Revealing the Role of Alternariol in the Local Steroidogenesis in Human Prostate Normal and Cancer Cells
Abstract
1. Introduction
2. Results
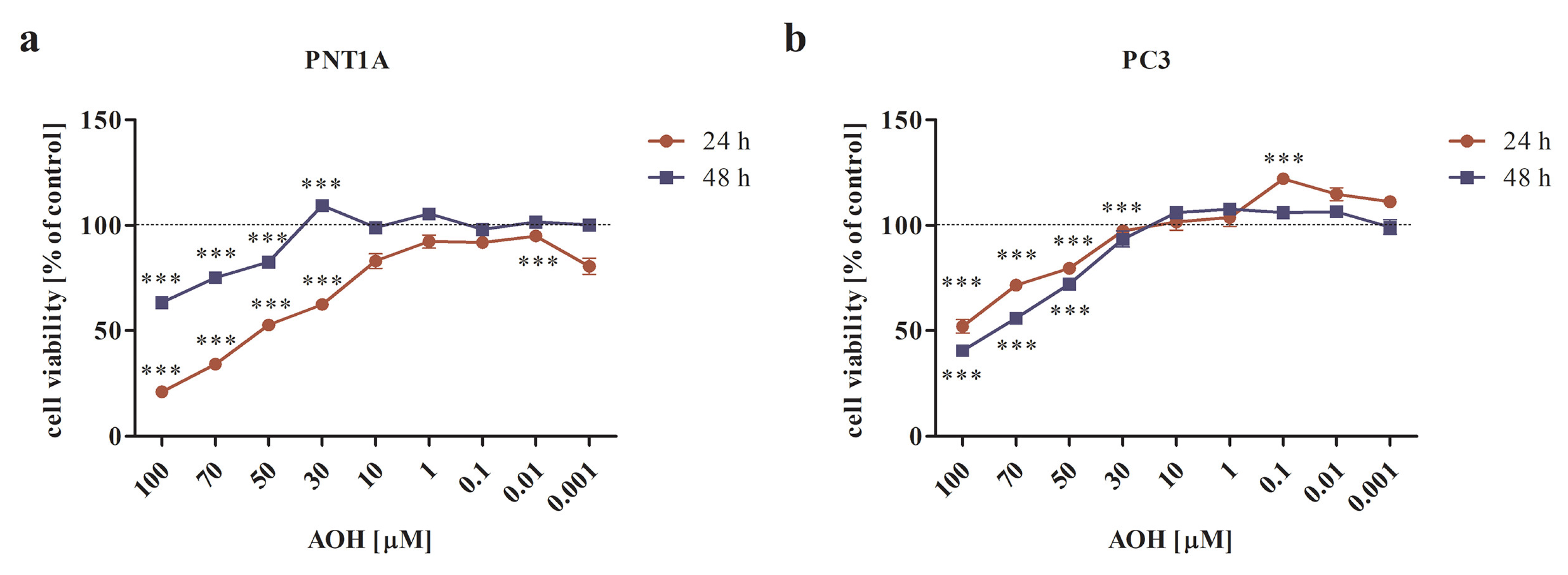
2.1. AOH Modulates the Viability of Prostate Normal and Cancer Cell Lines in a Dose- and Time-Dependent Manner
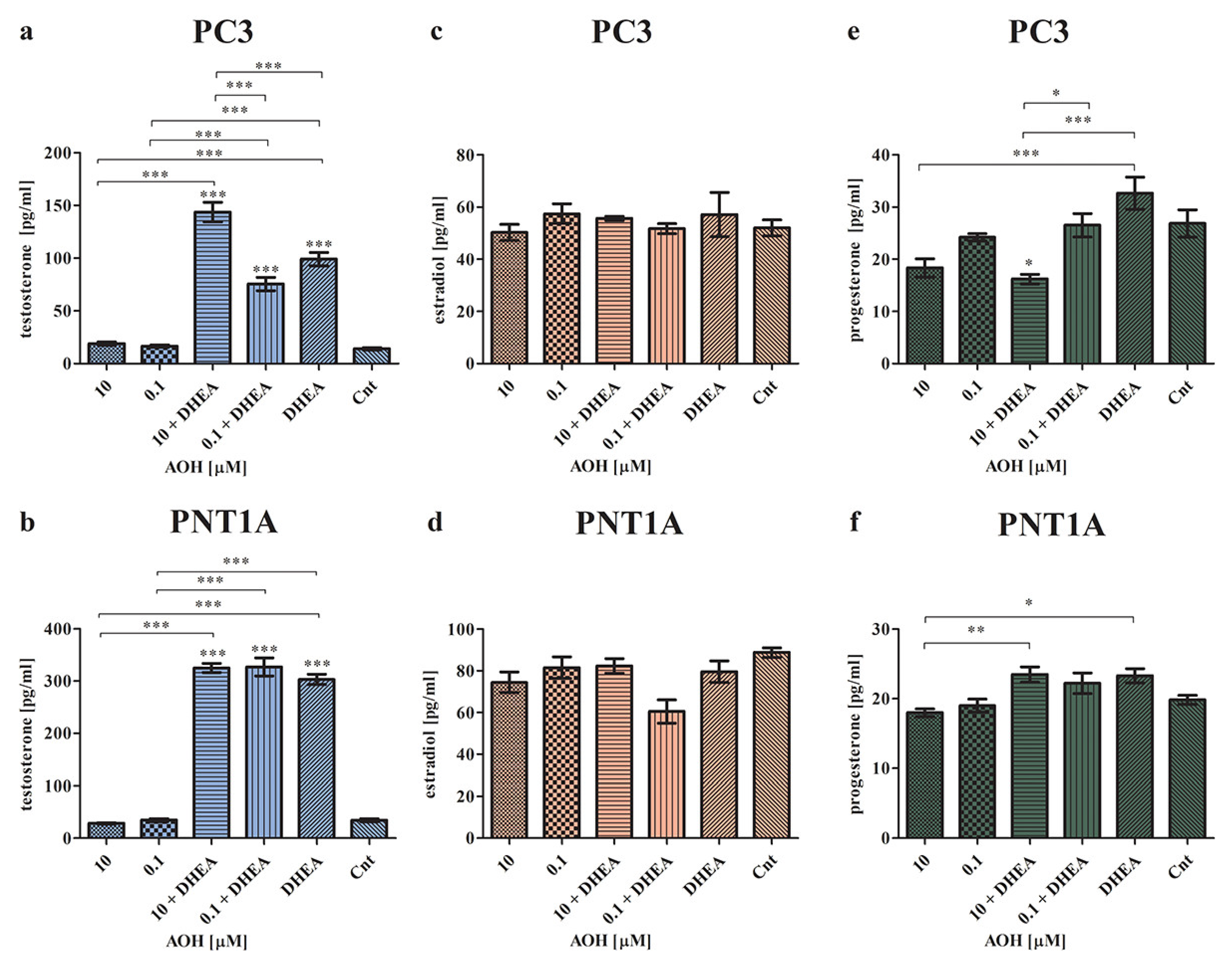
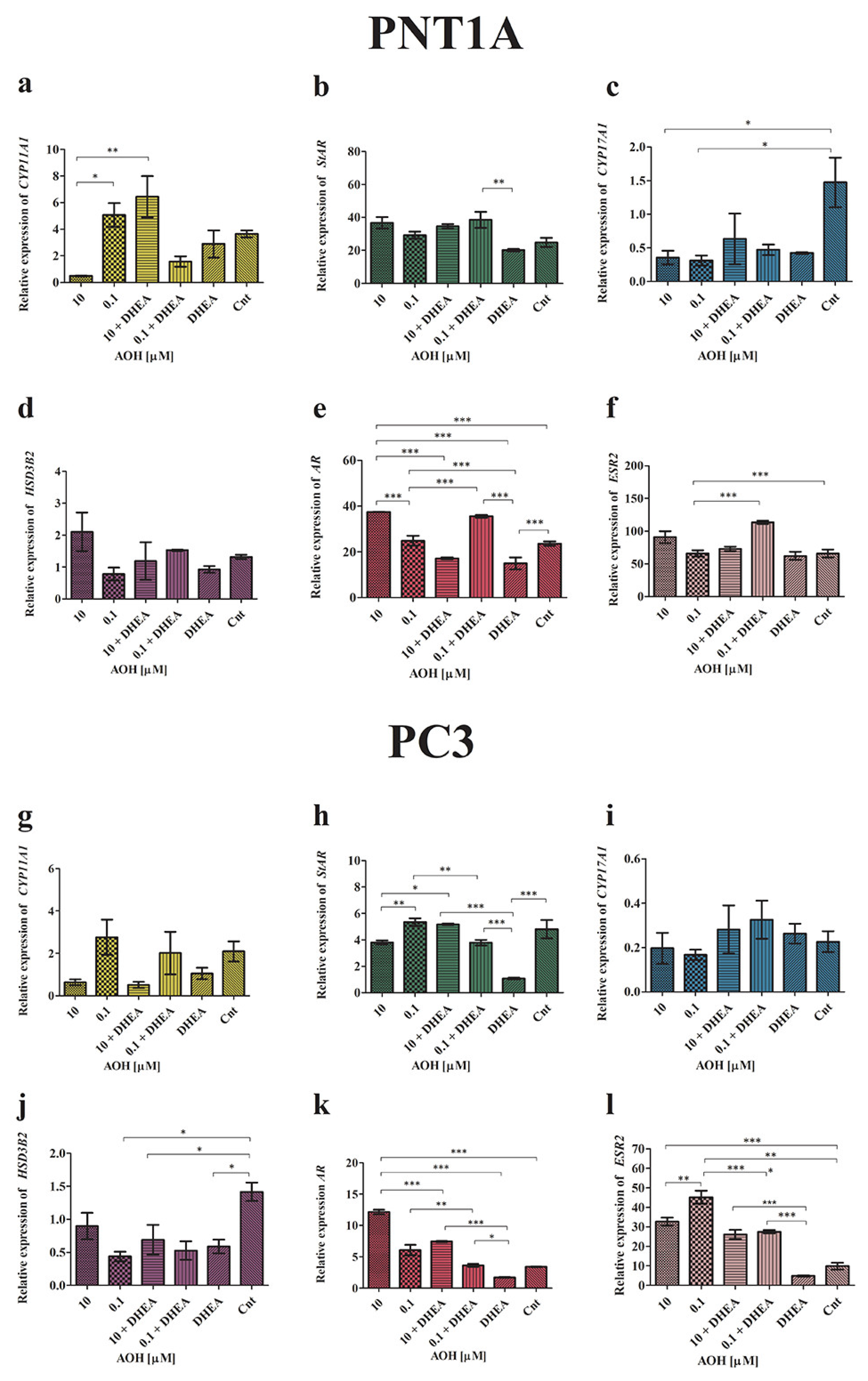
2.2. AOH Modulates the Local Steroidogenesis in Prostate Normal and Cancer Cells in the Presence of DHEA
2.3. AOH Affects the Expression of Caveolin-1 (CAV-1) in Prostate Cells
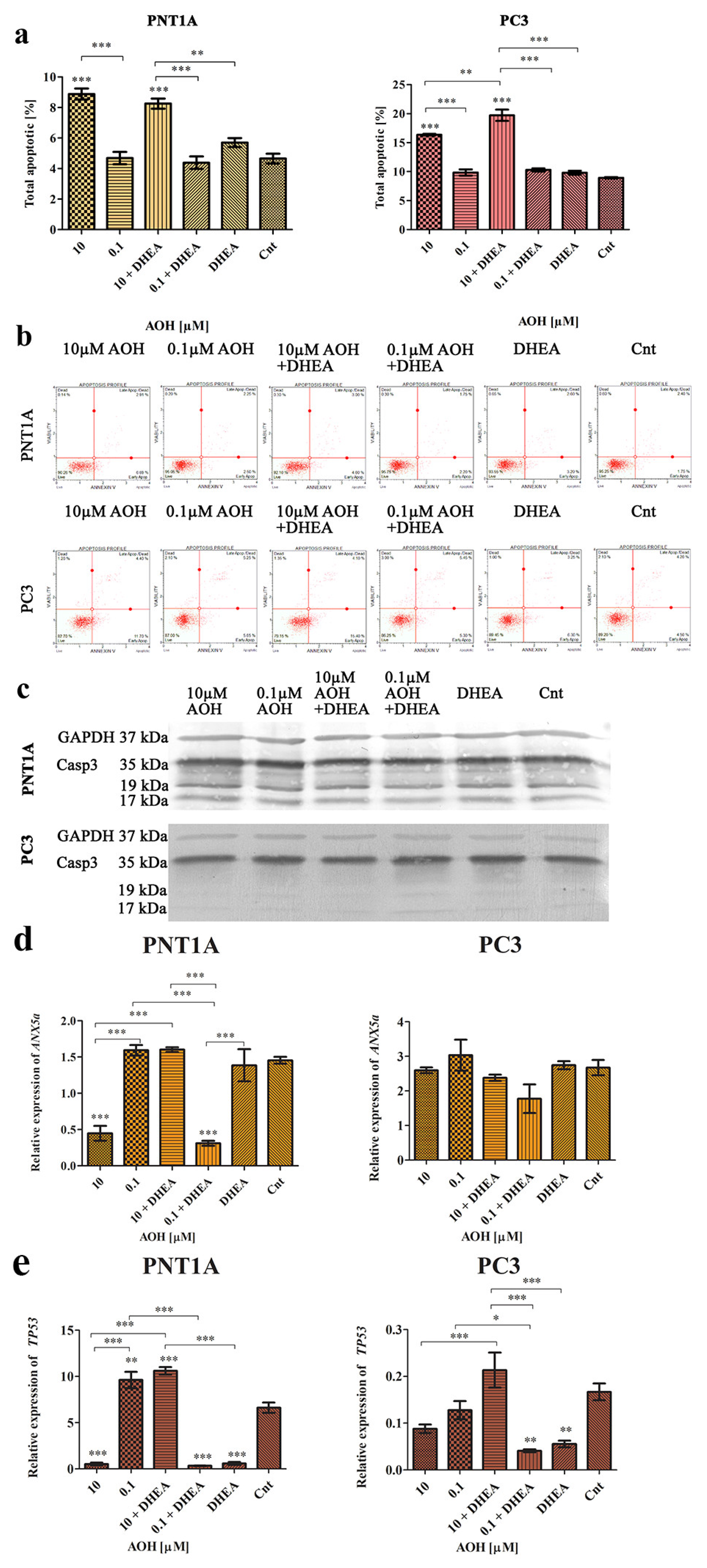
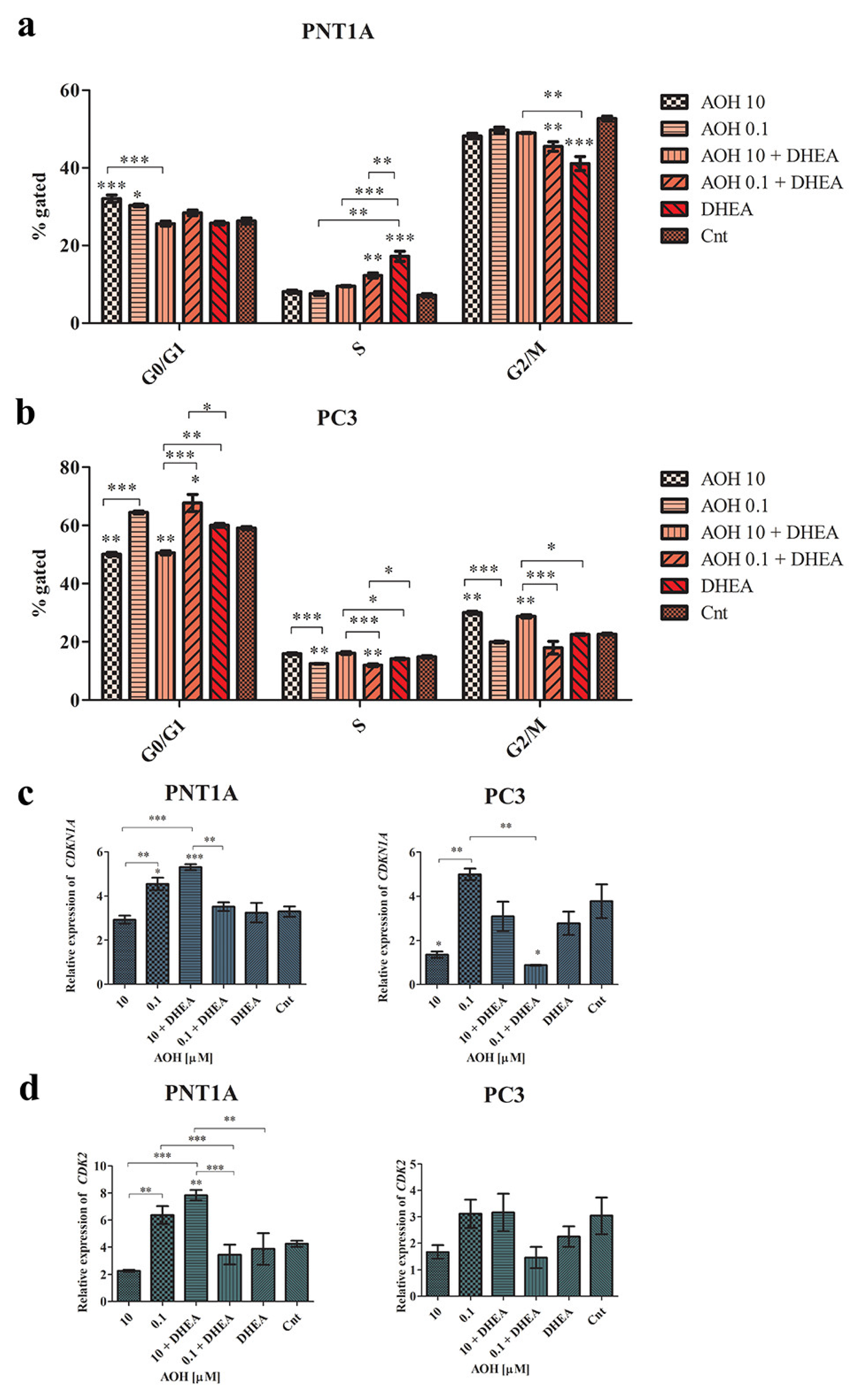
2.4. AOH Modulates Cell Cycle and Apoptosis in Prostate Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Experimental Treatments
4.2. Cell Viability Assay
4.3. Annexin V Staining Assay
4.4. Cell Cycle
4.5. Steroid Assays
4.6. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.7. Western Blot
4.8. Immunohistochemistry Staining and Confocal Microscopy
4.9. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef]
- Crudo, F.; Aichinger, G.; Dellafiora, L.; Kiss, E.; Mihajlovic, J.; Del Favero, G.; Berry, D.; Dall’Asta, C.; Marko, D. Persistence of the antagonistic effects of a natural mixture of Alternaria mycotoxins on the estrogen-like activity of human feces after anaerobic incubation. Toxicol. Lett. 2022, 358, 88–99. [Google Scholar] [CrossRef]
- Den Hollander, D.; Holvoet, C.; Demeyere, K.; De Zutter, N.; Audenaert, K.; Meyer, E.; Croubels, S. Cytotoxic Effects of Alternariol, Alternariol Monomethyl-Ether, and Tenuazonic Acid and Their Relevant Combined Mixtures on Human Enterocytes and Hepatocytes. Front. Microbiol. 2022, 13, 9243. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sørlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef]
- Kalayou, S.; Hamre, A.G.; Ndossi, D.; Connolly, L.; Sørlie, M.; Ropstad, E.; Verhaegen, S. Using SILAC proteomics to investigate the effect of the mycotoxin, alternariol, in the human H295R steroidogenesis model. Cell Biol. Toxicol. 2014, 30, 361–376. [Google Scholar] [CrossRef]
- Del Favero, G.; Mayer, R.M.; Dellafiora, L.; Janker, L.; Niederstaetter, L.; Dall’Asta, C.; Gerner, C.; Marko, D. Structural Similarity with Cholesterol Reveals Crucial Insights into Mechanisms Sustaining the Immunomodulatory Activity of the Mycotoxin Alternariol. Cells 2020, 9, 847. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Yen, A.E.; Weigel, N.L. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol. Ther. 2013, 140, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chung, Y.-M.; Sergeeva, O.; Kepe, V.; Berk, M.; Li, J.; Ko, H.-K.; Li, Z.; Petro, M.; DiFilippo, F.P.; et al. Loss of dihydrotestosterone-inactivation activity promotes prostate cancer castration resistance detectable by functional imaging. J. Biol. Chem. 2018, 293, 17829–17837. [Google Scholar] [CrossRef] [PubMed]
- Frycz, B.A.; Jagodziński, P.P. Expressions of genes encoding steroidogenic enzymes and their role in prostate carcinogenesis. J. Med. Sci. 2014, 83, 73–80. [Google Scholar] [CrossRef]
- Fokidis, H.B.; Adomat, H.H.; Kharmate, G.; Hosseini-Beheshti, E.; Guns, E.S.; Soma, K.K. Regulation of local steroidogenesis in the brain and in prostate cancer: Lessons learned from interdisciplinary collaboration. Front. Neuroendocrinol. 2015, 36, 108–129. [Google Scholar] [CrossRef]
- Škara, L.; Huđek Turković, A.; Pezelj, I.; Vrtarić, A.; Sinčić, N.; Krušlin, B.; Ulamec, M. Prostate Cancer—Focus on Cholesterol. Cancers 2021, 13, 4696. [Google Scholar] [CrossRef]
- Deb, S.; Pham, S.; Ming, D.-S.; Chin, M.; Adomat, H.; Hurtado-Coll, A.; Gleave, M.; Guns, E. Characterization of Precursor-Dependent Steroidogenesis in Human Prostate Cancer Models. Cancers 2018, 10, 343. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Uemura, H.; Umemoto, S.; Sakamaki, K.; Taguri, M.; Suzuki, K.; Shibata, Y.; Masumori, N.; Ichikawa, T.; Mizokami, A.; et al. Low serum dehydroepiandrosterone examined by liquid chromatography-tandem mass spectrometry correlates with poor prognosis in hormone-naïve prostate cancer. Prostate 2016, 76, 376–382. [Google Scholar] [CrossRef]
- Urbanek, K.A.; Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Sakowicz, A.; Piastowska-Ciesielska, A.W. In Vitro Analysis of Deoxynivalenol Influence on Steroidogenesis in Prostate. Toxins 2021, 13, 685. [Google Scholar] [CrossRef]
- Kowalska, K.; Kozieł, M.J.; Urbanek, K.A.; Habrowska-Górczyńska, D.E.; Domińska, K.; Piastowska-Ciesielska, A.W. Estrogen receptor β participates in alternariol-induced oxidative stress in normal prostate epithelial cells. Toxins 2021, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, H.; Li, Z.; Li, F. Interactions of caveolin-1 scaffolding and intramembrane regions containing a CRAC motif with cholesterol in lipid bilayers. Biochim. Biophys. Acta—Biomembr. 2014, 1838, 2588–2599. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.N.; Yamazaki, M.; Maruyama, S.; Abé, T.; Haga, K.; Kawaharada, M.; Izumi, K.; Kobayashi, T.; Tanuma, J. Cholesterol Is a Regulator of CAV1 Localization and Cell Migration in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 6035. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.C.; Tahir, S.A.; Li, L.; Watanabe, M.; Naruishi, K.; Yang, G.; Kadmon, D.; Logothetis, C.J.; Troncoso, P.; Ren, C.; et al. The role of caveolin-1 in prostate cancer: Clinical implications. Prostate Cancer Prostatic Dis. 2010, 13, 6–11. [Google Scholar] [CrossRef]
- Ayala, G.; Morello, M.; Frolov, A.; You, S.; Li, R.; Rosati, F.; Bartolucci, G.; Danza, G.; Adam, R.M.; Thompson, T.C.; et al. Loss of caveolin-1 in prostate cancer stroma correlates with reduced relapse-free survival and is functionally relevant to tumour progression. J. Pathol. 2013, 231, 77–87. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Wüstner, D. Intracellular cholesterol transport. J. Clin. Investig. 2002, 110, 891–898. [Google Scholar] [CrossRef]
- Kowalska, K.; Nowakowska, M.; Dominska, K.; Piastowska-Ciesielska, A.W. Coexpression of CAV-1, AT1-R and FOXM1 in prostate and breast cancer and normal cell lines and their influence on metastatic properties. Acta Biochim. 2016, 63, 493–499. [Google Scholar] [CrossRef]
- Ali, A.; Kulik, G. Signaling Pathways That Control Apoptosis in Prostate Cancer. Cancers 2021, 13, 937. [Google Scholar] [CrossRef]
- Small, E.J.; Ryan, C.J. The Case for Secondary Hormonal Therapies in the Chemotherapy Age. J. Urol. 2006, 176, S66–S71. [Google Scholar] [CrossRef]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol acts as a topoisomerase poison, preferentially affecting the IIα isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Wagner, J.; Metzler, M. Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem. Toxicol. 2006, 44, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Stypuła-Trębas, S.; Minta, M.; Radko, L.; Jedziniak, P.; Posyniak, A. Nonsteroidal mycotoxin alternariol is a full androgen agonist in the yeast reporter androgen bioassay. Environ. Toxicol. Pharmacol. 2017, 55, 208–211. [Google Scholar] [CrossRef]
- Dellafiora, L.; Warth, B.; Schmidt, V.; Del Favero, G.; Mikula, H.; Fröhlich, J.; Marko, D. An integrated in silico/in vitro approach to assess the xenoestrogenic potential of Alternaria mycotoxins and metabolites. Food Chem. 2018, 248, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, e04654. [Google Scholar] [CrossRef]
- Puntscher, H.; Aichinger, G.; Grabher, S.; Attakpah, E.; Krüger, F.; Tillmann, K.; Motschnig, T.; Hohenbichler, J.; Braun, D.; Plasenzotti, R.; et al. Bioavailability, metabolism, and excretion of a complex Alternaria culture extract versus altertoxin II: A comparative study in rats. Arch. Toxicol. 2019, 93, 3153–3167. [Google Scholar] [CrossRef]
- Ndossi, D.G.; Frizzell, C.; Tremoen, N.H.; Fæste, C.K.; Verhaegen, S.; Dahl, E.; Eriksen, G.S.; Sørlie, M.; Connolly, L.; Ropstad, E. An in vitro investigation of endocrine disrupting effects of trichothecenes deoxynivalenol (DON), T-2 and HT-2 toxins. Toxicol. Lett. 2012, 214, 268–278. [Google Scholar] [CrossRef]
- Rebhahn, V.I.C.; Kiss, E.; Marko, D.; Del Favero, G. Foodborne compounds that alter plasma membrane architecture can modify the response of intestinal cells to shear stress in vitro. Toxicol. Appl. Pharmacol. 2022, 446, 116034. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, F.-T.; Chan, W.-H. Alternariol exerts embryotoxic and immunotoxic effects on mouse blastocysts through ROS-mediated apoptotic processes. Toxicol. Res. 2021, 10, 719–732. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Juan-García, A.; Juan, C.; Font, G.; Ruiz, M.-J. Alternariol induce toxicity via cell death and mitochondrial damage on Caco-2 cells. Food Chem. Toxicol. 2016, 88, 32–39. [Google Scholar] [CrossRef]
- Bensassi, F.; Gallerne, C.; Sharaf El Dein, O.; Hajlaoui, M.R.; Bacha, H.; Lemaire, C. Cell death induced by the Alternaria mycotoxin Alternariol. Toxicol. Vitr. 2012, 26, 915–923. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Treatment | CAV-1 |
|---|---|---|
| PNT1A | 10 μM AOH | 0.62 |
| 0.1 μM AOH | 1.06 | |
| 100 nM DHEA | 1.08 | |
| 10 μM AOH + 100 nM DHEA | 1.61 | |
| 0.1 μM AOH + 100 nM DHEA | 1.48 | |
| Cnt | 1.00 | |
| PC3 | 10 μM AOH | 0.67 |
| 0.1 μM AOH | 0.44 | |
| 100 nM DHEA | 0.95 | |
| 10 μM AOH + 100 nM DHEA | 0.62 | |
| 0.1 μM AOH + 100 nM DHEA | 0.57 | |
| Cnt | 1.00 |
| Cell Line | Treatment | Casp3 | Cleaved Casp3 |
|---|---|---|---|
| PNT1A | 10 μM AOH | 0.94 | 0.70 |
| 0.1 μM AOH | 0.89 | 0.66 | |
| 100 nM DHEA | 1.33 | 1.03 | |
| 10 μM AOH + 100 nM DHEA | 0.92 | 0.71 | |
| 0.1 μM AOH + 100 nM DHEA | 1.31 | 0.80 | |
| Cnt | 1.00 | 1.00 | |
| PC3 | 10 μM AOH | 2.15 | 1.83 |
| 0.1 μM AOH | 1.07 | 1.42 | |
| 100 nM DHEA | 1.51 | 1.92 | |
| 10 μM AOH + 100 nM DHEA | 0.98 | 0.98 | |
| 0.1 μM AOH + 100 nM DHEA | 0.99 | 1.38 | |
| Cnt | 1.00 | 1.00 |
| Gene | Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| CYP11A1 | For CCAGAACGATTCCTCATCC | 126 |
| Rev CATCACCTCCTGGTTCAG | ||
| CYP17A1 | For GAAGTTATCATCAATCTGTGGG | 119 |
| Rev ACTGACGGTGAGATGAGC | ||
| Rev AAGATGTCTGGTTTGATGAGGAG | ||
| HSD3B2 | For CTTGGTGTCACTCACAGAGAG | 128 |
| Rev GTAGATGAAGACTGGCACACTG | ||
| Rev CACCTCCAATTGTGACATAA | ||
| STAR | For CATGGAGAGGCTCTATGAAGA | 128 |
| Rev CAGCCAGCTCGTGAGTAAT | ||
| ANXA5 | For ACCCTCTCGGCTTTATGATGCT | 116 |
| Rev TGGCTCTCAGTTCTTCAGGTGT | ||
| Rev TGCTGGACAGAAATGTGTACACTCCAGA | ||
| ESR2 | For ACACCTGGGCACCTTTCTCCTTTA | 90 |
| Rev TCTTGCTTCACACCAGGGACTCTT | ||
| AR | For GGGAGGTTACACCAAAGGGC | 102 |
| Rev AGAGACAGGGTAGACGGCAG | ||
| CAV-1 | Rev GAACTTGAAATTGGCACCAGG | 139 |
| For ACCCACTCTTTGAAGCTGTTG | ||
| CDK1N1A | Rev CTGAGACTAAGGCAGAAGATGT | 133 |
| For GACAGATTTCTACCACTCCAA | ||
| CDK2 | Rev GAGCAGAGGCATCCATGAATT | 126 |
| For TGCTTAAGGAGCTTAACCATCC | ||
| TP53 | Rev TTTATGGCGGGAGGTAGA | 102 |
| For TTGGAACTCAAGGATGCC | ||
| For GCAAGACTGTTAGCCCTCAA | ||
| RPLP0 | For ACGGATTACACCTTCCCACTTGCTAAAAGGTC | 69 |
| Rev AGCCACAAAGGCAGATGGATCAGCCAAG | ||
| RPS17 | For AAGCGCGTGTGCGAGGAGATCG | 87 |
| Rev TCGCTTCATCAGAT GCGTGACATAACCTG | ||
| H3F3A | For AGGACTTTAAAAGATCTGCGCTTCCAGAG | 74 |
| Rev ACCAGATAGGCCTCACTTGCCTCCTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanek, K.A.; Kowalska, K.; Habrowska-Górczyńska, D.E.; Kozieł, M.J.; Domińska, K.; Piastowska-Ciesielska, A.W. Revealing the Role of Alternariol in the Local Steroidogenesis in Human Prostate Normal and Cancer Cells. Int. J. Mol. Sci. 2023, 24, 9513. https://doi.org/10.3390/ijms24119513
Urbanek KA, Kowalska K, Habrowska-Górczyńska DE, Kozieł MJ, Domińska K, Piastowska-Ciesielska AW. Revealing the Role of Alternariol in the Local Steroidogenesis in Human Prostate Normal and Cancer Cells. International Journal of Molecular Sciences. 2023; 24(11):9513. https://doi.org/10.3390/ijms24119513
Chicago/Turabian StyleUrbanek, Kinga Anna, Karolina Kowalska, Dominika Ewa Habrowska-Górczyńska, Marta Justyna Kozieł, Kamila Domińska, and Agnieszka Wanda Piastowska-Ciesielska. 2023. "Revealing the Role of Alternariol in the Local Steroidogenesis in Human Prostate Normal and Cancer Cells" International Journal of Molecular Sciences 24, no. 11: 9513. https://doi.org/10.3390/ijms24119513
APA StyleUrbanek, K. A., Kowalska, K., Habrowska-Górczyńska, D. E., Kozieł, M. J., Domińska, K., & Piastowska-Ciesielska, A. W. (2023). Revealing the Role of Alternariol in the Local Steroidogenesis in Human Prostate Normal and Cancer Cells. International Journal of Molecular Sciences, 24(11), 9513. https://doi.org/10.3390/ijms24119513









