Effectiveness of Combinational Treatments for Alzheimer’s Disease with Human Neural Stem Cells and Microglial Cells Over-Expressing Functional Genes
Abstract
1. Introduction
2. Results
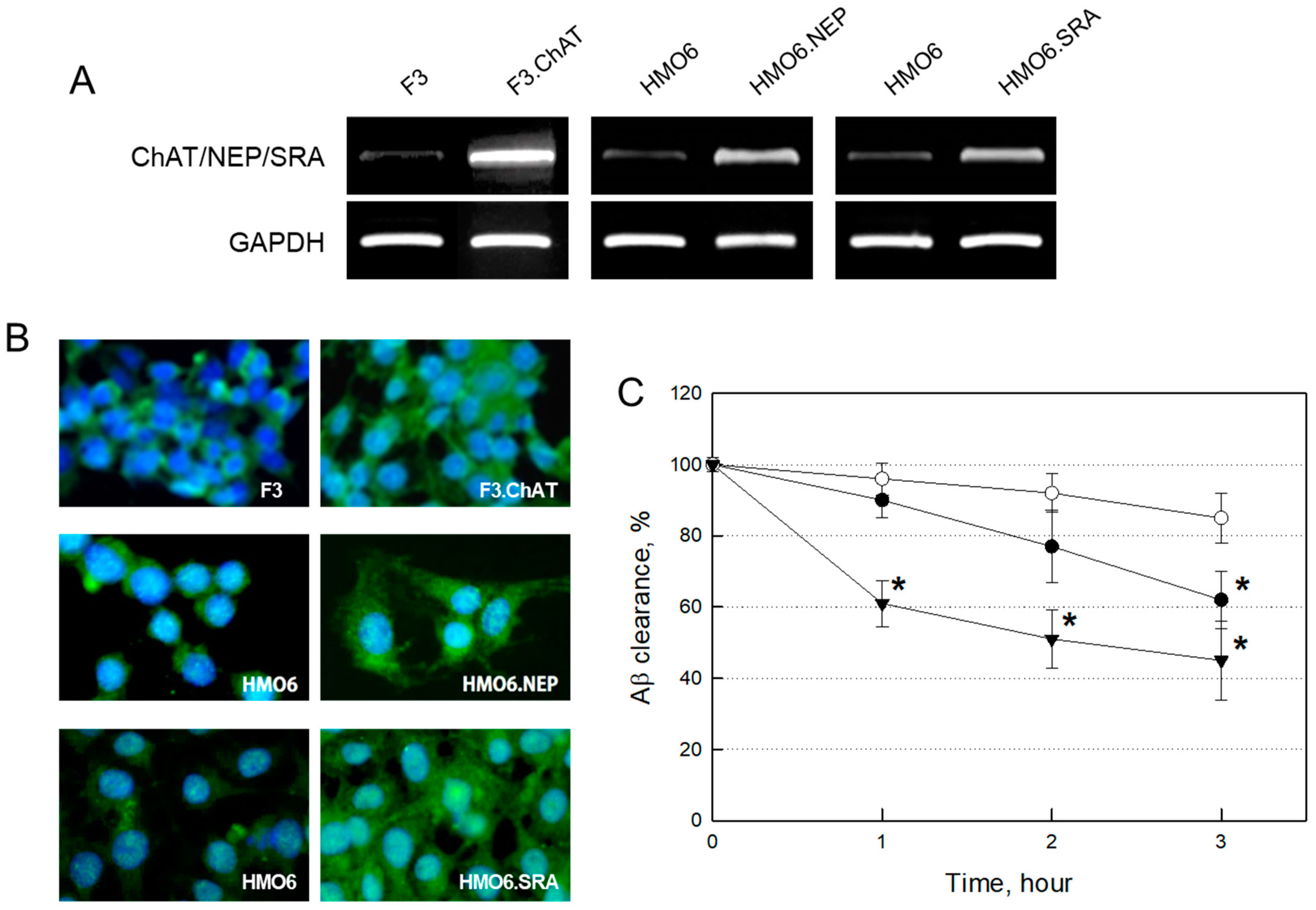
2.1. Establishment of NSCs and Microglial Cells Over-Expressing Functional Genes
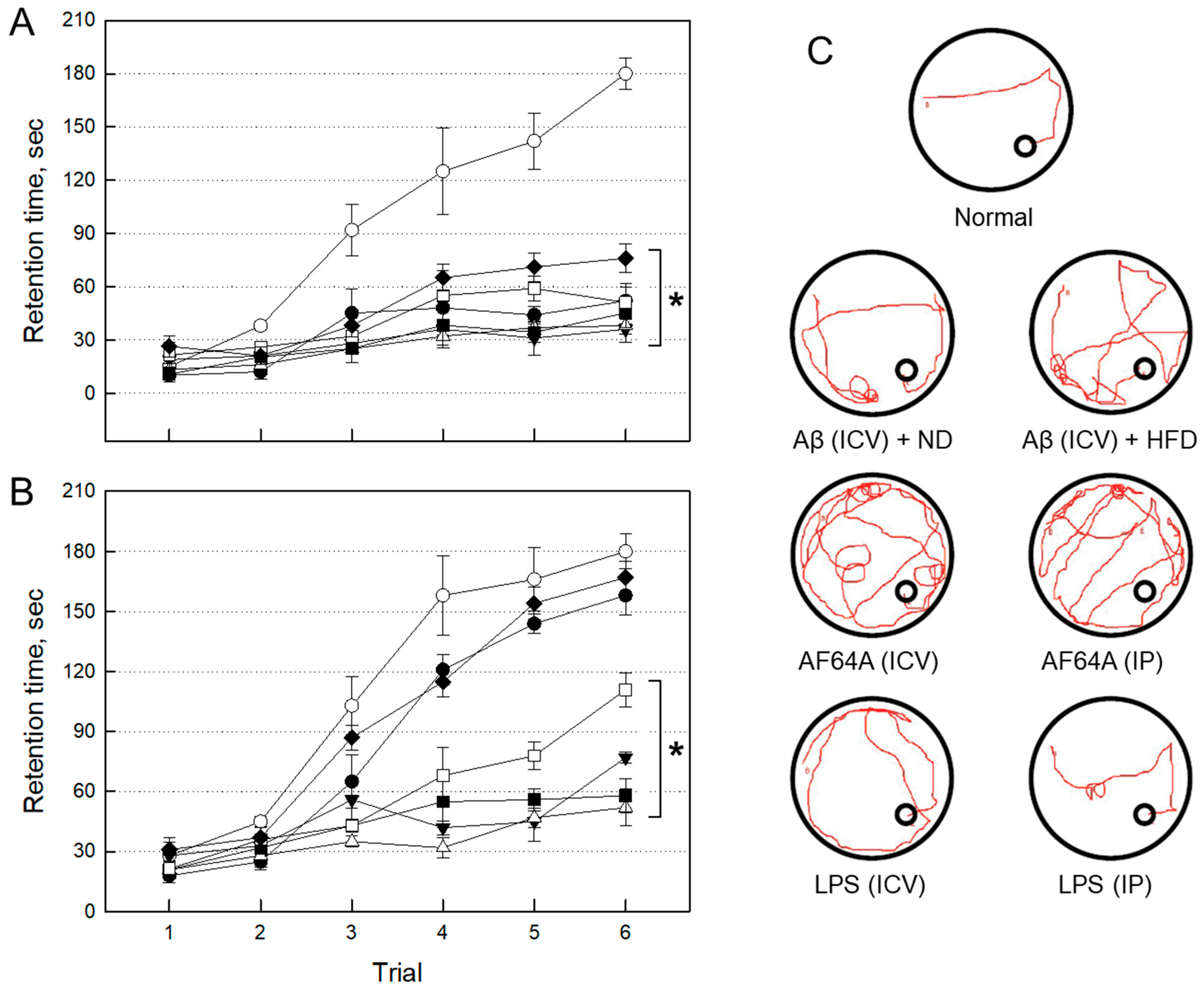
2.2. Establishment of AD Animal Models
2.2.1. Impairment of Cognitive Function by Neurotoxicants
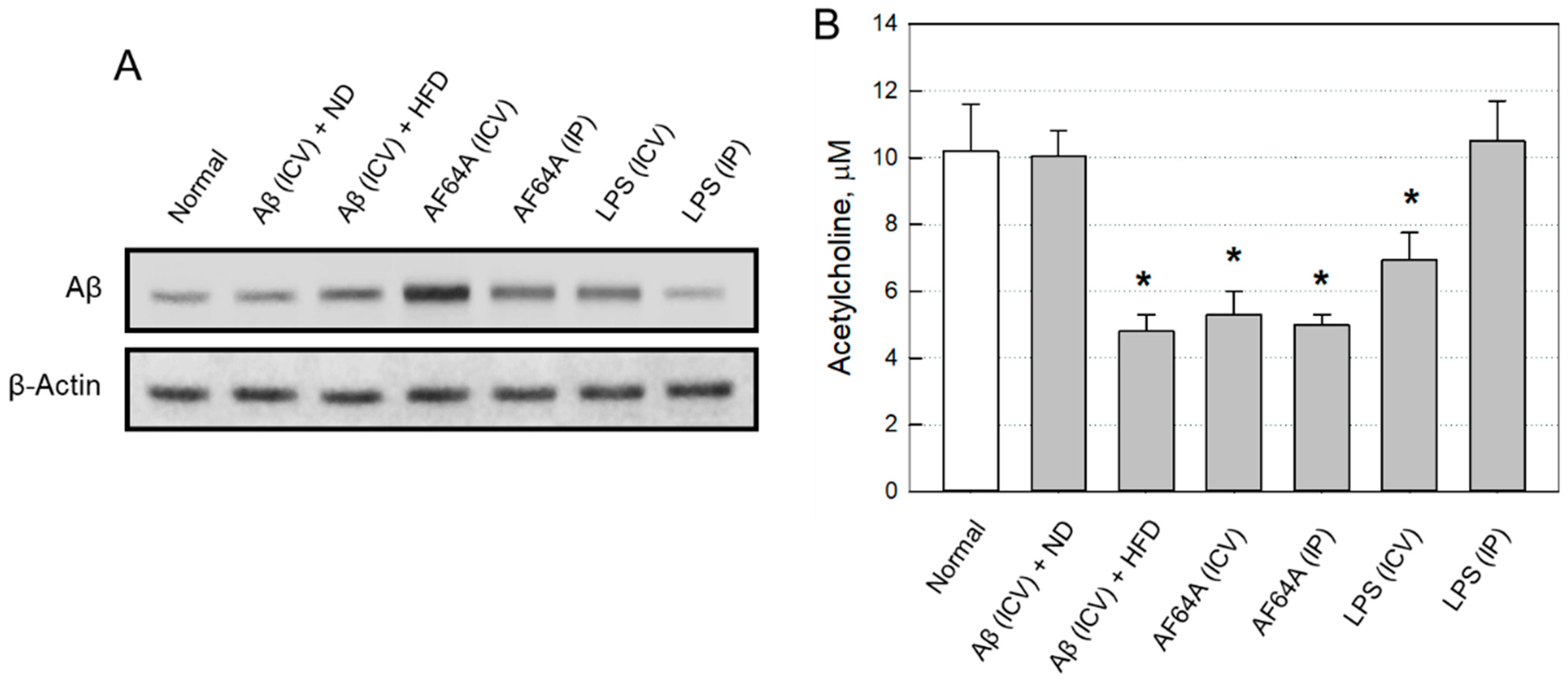
2.2.2. Changes in Brain Aβ and ACh
2.2.3. Selection of an AD Animal Model
2.3. Evaluation of Therapeutic Efficacies
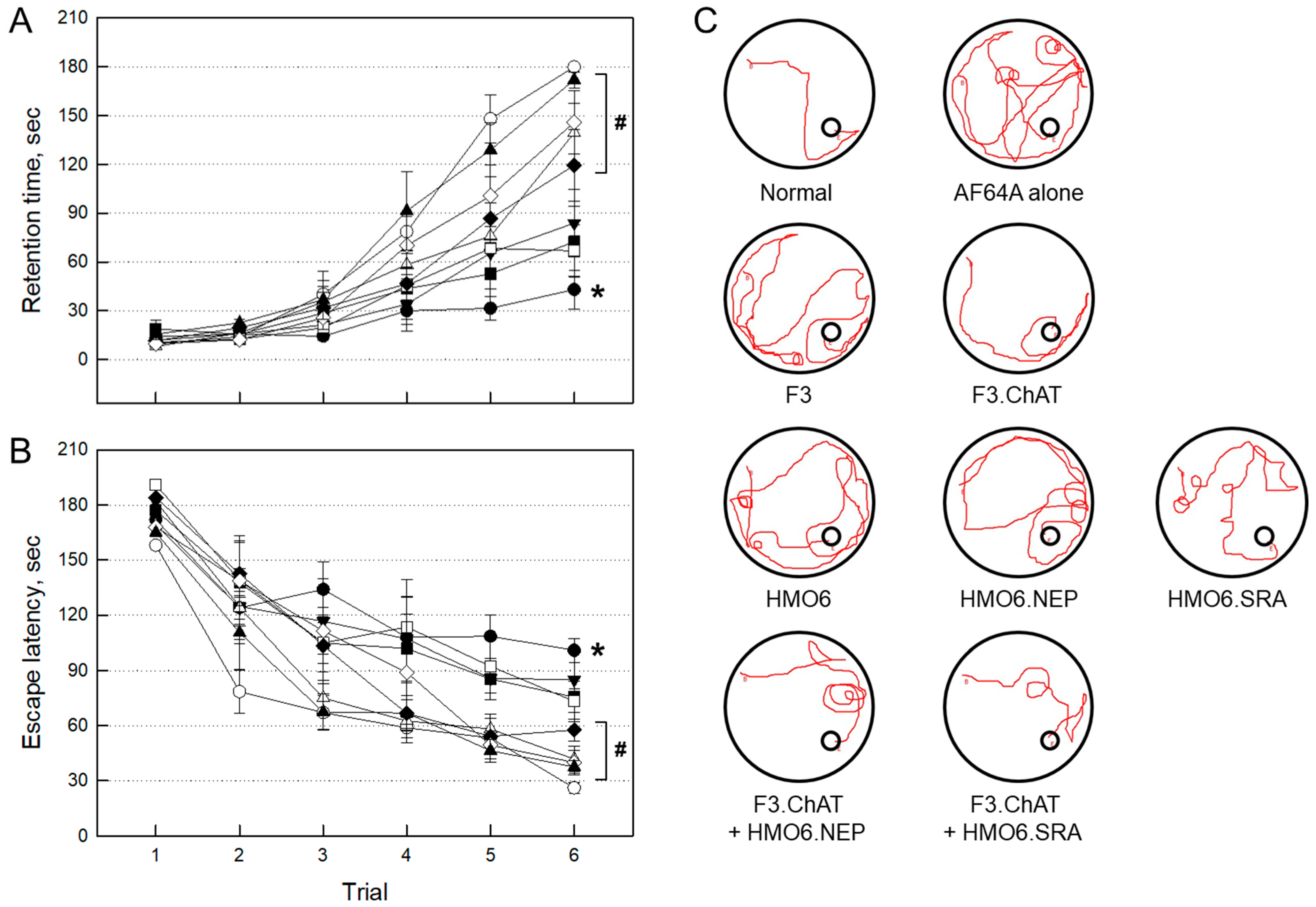
2.3.1. Improvement of Cognitive Function
2.3.2. Expression of Functional Genes in Transplanted Cells
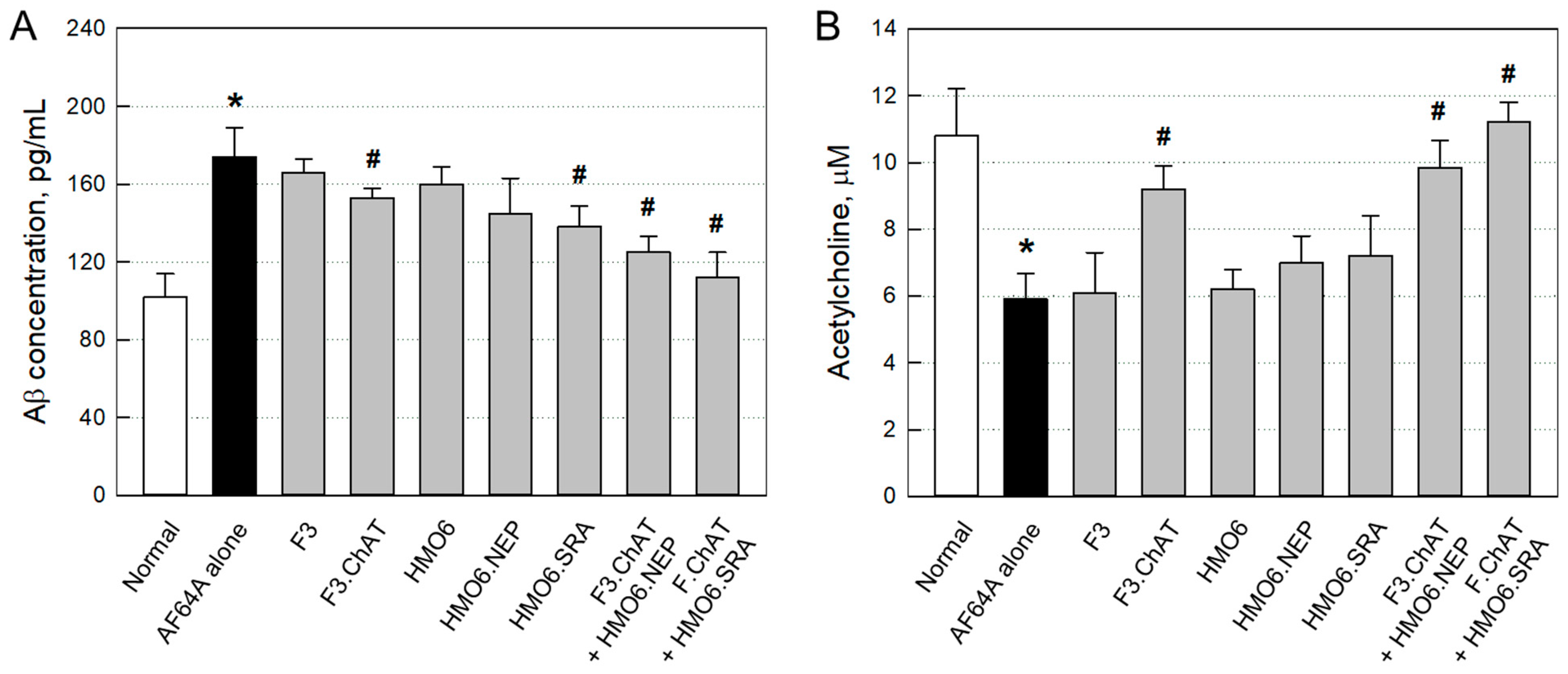
2.3.3. Aβ Elimination and ACh Recovery
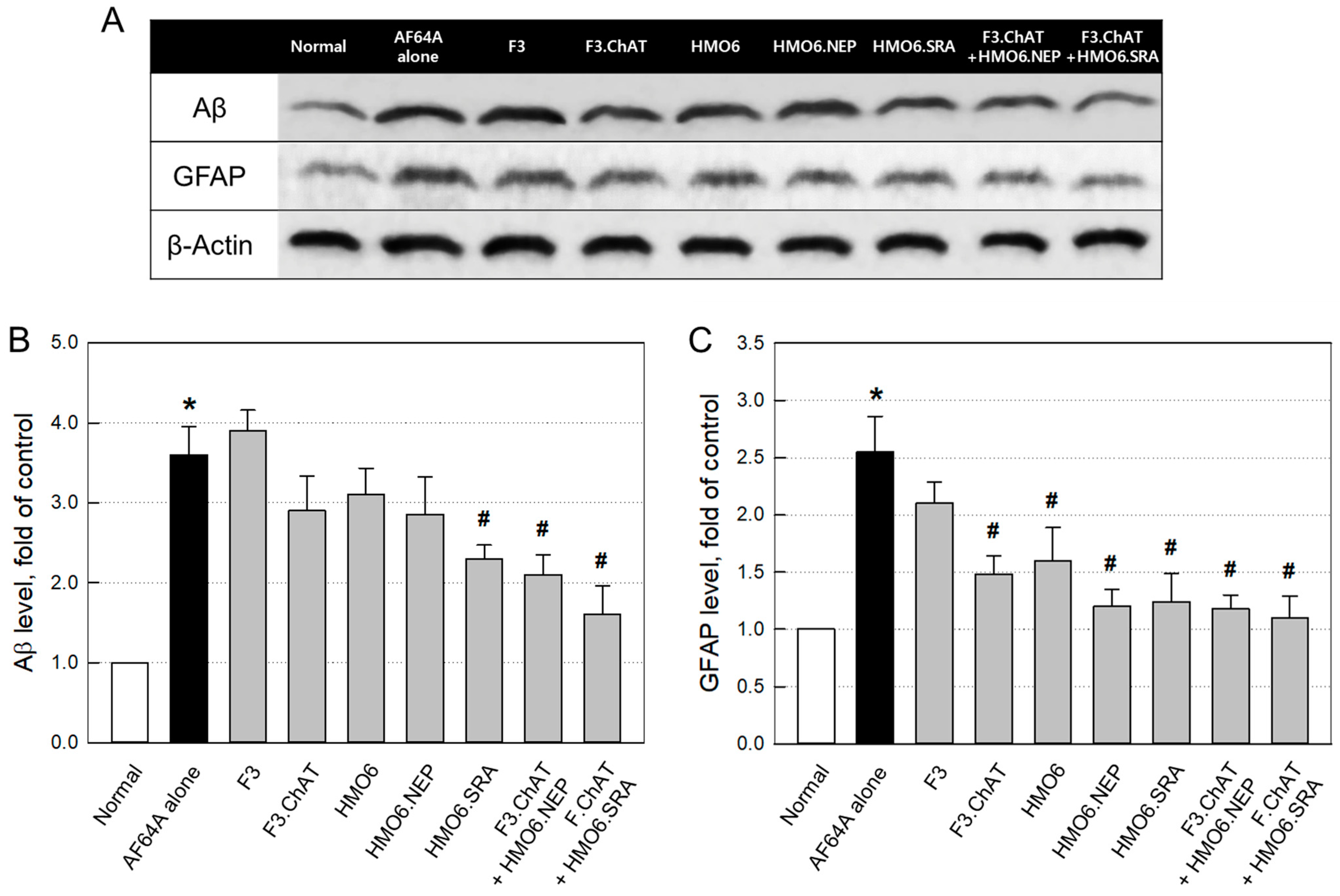
2.3.4. Aβ Elimination and Neuroprotection
3. Discussion
4. Materials and Methods
4.1. Establishment of NSCs and Microglial Cells
4.1.1. Cell Culture
4.1.2. Establishment of NSCs Encoding Functional Genes
4.1.3. Establishment of Microglial Cells Encoding Functional Genes
4.1.4. Immunocytochemical Analysis of Functional Proteins
4.1.5. Aβ Clearance by HMO6 Cells
4.2. Establishment of AD Animal Model
4.2.1. Animals
4.2.2. Experimental Design for AD Modeling
4.2.3. Cognitive Function Tests
4.2.4. Western Blot Analysis of Brain Aβ
4.2.5. Enzymatic Analysis of Brain ACh
4.3. Efficacy Evaluation of NSCs and Microglial Cells Encoding Functional Genes
4.3.1. Experimental Design for Efficacy Evaluation
4.3.2. Cognitive Function Tests
4.3.3. Immunohistochemical Analysis of hMito and Functional Proteins
4.3.4. ELISA and Enzymatic Analyses of Brain Aβ and ACh
4.3.5. Western Blot Analysis of Brain Aβ and GFAP
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, H.J.; Joo, S.S.; Bae, D.K.; Yang, G.; Yang, Y.H.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.B.; et al. Human neural stem cells over- expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp. Neurol. 2012, 234, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Kerridge, C.; Belyaev, N.D.; Nalivaeva, N.N.; Turner, A.J. The Aβ-clearance protein transthyretin, like neprilysin, is epigenetically regulated by the amyloid precursor protein intracellular domain. J. Neurochem. 2014, 130, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef]
- Jang, S.K.; Yu, J.M.; Kim, S.T.; Kim, G.H.; Park, D.W.; Lee, D.I.; Joo, S.S. An Aβ42 uptake and degradation via Rg3 requires an activation of caveolin, clathrin and Aβ-degrading enzymes in microglia. Eur. J. Pharmacol. 2015, 758, 1–10. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Chakrabartia, M.; Govindaraju, T. Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease. Chem. Commun. 2015, 51, 13434–13450. [Google Scholar] [CrossRef]
- Musial, A.; Bajda, M.; Malawska, B. Recent developments in cholinesterases inhibitors for Alzheimer’s disease treatment. Curr Med. Chem. 2007, 14, 2654–2679. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Kim, S.U.; Park, I.H.; Kim, T.H.; Kim, K.S.; Choi, H.B.; Hong, S.H.; Bang, J.H.; Lee, M.A.; Joo, I.S.; Lee, C.S.; et al. Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson disease. Neuropathology 2006, 26, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Park, D.; Ban, Y.H.; Cha, Y.; An, E.S.; Choi, J.; Choi, E.K.; Kim, Y.B. Improvement by human oligodendrocyte progenitor cells of neurobehavioral disorders in an experimental model of neonatal periventricular leukomalacia. Cell Transplant. 2018, 27, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, K.; Cha, Y.; Ban, Y.H.; Park, S.K.; Jeong, H.S.; Park, D.; Choi, E.K.; Kim, Y.B. Neuroprotective effects of human neural stem cells over-expressing choline acetyltransferase in a middle cerebral artery occlusion model. J. Chem. Neuroanat. 2020, 103, 101730. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Joo, S.S.; Kim, T.K.; Lee, S.H.; Kang, H.; Lee, H.J.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.B.; et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive runction of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yang, Y.H.; Bae, D.K.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.K.; Lee, S.W.; Kim, G.H.; et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol. Aging 2013, 34, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Choi, E.K.; Cho, T.H.; Joo, S.S.; Kim, Y.B. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Aβ Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2020, 21, 3958. [Google Scholar] [CrossRef]
- Miners, J.S.; Barua, N.; Kehoe, P.G.; Gill, S.; Love, S. Aβ-degrading enzymes: Potential for treatment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2011, 70, 944–959. [Google Scholar] [CrossRef]
- Yang, C.N.; Shiao, Y.J.; Shie, F.S.; Guo, B.S.; Chen, P.H.; Cho, C.Y.; Chen, Y.J.; Huang, F.L.; Tsay, H.J. Mechanism mediating oligomeric Aβ clearance by naive primary microglia. Neurobiol. Dis. 2011, 42, 221–230. [Google Scholar] [CrossRef]
- Lue, L.F.; Walker, D.G.; Brachova, L.; Beach, T.G.; Rogers, J.; Schmidt, A.M.; Stern, D.M.; Yan, S.D. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: Identification of a cellular activation mechanism. Exp. Neurol. 2001, 171, 29–45. [Google Scholar] [CrossRef]
- Marr, R.A.; Guan, H.; Rockenstein, E.; Kindy, M.; Gage, F.H.; Verma, I.; Masliah, E.; Hersh, L.B. Neprilysin regulates amyloid β peptide levels. J. Mol. Neurosci. 2004, 22, 5–11. [Google Scholar] [CrossRef]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Watanabe, K.; Sekiguchi, M.; Hosoki, E. Identification of the major Aβ1-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 2000, 6, 143–150. [Google Scholar] [CrossRef]
- Humpel, C. Intranasal neprilysin rapidly eliminates amyloid-beta plaques, but causes plaque compensations: The explanation why the amyloid-beta cascade may fail? Neural. Regen. Res. 2022, 17, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, W.; Min, F.; Li, Z.; Huang, J.; Huang, R. A nonhuman primate model of Alzheimer’s disease generated by intracranial injection of amyloid-β42 and thiorphan. Metab. Brain. Dis. 2010, 25, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Nakagawa, E.; Hatori, K.; Choi, H.B.; McLarnon, J.G.; Lee, M.A.; Kim, S.U. Generation and characterization of immortalized human microglial cell lines: Expression of cytokines and chemokines. Neurobiol. Dis. 2001, 8, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, Y.J.; Zhou, W.W.; Wang, S.W.; Xu, P.X.; Yu, X.L.; Liu, R.T. Activated scavenger receptor A promotes glial internalization of Aβ. PLoS ONE 2014, 9, e94197. [Google Scholar] [CrossRef]
- Yu, X.; Yi, H.; Gou, C.; Zuo, D.; Wang, Y.; Kim, H.L.; Subjeck, J.R.; Wang, X.Y. Pattern recognition scavenger receptor CD204 attenuates Toll-like receptor 4-induced NF-κB activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J. Biol. Chem. 2011, 286, 18795–18806. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T.; Kameyama, T. Impairment of active avoidance response in rats with continuous infusion of quinolinic acid into the lateral ventricle. J. Pharmacobiodyn. 1991, 14, 351–355. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, J. Animal models of dementia. Brain. Neurorehabil. 2011, 4, 21–29. [Google Scholar] [CrossRef]
- Stepanichev, M.Y.; Moiseeva, Y.V.; Lazareva, N.A.; Onufriev, M.V.; Gulyaeva, N.V. Single intracerebroventricular administration of amyloid-beta (25–35) peptide induces impairment in short-term rather than long-term memory in rats. Brain Res. Bull. 2003, 61, 197–205. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.Y.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef]
- Hwang, C.J.; Park, M.H.; Hwang, J.Y.; Kim, J.H.; Yun, N.Y.; Oh, S.Y.; Song, J.K.; Seo, H.O.; Kim, Y.B.; Hwang, D.Y.; et al. CCR5 deficiency accelerates lipopolysaccharide-induced astrogliosis, amyloid-beta deposit and impaired memory function. Oncotarget 2016, 7, 11984–11999. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Oh, J.; Kim, T.K.; Cho, Y.J.; Lee, S.H.; Bae, D.K.; Yang, Y.H.; Yang, G.; Hwang, S.Y.; Kim, Y.B. Augmentation by hypercholesterolemia of amyloid β peptide-induced learning and memory deficit. J. Biomed. Res. 2009, 10, 87–95. [Google Scholar]
- Jean, Y.Y.; Baleriola, J.; Fa, M.; Hengst, U.; Troy, C.M. Stereotaxic infusion of oligomeric amyloid-beta into the mouse hippocampus. J. Vis. Exp. 2015, 100, e52805. [Google Scholar]
- Levin-Allerhand, J.A.; Lominska, C.E.; Smith, J.D. Increased amyloid-levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J. Nutr. Health Aging 2002, 6, 315–319. [Google Scholar] [PubMed]
- Nicholson, A.M.; Ferreira, A. Cholesterol and neuronal susceptibility to beta-amyloid toxicity. Cogn. Sci. 2010, 5, 35–56. [Google Scholar]
- Lee, Y.K.; Yuk, D.Y.; Lee, J.W.; Lee, S.Y.; Ha, T.Y.; Oh, K.W.; Yun, Y.P.; Hong, J.T. (-)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res. 2009, 1250, 164–174. [Google Scholar] [CrossRef]
- Fan, Q.I.; Hanin, I. Effects of AF64A on gene expression of choline acetyltransferase (ChAT) in the septo-hippocampal pathway and striatum in vivo. Neurochem. Res. 1999, 24, 15–24. [Google Scholar] [CrossRef]
- Bessho, T.; Takashina, K.; Eguchi, J.; Komatsu, T.; Saito, K. MKC-231, a choline uptake enhancer: Long-lasting cognitive improvement after repeated administration in AF64A-treated rats. J. Neural. Transm. 2008, 115, 1019–1025. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.S.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef]
- Hersh, L.B.; Rodgers, D.W. Neprilysin and amyloid beta peptide degradation. Curr. Alzheimer Res. 2008, 5, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Landereth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural. Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.H.; Kim, J.H.; Lee, D.; Jeon, H.B.; Kwon, S.J.; Kim, S.M.; Yoo, Y.J.; Lee, E.H.; Choi, S.J.; et al. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012, 19, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Gao, G.; Feng, J.; Jin, Y.; Wang, C.; Mao, Q.; Jiang, J. Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: A prospective cohort study. Crit. Care 2015, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-coria, H.; Castello, N.A.; Muller, F.J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef]
- Narantuya, D.; Nagai, A.; Sheikh, A.M.; Masuda, J.; Kobayashi, S.; Yamaguchi, S.; Kim, S.U. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS ONE 2010, 5, e11746. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; de Vellis, J. Microglia in health and disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, H.J.; Cha, S.H.; Jeong, K.J.; Lee, Y.; Jeon, C.Y.; Yi, K.S.; Lim, I.; Cho, Z.H.; Chang, K.T.; et al. Long-term survival and differentiation of human neural stem cells in nonhuman primate brain with no immunosuppression. Cell Transplant. 2015, 24, 191–201. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, K.S.; Kim, E.J.; Choi, H.B.; Lee, K.H.; Park, I.H.; Ko, Y.; Jeong, S.W.; Kim, S.U. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007, 25, 1204–1212. [Google Scholar] [CrossRef]
- Kim, S.U.; Nagai, A.; Nakagawa, E.; Choi, H.B.; Bang, J.H.; Lee, H.J.; Lee, M.A.; Lee, Y.B.; Park, I.H. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol. Biol. 2008, 438, 103–121. [Google Scholar]
- Matsuo, A.; Bellier, J.P.; Hisano, T.; Aimi, Y.; Yasuhara, O.; Tooyama, I.; Saito, N.; Kimura, H. Rat choline acetyltransferase of the peripheral type differs from that of the common type in intracellular translocation. Neurochem. Int. 2005, 46, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Miwa, T.; Nishio, H.; Yagasaki, O. The biochemical and ultrastructural examinations in central cholinergic damage of the rat induced by the intraperitoneal administration of AF64A. Jpn. J. Pharmacol. 1990, 54, 415–423. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, Y.-H.; Park, D.; Choi, E.-K.; Kim, T.M.; Joo, S.S.; Kim, Y.-B. Effectiveness of Combinational Treatments for Alzheimer’s Disease with Human Neural Stem Cells and Microglial Cells Over-Expressing Functional Genes. Int. J. Mol. Sci. 2023, 24, 9561. https://doi.org/10.3390/ijms24119561
Ban Y-H, Park D, Choi E-K, Kim TM, Joo SS, Kim Y-B. Effectiveness of Combinational Treatments for Alzheimer’s Disease with Human Neural Stem Cells and Microglial Cells Over-Expressing Functional Genes. International Journal of Molecular Sciences. 2023; 24(11):9561. https://doi.org/10.3390/ijms24119561
Chicago/Turabian StyleBan, Young-Hwan, Dongsun Park, Ehn-Kyoung Choi, Tae Myoung Kim, Seong Soo Joo, and Yun-Bae Kim. 2023. "Effectiveness of Combinational Treatments for Alzheimer’s Disease with Human Neural Stem Cells and Microglial Cells Over-Expressing Functional Genes" International Journal of Molecular Sciences 24, no. 11: 9561. https://doi.org/10.3390/ijms24119561
APA StyleBan, Y.-H., Park, D., Choi, E.-K., Kim, T. M., Joo, S. S., & Kim, Y.-B. (2023). Effectiveness of Combinational Treatments for Alzheimer’s Disease with Human Neural Stem Cells and Microglial Cells Over-Expressing Functional Genes. International Journal of Molecular Sciences, 24(11), 9561. https://doi.org/10.3390/ijms24119561






