Omega-3 Fatty Acids in Arterial Hypertension: Is There Any Good News?
Abstract
1. Introduction
2. Biochemistry and Cellular Mechanisms of Polyunsaturated Fatty Acids (PUFAs)
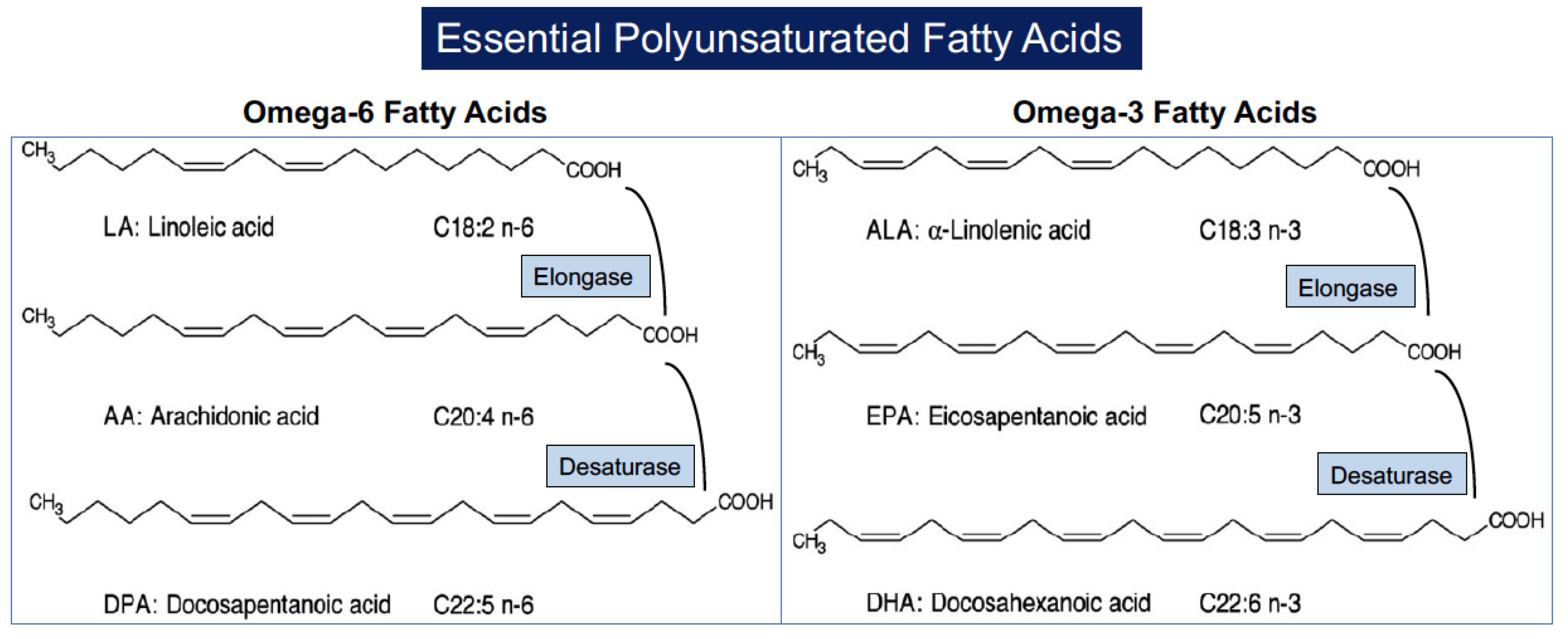
2.1. General Biochemistry and Biologic Sources of PUFAs
2.2. PUFAs and Cellular Membranes
2.3. PUFA and Intracellular Signaling
2.4. PUFAs and Its Metabolites: Role of Oxylipins
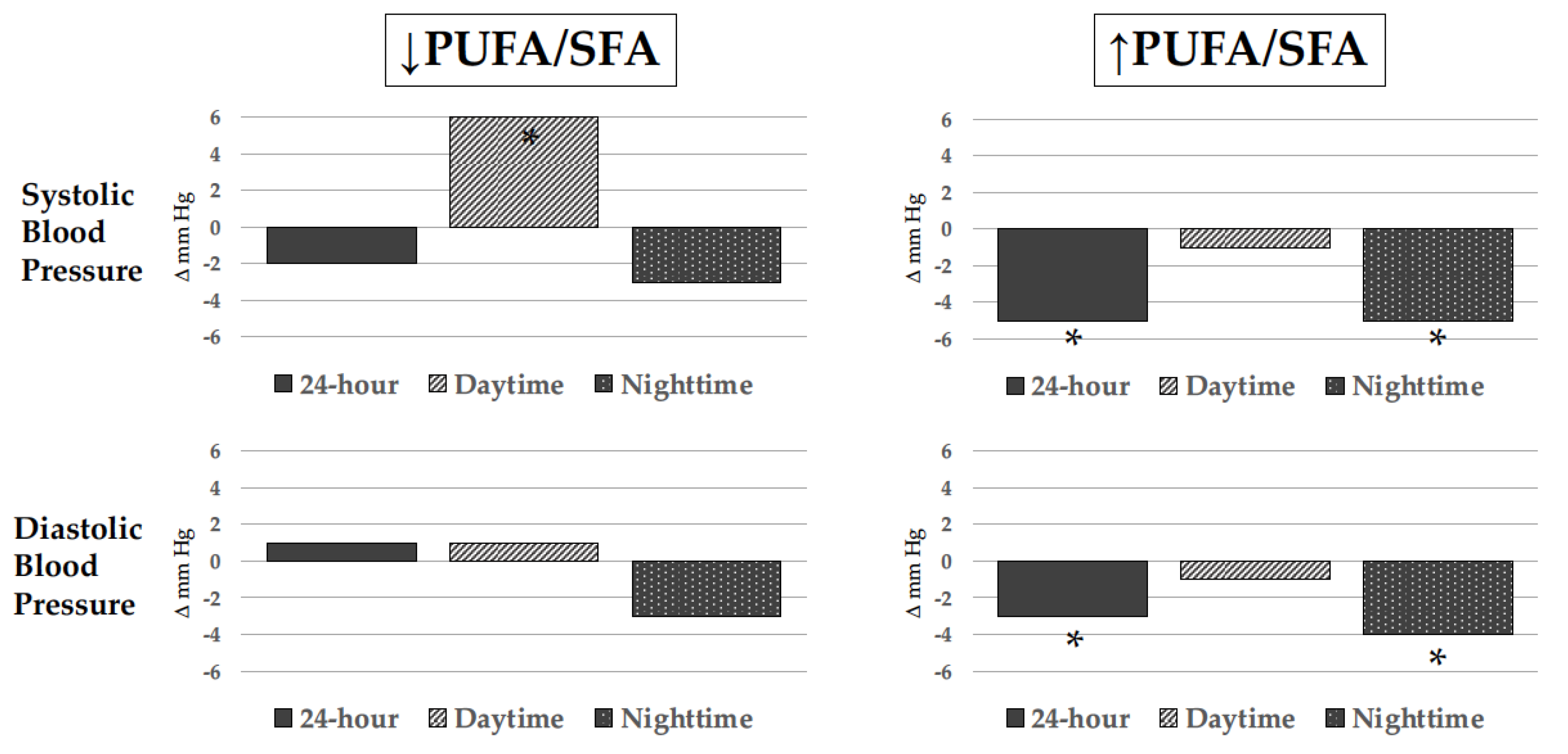
3. ω-3 PUFAs and Blood Pressure
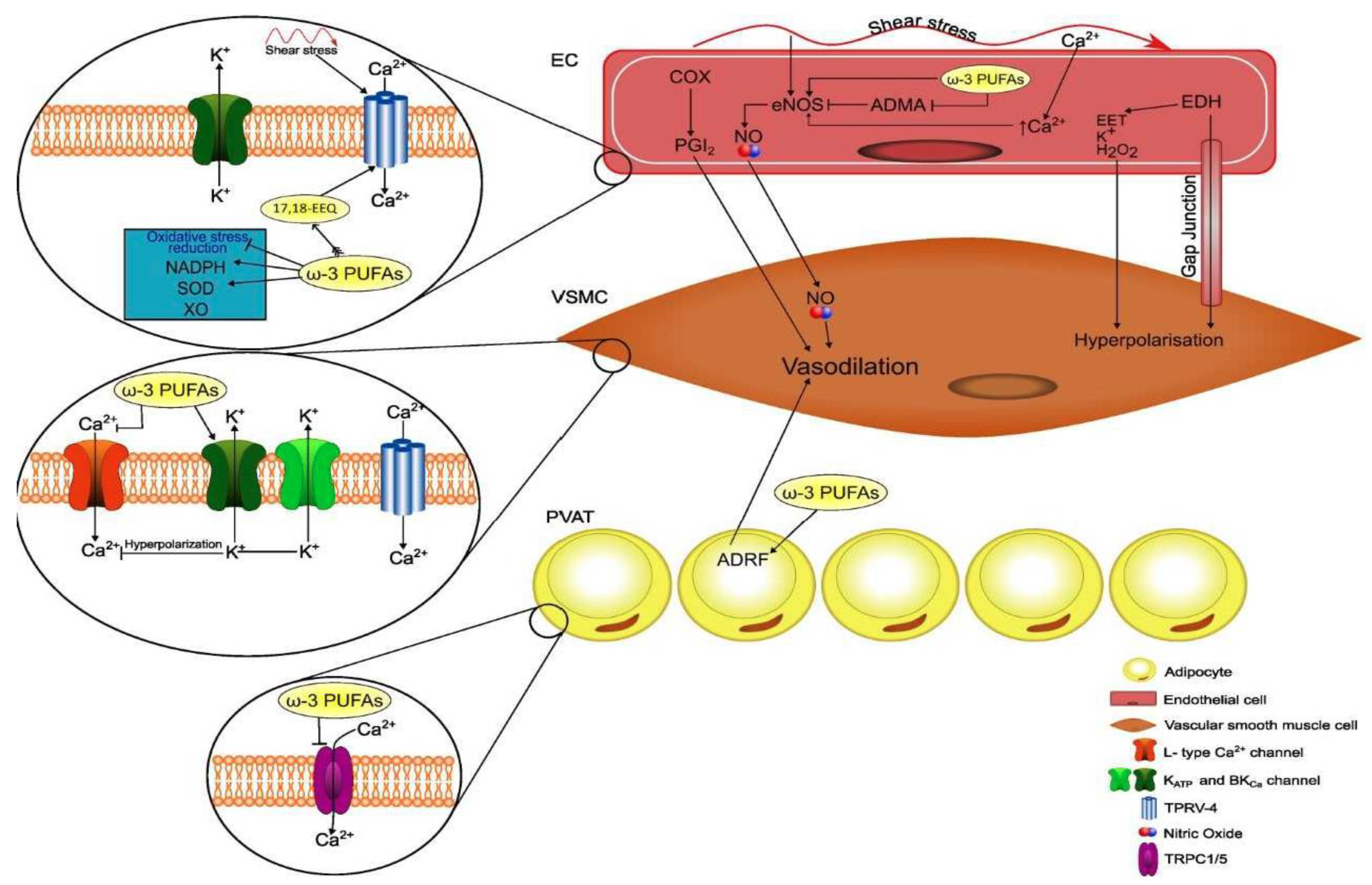
4. Antihypertensive Mechanisms of ω-3 PUFAs
4.1. ω-3 PUFAs and Cardiac Hemodynamics
4.2. ω-3 PUFAs and Regulation of Peripheral Vascular Resistance
4.2.1. ω-3 PUFAs and Endothelium-Dependent Regulation of Vascular Tone
4.2.2. ω-3 PUFAs and Endothelium-Independent Regulation of Vascular Tone
5. ω-3 PUFAs and the Risk of Hypertension Development
6. ω-3 PUFAs and Hypertension-Related Vascular Damage
6.1. Arterial Stiffness
6.2. Atherosclerosis and Plaque Formation
7. Cardiovascular Prevention Studies
7.1. Early Studies
7.2. Recent Studies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Erratum in: Eur. Heart J. 2019, 40, 475. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.J.; Collins, K.J.; Dennison Himmelfarb, C.; De Palma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115, Erratum in Hypertension 2018, 71, e140–e144. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Health Organization Obesity and Overweight Fact Sheet; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Ward, R. Familial aggregation and genetic epidemiology of blood pressure. In Hypertension: Pathophysiology, Diagnosis and Management; New York Raven Press: New York, NY, USA, 1990; Volume 1, pp. 81–100. [Google Scholar]
- Bang, H.O.; Dyerberg, J.; Hjoorne, T. The composition of food consumed by Greenland Eskimos. Acta Med. Scand. 1976, 200, 69–73. [Google Scholar] [CrossRef]
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in northwestern Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661. [Google Scholar] [CrossRef]
- Middaugh, J.P. Cardiovascular deaths among Alaskan Natives, 1980–1986. Am. J. Public Health 1990, 80, 282–285. [Google Scholar] [CrossRef]
- Newman, W.P.; Middaugh, J.P.; Propst, M.T.; Roger, D.R. Atherosclerosis in Alaska Natives and Non-Natives. Lancet 1993, 341, 1056–1057. [Google Scholar] [CrossRef]
- Weinberg, R.L.; Brook, R.D.; Rubenfire, M.; Eagle, K.A. Cardiovascular Impact of Nutritional Supplementation with Omega-3 Fatty Acids: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 593–608. [Google Scholar] [CrossRef]
- Colussi, G.L.; Baroselli, S.; Sechi, L. Omega-3 polyunsaturated fatty acids decrease plasma lipoprotein(a) levels in hypertensive subjects. Clin. Nutr. 2004, 23, 1246–1247. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’Donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: A systematic review and meta-regression analysis of randomized controlled trials. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: Findings from the China national stroke screening and prevention project (CNSSPP). BMC Public Health 2019, 19, 1500. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Budoff, M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am. J. Cardiol. 2016, 118, 138–145. [Google Scholar] [CrossRef]
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1352. [Google Scholar] [CrossRef]
- Thomsen, M.; Varbo, A.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Low nonfasting triglycerides and reduced all-cause mortality: A mendelian randomization study. Clin. Chem. 2014, 60, 737–746. [Google Scholar] [CrossRef]
- Sacks, F.M.; Carey, V.J.; Fruchart, J.C. Combination lipid therapy in type 2 diabetes. N. Engl. J. Med. 2010, 363, 692–695. [Google Scholar] [CrossRef]
- The ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef]
- Guyton, J.R.; Slee, A.E.; Anderson, T.; Fleg, J.L.; Goldberg, R.B.; Kashyap, M.L.; Marcovina, S.M.; Nash, S.D.; O’Brien, K.D.; Weintraub, W.S.; et al. Relationship of lipoproteins to cardiovascular events: The AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 2013, 62, 1580–1584. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans. US Department of Health and Human Services and US Department of Agriculture. 2015; Volume 7. Available online: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed on 5 April 2023).
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M. Fish oil omega-3 fatty acids and cardio-metabolic health, alone or with statins. Eur. J. Clin. Nutr. 2013, 67, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Gordon Hguyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Baroselli, S.; Nadalini, E.; Lapenna, R.; Chiuch, A.; Sechi, L.A. Omega-3 fatty acids: From biochemistry to their clinical use in the prevention of cardiovascular disease. Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 13–21. [Google Scholar] [CrossRef]
- Burdge, G.C. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Nettleton, J.A. Omega-3 fatty acids: Comparison of plant and seafood sources in human nutrition. J. Am. Diet. Assoc. 1991, 91, 331–337. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic and metabolism in young men. Br. J. Nutr. 2002, 88, 355–364. [Google Scholar] [CrossRef]
- Lin, Y.H.; Salem, N., Jr. Whole body distribution of deuterated linoleic and alpha-linolenic acids and their metabolites in the rat. J. Lipid Res. 2007, 48, 2709–2724. [Google Scholar] [CrossRef]
- Cook, H.; McMaster, C. Fatty acid desaturation and chain elongation in eukaryotes. New Compr. Biochem. 2002, 36, 181–204. [Google Scholar] [CrossRef]
- Wada, M.; De Long, C.J.; Hong, Y.H.; Rieke, C.J.; Song, I.; Sidhu, R.S.; Yuan, C.; Warnock, M.; Schmaier, A.H.; Yokoyama, C.; et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J. Biol. Chem. 2007, 282, 22254–22266. [Google Scholar] [CrossRef]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef]
- Liou, Y.A.; King, D.J.; Zibrik, D.; Innis, S.M. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J. Nutr. 2007, 137, 945–952. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The omega-3 index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- McDonnell, S.L.; French, C.B.; Baggerly, C.A.; Harris, W.S. Cross-sectional study of the combined associations of dietary and supplemental eicosapentaenoic acid + docosahexaenoic acid on omega-3 index. Nutr. Res. 2019, 71, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, M.; Rockwell, M.S.; Wentz, L.M. The influence of dietary and supplemental omega-3 fatty acids on the omega-3 index: A scoping review. Front. Nutr. 2023, 10, 1072653. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Mason, R.P. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by X-ray diffraction. Chem. Phys. Lipids 2018, 212, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Batten, S.E.; Harris, M.; Rockett Drew, B.; Shaikh Raza, S.; Stillwell, W.; Wassall, S.R. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J. 2012, 103, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.R.; Chattopadhyay, A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim. Biophys. Acta 2016, 1858, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Berkhout, M.; Vos, A.P.; Sijben, J.W.C.; Calder, P.C.; Garssen, J.; Van Helvoort, A. Supplementation with a fish oil-enriched, high-protein medicalfood leads to rapid incorporation of EPA into white blood cells and modulates immune responses within one week in healthy men and women. J. Nutr. 2011, 141, 964–970. [Google Scholar] [CrossRef]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef]
- Vidgren, H.M.; Agren, J.J.; Schwab, U.; Rissanen, T.; Hänninen, O.; Uusitupa, M.I. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 1997, 32, 697–705. [Google Scholar] [CrossRef]
- Laude, A.J.; Prior, I.A. Plasma membrane microdomains: Organization, function and trafficking. Mol. Membr. Biol. 2004, 21, 193–205. [Google Scholar] [CrossRef]
- Hancock, J.F. Lipid rafts: Contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 2006, 7, 456–462. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Andersonl, R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998, 67, 199–225. [Google Scholar] [CrossRef]
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 770–776. [Google Scholar] [CrossRef]
- Ikonen, E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001, 13, 470–477. [Google Scholar] [CrossRef]
- Layne, J.; Majkova, Z.; Smart, E.J.; Toborek, M.; Hennig, B. Caveolae: A regulatory platform for nutritional modulation of inflammatory diseases. J. Nutr. Biochem. 2011, 22, 807–811. [Google Scholar] [CrossRef]
- Dart, C. Lipid microdomains and the regulation of ion channel function. J. Physiol. 2010, 588, 3169–3178. [Google Scholar] [CrossRef]
- Grossfield, A.; Feller, S.E.; Pitman, M.C. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. USA 2006, 103, 4888–4893. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Ke, Q.; Wang, S.Y.; Auktor, K.; Yang, Y.; Wang, G.K.; Morgan, J.P.; Leaf, A. Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na. channels. Proc. Natl. Acad. Sci. USA 2001, 98, 3606–3611. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Im, D.S. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur. J. Pharmacol. 2016, 785, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castilo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Haneklaus, M.; O’Neill, L.A.; Coll, R.C. Modulatory mechanisms controlling the NLRP3 inammasome in inammation: Recent developments. Curr. Opin. Immunol. 2013, 25, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr. Opin. Lipidol. 2008, 19, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef]
- Hertz, R.; Magenheim, J.; Berman, I.; Bar-Tana, J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature 1998, 392, 512–516. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Spencer, T.E.; Wang, N.; Moyer, M.P.; Chapkin, R.S. Chemopreventive n-3 fatty acids activate RXRalpha in colonocytes. Carcinogenesis 2003, 24, 1541–1548. [Google Scholar] [CrossRef]
- de Urquiza, A.M.; Liu, S.; Sjoberg, M.; Zetterström, R.H.; Griffiths, W.; Sjövall, J.; Perlmann, T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 2000, 290, 2140–2144. [Google Scholar] [CrossRef]
- Schroeder, F.; Petrescu, A.D.; Huang, H.; Atshaves, B.P.; McIntosh, A.L.; Martin, G.G.; Hosteler, H.A.; Vespa, A.; Landrock, D.; Landrock, K.K.; et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 2008, 43, 1–17. [Google Scholar] [CrossRef]
- Wolfrum, C.; Borrmann, C.M.; Borchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha- and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328. [Google Scholar] [CrossRef]
- Needleman, P.; Truk, J.; Jakschik, B.A.; Morrison, A.R.; Lefkowith, J.B. Arachidonic acid metabolism. Annu. Rev. Biochem. 1986, 55, 69–102. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Biscione, F.; Pignalberi, C.; Totteri, A.; Messina, F.; Altamura, G. Cardiovascular effects of omega-3 free fatty acids. Curr. Vasc. Pharmacol. 2007, 5, 163–172. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhytmic, anty-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008, 7, 37. [Google Scholar] [CrossRef]
- Westphal, C.; Konkel, A.; Schunck, W.-H. Cytochrome P-450 enzymes in the bioactivcation of polyunsaturated fatty acids and their role in cardiovascular disease. Adv. Exp. Med. Biol. 2015, 851, 151–187. [Google Scholar] [CrossRef]
- Schunk, W.-H. EPA and/or DHA? A test question on the principles and opportunities in utilizing the therapeutic potential of omega-3 fatty acids. J. Lipid Res. 2016, 57, 1608–1611. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inammation: Dual anti-inammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Wang, H.J.; Jung, T.W.; Kim, J.W.; Kim, J.A.; Lee, Y.B.; Hong, S.H.; Roh, E.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Protectin DX prevents H2O2-mediated oxidative stress in vascular endothelial cells via an AMPK-dependent mechanism. Cell Signal 2019, 53, 14–21. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Y.; Zhao, F.; Wang, J. Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 9634803. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Miller, E.R., 3rd; Seidler, A.J.; Whelton, P.K. Does supplementation of diet with ‘fish oil’ reduce blood pressure? A meta-analysis of controlled clinical trials. Arch. Intern. Med. 1993, 153, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.; Kok, F.J. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.O.; Mason, J.M.; Nicolson, D.J.; Campbell, F.; Beyer, F.C.; Beyer, F.R.; Cook, J.V.; Williams, B.; Ford, G.A. Lifestyle interventions to reduce raised blood pressure: A systematic review of randomized controlled trials. J. Hypertens. 2006, 24, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Dickinson, H.O.; Critchley, J.A.; Ford, G.A.; Bradburn, M. A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur. J. Prev. Cardiol. 2013, 20, 107–120. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-chain omega-3 fatty acids eicosapen-taenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- AbuMweis, S.; Jew, S.; Tayyem, R.; Agraib, L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: A meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet. 2018, 31, 67–84. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, K.L.; Li, J.M.; Li, D. Effects of EPA and DHA on blood pressure and inflammatory factors: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 3380–3393. [Google Scholar] [CrossRef]
- Musazadeh, V.; Kavyani, Z.; Naghshbandi, B.; Dehghan, P.; Vajdi, M. The beneficial effects of omega-3 polyunsaturated fatty acids on controlling blood pressure: An umbrella meta-analysis. Front. Nutr. 2022, 18, 85451. [Google Scholar] [CrossRef]
- Minihane, A.M.; Armah, C.K.; Miles, E.A.; Madden, J.M.; Clark, A.B.; Caslake, M.J.; Packard, C.J.; Kofler, B.M.; Lietz, G.; Curtis, P.J.; et al. Consumption of fish oil providing amounts of eicosapentaenoic acid and docosahexaenoic acid that can be obtained from the diet reduces blood pressure in adults with systolic hypertension: A retrospective analysis. J. Nutr. 2016, 146, 516–523. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Dialti, V.; Pezzutto, F.; Mos, L.; Sechi, L.A. Fish meal supplementation and ambulatory blood pressure in patients with hypertension: Relevance of baseline membrane fatty acid composition. Am. J. Hypertens. 2014, 27, 471–481. [Google Scholar] [CrossRef]
- Yang, B.; Shi, M.Q.; Li, Z.H.; Yang, J.J.; Li, D. Fish, Long-chain n-3 PUFA and incidence of elevated blood pressure: A meta-analysis of prospective cohort studies. Nutrients 2016, 8, 58. [Google Scholar] [CrossRef]
- Chen, J.; Sun, B.; Zhang, D. Association of dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007–2014. Nutrients 2019, 11, 1232. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta- analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- Hardy, S.T.; Loehr, L.R.; Butler, K.R.; Chakladar, S.; Chang, P.P.; Folsom, A.R.; Heiss, G.; MacLehose, R.F.; Matsushita, K.; Avery, C.L. Reducing the blood pressure-related burden of cardiovascular disease: Impact of achievable improvements in blood pressure prevention and control. J. Am. Heart Assoc. 2015, 4, e002276. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Gottdiener, J.S.; Siscovick, D.S. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am. J. Cardiol. 2016, 97, 216–222. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Geelen, A.; Brouwer, I.A.; Geleijnse, J.M.; Zock, P.L.; Katan, M.B. Effect of fish oil on heart rate in humans: A meta-analysis of randomized controlled trials. Circulation 2005, 112, 1945–1952. [Google Scholar] [CrossRef]
- Ninio, D.M.; Hill, A.M.; Howe, P.R.; Buckley, J.D.; Saint, D.A. Docosahexaenoic acid-rich fish oil improves heart rate variability and heart rate responses to exercise in overweight adults. Br. J. Nutr. 2008, 100, 1097–1103. [Google Scholar] [CrossRef]
- Macartney, M.J.; Hingley, L.; Brown, M.A.; Peoples, G.E.; McLennan, P.L. Intrinsic heart rate recovery after dynamic exercise is improved with an increased omega-3 index in healthy males. Br. J. Nutr. 2014, 112, 1984–1992. [Google Scholar] [CrossRef]
- McLennan, P.L.; Barnden, L.R.; Bridle, T.M.; Abeywardena, M.Y.; Charnock, J.S. Dietary fat modulation of left ventricular ejection fraction in the marmoset due to enhanced filling. Cardiovasc. Res. 1992, 26, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Grimsgaard, S.; Bønaa, K.H.; Hansen, J.B.; Myhre, E.S.P. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am. J. Clin. Nutr. 1998, 68, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Elherik, K.; Bolton-Smith, C.; Barr, R.; Hill, A.; Murrie, I.; Belch, J.J. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc. Res. 2003, 59, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Siniarski, A.; Haberka, M.; Mostowik, M.; Gołębiowska-Wiatrak, R.; Poręba, M.; Malinowski, K.P.; Gąsior, Z.; Konduracka, E.; Nessler, J.; Gajos, G. Treatment with omega-3 polyunsaturated fatty acids does not improve endothelial function in patients with type 2 diabetes and very high cardiovascular risk: A randomized, double-blind, placebo-controlled study (Omega-FMD). Atherosclerosis 2018, 271, 148–155. [Google Scholar] [CrossRef]
- Nyby, M.D.; Hori, M.T.; Ormsby, B.; Gabrielian, A.; Tuck, M.L. Eicosapentaenoic acid inhibits Ca2+ mobilization and PKC activity in vascular smooth muscle cells. Am. J. Hypertens. 2003, 16, 708–714. [Google Scholar] [CrossRef]
- Daci, A.; Özen, G.; Uyar, İ.; Civelek, E.; Yildirim, F.İ.A.; Durman, D.K.; Teskin, Ö.; Norel, X.; Uydeş-Doğan, B.S.; Topal, G. Omega-3 polyunsaturated fatty acids reduce vascular tone and inflammation in human saphenous vein. Prostaglandins Other Lipid Mediat. 2017, 133, 29–34. [Google Scholar] [CrossRef]
- Ayer, J.G.; Harmer, J.A.; Xuan, W.; Toelle, B.; Webb, K.; Almqvist, C.; Marks, G.B.; Celermajer, D.S. Dietary supplementation with n23 polyunsaturated fatty acids in early childhood: Effects on blood pressure and arterial structure and function at age 8 y 1–3. Am. J. Clin. Nutr. 2009, 90, 438–446. [Google Scholar] [CrossRef]
- Sanders, T.A.B.; Hall, W.L.; Maniou, Z.; Lewis, F.; Seed, P.T.; Chowienczyk, P.J. Effect of low doses of long-chain n-3 PUFAs on endothelial function and arterial stiffness: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 973–980. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Harris, W.S.; Vanden Heuvel, J.P.; Wagner, P.R.; West, S.G. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with. Am. J. Clin. Nutr. 2011, 3, 243–252. [Google Scholar] [CrossRef]
- Rossi, G.P.; Seccia, T.M.; Barton, M.; Danser, A.H.J.; de Leeuw, P.W.; Dhaun, N.; Rizzoni, D.; Rossignol, P.; Ruilope, L.M.; van den Meiracker, A.; et al. Endothelial factors in the pathogenesis and treatment of chronic kidney disease Part II: Role in disease conditions: A joint consensus statement from the European Society of Hypertension Working Group on Endothelin and Endothelial Factors and the Japanese Society of Hypertension. J. Hypertens. 2018, 36, 462–471. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta–Mol. Basis Dis. 2013, 1832, 2216–2231. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Ludmer, P.L.; Selwyn, A.P.; Shook, T.L.; Wayne, R.R.; Mudge, G.H.; Wayne Alexander, R.; Ganz, P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986, 315, 1046–1051. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flowmediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef]
- Omura, M.; Kobayashi, S.; Mizukami, Y.; Mogami, K.; Todoroki-Ikeda, N.; Miyake, T.; Matsuzaki, M. Eicosapentaenoic acid (EPA) induces Ca(2+)- independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001, 487, 361–366. [Google Scholar] [CrossRef]
- Lawson, D.L.; Mehta, J.L.; Saldeen, K.; Saldeen, T.G. Omega-3 polyunsaturated fatty acids augment endothelium-dependent vasorelaxation by enhanced release of EDRF and vasodilator prostaglandins. Eicosanoids 1991, 4, 217–223. [Google Scholar]
- Raimondi, L.; Lodovici, M.; Visioli, F.; Sartiani, L.; Cioni, C.; Alfrano, C.; Banchelli, G.; Pirisino, R.; Cecchi, E.; Cerbai, E.; et al. n-3 polyunsaturated fatty acids supplementation decreases asymmetric dimethyl arginine and arachidonate accumulation in aging spontaneously hypertensive rats. Eur. J. Nutr. 2005, 44, 327–333. [Google Scholar] [CrossRef]
- Niazi, Z.R.; Silva, G.C.; Ribeiro, T.P.; León-González, A.J.; Kassem, M.; Mirajkar, A.; Alvi, A.; Abbas, M.; Zgheel, F.; Schini-Kerth, V.B.; et al. EPA:DHA 6:1 prevents angiotensin II- induced hypertension and endothelial dysfunction in rats: Role of NADPH oxidase- and COX- derived oxidative stress. Hypertens. Res. 2017, 40, 966–975. [Google Scholar] [CrossRef]
- Suzuki, H.; De Lano, F.A.; Parks, D.A.; Jamshidi, N.; Granger, D.N.; Ishii, H.; Suematsu, M.; Zweifach, B.W.; Schmid-Schönbein, G.W. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl. Acad. Sci. USA 1998, 95, 4754–4759. [Google Scholar] [CrossRef]
- Erdogan, H.; Fadillioglu, E.; Ozgocmen, S.; Sogut, S.; Ozyurt, B.; Akyol, O.; Ardicoglu, O. Effect of fish oil supplementation on plasma oxidant/ antioxidant status in rats. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Mayneris-Perxachs, J.; Lovegrove, J.A.; Todd, S.; Yaqoob, P. Fish-oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am. J. Clin. Nutr. 2014, 100, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Limbu, R.; Cottrell, G.S.; McNeish, A.J. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS ONE 2018, 13, e0192484. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Chino, D.; Kobayashi, T.; Obara, K.; Miyauchi, S.; Tanaka, Y. Selective and potent inhibitory effect of docosahexaenoic acid (DHA) on U46619-induced contraction in rat aorta. J. Smooth Muscle Res. 2013, 49, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.U.; Kathirvel, K.; Choudhury, S.; Garg, S.K.; Mishra, S.K. Eicosapentaenoic acid-induced endothelium-dependent and independent relaxation of sheep pulmonary artery. Eur. J. Pharmacol. 2010, 636, 108–113. [Google Scholar] [CrossRef]
- Wang, R.X.; Chai, Q.; Lu, T.; Lee, H.C. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc. Res. 2011, 90, 344–352. [Google Scholar] [CrossRef]
- Engler, M.B.; Engler, M.M. Docosahexaenoic acid–induced vasorelaxation in hypertensive rats: Mechanisms of action. Biol. Res. Nurs. 2000, 2, 85–95. [Google Scholar] [CrossRef]
- Sato, K.; Chino, D.; Nishioka, N.; Kanai, K.; Aoki, M.; Obara, K.; Miyauchi, S.; Tanaka, Y. Pharmacological evidence showing significant roles for potassium channels and CYP epoxygenase metabolites in the relaxant effects of docosahexaenoic acid on the rat aorta contracted with U46619. Biol. Pharm. Bull. 2014, 37, 394–403. [Google Scholar] [CrossRef]
- Villalpando, D.M.; Navarro, R.; Del Campo, L.; Largo, C.; Munoz, D.; Tabernero, M.; Baeza, R.; Otero, C.; Garcìa, H.S.; Ferrer, M. Effect of dietary docosahexaenoic acid supplementation on the participation of vasodilator factors in aorta from orchidectomized rats. PLoS ONE 2015, 10, e0142039. [Google Scholar] [CrossRef]
- Riedel, M.J.; Light, P.E. Saturated and cis/trans unsaturated acyl CoA esters differentially regulate wild-type and polymorphic beta-cell ATP-sensitive K+ channels. Diabetes 2005, 54, 2070–2079. [Google Scholar] [CrossRef]
- Mies, F.; Shlyonsky, V.; Goolaerts, A.; Sariban-Sohraby, S. Modulation of epithelial Na+ channel activity by long-chain n-3 fatty acids. American Journal of Physiology. Ren. Physiol. 2004, 287, F850–F855. [Google Scholar] [CrossRef]
- Tai, C.C.; Chen, C.Y.; Lee, H.S.; Wang, Y.C.; Li, T.K.; Mersamm, H.J.; Ding, S.T.; Wang, P.H. Docosahexaenoic acid enhances hepatic serum amyloid A expression via protein kinase A-dependent mechanism. J. Biol. Chem. 2009, 284, 32239–32247. [Google Scholar] [CrossRef]
- Gillum, R.F.; Mussolino, M.E.; Madans, J.H. Fish consumption and hypertension incidence in African Americans and whites: The NHANES I Epidemiologic Follow-up Study. J. Natl. Med. Assoc. 2001, 93, 124–128. [Google Scholar]
- Steffen, L.M.; Kroenke, C.H.; Yu, X.; Pereira, M.A.; Slattery, M.L.; Van Horn, L.; Gross, M.D.; Jacobs, D.R. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Clin. Nutr. 2005, 82, 1169–1177. [Google Scholar] [CrossRef]
- Baik, I.; Abbott, R.D.; Curb, J.D.; Shin, C. Intake of fish and n-3 fatty acids and future risk of metabolic syndrome. J. Am. Diet. Assoc. 2010, 110, 1018–1026. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Mos, L.; Sechi, L.A. The metabolic syndrome and the membrane content of polyunsaturated fatty acids in hypertensive patients. Metab. Syndr. Relat. Disord. 2015, 13, 343–351. [Google Scholar] [CrossRef]
- Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N. Engl. J. Med. 1989, 321, 129–135. [Google Scholar] [CrossRef]
- Christen, W.G.; Gaziano, J.M.; Hennekens, C.H. Design of Physicians’ Health Study II–a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann. Epidemiol. 2000, 10, 125–134. [Google Scholar] [CrossRef]
- Diez, J. Arterial stiffness and extracellular matrix. Adv. Cardiol. 2007, 44, 76–95. [Google Scholar] [CrossRef]
- Pase, M.P.; Grima, N.A.; Sarris, J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br. J. Nutr. 2011, 106, 974–980. [Google Scholar] [CrossRef]
- Pase, M.P.; Grima, N.; Cockerell, R.; Stough, C.; Scholey, A.; Sali, A.; Pipingas, A. The effects of long-chain omega-3 fish oils and multivitamins on cognitive and cardiovascular function: A randomized, controlled clinical trial. J. Am. Coll. Nutr. 2015, 34, 21–31. [Google Scholar] [CrossRef]
- Yeboah, J.; Crouse, J.R.; Hsu, F.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multiethnic study of atherosclerosis. Circulation 2009, 120, 502–550. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Dialti, V.; Mos, L.; Sechi, L.A. The vascular response to vasodilators is related to the membrane content of polyunsaturated fatty acids in hypertensive patients. J. Hypertens. 2015, 33, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Dialti, V.; Mos, L.; Sechi, L.A. Effects of the consumption of fish meals on the carotid IntimaMedia thickness in patients with hypertension: A prospective study. J. Atheroscler. Thromb. 2014, 21, 941–956. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Mayer-Davis, E.; Swords Jenny, N.; Jiang, R.; Ouyang, P.; Steffen, L.M.; Siscovick, D.; Wu, C.; et al. Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am. J. Clin. Nutr. 2008, 88, 1111–1118. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, B.; Wang, P.; Chen, C.; Chen, Y.; Su, Y. Erythrocyte membrane n-3 fatty acid levels and carotid atherosclerosis in Chinese men and women. Atherosclerosis 2014, 232, 79–85. [Google Scholar] [CrossRef]
- Hjerkinn, E.M.; Abdelnoor, M.; Breivik, L.; Bergengen, L.; Ellingsen, I.; Seljeflot, I.; Aase, O.; Klemsdal, T.O.; Hjermann, I.; Arnesen, H. Effect of diet or very long chain omega-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intimamedia thickness and by pulse wave propagation in elderly men with hypercholester- olaemia. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 325–333. [Google Scholar] [CrossRef]
- Thies, F.; Garry, J.M.C.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.J.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Cawood, A.L.; Ding, R.; Napper, F.L.; Young, R.H.; Williams, J.A.; Ward, M.J.A.; Gudmundsen, O.; Vige, R.; Payne, S.P.K.; Ye, S.; et al. Eicosapentaenoic acid (EPA) from highly concentrated n − 3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 2010, 212, 252–259. [Google Scholar] [CrossRef]
- Yamano, T.; Kubo, T.; Shiono, Y.; Shimamura, K.; Orii, M.; Tanimoto, T.; Matsuo, Y.; Ino, Y.; Kitabata, H.; Yamaguchi, T.; et al. Impact of eicosapentaenoic acid treatment on the fibrous cap thickness in patients with coronary atherosclerotic plaque: An optical coherence tomography study. J. Atheroscler. Thromb. 2015, 22, 52–61. [Google Scholar] [CrossRef]
- Venturini, D.; Simão, A.N.C.; Urbano, M.R.; Dichi, I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition 2015, 31, 834–840. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Senanayake, V.K.; Pu, S.; Jenkins, D.J.A.; Connelly, P.W.; Lamarche, B.; Couture, P.; Charest, A.; Baril-Gravel, L.; West, S.G.; et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 88–97. [Google Scholar] [CrossRef]
- Shinozaki, K.; Kambayashi, J.; Kawasaki, T.; Uemura, Y.; Sakon, M.; Shiba, E.; Shibuya, T.; Nakamura, T.; Mori, T. The long-term effect of eicosapentaenoic acid on serum levels of lipoprotein (a) and lipids in patients with vascular disease. J. Atheroscler. Thromb. 1996, 2, 107–109. [Google Scholar] [CrossRef]
- Nishio, R.; Shinke, T.; Otake, H.; Nakagawa, M.; Nagoshi, R.; Inoue, T.; Kozuki, A.; Hariki, H.; Osue, T.; Taniguchi, Y.; et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis 2014, 234, 114–119. [Google Scholar] [CrossRef]
- Block, R.C.; Kakinami, L.; Jonovich, M.; Antonetti, I.; Lawrence, P.; Meednu, N.; Calderon-Artero, P.; Mousa, S.A.; Brenna, J.T.; Georas, S. The combination of EPA+DHA and low-dose aspirin ingestion reduces platelet function acutely whereas each alone may not in healthy humans. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 143–151. [Google Scholar] [CrossRef]
- Gruppo Italiano per lo Studio della Sopravvivenza Nell’infarto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Yokoyama, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; Kita, T.; et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ. J. 2009, 73, 1283–1290. [Google Scholar] [CrossRef]
- Sasaki, J.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Itakura, H.; Hishida, H.; Kita, T.; et al. Relationship between coronary artery disease and non-HDL-C, and efect of highly purifed EPA on the risk of coronary artery disease in hypercholesterolemic patients treated with statins: Sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). J. Atheroscler. Thromb. 2012, 19, 194–204. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- ORIGIN Trial Investigators; Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Díaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Risk and Prevention Study Collaborative Group; Roncaglioni, M.C.; Tombesi, M.; Avanzini, F.; Barlera, S.; Caimi, V.; Longoni, P.; Marzona, I.; Milani, V.; Silletta, M.G.; et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs. Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- ASCEND Study Collaborative Group; Murawska, A.; Young, A.; Lay, M.; Chen, F.; Sammons, E.; Waters, E.; Adler, A.; Bodansky, J.; Farmer, A.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
| Cardiac Hemodynamics | Endothelium-Dependent Regulation of Vascular Tone | Endothelium-Independent Regulation of Vascular Tone |
|---|---|---|
| Slowing of heart rate | Increased nitric oxide generation | Inhibition of L-type Ca2+ channels |
| Increased stroke volume | Decreased oxidative stress | Activation of K+ channels |
| Increased regeneration of endothelial cells | Activation of TPRV4 channel | |
| Inhibition of TRPC1/5 channels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brosolo, G.; Da Porto, A.; Marcante, S.; Picci, A.; Capilupi, F.; Capilupi, P.; Bertin, N.; Vivarelli, C.; Bulfone, L.; Vacca, A.; et al. Omega-3 Fatty Acids in Arterial Hypertension: Is There Any Good News? Int. J. Mol. Sci. 2023, 24, 9520. https://doi.org/10.3390/ijms24119520
Brosolo G, Da Porto A, Marcante S, Picci A, Capilupi F, Capilupi P, Bertin N, Vivarelli C, Bulfone L, Vacca A, et al. Omega-3 Fatty Acids in Arterial Hypertension: Is There Any Good News? International Journal of Molecular Sciences. 2023; 24(11):9520. https://doi.org/10.3390/ijms24119520
Chicago/Turabian StyleBrosolo, Gabriele, Andrea Da Porto, Stefano Marcante, Alessandro Picci, Filippo Capilupi, Patrizio Capilupi, Nicole Bertin, Cinzia Vivarelli, Luca Bulfone, Antonio Vacca, and et al. 2023. "Omega-3 Fatty Acids in Arterial Hypertension: Is There Any Good News?" International Journal of Molecular Sciences 24, no. 11: 9520. https://doi.org/10.3390/ijms24119520
APA StyleBrosolo, G., Da Porto, A., Marcante, S., Picci, A., Capilupi, F., Capilupi, P., Bertin, N., Vivarelli, C., Bulfone, L., Vacca, A., Catena, C., & Sechi, L. A. (2023). Omega-3 Fatty Acids in Arterial Hypertension: Is There Any Good News? International Journal of Molecular Sciences, 24(11), 9520. https://doi.org/10.3390/ijms24119520





