Abstract
Methotrexate (MTX) is a folic acid analog and has been used to treat a wide variety of malignant and non-malignant diseases. The wide use of these substances has led to the continuous discharge of the parent compound and its metabolites in wastewater. In conventional wastewater treatment plants, the removal or degradation of drugs is not complete. In order to study the MTX degradation by photolysis and photocatalysis processes, two reactors were used with TiO2 as a catalyst and UV-C lamps as a radiation source. H2O2 addition was also studied (absence and 3 mM/L), and different initial pHs (3.5, 7, and 9.5) were tested to define the best degradation parameters. Results were analyzed by means of ANOVA and the Tukey test. Results show that photolysis in acidic conditions with 3 mM of H2O2 added is the best condition for MTX degradation in these reactors, with a kinetic constant of 0.028 min−1. According to the ANOVA test, all considered factors (process, pH, H2O2 addition, and experimentation time) caused statistically significant differences in the MTX degradation results.
1. Introduction
The Environmental Protection Agency (EPA) defines emerging pollutants (EPs) as new types of chemical compounds that are not regulated and whose impact is not fully understood [1,2]. These persistent chemical compounds are not monitored in the environment, but they can produce adverse health effects [3]. Due to the above, the NORMAN database was created to monitor and bio-monitor emerging substances in the environment, aimed at laboratories, research centers, and organizations specialized in monitoring EPs [4]. The NORMAN database has identified more than 700 compounds that have been classified into various groups such as drugs, personal care products, disinfectants, detergents, pesticides, hormones, and drugs, among others [2,3,4].
One of the groups of EPs that has gained great importance are drugs that are widely used in medicine (human and veterinary) and aquaculture. The wide use of these substances has led to the continuous discharge of the parent compound and its metabolites in wastewater [5]. In conventional wastewater treatment plants (PTAR), the removal or degradation of drugs is not complete, and concentrations of 0.008 to 55.78 μg/L have been detected. The accumulation of these compounds can cause reproductive problems and the inhibition of cell division in aquatic or terrestrial organisms that come into contact with them [6].
Drugs for the treatment of cancer, also called cytostatic drugs (CD), represent a worrying health risk due to the increase in their demand due to the increase in cases of patients with this condition. Its carcinogenic, genotoxic, mutagenic, and cytotoxic characteristics at low concentrations, together with its low biodegradability, have aroused the interest of researchers to monitor its presence in the environment and its toxic potential [7,8,9,10].
Methotrexate (MTX) is a folic acid analog [11] and has been used to treat a wide variety of malignant and non-malignant diseases [12]. MTX was originally produced as an anticancer drug, and its ability to prevent cell proliferation has been tested in various types of cancer, such as leukemia, osteosarcoma, lymphoma, breast, and bladder cancer, and has even been tested in the treatment of some brain tumors [11,12,13,14]. The initial doses for the treatment of various types of cancer in adults administered in Mexico are 50 mg/m2 of body surface by the intravenous or intramuscular route and from 5 to 10 mg/m2 of body surface by the intrathecal route [15,16]. In some European countries, the United Kingdom, and the United States of America, doses can range from 20 to 40 mg/m2/day administered orally [17]. In addition to its chemotherapeutic use, MTX has anti-inflammatory properties, which is why it is used in the treatment of skin and joint disorders, especially moderate psoriasis and rheumatoid arthritis [18,19], where the doses used to treat these conditions are 30 mg/week and 20 mg/week, respectively [20,21].
It is well known that MTX is not completely metabolized once it is consumed, and up to 90% of the parent compound can be excreted through feces and urine after 24 h. Being a compound resistant to biodegradation, it can enter the water cycle through domestic and hospital wastewater, and its presence in drinking water has even been reported [8,9,22]. Due to its characteristics and its wide use in different conditions in high doses, the presence and degradation of the cytostatic drug MTX present in residual water are extremely important.
The most promising and popular technique for degrading recalcitrant contaminants of pharmaceutical origin is heterogeneous photocatalysis (HPC), due to its ability to produce hydroxyl radicals (∙OH) from the photocatalyst involved in the process. ∙OH are highly oxidizing agents (E° = 2.8 eV) that can carry contaminants to mineralization, producing harmless compounds such as CO2 and H2O [23,24,25,26]. The photocatalyst is the central part of the photocatalytic process, and most are metallic oxides such as TiO2, which is the most commonly used in environmental applications because it is biologically inert and resistant to chemical corrosion; it is also low cost, has a good ability to absorb light, and can be used at room temperature and pressure [27,28].
HPC can be defined as a series of simultaneous redox reactions on the surface of the photocatalyst. When irradiated with UV light with an energy equal to or greater than the bandgap, valence band electrons pass to the conduction band () leaving an empty area with a positive charge known as a hole (). While reduces dissolved oxygen in the solution to produce ∙OH, is positive enough to convert water adsorbed on the photocatalyst to generate ∙OH [23,24,25,26].
The degradation of MTX by advanced oxidation processes has been studied in the past, but there is still a need to find an effective, innovative, and cheap method to degrade methotrexate. The objective of this work was to study MTX degradation through the application of photolysis and heterogeneous photocatalysis processes using UV-C lamps as radiation sources under different operating conditions (acid, neutral, and basic pH). The effect of adding hydrogen peroxide (H2O2) to processes as an oxidizing agent was also studied in order to determine the statistically significant factors that influence the process results.
2. Results and Discussion
Methotrexate is a folic acid analog and has been continually discharged into the environment as it is not considered in common treatments employed by wastewater treatment plants. Results here presented show that photolysis and photocatalysis can be efficiently employed to degrade MTX present in wastewater.
2.1. MTX Photolityc Degradation
Control experiments were performed in the absence of radiation, showing a maximum degradation of less than 1% after 120 min of reaction; therefore, the presence of H2O2 is not sufficient to degrade MTX if UV light is not involved in the process.
In the past, Lai et al. [10] (2017) stated that UV radiation does not break the MTX molecule, coinciding with our results, where photolysis alone showed very low degradation.
The degradation of MTX after two hours of UV radiation in the absence of H2O2 was less than 17% in the three magnitudes of pH evaluated. Figure 1 only shows the experiments to degrade MTX in the presence of H2O2 at a concentration of 3 mM/L, where the best results were obtained at a pH of 3.5, reaching a total degradation of 82.64%.
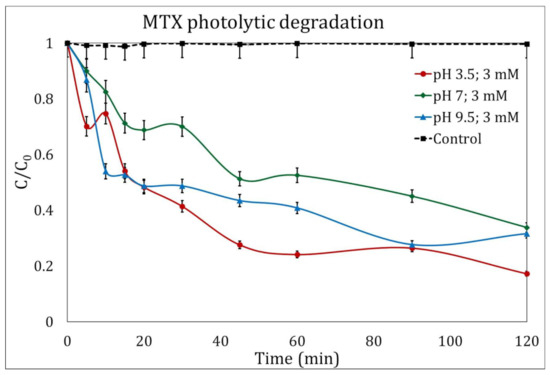
Figure 1.
Photolytic degradation of methotrexate (MTX) in the presence of H2O2 (3 mM/L) at different pH magnitudes: 3.5 (circles), 7 (diamonds), and 9.5 (triangles). The control experiment is represented by the dotted line.
Direct photolysis of molecules is possible without the addition of any reagents, as a 254 nm photon possesses 4.89 eV of energy, enough to produce homolytic or heterolytic breakages of the molecules [29]. Nevertheless, such a reaction is too slow in this case to compete with a UV/H2O2 process.
Kinetic analysis showed kinetic constants of 0.028 min−1, 0.013 min−1 and 0.027 min−1 for the photolysis process when adding 3 mM of peroxide for experiments with initial pH of 3.5, 7 and 9.5, respectively. Calculated kinetic constants for photolytic MTX degradation without H2O2 addition was always lower.
Somensi et al. calculated rate constants for MTX degradation through ozonolysis of 0.3373 min−1 and 0.4163 min−1 through the sonolysis/ozonolysis process, both performed under pH 7 [30].
2.2. MTX Photocatalytic Degradation
Control experiments carried out with the presence of TiO2 and H2O2 in the absence of UV radiation showed a maximum degradation of 1.5% after 120 min of reaction, demonstrating that the presence of the photocatalyst is not enough to degrade MTX. Moreover, MTX does not absorb on the photocatalysts, as stated by Lai et al. [10].
The photocatalysis experiments showed a similar behavior to the photolysis experiments (Section 2.1), and the addition of H2O2 (3 mM/L) greatly improved the efficiency of pollutant degradation. After 120 min of reaction, a maximum of 65.73% was reached at a pH of 3.5 (Figure 2). Similar results were obtained by Espinosa et al. [31], who achieved a 73% degradation of MTX at a pH of 3 and radiation with a wavelength of 254 nm.
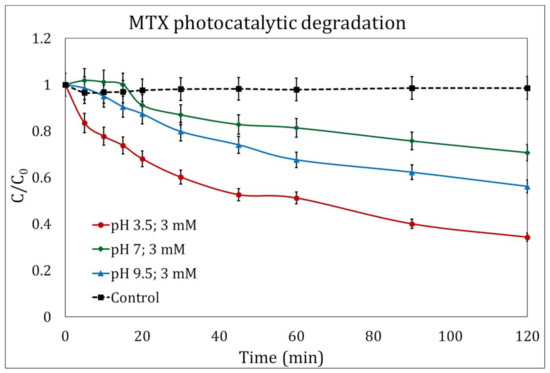
Figure 2.
Photocatalytic degradation of methotrexate (MTX) in the presence of H2O2 (3 mM/L) at different pH magnitudes: 3.5 (circles), 7 (diamonds), and 9.5 (triangles). The control experiment is represented by the dotted line.
Remarkably, photocatalytic kinetic constants were lower than those calculated for the photolytic process, with values for experiments when H2O2 was added of 0.016 min−1, 0.005 min−1, and 0.008 min−1 for initial pH values of 3.5, 7, and 9.5, respectively.
In the past, Alinejad et al. [32] used ozonation processes and catalytic ozonation processes with a nitrate magnesium oxide nano-catalyst to degrade MTX; in such research, acidic and alkaline pH showed better degradation when compared to neutral initial pH. The best results were obtained at a pH near 8, reaching 87% degradation.
2.3. Statistic Analysis
From the degradation data, statistical analysis was performed to find the factors that influence the statistical significance of the degradation response variable.
The following boxplots show the means of degradation with respect to the factors process (Figure 3), pH (Figure 4), presence of hydrogen peroxide (Figure 5), and experimentation time (Figure 6).
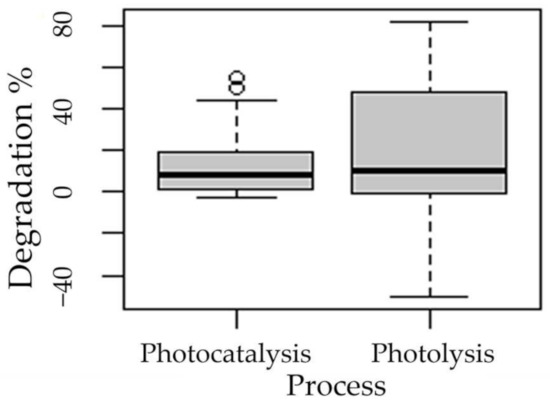
Figure 3.
Boxplot of the degradation of methotrexate with respect to the process factor.
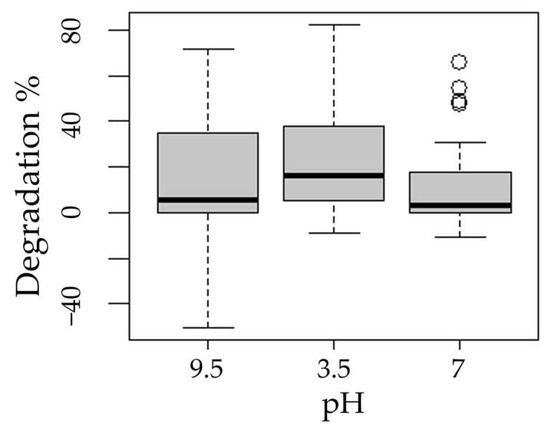
Figure 4.
Boxplot of methotrexate degradation with respect to pH factor.
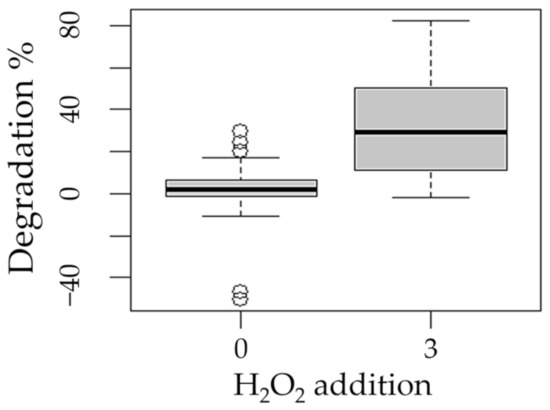
Figure 5.
Boxplot of the degradation of methotrexate with respect to the factor addition of hydrogen peroxide (0 and 3 mM/L).
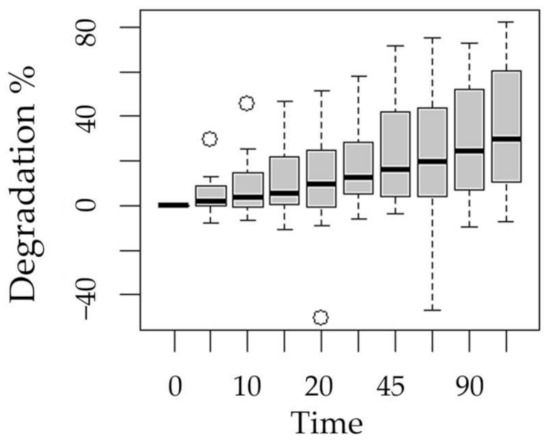
Figure 6.
Boxplot of methotrexate degradation with respect to the experimentation time factor.
Direct irradiation of pollutant-containing water leads to the promotion of a pollutant molecule from the fundamental state to an excited singlet state, which may then intersystem cross to produce triplets. Such excited states can undergo homolysis, heterolysis, or photoionization, among other processes. In most cases, homolytic rupture produces radicals (Equation (1)):
These radicals initiate chain reactions to produce the final, low-weight products; in the presence of oxygen, additional reactions generating the superoxide radical are possible [29].
In the past, Kanjal et al. [33] found MTX photolysis degradation as low as 9% after 30 min of experimentation; in this research, 17% degradation under acidic conditions was achieved.
One of the disadvantages of photocatalysis is the high probability of recombination of the electron hole generated on the surface of the catalyst. Besides, as there is no physical separation between the anodic reaction site (oxidation by holes) and the cathodic one (reduction by electrons), back reactions can be of importance. Low efficiency is one of the most severe limitations of heterogeneous photocatalysis [29]. To avoid such recombination and improve HO∙ generation in situ, oxidative agents, such as H2O2, are added to the reaction [34].
In photolytic processes (UV and UV/H2O2), two degradation pathways are possible: direct photolytic breakage of pollutant and chain reactions started by the breakage of H2O2 in order to form HO∙ as portrayed in Equation (1) (when H2O2 was added). So, both possible degradation pathways compete for available UV radiation.
In photocatalysis (UV/TiO2 and UV/TiO2/H2O2), besides the pathways presented in photolysis, radiation excites the photocatalyst to form the electron hole (Equation (2)):
This fact could explain the results obtained in this study, where calculated reaction constants were higher for photolysis than those for photocatalysis (Section 2.2).
MTX has an extremely low solubility in water [35], is a weak dicarboxylic acid with a molecular weight of 454.5 g/mol and pKa values of 4.7 and 5.5 [36], and thus the isoelectric point for MTX was calculated to be 5.1. Compared with the 6.5 isoelectric point for TiO2, it is clear that the solution’s initial pH yields different results when studied. According to Babyszko et al. [37], the TiO2 surface remains positive under acidic pH, so it was expected that electrostatic repulsion, with both MTX and TiO2 positively charged, would decrease degradation given that heterogeneous photocatalysis is a surface phenomenon, but this was not the case.
The Tukey test applied to the pH factor showed significant differences between the low and high, neutral and high levels, but not in the neutral and low combination. Different effects of solution pH were expected, as, according to Alinejad et al. [32], solution pH is an important parameter given that most semiconductor oxides present amphoteric behavior.
Reguzzoni reported in 2017 [38] that the degradation of MTX with TiO2-P25/UV is not sensitive to pH, even when considering the zero charge point of the photocatalyst, which should favor attraction at low pH. In his research, it was established that the acidic pH hinders the degradation due to the coverage of the MTX on the TiO2 surface, inhibiting the photoactivation of the catalyst. In the present study, acidic pH actually improved photodegradation, so no evidence of such coverage was found.
According to Litter [29], the photochemical process is more efficient in alkaline media because the concentration of the conjugate anion of hydrogen peroxide increases with pH, and this species has a higher absorption coefficient than H2O2, favoring light absorption and increasing HO∙ production. In this study, slightly better results were demonstrated by acidic media.
When HNO3 (added in order to lower the pH of the solution for experiments with an acidic initial pH) dissociates in water, it yields and , which, when excited under radiation, result in the formation of HO∙ [39]. Such a hydroxyl radical formation pathway could explain the better results obtained under acidic pH by posing an advantage over processes where no HNO3 was added.
When irradiated with short UV radiation, H2O2 can form hydroxyl radicals, according to the following equation (Equation (3)) [29,34,40,41]:
So the additional path for hydroxyl radical formation was an important part of the experiments where H2O2 was added.
In UV/H2O2 or UV/TiO2/H2O2 processes, hydroxyl radicals produced by hydrogen peroxide photolysis react with organic pollutants present. When the pollutants are acidic compounds, they may exist in a protonated form (R1H), and in addition to oxidation by hydroxyl radicals, they might be subject to direct photolysis under UV radiation (Equations (4)–(6)) [41]:
Normally, H2O2 addition improves pollutant degradation, but when concentration is too high, it competes with pollutants by forming hydroxyl radicals, slowing down degradation by the formation of less reactive hydroperoxy radicals (Equation (7)) [29,41]:
The statistical analysis showed that all factors considered (process, initial pH, peroxide addition, and experimentation time) were significant for the MXT degradation (p < 0.05) (Table 1).

Table 1.
Results of the ANOVA, considering the degradation of methotrexate as a response variable, and the factors process, pH, addition of peroxide, and time as independent variables.
2.4. Comparison of MTX Degradation, Residual H2O2 and TOC
The residual hydrogen peroxide in the photolysis experiments was 57.5%, while in the photocatalysis processes, the H2O2 consumption rose to 76%.
Figure 7 and Figure 8 show the decrease in the concentration of the oxidizing agent during the duration of the experiments, and it can be observed that the initial concentration of H2O2 for these processes, at the experimental conditions presented here and 120 min of UV radiation, can be reduced to 2.3 mM/L for photocatalysis and 1.8 mM/L for photolysis.
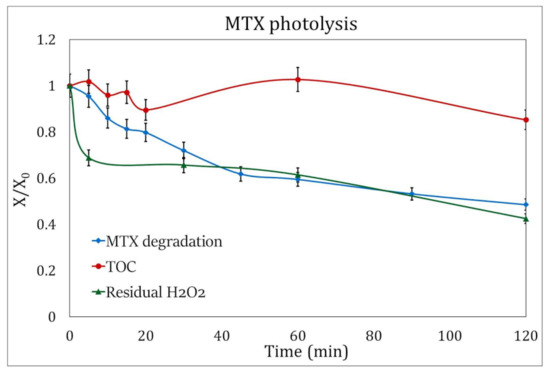
Figure 7.
H2O2 consumption (green triangles) and evolution of TOC (red circles) during the degradation of MTX by photolysis (blue squares). The analysis was done under the best experimental conditions: pH 3.5 and addition of 3 mM H2O2.
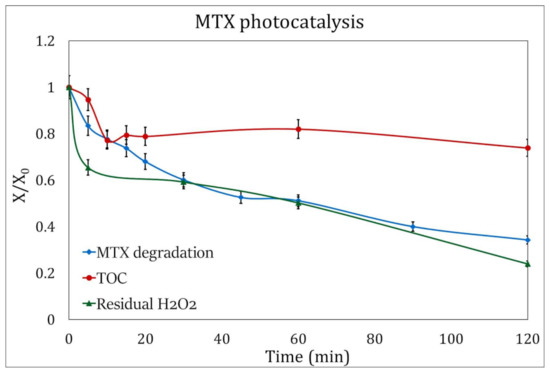
Figure 8.
H2O2 consumption (green triangles) and TOC evolution (red circles) during MTX degradation by photocatalysis (blue squares). The analysis was performed under the best experimental conditions: pH 3.5 and addition of 3 mM H2O2.
The UV lamps used in this study, with an emission peak of 254 nm, do not represent an effective procedure for the total mineralization of pollutants [39], so low TOC removal was to be expected for the experiments.
2.5. MTX Degradation by Products
The analysis carried out by means of HPLC-MS of the samples obtained from the degradation of MTX by photolysis and photocatalysis at a pH of 3.5 and a concentration of 3 mM/L of H2O2 showed the existence of four possible degradation by-products.
There are three main mechanisms involved when a pollutant is attacked by a hydroxyl radical: hydrogen abstraction (generally the first step for acidic pollutants), OH addition or substitution, and electron transfer (Equations (8) and (9)) [29]:
If the target is an aromatic pollutant, the first step is ring hydroxylation, but further HO∙ reactions lead to the opening of the ring and the formation of open conjugated structures [29].
The formation of each by-product from MTX could follow different pathways: a ketonization at the carbon of position 11 produces by-product 1 with a higher molecular weight than the original compound (470.44 g/mol for by-product 1 versus 454.44 for the original MTX molecule). The cleavage at the nitrogen at position 21 removes the carboxylic groups from the molecule, forming by-product 2. The formation of a third and fourth by-product as a result of the cleavage at the nitrogen at position 12.
3. Materials and Methods
3.1. Sample Preparation and Reagents
The samples were produced by dissolving the drug MTX (Trxilem®, Lemery S.A. de C.V., Tlalpan, Mexico City, Mexico) in distilled water up to the desired concentration. For the photolysis experiments, 50 L samples were prepared, while for photocatalysis, the samples had a volume of 25 L, both with a 5 mg/L initial concentration of MTX. H2O2 was obtained from Labbox Labware (CAS: 7722-84-1, Barcelona, Catalonia, Spain). Titanium oxysulfate was purchased from Sigma-Aldrich (CAS: 13825-74-6, St. Louis, MO, USA). The MTX reagent for the calibration curves was purchased from Sigma-Aldrich (CAS: 59-05-2, St. Louis, MO, USA).
3.2. MTX Degradation via Photolysis
The system where the reactor is located consists of a 200 L tank, a pump with a power of 1 hp that allows recirculating the sample through the entire system, and a 50 μm filter. The main part of the system is a stainless-steel compartment that has a low-pressure mercury lamp (radiation peak of 254 nm, T5 Philips, Amsterdam, The Netherlands) as a source of UV radiation. A quartz tube surrounds the UV lamp to prevent it from coming into direct contact with the sample. Both components are in the center of the reactor, and in this way, the radiation is reflected by the polished inner surface of the stainless-steel body of the reactor to the photocatalyst and the sample. For photocatalysis experiments, the reactor has four stainless steel cones that contain a SiO2 mesh where the TiO2 is impregnated. For photolysis processes, as in this study, the cones are removed to avoid the presence of the photocatalyst (Figure 9) [42].
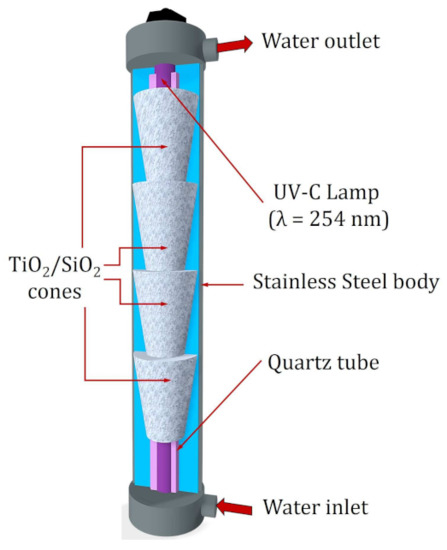
Figure 9.
Components of the reactor used in the degradation of MTX by photolysis. Interior stainless-steel cones with the TiO2/SiO2 mesh were removed for photolysis experiments.
The experiments were carried out at a constant flow rate of 650 L/h. Once the recirculation through the system began, the pH was adjusted with a 65% HNO3 solution or a 0.1 M NaOH solution to obtain the desired magnitudes. (pH: 3.5, 7, and 9.5), which were selected considering the isoelectric point of TiO2 (6.5).
The effect of H2O2 on the degradation of MTX was also analyzed; for this objective, the experiments were carried out either adding 3 mM/L of H2O2 or without H2O2 addition at each magnitude of pH.
After adjusting the conditions in each experimental run (pH and H2O2 dose added when required), the initial sample (0 min) was taken and the lamp was turned on. During the experiments, sampling had the following time distribution: 5, 10, 15, 20, 30, 45, 60, 90, and 120 min, from which the analyses and quantification of MTX degradation were performed.
3.3. MTX Degradation via Photocatalysis
The photocatalysis experiments were carried out, testing the same factors as the photolytic processes: pH levels (3.5, 7, and 9.5) and H2O2 doses (absence and 3 mM/L). Samples were taken at the same experimentation times as established for photolysis. The flow rate was set to 500 L/h for photocatalysis experiments.
The reactor used for photocatalysis was the commercial model AOP1 (Bright-Water Environmental, Harleston, Norfolk, UK). Figure 10 shows the reactor, made up of a titanium cylinder with dimensions of 75 mm in diameter and 475 mm in length, covered on the inside by a layer of TiO2. A 254 nm radiation-emitting lamp was used as a radiation source.
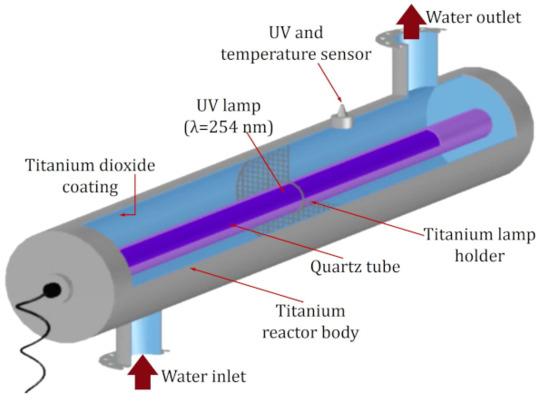
Figure 10.
Internal structure of the AOP1 model reactor, used for the MTX degradation experiments by means of photocatalysis.
3.4. Control Experiments
In order to evaluate the performance of the photolysis and photocatalysis processes in the degradation of MTX, the effect of H2O2 and the TiO2/H2O2 interaction on the contaminant must be ruled out; therefore, control experiments were carried out in the absence of radiation. The solutions were treated as described in Section 3.2 and Section 3.3, adding 3 mM/L H2O2, and the pH was adjusted to 6.5, the same as the TiO2 isoelectric point, also avoiding charge interaction. In these experiments, the lamp was not turned on.
3.5. Design of Experiments and Statistical Analysis
The MTX degradation was investigated as a function of four factors: process, initial pH, and H2O2 addition. Besides, experimentation time was considered as samples were taken at different moments during each experiment (0, 5, 10, 15, 20, 30, 45, 60, 90, or 120 min). The experimental design then consists of a 2 × 3 × 3 factorial design, with sampling at different times and at least 2 duplicates (Table 2).

Table 2.
Experimental parameters and level values for the design of experiments for MTX degradation.
The statistical analysis of the degradation data was performed using the R Studio language. To analyze the data, a multiple ANOVA was used, considering MTX degradation as the response variable and, as factors, the process, the initial pH, the addition of peroxide, and the experimentation time in which the sample was taken (Table 2). In addition, a Tukey test was performed to identify the levels of each factor with significantly different means.
3.6. MTX, Residual H2O2 and TOC Concentration Analysis
Samples were taken directly at the outlet of the solution tank just in connection with the rest of the system and analyzed by UV/vis spectrophotometry (T80+ UV/vis spectrophotometer, PG Instruments Ltd., Alma Park, Leicestershire, UK) at a wavelength of 303 nm to follow the degradation of MTX. The calibration curve was made with the MTX reagent level diluted in deionized water, and an R2 of 0.9996 was obtained.
The consumption of H2O2 during the experiments was measured at times of 5, 30, 60, and 120 min of reaction. The method established by Klamerth [40] was followed to carry out the quantitative analysis of residual H2O2, which is based on adding titanium oxysulfate to the samples, which causes a yellow color that varies in intensity in relation to the H2O2 concentration. The solutions were measured at a wavelength of 410 nm. The calibration curve was developed using H2O2 solutions with concentrations ranging from 0 to 3 mM/L.
The TOC of samples taken at different experimentation times was measured by direct injection of samples filtered with 0.2 mm syringe-driven filters into a Shimadzu 5000A TOC analyzer (Shimadzu Scientific Instruments Inc., Columbia, MD, USA).
3.7. MTX Formation By-Products
Samples at 0 and 120 min were analyzed by HPLC-MS to determine the chemical structure of possible MTX degradation byproducts. For comparison purposes, different equipment was used: (a) an Agilent 1100 HPLC with a UV detector coupled to an Agilent Trap XCT mass spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA); and (b) an HPLC Surveyor MS with an LTQ spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
4. Conclusions
Methotrexate is a folic acid analog and has been continually discharged into the environment as it is not considered in common treatments employed by wastewater treatment plants.
The photolytic degradation of MTX after two hours of UV radiation in the absence of H2O2 was less than 17% in the three magnitudes of pH evaluated. The MTX degradation by photolysis in the presence of 3 mM/L H2O2 showed the best results obtained at a pH of 3.5, reaching a total degradation of 82.64%.
Kinetic analysis showed kinetic constants of 0.028 min−1, 0.013 min−1, and 0.027 min−1 for the photolysis process when adding 3 mM of peroxide for experiments with initial pHs of 3.5, 7, and 9.5, respectively. Calculated kinetic constants for photolytic MTX degradation without H2O2 addition were always lower.
The photocatalysis experiments showed a similar behavior to the photolysis experiments; the addition of H2O2 (3 mM/L) greatly improved the efficiency of pollutant degradation. After 120 min of reaction, a maximum of 65.73% was reached at a pH of 3.5.
Remarkably, photocatalytic kinetic constants were lower than those calculated for the photolytic process, with values for experiments when H2O2 was added of 0.016 min−1, 0.005 min−1, and 0.008 min−1 for initial pH values of 3.5, 7, and 9.5, respectively.
The statistical analysis showed that all factors considered (process, initial pH, peroxide addition, and experimentation time) were significant for MXT degradation (p < 0.05).
The residual hydrogen peroxide for MTX degradation in the photolysis experiments was 57.5%, while in the photocatalysis processes the H2O2 consumption rose to 76%, so at the experimental conditions presented in this work and 120 min of UV radiation, it can be reduced to 2.3 mM/L for photocatalysis and 1.8 mM/L for photolysis.
Author Contributions
Conceptualization, L.A.G.-B. and J.B.P.-N.; formal analysis, L.A.G.-B., J.C.G.-P. and C.M.N.-N.; funding acquisition, J.B.P.-N.; investigation, L.A.G.-B. and J.C.G.-P.; methodology, L.A.G.-B. and J.C.G.-P.; resources, J.B.P.-N.; supervision, J.B.P.-N.; validation, C.M.N.-N. and J.B.P.-N.; visualization, L.A.G.-B. and C.M.N.-N.; writing—original draft, L.A.G.-B.; writing—review and editing, C.M.N.-N. and J.B.P.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Politécnico Nacional, project number SIP: 20200670. The APC was funded by Instituto Politécnico Nacional.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Authors thank Manuel García-Roig, from the University of Salamanca, Spain, for his invaluable support for the realization of the present study and for his collaboration and friendship through the years. The authors would like to acknowledge the Centro de Investigación y Desarrollo Tecnológico del Agua of the University of Salamanca, Spain, for facilitating the use of its facilities to carry out experimentation, and Servicio General de Espectrometría de Masas (SGEM-USAL) for sample analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent Trends in Disposal and Treatment Technologies of Emerging-Pollutants—A Critical Review. TrAC Trends Anal. Chem. 2020, 122, 115744. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging Pollutants in Water Environment: Occurrence, Monitoring, Fate, and Risk Assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Vasilachi, I.; Asiminicesei, D.; Fertu, D.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging Pollutants in the Urban Water Cycle in Latin America: A Review of the Current Literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Moreno Ríos, A.L.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Silva Oliveira, L.F. Pharmaceuticals as Emerging Pollutants: Case Naproxen an Overview. Chemosphere 2022, 291, 132822. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Yukesh Kannah, R.; Parthiba Karthikeyan, O.; Kumar, G.; Kumar Tyagi, V.; Rajesh Banu, J. Algal-Based System for Removal of Emerging Pollutants from Wastewater: A Review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Sarro, M.; Rosato, V.; Aigotti, R.; Baiocchi, C.; Minero, C. Photocatalytic Degradation of Selected Anticancer Drugs and Identification of Their Transformation Products in Water by Liquid Chromatography–High Resolution Mass Spectrometry. J. Chromatogr. A 2014, 1362, 135–144. [Google Scholar] [CrossRef]
- Roig, B.; Marquenet, B.; Delpla, I.; Bessonneau, V.; Sellier, A.; Leder, C.; Thomas, O.; Bolek, R.; Kummerer, K. Monitoring of Methotrexate Chlorination in Water. Water Res. 2014, 57, 67–75. [Google Scholar] [CrossRef]
- Lutterbeck, C.A.; Baginska, E.; Machado, Ê.L.; Kümmerer, K. Removal of the Anti-Cancer Drug Methotrexate from Water by Advanced Oxidation Processes: Aerobic Biodegradation and Toxicity Studies after Treatment. Chemosphere 2015, 141, 290–296. [Google Scholar] [CrossRef]
- Lai, W.W.-P.; Hsu, M.-H.; Lin, A.Y.-C. The Role of Bicarbonate Anions in Methotrexate Degradation via UV/TiO2: Mechanisms, Reactivity and Increased Toxicity. Water Res. 2017, 112, 157–166. [Google Scholar] [CrossRef]
- Attari, E.; Nosrati, H.; Danafar, H.; Kheiri Manjili, H. Methotrexate Anticancer Drug Delivery to Breast Cancer Cell Lines by Iron Oxide Magnetic Based Nanocarrier. J. Biomed. Mater. Res. A 2019, 107, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Y.; Chen, Y.; Hung, Y.; Yiang, G. Anticancer Effects of Methotrexate in Combination with A-tocopherol and A-tocopherol Succinate on Triple-negative Breast Cancer. Oncol. Rep. 2019, 41, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Levêque, D.; Becker, G.; Toussaint, E.; Fornecker, L.-M.; Paillard, C. Clinical Pharmacokinetics of Methotrexate in Oncology. Int. J. Pharmacokinet. 2017, 2, 137–147. [Google Scholar] [CrossRef]
- Consejo de Salubridad General; Comisión Interinstitucional del Cuadro Básico y Catálogo de Insumos del Sector Salud. Cuadro Básico y Catálogo de Medicamentos, 2017th ed.; Consejo de Salubridad General: Ciudad de México, Mexico, 2017. [Google Scholar]
- Instituto Mexicano del Seguro Social. Cuadro Básico de Medicamentos Instituto Mexicano Del Seguro Social; Dirección de Prestaciones Médicas, Unidad de Atención Médica, Coordinación de Unidades de Medicas de Alta Especialidad, Eds.; Instituto Mexicano del Seguro Social: Ciudad de México, Mexico, 2019. [Google Scholar]
- Schmiegelow, K.; Nielsen, S.N.; Frandsen, T.L.; Nersting, J. Mercaptopurine/Methotrexate Maintenance Therapy of Childhood Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2014, 36, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Vena, G.A.; Cassano, N.; Iannone, F. Update on Subcutaneous Methotrexate for Inflammatory Arthritis and Psoriasis. Ther. Clin. Risk Manag. 2018, 14, 105–116. [Google Scholar] [CrossRef]
- Yélamos, O.; Puig, L. Systemic Methotrexate for the Treatment of Psoriasis. Expert Rev. Clin. Immunol. 2015, 11, 553–563. [Google Scholar] [CrossRef]
- Methotrexate: Dosing and Uses, Medscape.com. Available online: https://reference.medscape.com/drug/trexall-otrexup-methotrexate-343201 (accessed on 25 March 2023).
- Methotrexate Dosage, Drugs.com. Available online: https://www.drugs.com/dosage/methotrexate.html#Usual_Adult_Dose_for_Psoriasis (accessed on 25 March 2023).
- Kosjek, T.; Negreira, N.; de Alda, M.L.; Barceló, D. Aerobic Activated Sludge Transformation of Methotrexate: Identification of Biotransformation Products. Chemosphere 2015, 119, S42–S50. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, R.; Choi, H.; Bhattacharjee, C. Involvement of Process Parameters and Various Modes of Application of TiO2 Nanoparticles in Heterogeneous Photocatalysis of Pharmaceutical Wastes—A Short Review. RSC Adv. 2014, 4, 57250–57266. [Google Scholar] [CrossRef]
- Chekir, N.; Tassalit, D.; Benhabiles, O.; Kasbadji Merzouk, N.; Ghenna, M.; Abdessemed, A.; Issaadi, R. A Comparative Study of Tartrazine Degradation Using UV and Solar Fixed Bed Reactors. Int. J. Hydrogen Energy 2017, 42, 8948–8954. [Google Scholar] [CrossRef]
- Lee, C.M.; Palaniandy, P.; Dahlan, I. Pharmaceutical Residues in Aquatic Environment and Water Remediation by TiO2 Heterogeneous Photocatalysis: A Review. Environ. Earth Sci. 2017, 76, 611. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. UV LED Sources for Heterogeneous Photocatalysis. In Environmental Photochemistry Part III; Springer: Berlin/Heidelberg, Germany, 2015; pp. 159–179. [Google Scholar]
- Yasmina, M.; Mourad, K.; Mohammed, S.H.; Khaoula, C. Treatment Heterogeneous Photocatalysis; Factors Influencing the Photocatalytic Degradation by TiO2. Energy Procedia 2014, 50, 559–566. [Google Scholar] [CrossRef]
- Litter, M.I. Introduction to Photochemical Advanced Oxidation Processes for Water Treatment. In Environmental Photochemistry Part II; Springer: Berlin/Heidelberg, Germany, 2005; pp. 325–366. [Google Scholar]
- Somensi, C.A.; Simionatto, E.L.; Dalmarco, J.B.; Gaspareto, P.; Radetski, C.M. A Comparison between Ozonolysis and Sonolysis/Ozonolysis Treatments for the Degradation of the Cytostatic Drugs Methotrexate and Doxorubicin: Kinetic and Efficiency Approaches. J. Environ. Sci. Health Part A 2012, 47, 1543–1550. [Google Scholar] [CrossRef]
- Espinosa, A.; Nélieu, S.; Lieben, P.; Skarbek, C.; Labruère, R.; Benoit, P. Photodegradation of Methotrexate in Aqueous Solution: Degradation Kinetics and Identification of Transformation Products. Environ. Sci. Pollut. Res. 2022, 29, 6060–6071. [Google Scholar] [CrossRef]
- Alinejad, A.; Akbari, H.; Ghaderpoori, M.; Jeihooni, A.K.; Adibzadeh, A. Catalytic Ozonation Process Using a MgO Nano-Catalyst to Degrade Methotrexate from Aqueous Solutions and Cytotoxicity Studies in Human Lung Epithelial Cells (A549) after Treatment. RSC Adv. 2019, 9, 8204–8214. [Google Scholar] [CrossRef]
- Kanjal, M.I.; Muneer, M.; Abdelhaleem, A.; Chu, W. Degradation of Methotrexate by UV/Peroxymonosulfate: Kinetics, Effect of Operational Parameters and Mechanism. Chin. J. Chem. Eng. 2020, 28, 2658–2667. [Google Scholar] [CrossRef]
- Catalkaya, E.C.; Kargi, F. Color, TOC and AOX Removals from Pulp Mill Effluent by Advanced Oxidation Processes: A Comparative Study. J. Hazard. Mater. 2007, 139, 244–253. [Google Scholar] [CrossRef]
- Kasim, N.A.; Whitehouse, M.; Ramachandran, C.; Bermejo, M.; Lennernäs, H.; Hussain, A.S.; Junginger, H.E.; Stavchansky, S.A.; Midha, K.K.; Shah, V.P.; et al. Molecular Properties of WHO Essential Drugs and Provisional Biopharmaceutical Classification. Mol. Pharm. 2004, 1, 85–96. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeong, S.-H.; Lee, Y.-B. Preparation and In Vitro/In Vivo Characterization of Polymeric Nanoparticles Containing Methotrexate to Improve Lymphatic Delivery. Int. J. Mol. Sci. 2019, 20, 3312. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Sadłowski, M.; Kusiak-Nejman, E.; Morawski, A.W. Synthesis and Characterization of SiO2/TiO2 as Photocatalyst on Methylene Blue Degradation. Catalysts 2022, 12, 1372. [Google Scholar] [CrossRef]
- Reguzzoni, G. Degradation of Cytotoxic Compounds by TiO2-UV Photocatalysis; Politecnico de Milano: Milan, Italy, 2017. [Google Scholar]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Klamerth, N. Application of a Solar Photo-Fenton for the Treatment of Contaminants in Municipal Wastewater Effluents. Ph.D. Thesis, University of Almería, Almería, Spain, 2011. [Google Scholar]
- Crittenden, J.C.; Hu, S.; Hand, D.W.; Green, S.A. A Kinetic Model for H2O2/UV Process in a Completely Mixed Batch Reactor. Water Res. 1999, 33, 2315–2328. [Google Scholar] [CrossRef]
- Pantoja-Espinoza, J.C.; Proal-Nájera, J.B.; García-Roig, M.; Cháirez-Hernández, I.; Osorio-Revilla, G.I. Comparative Efficiencies of Coliform Bacteria Inactivation in Municipal Wastewater by Photolysis (UV) and Photocatalysis (UV/TiO2/SiO2). Case: Treatment Wastewater Plant of Salamanca, Spain. Rev. Mex. Ing. Quim. 2015, 14, 119–135. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).