 3329. [Google Scholar] [CrossRef]
- Spelier, S.; van Doorn, E.P.M.; van der Ent, C.K.; Beekman, J.M.; Koppens, M.A.J. Readthrough Compounds for Nonsense Mutations: Bridging the Translational Gap. Trends Mol. Med. 2023, 29, 297–314. [Google Scholar] [CrossRef]
- Pibiri, I.; Lentini, L.; Tutone, M.; Melfi, R.; Pace, A.; Di Leonardo, A. Exploring the Readthrough of Nonsense Mutations by Non-Acidic Ataluren Analogues Selected by Ligand-Based Virtual Screening. Eur. J. Med. Chem. 2016, 122, 429–435. [Google Scholar] [CrossRef]
- Pibiri, I.; Lentini, L.; Melfi, R.; Tutone, M.; Baldassano, S.; Ricco Galluzzo, P.; Di Leonardo, A.; Pace, A. Rescuing the CFTR Protein Function: Introducing 1,3,4-Oxadiazoles as Translational Readthrough Inducing Drugs. Eur. J. Med. Chem. 2018, 159, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Tutone, M.; Pibiri, I.; Lentini, L.; Pace, A.; Almerico, A.M. Deciphering the Nonsense Readthrough Mechanism of Action of Ataluren: An In Silico Compared Study. ACS Med. Chem. Lett. 2019, 10, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Tutone, M.; Pibiri, I.; Perriera, R.; Campofelice, A.; Culletta, G.; Melfi, R.; Pace, A.; Almerico, A.M.; Lentini, L. Pharmacophore-Based Design of New Chemical Scaffolds as Translational Readthrough-Inducing Drugs (TRIDs). ACS Med. Chem. Lett. 2020, 11, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Melfi, R.; Tutone, M.; Di Leonardo, A.; Pace, A.; Lentini, L. Targeting Nonsense: Optimization of 1,2,4-Oxadiazole Trids to Rescue Cftr Expression and Functionality in Cystic Fibrosis Cell Model Systems. Int. J. Mol. Sci. 2020, 21, 6420. [Google Scholar] [CrossRef]
- Corrao, F.; Zizzo, M.G.; Tutone, M.; Melfi, R.; Fiduccia, I.; Carollo, P.S.; Leonardo, A.D.; Caldara, G.; Perriera, R.; Pace, A.; et al. Nonsense Codons Suppression. An Acute Toxicity Study of Three Optimized TRIDs in Murine Model, Safety and Tolerability Evaluation. Biomed. Pharmacother. 2022, 156, 113886. [Google Scholar] [CrossRef]
- Ng, M.Y.; Zhang, H.; Weil, A.; Singh, V.; Jamiolkowski, R.; Baradaran-Heravi, A.; Roberge, M.; Jacobson, A.; Friesen, W.; Welch, E.; et al. New in Vitro Assay Measuring Direct Interaction of Nonsense Suppressors with the Eukaryotic Protein Synthesis Machinery. ACS Med. Chem. Lett. 2018, 9, 1285–1291. [Google Scholar] [CrossRef]
- Trzaska, C.; Amand, S.; Bailly, C.; Leroy, C.; Marchand, V.; Duvernois-Berthet, E.; Saliou, J.M.; Benhabiles, H.; Werkmeister, E.; Chassat, T.; et al. 2,6-Diaminopurine as a Highly Potent Corrector of UGA Nonsense Mutations. Nat. Commun. 2020, 11, 1509. [Google Scholar] [CrossRef]
- Zeitlin, P.L.; Lu, L.; Rhim, J.; Cutting, G.; Stetten, G.; Kieffer, K.A.; Craig, R.; Guggino, W.B. A Cystic Fibrosis Bronchial Epithelial Cell Line: Immortalization by Adeno-12-SV40 Infection. Am. J. Respir. Cell. Mol. Biol. 1991, 4, 313–319. [Google Scholar] [CrossRef]
- Lentini, L.; Melfi, R.; Di Leonardo, A.; Spinello, A.; Barone, G.; Pace, A.; Palumbo Piccionello, A.; Pibiri, I. Toward a Rationale for the PTC124 (Ataluren) Promoted Readthrough of Premature Stop Codons: A Computational Approach and GFP-Reporter Cell-Based Assay. Mol. Pharm. 2014, 11, 653–664. [Google Scholar] [CrossRef]
- Welch, E.M.; Barton, E.R.; Zhuo, J.; Tomizawa, Y.; Friesen, W.J.; Trifillis, P.; Paushkin, S.; Patel, M.; Trotta, C.R.; Hwang, S.; et al. PTC124 Targets Genetic Disorders Caused by Nonsense Mutations. Nature 2007, 447, 87–91. [Google Scholar] [CrossRef]
- Auld, D.S.; Thorne, N.; Maguire, W.F.; Inglese, J. Mechanism of PTC124 Activity in Cell-Based Luciferase Assays of Nonsense Codon Suppression. Proc. Natl. Acad. Sci. USA 2009, 106, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Lentini, L.; Melfi, R.; Gallucci, G.; Pace, A.; Spinello, A.; Barone, G.; Di Leonardo, A. Enhancement of Premature Stop Codon Readthrough in the CFTR Gene by Ataluren (PTC124) Derivatives. Eur. J. Med. Chem. 2015, 101, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Lentini, L.; Melfi, R.; Cancemi, P.; Pibiri, I.; Di Leonardo, A. Caffeine Boosts Ataluren’s Readthrough Activity. Heliyon 2019, 5, e01963. [Google Scholar] [CrossRef]
- Schrödinger Maestro|Schrödinger. Schrödinger Release 2018-2, Version 11.8; Schrödinger, LLC: New York, NY, USA, 2018. [Google Scholar]
- Pérez-Sánchez, H.; Thirumal Kumar, D.; George Priya Doss, C.; Rodríguez-Schmidt, R.; Cerón-Carrasco, J.P.; Peña-García, J.; Ye, Z.W.; Yuan, S.; Günther, S. Prediction and Characterization of Influenza Virus Polymerase Inhibitors through Blind Docking and Ligand Based Virtual Screening. J. Mol. Liq. 2021, 321, 114784. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead Finder: An Approach to Improve Accuracy of Protein-Ligand Docking, Binding Energy Estimation, and Virtual Screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- D.E. Shaw Research. Schrödinger Release 2020-4: Desmond Molecular Dynamics System; Maestro-Desmond Interoperability Tools, Schrödinger: New York, NY, USA, 2020. [Google Scholar]
- Allegra, M.; Tutone, M.; Tesoriere, L.; Attanzio, A.; Culletta, G.; Almerico, A.M. Evaluation of the IKKβ Binding of Indicaxanthin by Induced-Fit Docking, Binding Pose Metadynamics, and Molecular Dynamics. Front. Pharmacol. 2021, 12, 701568. [Google Scholar] [CrossRef]
- Culletta, G.; Allegra, M.; Almerico, A.M.; Restivo, I.; Tutone, M. In Silico Design, Synthesis and Biological Evaluation of Anticancer Arylsulfonamide Endowed with Anti-Telomerase Activity. Pharmaceuticals 2022, 15, 82. [Google Scholar] [CrossRef]
- Culletta, G.; Zappalà, M.; Ettari, R.; Almerico, A.M.; Tutone, M. Immunoproteasome and Non-Covalent Inhibition: Exploration by Advanced Molecular Dynamics and Docking Methods. Molecules 2021, 26, 4046. [Google Scholar] [CrossRef]
- Tutone, M.; Virzì, A.; Almerico, A.M. Reverse Screening on Indicaxanthin from Opuntia Ficus-Indica as Natural Chemoactive and Chemopreventive Agent. J. Theor. Biol. 2018, 455, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA Methods to Estimate Ligand-Binding Affinities. Expert. Opin. Drug. Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A Hierarchical Approach to All-Atom Protein Loop Prediction. Proteins: Struct. Funct. Genet. 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.; Spelier, S.; Essonghe, N.C.; Poix, V.; Kong, R.; Gizzi, P.; Bourban, C.; Amand, S.; Bailly, C.; Guilbert, R.; et al. Use of 2,6-Diaminopurine as a Potent Suppressor of UGA Premature Stop Codons in Cystic Fibrosis. Mol. Ther. 2023, 31, 970–985. [Google Scholar] [CrossRef]
| Docking | MD | |||||||
|---|---|---|---|---|---|---|---|---|
| Replica 1 | Replica 2 | Replica 3 | ||||||
| Cpd * | Residue | Int. ** | Residue | Int. ** | Residue | Int. ** | Residue | Int. ** |
| DAP | Trp55 | Pi–pi | Asp116 | HB | Asp116 | HB | Asp116 | HB |
| Lys156 | Pi–cation | Trp55 | HPhob | Trp55 | HPhob | Trp55 | HPhob | |
| Asp116 | vdW | Asp47 | HB | Asp47 | HB | Asp47 | HB | |
| Asp47 | vdW | Leu48 | HPhob | Leu48 | HPhob | Leu48 | HPhob | |
| Gly53 | HB | Gly53 | HB | |||||
| Ser54 | HB | |||||||
| NV848 | Trp55 | HB, pi–cation | Trp55 | HB, HPhob | Asp47 | WB, HPhob | Lys28 | HB, ionic |
| Gly53 | vdW | Gly53 | HB | Leu48 | HB, HPhob, and Ionic | Gly53 | HB | |
| Ser54 | vdW | Ser54 | HB | Gly53 | HB | Ser54 | HB | |
| Asp116 | vdW | Asp116 | WB | Ser54 | HB, Hphobic | Trp55 | HB, Hphob | |
| Cys115 | WB, HB | Asp116 | HB, WB | |||||
| Asp116 | WB, HB | Lys156 | Hphob | |||||
| NV914 | Asp75 | vdW | Cys49 | HB, Hphob | Cys49 | HB, Hphob | Cys49 | WB, HB |
| Asp116 | vdW | Leu76 | HB, HPhob | Leu76 | HPhob | Asp91 | WB | |
| Lys156 | vdW | Asp91 | WB | Ile92 | HPhob, WB | Ala118 | HB, WB | |
| Ala118 | vdW, HB | Ala118 | HB, WB | Ala118 | HB, WB | Asp120 | HB, WB | |
| Cys49 | vdW | |||||||
| NV930 | Asp75 | vdW | Leu48 | HPhob | Asp91 | HB, Hphobic. WB | Ile92 | Hphobic, WB |
| Ala118 | HB, vdW | Cys49 | HB, HPhob | Ile92 | WB | Ala118 | HB, Hphobic, and WB | |
| Lys156 | vdW | Val74 | HPhob | Tyr130 | Hphobic | Leu135 | Hphobic | |
| Asp116 | vdW | Asp75 | WB | Gln134 | HB, WB | Ala139 | ||
| Cys49 | vdW | Ile92 | HB, HPhob | Leu135 | Hphobic | |||
| Leu135 | vdW | Leu135 | HPhob | Tyr218 | Hphobic, WB | |||
| Ile142 | HPhob | |||||||
| PTC124 | Ser25 | HB, vdW | Arg24 | HB, WB | Arg24 | HB, WB | Ser25 | HB, WB |
| Arg24 | Salt bridge | Ser25 | HB, WB | Ser25 | HB, WB | Arg24 | HB, WB | |
| Lys28 | Salt bridge | Pro52 | Hphobic | Lys28 | HB, WB, Ionic, and Hphobic | Lys28 | HB, WB, and Hphobic | |
| Lys156 | Pi-cation | Trp55 | Hphobic | Trp55 | Hphobic | Trp55 | Hphobic | |
| Cys49 | vdW | Ala118 | Hphobic | Lys156 | HB, Hphobic, ionic, and WB | Lys156 | Hphobic, ionic, and WB | |
| Pro52 | vdW | Arg186 | HB, ionic, and WB | Arg186 | HB, ionic, and WB | |||
| Gly53 | vdW | |||||||
| Trp55 | vdW | |||||||
| Arg186 | Salt bridge | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carollo, P.S.; Tutone, M.; Culletta, G.; Fiduccia, I.; Corrao, F.; Pibiri, I.; Di Leonardo, A.; Zizzo, M.G.; Melfi, R.; Pace, A.; et al. Investigating the Inhibition of FTSJ1, a Tryptophan tRNA-Specific 2′-O-Methyltransferase by NV TRIDs, as a Mechanism of Readthrough in Nonsense Mutated CFTR. Int. J. Mol. Sci. 2023, 24, 9609. https://doi.org/10.3390/ijms24119609
Carollo PS, Tutone M, Culletta G, Fiduccia I, Corrao F, Pibiri I, Di Leonardo A, Zizzo MG, Melfi R, Pace A, et al. Investigating the Inhibition of FTSJ1, a Tryptophan tRNA-Specific 2′-O-Methyltransferase by NV TRIDs, as a Mechanism of Readthrough in Nonsense Mutated CFTR. International Journal of Molecular Sciences. 2023; 24(11):9609. https://doi.org/10.3390/ijms24119609
Chicago/Turabian StyleCarollo, Pietro Salvatore, Marco Tutone, Giulia Culletta, Ignazio Fiduccia, Federica Corrao, Ivana Pibiri, Aldo Di Leonardo, Maria Grazia Zizzo, Raffaella Melfi, Andrea Pace, and et al. 2023. "Investigating the Inhibition of FTSJ1, a Tryptophan tRNA-Specific 2′-O-Methyltransferase by NV TRIDs, as a Mechanism of Readthrough in Nonsense Mutated CFTR" International Journal of Molecular Sciences 24, no. 11: 9609. https://doi.org/10.3390/ijms24119609
APA StyleCarollo, P. S., Tutone, M., Culletta, G., Fiduccia, I., Corrao, F., Pibiri, I., Di Leonardo, A., Zizzo, M. G., Melfi, R., Pace, A., Almerico, A. M., & Lentini, L. (2023). Investigating the Inhibition of FTSJ1, a Tryptophan tRNA-Specific 2′-O-Methyltransferase by NV TRIDs, as a Mechanism of Readthrough in Nonsense Mutated CFTR. International Journal of Molecular Sciences, 24(11), 9609. https://doi.org/10.3390/ijms24119609










