Genome-Wide Analysis of WRKY Transcription Factors Involved in Abiotic Stress and ABA Response in Caragana korshinskii
Abstract
1. Introduction
2. Results
2.1. Identification and Chromsome Distribution of WRKY Genes from C. korshinskii
2.2. Phylogenetic and Multiple Sequence Alignment of the CkWRKYs and AtWRKYs
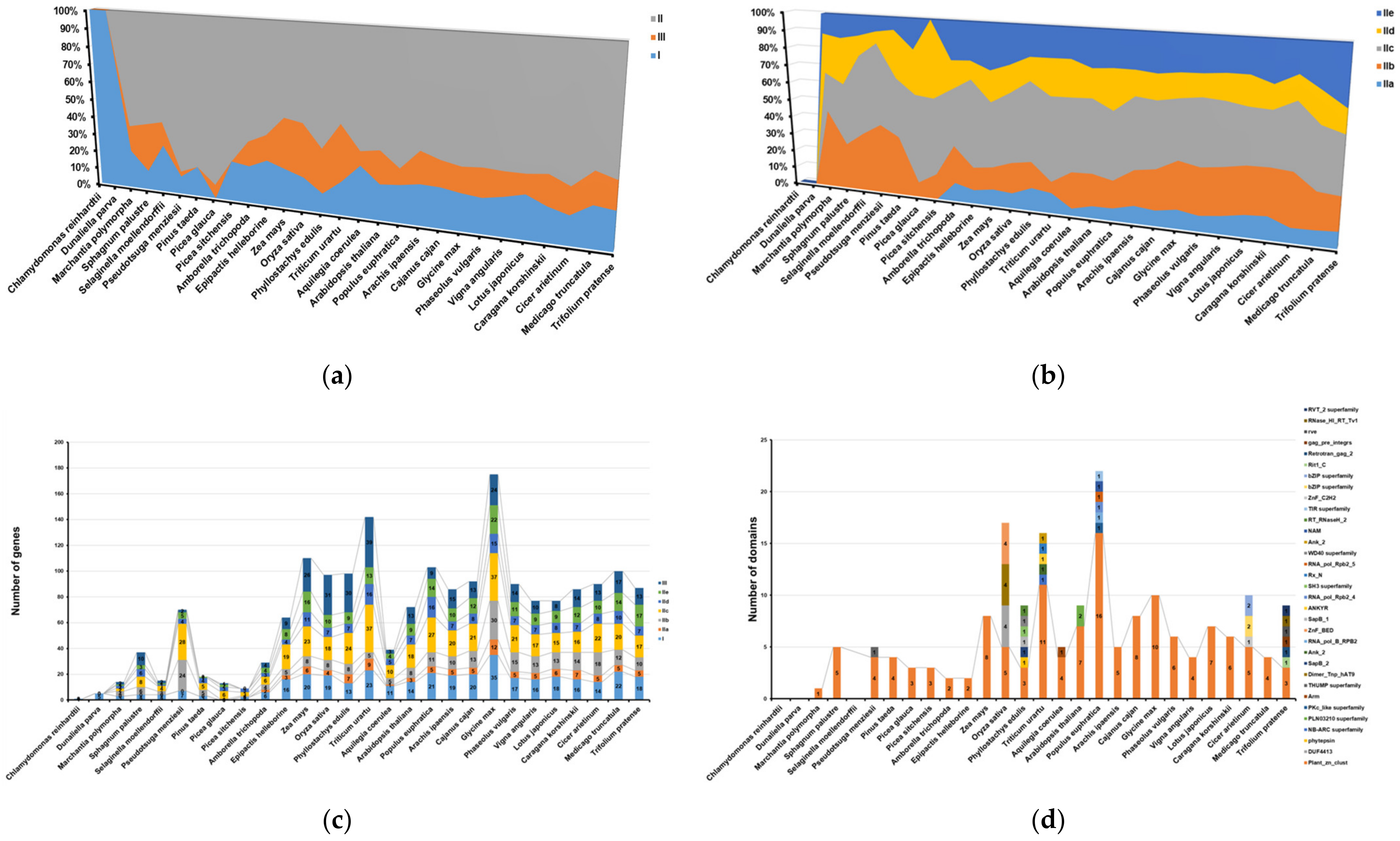
2.3. The Conserved Motif Analysis
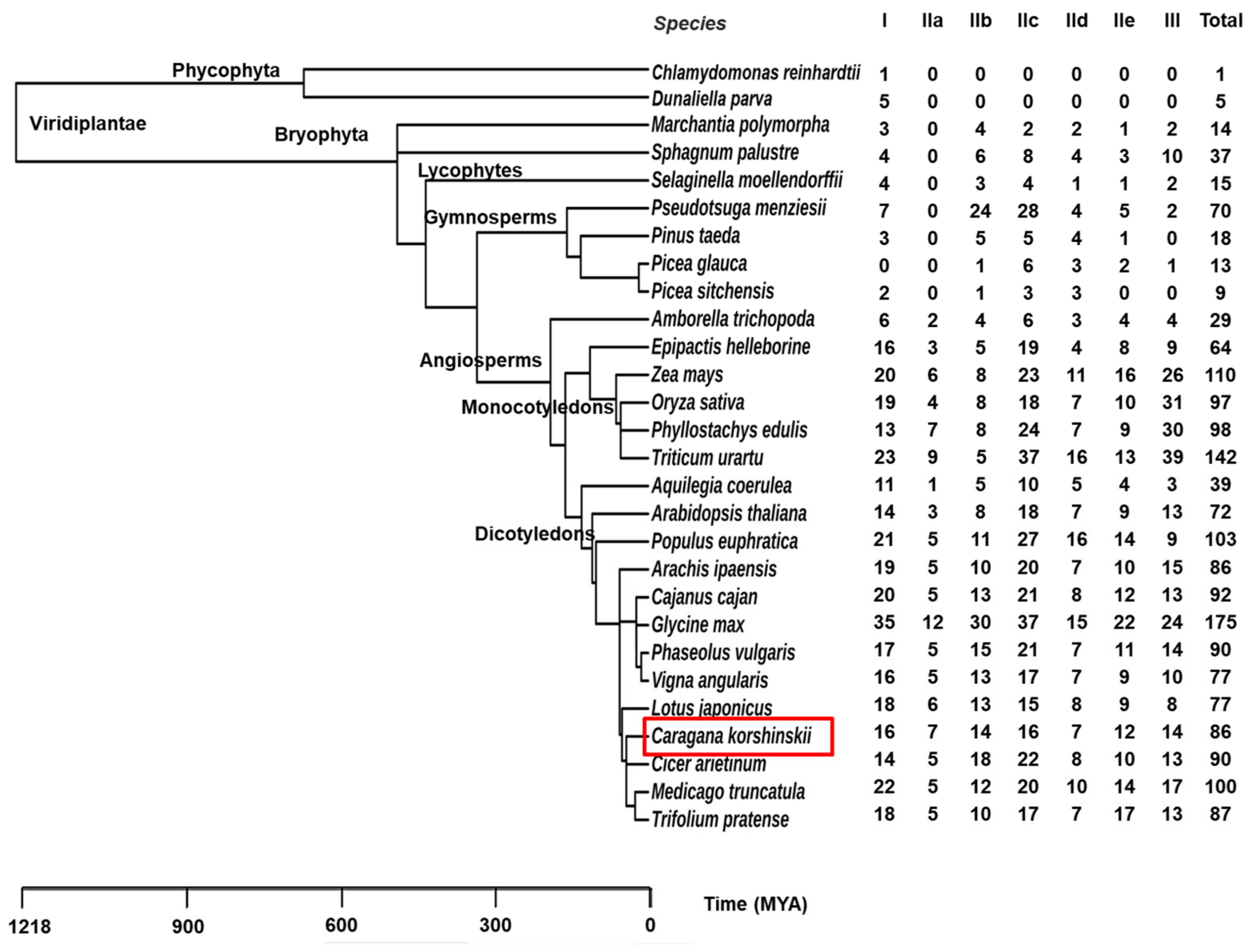
2.4. Evolution Analysis of WRKYs among Different Species
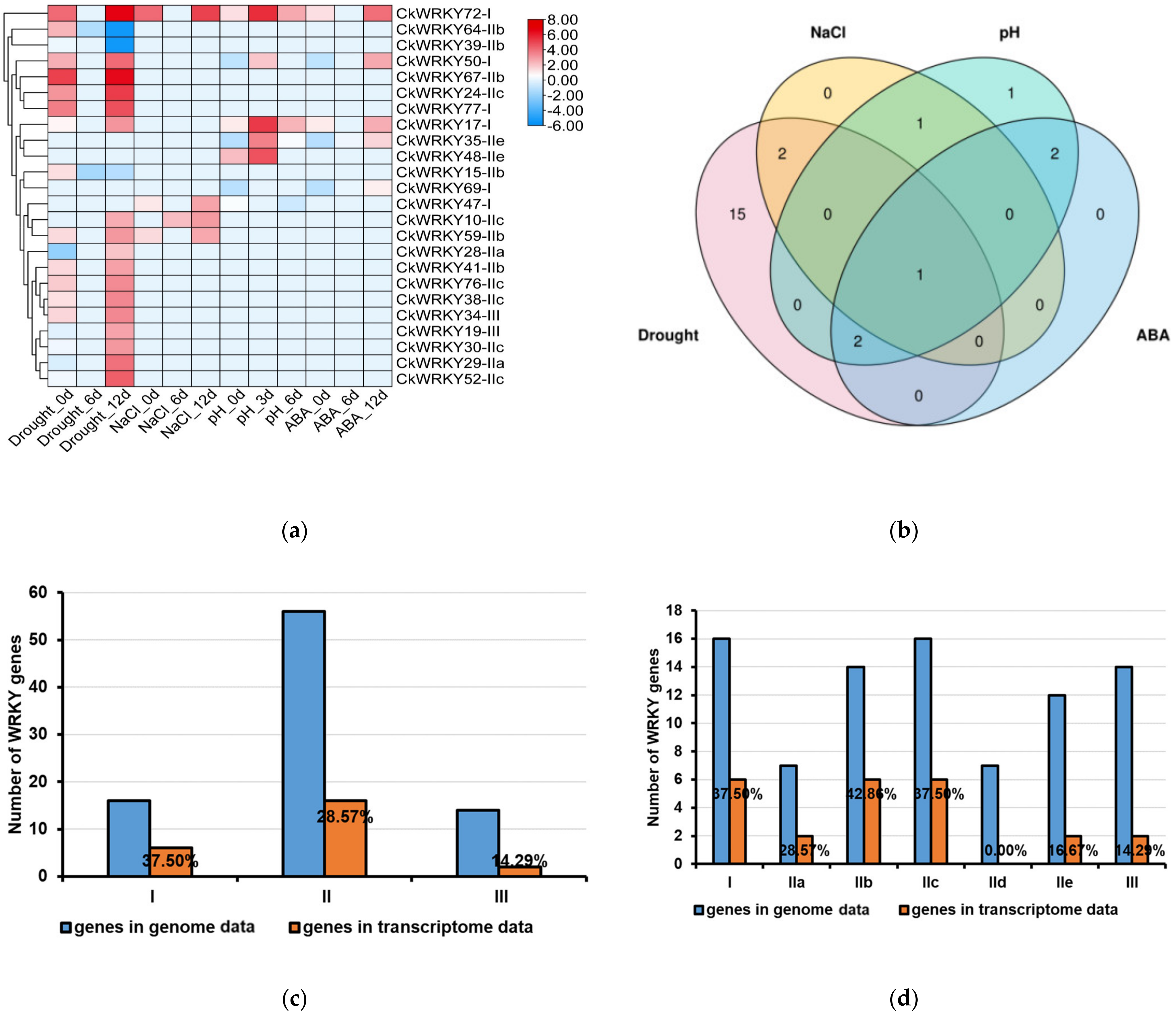
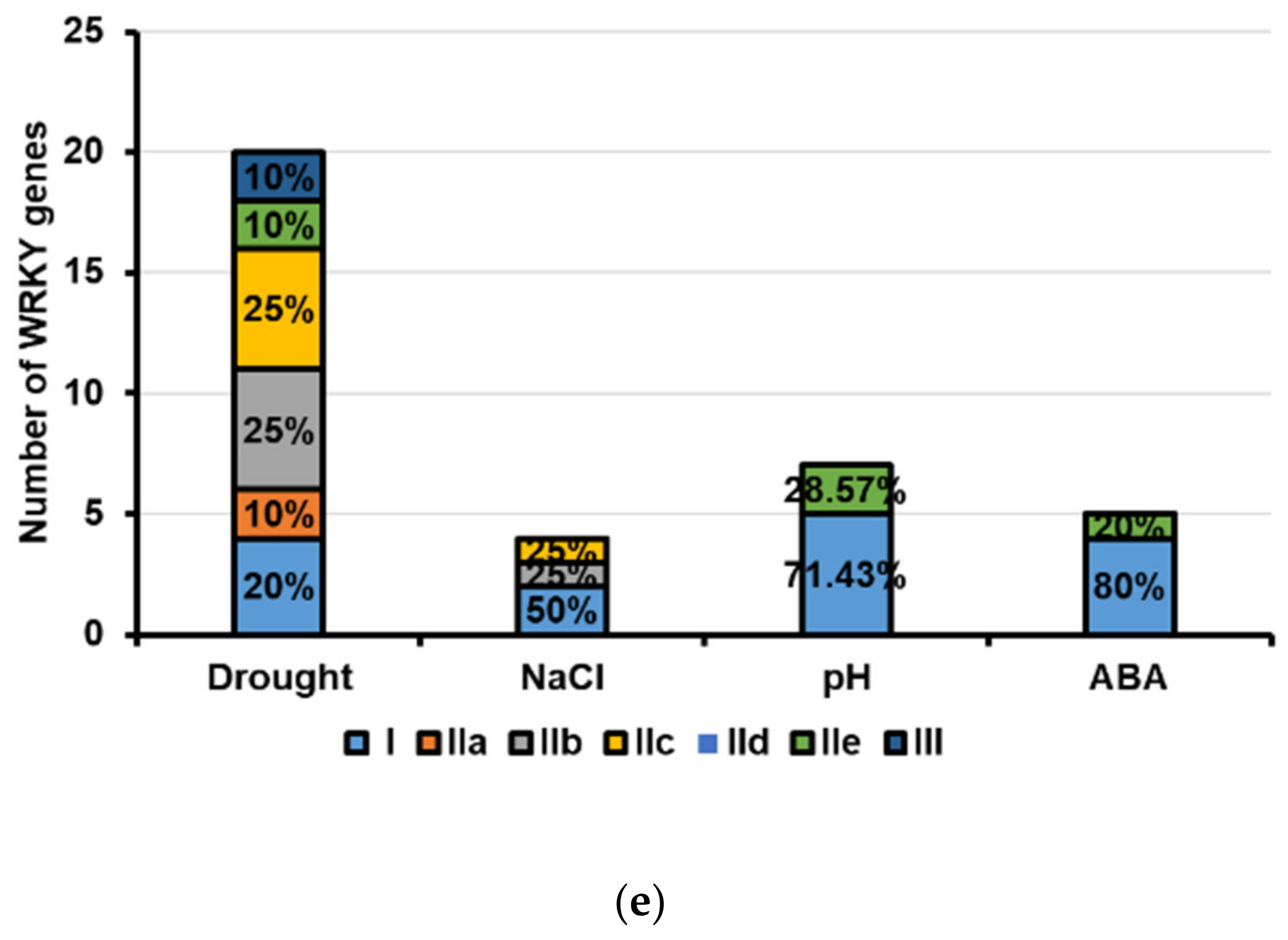
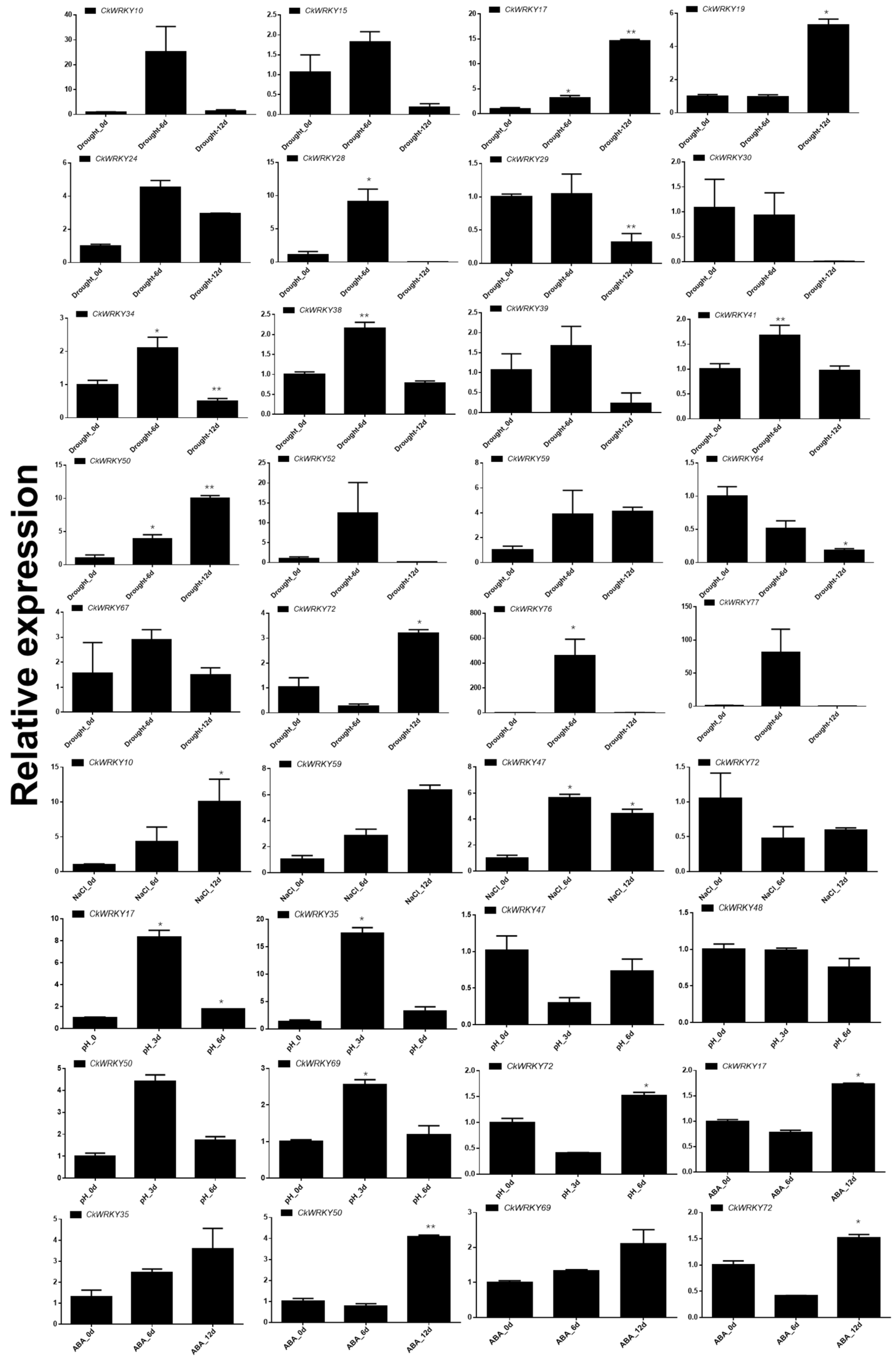
2.5. Expression Profiles of CkWRKYs under Drought, Salt, Alkali and ABA Treatments
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification and Distribution of CkWRKYs
4.2. Phylogenetic, Conserved Domains and Motif Analysis
4.3. Evolution Analysis
4.4. Abiotic Stress and ABA Treatments
4.5. Heatmap Analysis and RT-qPCR Verification of the Transcriptome Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kosova, K.; Vitamvas, P.; Prasil, I.T. Wheat and barley dehydrins under cold, drought, and salinity-what can LEA-II proteins tell us about plant stress response? Front. Plant Sci. 2014, 5, 343. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, C.; Yang, J.; Tian, Z.; Jiang, R.; Yang, F.; Jiao, K.; Qi, M.; Liu, L.; Zhang, B.; et al. Comprehensive Transcriptome Analysis of Responses during Cold Stress in Wheat (Triticum aestivum L.). Genes 2023, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta-Gene Regul. Mech. 2012, 1819, 120–128. [Google Scholar] [CrossRef]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and Mechanism of WRKY Transcription Factors in Abiotic Stress Responses of Plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef]
- Eulgem, T.; Somssich, I.E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007, 10, 366–371. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Ulker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar] [CrossRef]
- Tripathi, P.; Rabara, R.C.; Rushton, P.J. A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta 2014, 239, 255–266. [Google Scholar] [CrossRef]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. MGG 1994, 244, 563–571. [Google Scholar] [CrossRef]
- Kumari, S.; Kanth, B.K.; Ahn, J.Y.; Kim, J.H.; Lee, G.J. Genome-Wide Transcriptomic Identification and Functional Insight of Lily WRKY Genes Responding to Botrytis Fungal Disease. Plants 2021, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Xu, Z.S.; Tian, C.; Huang, Y.; Wang, F.; Xiong, A.S. Genomic identification of WRKY transcription factors in carrot (Daucus carota) and analysis of evolution and homologous groups for plants. Sci. Rep. 2016, 6, 23101. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Zhang, X.; Pang, C.; Song, M.; Wei, H.; Fan, S.; Yu, S. Genome-wide analysis of the WRKY gene family in cotton. Mol. Genet. Genom. MGG 2014, 289, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wang, Z.; Wang, J.; Miao, H.; Zhang, J.; Xu, B.; Liu, J.; Jin, Z.; Liu, J. Genome-Wide Analysis of the Banana WRKY Transcription Factor Gene Family Closely Related to Fruit Ripening and Stress. Plants 2022, 11, 662. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Wang, L.; Liu, X.; Liu, Y.; Phillips, J.; Deng, X. A WRKY transcription factor participates in dehydration tolerance in Boea hygrometrica by binding to the W-box elements of the galactinol synthase (BhGolS1) promoter. Planta 2009, 230, 1155–1166. [Google Scholar] [CrossRef]
- Yang, Z.; Chi, X.; Guo, F.; Jin, X.; Luo, H.; Hawar, A.; Chen, Y.; Feng, K.; Wang, B.; Qi, J.; et al. SbWRKY30 enhances the drought tolerance of plants and regulates a drought stress-responsive gene, SbRD19, in sorghum. J. Plant Physiol. 2020, 246, 153142. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Gao, C.; Wang, L.; Wang, Y.; Sun, J.; Jiang, J.; Chen, F.; Chen, S. Chrysanthemum WRKY gene CmWRKY17 negatively regulates salt stress tolerance in transgenic chrysanthemum and Arabidopsis plants. Plant Cell Rep. 2015, 34, 1365–1378. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Xu, S.; Zhang, Z.; Xu, Y.; Zhang, J.; Chong, K. OsMADS57 together with OsTB1 coordinates transcription of its target OsWRKY94 and D14 to switch its organogenesis to defense for cold adaptation in rice. New Phytol. 2018, 218, 219–231. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Chang, H.S.; Gupta, R.; Wang, X.; Zhu, T.; Luan, S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002, 129, 661–677. [Google Scholar] [CrossRef]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. Cell Mol. Biol. 2007, 50, 347–363. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Bot. 2009, 65, 35–47. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Yu, D. Arabidopsis WRKY Transcription Factors WRKY12 and WRKY13 Oppositely Regulate Flowering under Short-Day Conditions. Mol. Plant 2016, 9, 1492–1503. [Google Scholar] [CrossRef]
- Discovery of WRKY transcription factors through transcriptome analysis and characterization of a novel methyl jasmonate-inducible PqWRKY1 gene from Panax quinquefolius. Plant Cell Tissue Organ Cult. 2013, 114, 269–277. [CrossRef]
- Chena, J.; Nolana, T.; Yea, H.; Zhanga, M.; Tongc, H. 3 Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors 4 Are Involved in Brassinosteroid-Regulated Plant Growth and Drought 5 Responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Mao, M.; Wan, D.; Yang, Q.; Yang, F.; Mandlaa; Li, G.; Wang, R. Identification of the WRKY gene family and functional analysis of two genes in Caragana intermedia. BMC Plant Biol. 2018, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, J.; Wang, L.; Wang, S. Genome-Wide Investigation of WRKY Transcription Factors Involved in Terminal Drought Stress Response in Common Bean. Front. Plant Sci. 2017, 8, 380. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Z.; Zhang, L.; Yao, W.; Xu, Z.; Liao, B.; Mi, Y.; Gao, H.; Jiang, C.; Duan, L.; et al. Genomic Characterization of WRKY Transcription Factors Related to Andrographolide Biosynthesis in Andrographis paniculata. Front. Genet. 2020, 11, 601689. [Google Scholar] [CrossRef]
- Song, H.; Sun, W.; Yang, G.; Sun, J. WRKY transcription factors in legumes. BMC Plant Biol. 2018, 18, 243. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Park, Y.H.; Bae, H. Novel Genomic and Evolutionary Insight of WRKY Transcription Factors in Plant Lineage. Sci. Rep. 2016, 6, 37309. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Wang, H.; Guo, Z. WRKY transcription factors: Evolution, binding, and action. Phytopathol. Res. 2019, 1, 13. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, Y.; Jiang, Q.; Yang, J.; Zhao, W.; Taylor, I.A.; Peng, Y.-L.; Wang, D.; Liu, J. Structural basis of dimerization and dual W-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 2019, 47, 4308–4318. [Google Scholar] [CrossRef]
- Englbrecht, C.C.; Schoof, H.; Bohm, S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Lee, J.H.; Yoo, J.H.; Moon, B.C.; Choi, M.S.; Kang, Y.H.; Lee, S.M.; Kim, H.S.; Kang, K.Y.; Chung, W.S.; et al. WRKY group IId transcription factors interact with calmodulin. FEBS Lett. 2005, 579, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Guo, Z.J.; Wang, H.H.; Li, J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. Dna Res. 2005, 12, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, P.; Lin, J.Y.; Zhao, C.; Bi, Y.; Wang, X. Genome-Wide Identification and Characterization of WRKY Gene Family in Peanut. Front. Plant Sci. 2016, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.M.; Iyer, L.M.; Balaji, S.; Aravind, L. The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons. Nucleic Acids Res. 2006, 34, 6505–6520. [Google Scholar] [CrossRef]
- Qiao, Z.; Li, C.L.; Zhang, W. WRKY1 regulates stomatal movement in drought-stressed Arabidopsis thaliana. Plant Mol. Biol. 2016, 91, 53–65. [Google Scholar] [CrossRef]
- Lim, C.; Kang, K.; Shim, Y.; Yoo, S.C.; Paek, N.C. Inactivating transcription factor OsWRKY5 enhances drought tolerance through abscisic acid signaling pathways. Plant Physiol. 2022, 188, 1900–1916. [Google Scholar] [CrossRef]
- Hu, Q.; Ao, C.; Wang, X.; Wu, Y.; Du, X. GhWRKY1-like, a WRKY transcription factor, mediates drought tolerance in Arabidopsis via modulating ABA biosynthesis. BMC Plant Biol. 2021, 21, 458. [Google Scholar] [CrossRef]
- Niu, Y.; Li, X.; Xu, C.; Ajab, Z.; Liu, Q.; Majeed, Z.; Guan, Q. Analysis of drought and salt-alkali tolerance in tobacco by overexpressing WRKY39 gene from Populus trichocarpa. Plant Signal. Behav. 2021, 16, 1918885. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Hu, X.; Liu, H.; Lin, Y. W-box and G-box elements play important roles in early senescence of rice flag leaf. Sci. Rep. 2016, 6, 20881. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, F.; Wu, S.; Li, J.; Xu, W. Cotton GhMYB7 is predominantly expressed in developing fibers and regulates secondary cell wall biosynthesis in transgenic Arabidopsis. Sci. China Life Sci. 2016, 59, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Chi, Y.; Fan, B.; Chen, Z. Characterization of Soybean WRKY Gene Family and Identification of Soybean WRKY Genes that Promote Resistance to Soybean Cyst Nematode. Sci. Rep. 2017, 7, 17804. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Jin, X.; Bao, Q.; Mei, C.; Zhou, Q.; Min, X.; Liu, Z. WRKY Transcription Factors in Medicago sativa L.: Genome-Wide Identification and Expression Analysis Under Abiotic Stress. DNA Cell Biol. 2020, 39, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Zhibiao, N. Genome-wide identification and analysis of WRKY transcription factors in Medicago truncatula. Hereditas 2014, 36, 152–168. [Google Scholar] [CrossRef]
- Yue, H.; Wang, M.; Liu, S.; Du, X.; Song, W.; Nie, X. Transcriptome-wide identification and expression profiles of the WRKY transcription factor family in Broomcorn millet (Panicum miliaceum L.). BMC Genom. 2016, 17, 343. [Google Scholar] [CrossRef]
- Wei, W.; Hu, Y.; Han, Y.T.; Zhang, K.; Zhao, F.L.; Feng, J.Y. The WRKY transcription factors in the diploid woodland strawberry Fragaria vesca: Identification and expression analysis under biotic and abiotic stresses. Plant Physiol. Biochem. 2016, 105, 129–144. [Google Scholar] [CrossRef]
- Song, H.; Wang, P.; Hou, L.; Zhao, S.; Wang, X. Global Analysis of WRKY Genes and Their Response to Dehydration and Salt Stress in Soybean. Front. Plant Sci. 2016, 7, 9. [Google Scholar] [CrossRef]
- He, G.H.; Xu, J.Y.; Wang, Y.X.; Liu, J.M.; Li, P.S.; Chen, M.; Ma, Y.Z.; Xu, Z.S. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Yin, G.; Xu, H.; Xiao, S.; Qin, Y.; Li, Y.; Yan, Y.; Hu, Y. The large soybean (Glycine max) WRKY TF family expanded by segmental duplication events and subsequent divergent selection among subgroups. BMC Plant Biol. 2013, 13, 148. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Ma, R.; Chang, Y.; Qin, X.; Xu, J.; Fu, Y. Genome-wide transcriptome analysis and characterization of the cytochrome P450 flavonoid biosynthesis genes in pigeon pea (Cajanus cajan). Planta 2022, 255, 120. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xi, B.; Xiaoli, S.; Dan, Z.; Baohui, L.; Wei, J.; Hua, C.; Lei, C.; Jing, W.; Mengran, H. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J. Exp. Bot. 2013, 64, 2155–2169. [Google Scholar]
- Qin, Y.; Tian, Y.; Liu, X. A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015, 464, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Y.; Xing, F.; Wang, N.; Wen, F.; Zhu, C. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma 2016, 253, 1265–1281. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, S.; Xu, L.; Zhu, L.; Wang, D.; Liu, Y.; Liu, S.; Hao, Z.; Lu, Y.; Yang, L.; et al. Genome-wide identification of the Liriodendron chinense WRKY gene family and its diverse roles in response to multiple abiotic stress. BMC Plant Biol. 2022, 22, 25. [Google Scholar] [CrossRef]
- Rinerson, C.I.; Rabara, R.C.; Tripathi, P.; Shen, Q.J.; Rushton, P.J. The evolution of WRKY transcription factors. BMC Plant Biol. 2015, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Chang, X.; Zhi, Y.; Wang, L.; Xing, G.; Song, W.; Nie, X. Evolution and Identification of the WRKY Gene Family in Quinoa (Chenopodium quinoa). Genes 2019, 10, 131. [Google Scholar] [CrossRef]
- Gang, L.; Shizhuo, Y.; Diqiu, Y.; Yanjuan, J. Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell 2014, 26, 230–245. [Google Scholar]
- Fan, X.; Guo, Q.; Xu, P.; Gong, Y.; Shu, H.; Yang, Y.; Ni, W.; Zhang, X.; Shen, X. Transcriptome-wide identification of salt-responsive members of the WRKY gene family in Gossypium aridum. PLoS ONE 2015, 10, e0126148. [Google Scholar] [CrossRef]
- Chang, X.; Yang, Z.; Zhang, X.; Zhang, F.; Huang, X.; Han, X. Transcriptome-wide identification of WRKY transcription factors and their expression profiles under different stress in Cynanchum thesioides. PeerJ 2022, 10, e14436. [Google Scholar] [CrossRef]
- Okay, S.; Derelli, E.; Unver, T. Transcriptome-wide identification of bread wheat WRKY transcription factors in response to drought stress. Mol. Genet. Genom. 2014, 289, 765–781. [Google Scholar] [CrossRef]
- Li, Z.; Liang, F.; Zhang, T.; Fu, N.; Pei, X.; Long, Y. Enhanced tolerance to drought stress resulting from Caragana korshinskii CkWRKY33 in transgenic Arabidopsis thaliana. BMC Genom. Data 2021, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yu, F.; Gao, Z.; An, H.; Cao, X.; Guo, X. GhWRKY3, a novel cotton (Gossypium hirsutum L.) WRKY gene, is involved in diverse stress responses. Mol. Biol. Rep. 2011, 38, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ren, Y.; Wang, D.; Farooq, T.; He, Z.; Zhang, C.; Li, S.; Yang, X.; Zhou, X. A group I WRKY transcription factor regulates mulberry mosaic dwarf-associated virus-triggered cell death in Nicotiana benthamiana. Mol. Plant Pathol. 2022, 23, 237–253. [Google Scholar] [CrossRef]
- Wu, X.; Xu, J.; Meng, X.; Fang, X.; Xia, M.; Zhang, J.; Cao, S.; Fan, T. Linker histone variant HIS1-3 and WRKY1 oppositely regulate salt stress tolerance in Arabidopsis. Plant Physiol. 2022, 189, 1833–1847. [Google Scholar] [CrossRef]
- Heerah, S.; Katari, M.; Penjor, R.; Coruzzi, G.; Marshall-Colon, A. WRKY1 Mediates Transcriptional Regulation of Light and Nitrogen Signaling Pathways. Plant Physiol. 2019, 181, 1371–1388. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yin, J.; Li, G.; Qi, L.; Yang, F.; Wang, R.; Li, G. Reference gene selection for qRT-PCR in Caragana korshinskii Kom. under different stress conditions. Mol. Biol. Rep. 2014, 41, 2325–2334. [Google Scholar] [CrossRef]
- Wan, D.; Zhang, J.; Wang, R.; Li, G.; Wan, Y. Cloning and Bioinformatics Analysis of Caragana intermedia WRKY12 gene. Mol. Plant Breed. 2020, 18, 9. (In Chinese) [Google Scholar]
- Liu, J.; Wan, Y.; Liu, Y.; Wang, R.; Li, G. Cloning and Bioinformatics Analysis of Caragana intermedia WRKY30. Mol. Plant Breed. 2019, 17, 10. (In Chinese) [Google Scholar]
- Liu, J.; Wang, R.; Li, G.; Wan, Y. Cloning and prokaryotic expression of WRKY48 from Caragana intermedia. Open Life Sci. 2022, 17, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mao, M. Functional analysis of Two WRKY Genes in Caragana intermedia. 2017, Inner Mongolia Agricultural University. (In Chinese)
- Bai, M.; Wan, Y.; Li, G.; Wang, R. Cloning and Bioinformatics Analysis of CiWRKY17 Gene. Genom. Appl. Biol. 2020, 39, 9. (In Chinese) [Google Scholar]
- Yang, Q.; Zhang, T.; Wang, Y.; Li, G.; Ying, J.; Han, X.; Qi, L.; Li, G.; Wang, R. Construction of a Suppression Subtractive Hybridization Library of Caragana korshinskii Under Drought Stress and Cloning of CkWRKY1 Gene. Sci. Silvae Sin. 2013, 49, 62–68. (In Chinese) [Google Scholar]
- Wan, Y. Identification of the WRKY Gene Family and Functional Analysis of Two CiWRKYs in Caragana intermedia. 2018, Inner Mongolia Agricultural University. (In Chinese)
- Wan, Y.; Mao, M.; Wan, D.; Liu, J.; Wang, G.; Li, G.; Wang, R. Caragana intermedia WRKY75 Altered Arabidopsis thaliana Tolerance to Salt Stress and ABA. Acta Bot. Boreali-Occident. Sin. 2018, 38, 9. (In Chinese) [Google Scholar]
- Mao, M.; Wan, Y.; Li, G.; Yang, Q.; Wang, R. Cloning and Expression Analysis of CiWRKY2 Gene in Caragana intermedia. Mol. Plant Breed. 2017, 15, 9. (In Chinese) [Google Scholar]
- Zhang, W.; Li, S.; Liu, Y.; Wang, R.; Li, G.; Yang, Q.; Wan, Y. Cloning and Bioinformatics Analysis of Caragana intermedia WRKY15. Chin. J. Grassl. 2019, 9. (In Chinese) [Google Scholar]
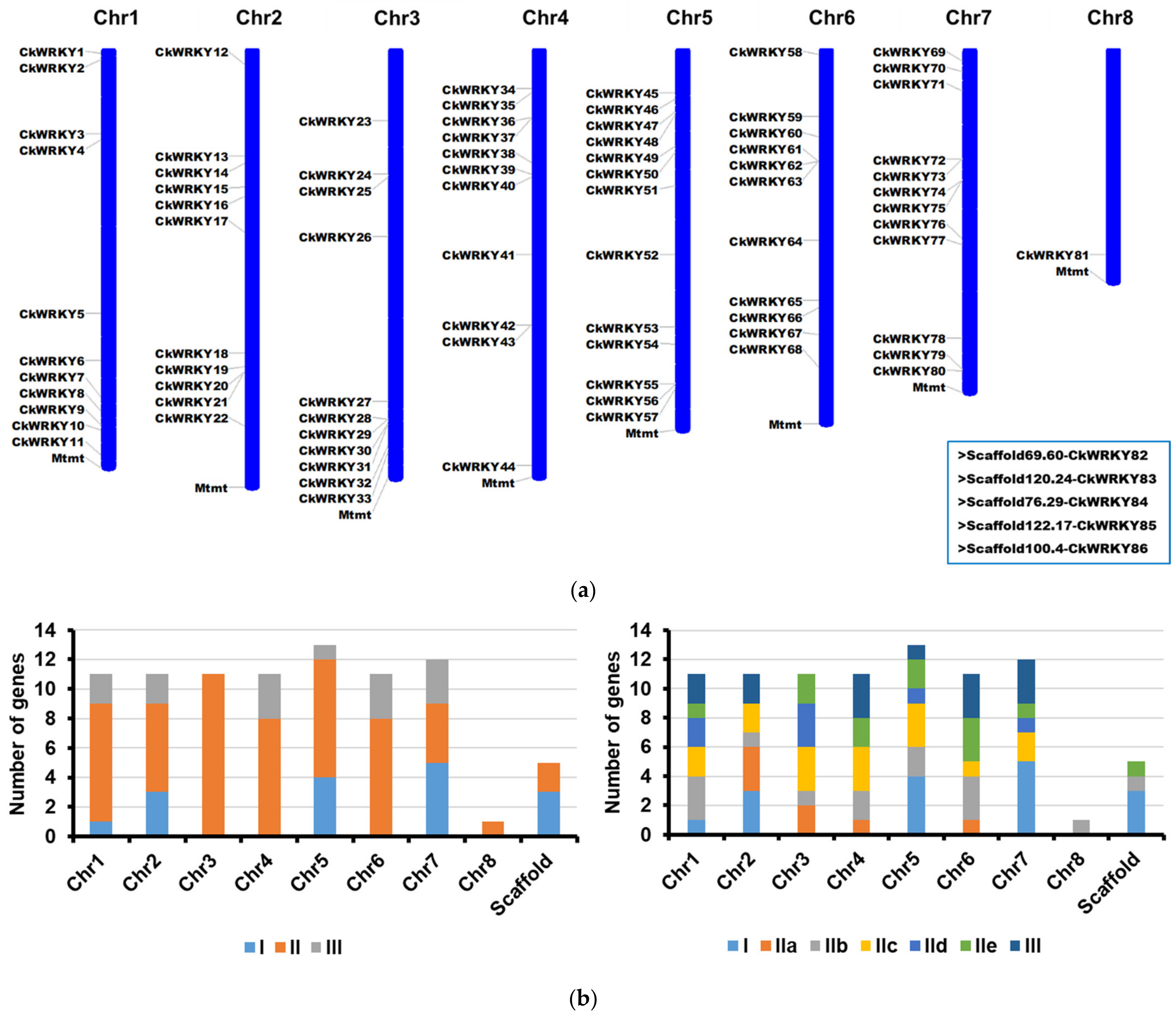
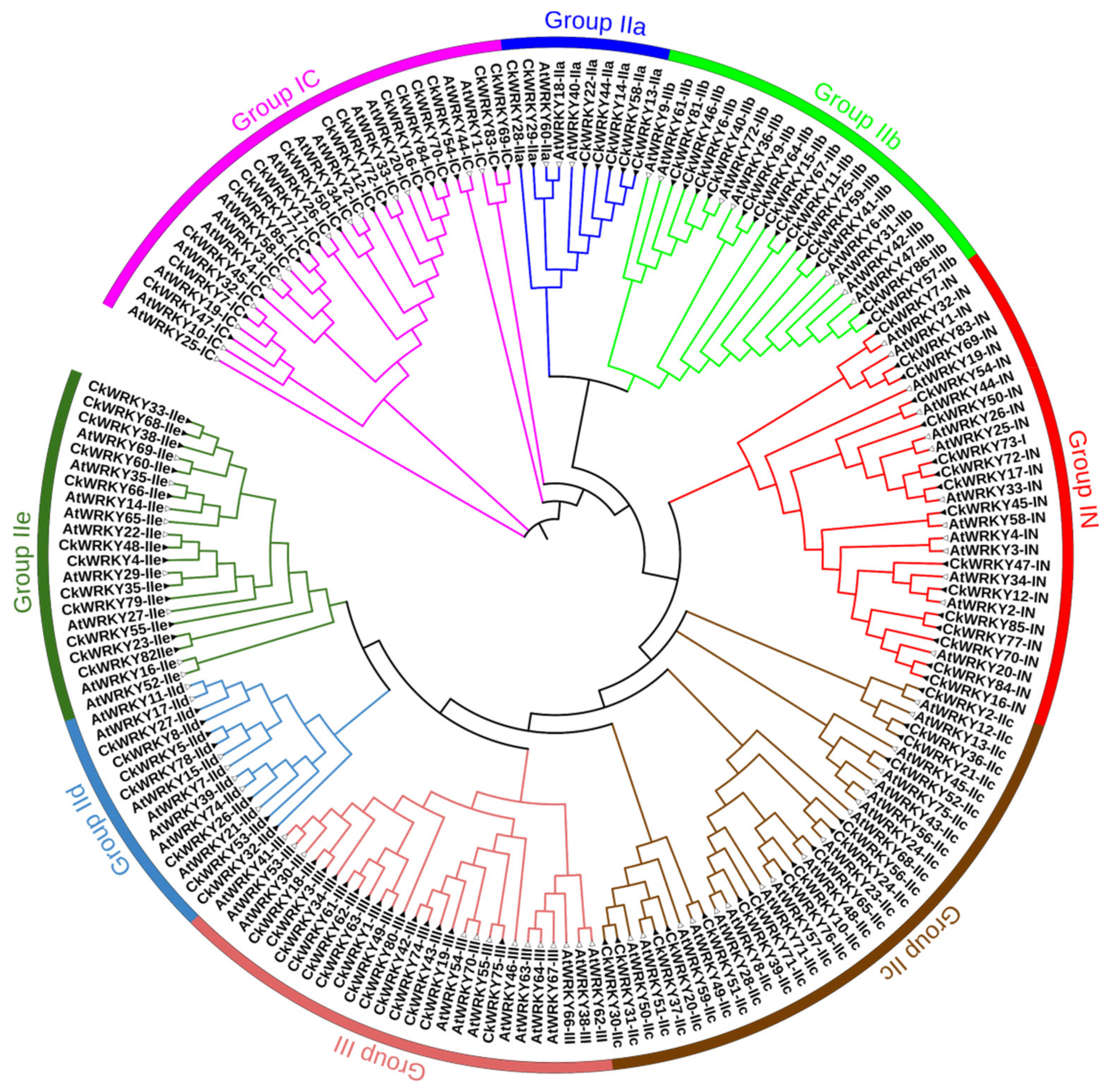
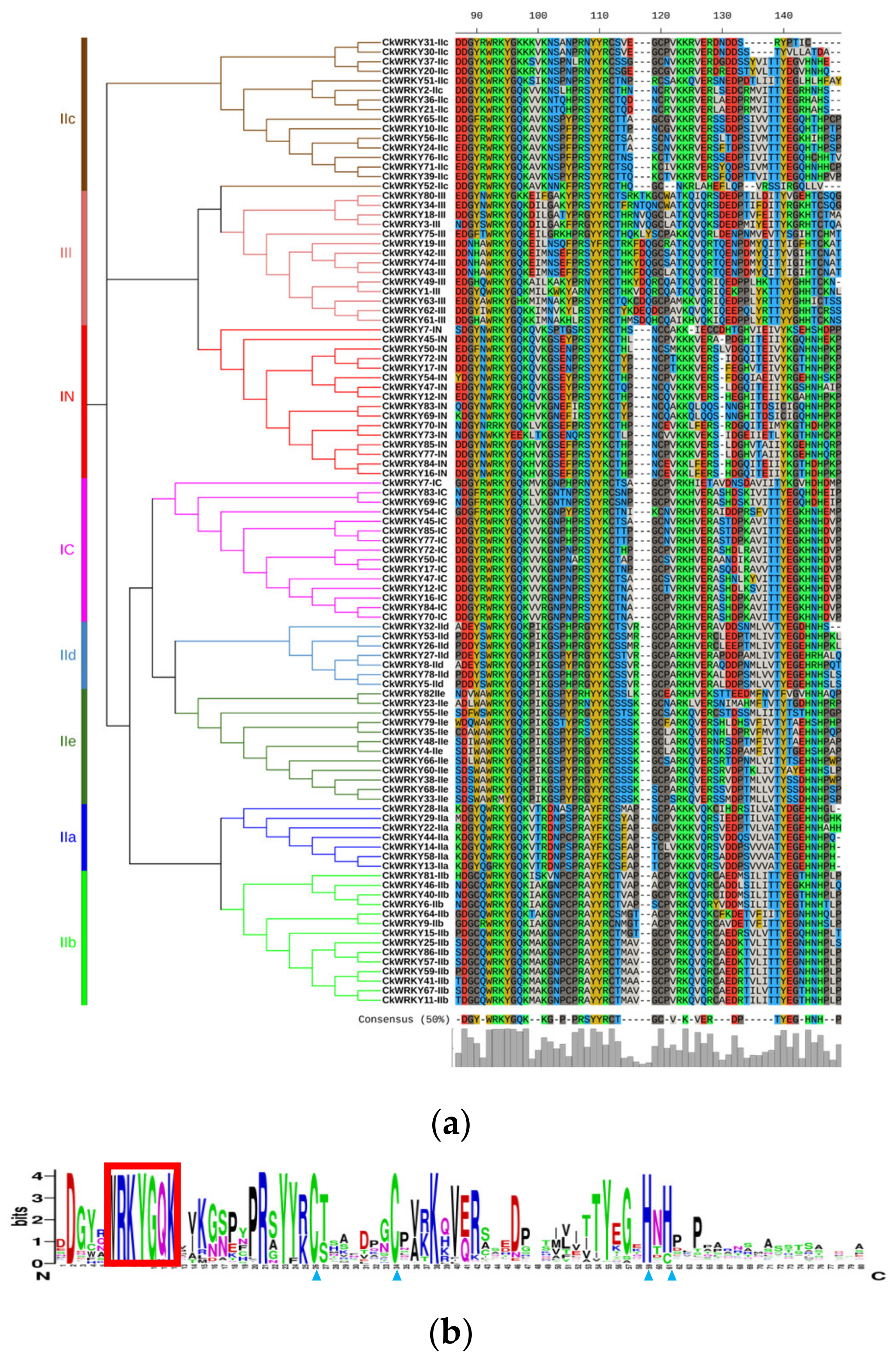
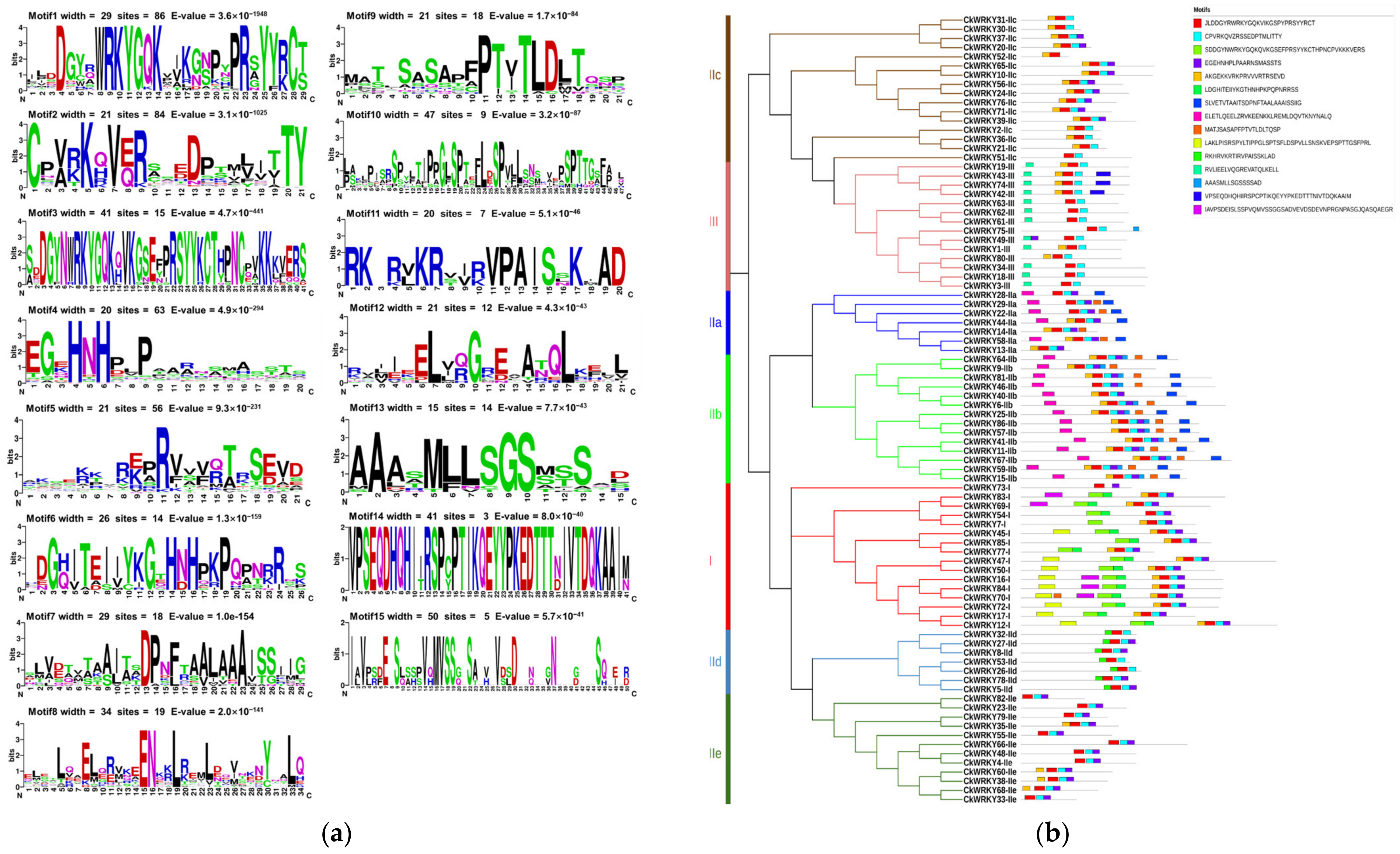

| Chromosome | Gene Name | ORF (aa) | pI | MW (kDa) | Conserved Heptapeptide | Zinc-Finger Type | Domain Number | Group/ Subgroup | Ortholog in A. thaliana |
|---|---|---|---|---|---|---|---|---|---|
| Chr1 | CkWRKY1 | 292 | 5.94 | 33.77 | WRKYGQK | C2HC | 1 | III | AtWRKY70 |
| CkWRKY2 | 232 | 7.11 | 26.32 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY12 | |
| CkWRKY3 | 361 | 5.37 | 40.77 | WRKYGQK | C2HC | 1 | III | AtWRKY46 | |
| CkWRKY4 | 333 | 5.72 | 36.37 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY27 | |
| CkWRKY5 | 336 | 9.67 | 36.59 | WRKYGQK | C2H2 | 1 | IId | AtWRKY15 | |
| CkWRKY6 | 594 | 6.12 | 64.39 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY72 | |
| CkWRKY7 | 507 | 7.64 | 54.68 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY32 | |
| CkWRKY8 | 312 | 9.81 | 33.88 | WRKYGQK | C2H2 | 1 | IId | AtWRKY11 | |
| CkWRKY9 | 391 | 8.98 | 42.96 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY42 | |
| CkWRKY10 | 383 | 6.15 | 41.86 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY48 | |
| CkWRKY11 | 504 | 5.54 | 54.71 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY42 | |
| Chr2 | CkWRKY12 | 223 | 8.16 | 24.85 | WRKYGQK | C2H2 | 1 | IIa | AtWRKY40 |
| CkWRKY13 | 144 | 5.45 | 16.16 | GRKYGQK | C2H2 | 1 | IIa | AtWRKY60 | |
| CkWRKY14 | 744 | 5.77 | 80.62 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY2 | |
| CkWRKY15 | 482 | 6.08 | 52.90 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY6 | |
| CkWRKY16 | 587 | 6.48 | 63.72 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY20 | |
| CkWRKY17 | 506 | 7.69 | 56.17 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY33 | |
| CkWRKY18 | 368 | 5.35 | 41.30 | WRKYGQK | C2HC | 1 | III | AtWRKY41 | |
| CkWRKY19 | 313 | 4.88 | 35.23 | WRKYGQK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY20 | 205 | 7.72 | 23.28 | WRKYGKK | C2H2 | 1 | IIc | AtWRKY51 | |
| CkWRKY21 | 250 | 8.84 | 27.87 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY13 | |
| CkWRKY22 | 299 | 7.11 | 33.66 | WRKYGQK | C2H2 | 1 | IIa | AtWRKY40 | |
| Chr3 | CkWRKY23 | 307 | 6.05 | 33.80 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY27 |
| CkWRKY24 | 314 | 7.17 | 35.06 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY23 | |
| CkWRKY25 | 429 | 8.9 | 47.18 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY47 | |
| CkWRKY26 | 350 | 9.69 | 39.23 | WRKYGQK | C2H2 | 1 | IId | AtWRKY21 | |
| CkWRKY27 | 339 | 9.51 | 36.64 | WRKYGQK | C2H2 | 1 | IId | AtWRKY17 | |
| CkWRKY28 | 260 | 8.19 | 29.15 | WRKYGQK | C2H2 | 1 | IIa | AtWRKY40 | |
| CkWRKY29 | 275 | 7.03 | 30.97 | WRKYGQK | C2H2 | 1 | IIa | AtWRKY40 | |
| CkWRKY30 | 175 | 5.49 | 20.40 | WRKYGKK | - | 1 | IIc | AtWRKY50 | |
| CkWRKY31 | 155 | 5.81 | 18.30 | WRKYGKK | - | 1 | IIc | AtWRKY50 | |
| CkWRKY32 | 336 | 9.57 | 37.09 | WRKYGQK | C2H2 | 1 | IId | AtWRKY7 | |
| CkWRKY33 | 162 | 4.82 | 17.80 | WRMYGQK | C2H2 | 1 | IIe | AtWRKY65 | |
| Chr4 | CkWRKY34 | 361 | 5.35 | 41.13 | WRKYGQK | C2HC | 1 | III | AtWRKY41 |
| CkWRKY35 | 284 | 5.22 | 32.43 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY27 | |
| CkWRKY36 | 233 | 8.96 | 26.64 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY13 | |
| CkWRKY37 | 186 | 6.65 | 21.55 | WRKYGKK | C2H2 | 1 | IIc | AtWRKY51 | |
| CkWRKY38 | 252 | 5.77 | 28.30 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY65 | |
| CkWRKY39 | 333 | 6.18 | 36.99 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY71 | |
| CkWRKY40 | 482 | 5.39 | 53.20 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY9 | |
| CkWRKY41 | 558 | 5.65 | 60.72 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY6 | |
| CkWRKY42 | 300 | 6.25 | 33.56 | WRKYGQK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY43 | 317 | 5.94 | 35.47 | WRKYGQK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY44 | 311 | 8.59 | 34.92 | WRKYGQK | C2H2 | 1 | IIa | AtWRKY40 | |
| Chr5 | CkWRKY45 | 512 | 7.02 | 55.86 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY4 |
| CkWRKY46 | 564 | 7.32 | 62.55 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY72 | |
| CkWRKY47 | 741 | 5.57 | 81.33 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY20 | |
| CkWRKY48 | 335 | 5.84 | 36.67 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY27 | |
| CkWRKY49 | 307 | 6.02 | 34.66 | WRKYGQK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY50 | 563 | 6.3 | 62.42 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY33 | |
| CkWRKY51 | 322 | 5.2 | 35.79 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY49 | |
| CkWRKY52 | 139 | 10.14 | 15.44 | WRKYGQK | - | 1 | IIc | AtWRKY75 | |
| CkWRKY53 | 318 | 9.62 | 35.76 | WRKYGQK | C2H2 | 1 | IId | AtWRKY21 | |
| CkWRKY54 | 448 | 8.91 | 50.16 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY44 | |
| CkWRKY55 | 265 | 4.85 | 30.29 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY35 | |
| CkWRKY56 | 296 | 8.62 | 33.14 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY23 | |
| CkWRKY57 | 518 | 6.89 | 56.40 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY42 | |
| Chr6 | CkWRKY58 | 307 | 8.75 | 34.15 | WRKYGQK | C2H2 | 1 | IIa | AtWRKY40 |
| CkWRKY59 | 469 | 6.28 | 50.69 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY6 | |
| CkWRKY60 | 266 | 5.87 | 28.92 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY69 | |
| CkWRKY61 | 299 | 5.56 | 34.11 | WRKYGQK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY62 | 313 | 5.57 | 35.40 | WRKYGQK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY63 | 284 | 7.59 | 32.77 | WRKYGHK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY64 | 456 | 7.57 | 50.70 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY42 | |
| CkWRKY65 | 389 | 6.4 | 42.37 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY48 | |
| CkWRKY66 | 483 | 6.11 | 53.23 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY35 | |
| CkWRKY67 | 610 | 6.33 | 65.55 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY6 | |
| CkWRKY68 | 224 | 6.02 | 24.51 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY65 | |
| Chr7 | CkWRKY69 | 550 | 6.01 | 60.78 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY1 |
| CkWRKY70 | 579 | 7.67 | 62.70 | RRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY20 | |
| CkWRKY71 | 264 | 8.16 | 29.90 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY71 | |
| CkWRKY72 | 574 | 7.21 | 63.30 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY33 | |
| CkWRKY73 | 321 | 9.33 | 36.85 | WKKYEEK | C2H2 | 1 | I | AtWRKY33 | |
| CkWRKY74 | 315 | 5.55 | 35.15 | WRKYGQK | C2HC | 1 | III | AtWRKY70 | |
| CkWRKY75 | 352 | 6.27 | 38.56 | WRKYGQK | C2HC | 1 | III | AtWRKY55 | |
| CkWRKY76 | 278 | 6.61 | 30.74 | WRKYGQK | C2H2 | 1 | IIc | AtWRKY57 | |
| CkWRKY77 | 387 | 7.76 | 42.48 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY3 | |
| CkWRKY78 | 312 | 9.53 | 34.35 | WRKYGQK | C2H2 | 1 | IId | AtWRKY15 | |
| CkWRKY79 | 252 | 8.67 | 28.53 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY29 | |
| CkWRKY80 | 292 | 6.71 | 33.07 | WRKYGKK | C2HC | 1 | III | AtWRKY41 | |
| Chr8 | CkWRKY81 | 554 | 6.83 | 60.54 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY72 |
| Scaffold | CkWRKY82 | 186 | 6.06 | 20.79 | WRKYGQK | C2H2 | 1 | IIe | AtWRKY27 |
| CkWRKY83 | 593 | 6.26 | 65.67 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY1 | |
| CkWRKY84 | 587 | 6.48 | 63.74 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY20 | |
| CkWRKY85 | 552 | 5.64 | 59.82 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | AtWRKY3 | |
| CkWRKY86 | 517 | 6.91 | 56.32 | WRKYGQK | C2H2 | 1 | IIb | AtWRKY47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, G.; Wang, R.; Wang, G.; Wan, Y. Genome-Wide Analysis of WRKY Transcription Factors Involved in Abiotic Stress and ABA Response in Caragana korshinskii. Int. J. Mol. Sci. 2023, 24, 9519. https://doi.org/10.3390/ijms24119519
Liu J, Li G, Wang R, Wang G, Wan Y. Genome-Wide Analysis of WRKY Transcription Factors Involved in Abiotic Stress and ABA Response in Caragana korshinskii. International Journal of Molecular Sciences. 2023; 24(11):9519. https://doi.org/10.3390/ijms24119519
Chicago/Turabian StyleLiu, Jinhua, Guojing Li, Ruigang Wang, Guangxia Wang, and Yongqing Wan. 2023. "Genome-Wide Analysis of WRKY Transcription Factors Involved in Abiotic Stress and ABA Response in Caragana korshinskii" International Journal of Molecular Sciences 24, no. 11: 9519. https://doi.org/10.3390/ijms24119519
APA StyleLiu, J., Li, G., Wang, R., Wang, G., & Wan, Y. (2023). Genome-Wide Analysis of WRKY Transcription Factors Involved in Abiotic Stress and ABA Response in Caragana korshinskii. International Journal of Molecular Sciences, 24(11), 9519. https://doi.org/10.3390/ijms24119519






