Apoptosis Genes as a Key to Identification of Inverse Comorbidity of Huntington’s Disease and Cancer
Abstract
1. Introduction
2. Results
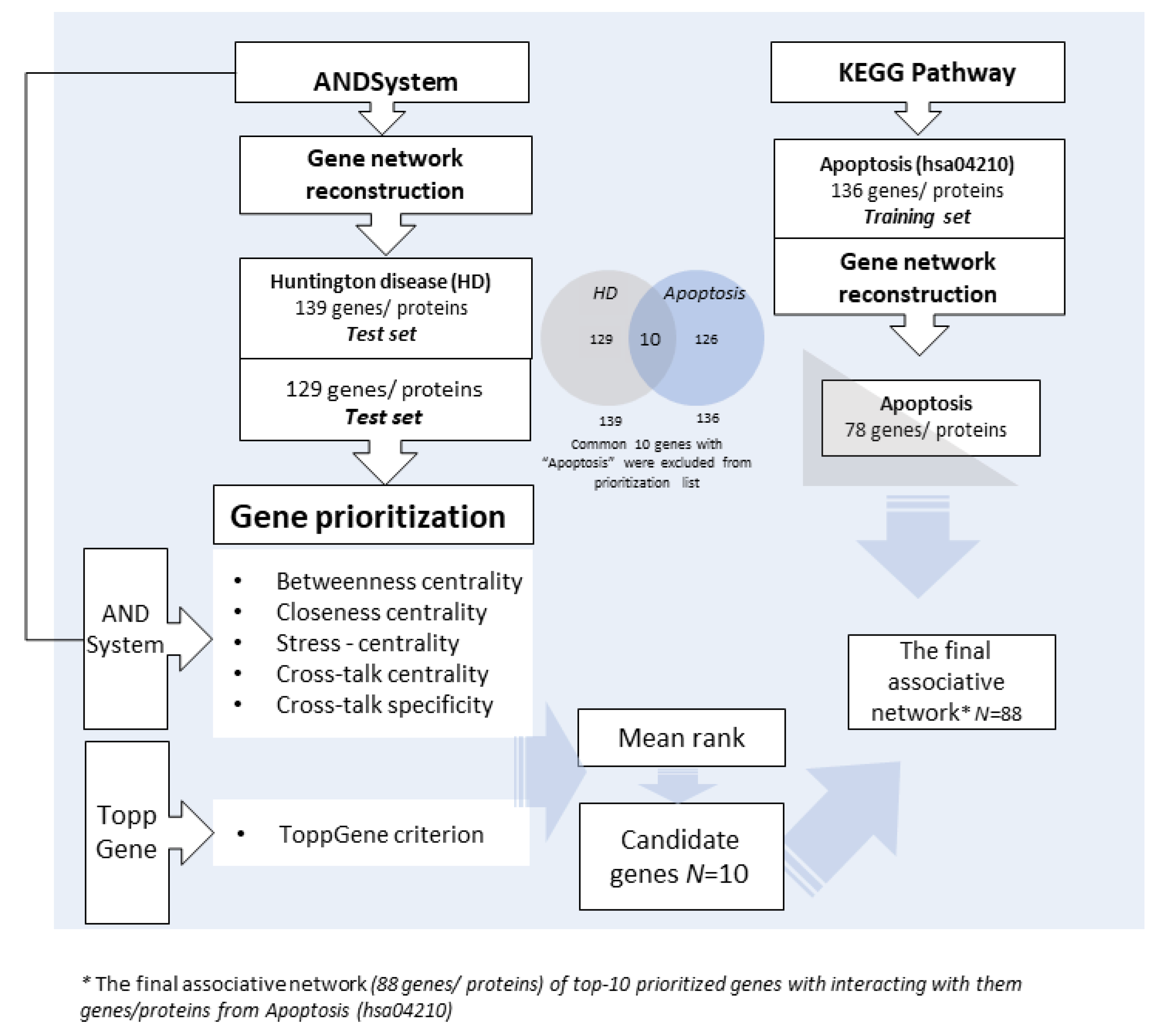
2.1. Associative Networks and Gene Prioritization
2.2. KEGG Functional Enrichment Analysis
2.3. Genes of the Final Associative Network in GWAS
2.4. Expression of Genes of the Final Associative Network in the Blood of Patients with HD and Breast/Prostate Cancer
2.5. Identifying Tissue-Specific Functional Modules of Genes of the Final Associative Network
3. Discussion
4. Materials and Methods
4.1. Reconstruction of Genetic Associative Networks
4.2. Prioritization of Candidate Genes of Inverse Comorbidity
4.3. Functional Enrichment Analysis
4.4. Analysis of GWAS Results
4.5. Identification of Differentially Expressed Genes of the Final Associative Network
4.6. Functional Modules of the Final List of Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Guarin, D.; Mohammadzadehhonarvar, N.; Chen, X.; Gao, X. Parkinson’s disease and cancer: A systematic review and meta-analysis of over 17 million participants. BMJ Open 2021, 11, e046329. [Google Scholar] [CrossRef] [PubMed]
- Catalá-López, F.; Suárez-Pinilla, M.; Suárez-Pinilla, P.; Valderas, J.M.; Gómez-Beneyto, M.; Martinez, S.; Balanzá-Martínez, V.; Climent, J.; Valencia, A.; McGrath, J.; et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: A meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother. Psychosom. 2014, 83, 89–105. [Google Scholar] [CrossRef]
- Tabarés-Seisdedos, R.; Rubenstein, J.L. Inverse cancer comorbidity: A serendipitous opportunity to gain insight into CNS disorders. Nat. Rev. Neurosci. 2013, 14, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Puzyrev, V.P. Genetic bases of human comorbidity. Russ. J. Genet. 2015, 51, 408–417. [Google Scholar] [CrossRef]
- Ibáñez, K.; Boullosa, C.; Tabarés-Seisdedos, R.; Baudot, A.; Valencia, A. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 2014, 10, e1004173. [Google Scholar] [CrossRef]
- Mencke, P.; Hanss, Z.; Boussaad, I.; Sugier, P.E.; Elbaz, A.; Krüger, R. Bidirectional relation between Parkinson’s disease and glioblastoma multiforme. Front. Neurol. 2020, 11, 898. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hickey, M.A.; Chesselet, M.F. Apoptosis in Huntington’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 255–265. [Google Scholar] [CrossRef]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A Novel Gene Containing a Trinucleotide Repeat That Is Expanded and Unstable on Huntington’s Disease Chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- McNulty, P.; Pilcher, R.; Ramesh, R.; Necuiniate, R.; Hughes, A.; Farewell, D.; Holmans, P.; Jones, L.; REGISTRY Investigators of the European Huntington’s Disease Network. Reduced Cancer Incidence in Huntington’s Disease: Analysis in the Registry Study. J. Huntingt. Dis. 2018, 7, 209–222. [Google Scholar] [CrossRef]
- Coarelli, G.; Diallo, A.; Thion, M.S.; Rinaldi, D.; Calvas, F.; Boukbiza, O.L.; Tataru, A.; Charles, P.; Tranchant, C.; Marelli, C.; et al. Low cancer prevalence in polyglutamine expansion diseases. Neurology 2017, 88, 1114–1119. [Google Scholar] [CrossRef]
- Ji, J.; Sundquist, K.; Sundquist, J. Cancer incidence in patients with polyglutamine diseases: A population-based study in Sweden. Lancet Oncol. 2012, 13, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Goldacre, R.; Goldacre, M.J. Reduced cancer incidence in Huntington’s disease: Record linkage study clue to an evolutionary trade-off? Clin. Genet. 2013, 83, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.A.; Fenger, K.; Olsen, J.H. Significantly lower incidence of cancer among patients with Huntington disease: An apoptotic effect of an expanded polyglutamine tract? Cancer 1999, 86, 1342–1346. [Google Scholar] [CrossRef]
- Moreira Sousa, C.; McGuire, J.R.; Thion, M.S.; Gentien, D.; de la Grange, P.; Tezenas du Montcel, S.; Vincent-Salomon, A.; Durr, A.; Humbert, S. The Huntington disease protein accelerates breast tumor development and metastasis through ErbB2/HER2 signaling. EMBO Mol. Med. 2013, 5, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Thion, M.S.; McGuire, J.R.; Sousa, C.M.; Fuhrmann, L.; Fitamant, J.; Leboucher, S.; Vacher, S.; du Montcel, S.T.; Bièche, I.; Bernet, A.; et al. Unraveling the Role of Huntingtin in Breast Cancer Metastasis. J. Natl. Cancer Inst. 2015, 107, djv208. [Google Scholar] [CrossRef]
- Thion, M.S.; Tézenas du Montcel, S.; Golmard, J.L.; Vacher, S.; Barjhoux, L.; Sornin, V.; Cazeneuve, C.; Bièche, I.; Sinilnikova, O.; Stoppa-Lyonnet, D.; et al. CAG repeat size in Huntingtin alleles is associated with cancer prognosis. Eur. J. Hum. Genet. 2016, 24, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Murmann, A.E.; Gao, Q.Q.; Putzbach, W.E.; Patel, M.; Bartom, E.T.; Law, C.Y.; Bridgeman, B.; Chen, S.; McMahon, K.M.; Thaxton, C.S.; et al. Small interfering RNAs based on huntingtin trinucleotide repeats are highly toxic to cancer cells. EMBO Rep. 2018, 19, e45336. [Google Scholar] [CrossRef]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Hu, J.X.; Thomas, C.E.; Brunak, S. Network biology concepts in complex disease comorbidities. Nat. Rev. Genet. 2016, 17, 615–629. [Google Scholar] [CrossRef]
- Sánchez-Valle, J.; Tejero, H.; Ibáñez, K.; Portero, J.L.; Krallinger, M.; Al-Shahrour, F.; Tabarés-Seisdedos, R.; Baudot, A.; Valencia, A. A molecular hypothesis to explain direct and inverse co-morbidities between Alzheimer’s Disease, Glioblastoma and Lung cancer. Sci. Rep. 2017, 7, 4474. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, C.; Venturin, M.; Sojic, A.; Jesuthasan, N.; Orro, A.; Spinelli, R.; Musicco, M.; De Bellis, G.; Adorni, F. Candidate Genes and MiRNAs Linked to the Inverse Relationship Between Cancer and Alzheimer’s Disease: Insights From Data Mining and Enrichment Analysis. Front. Genet. 2019, 10, 846. [Google Scholar] [CrossRef]
- Greco, A.; Sanchez Valle, J.; Pancaldi, V.; Baudot, A.; Barillot, E.; Caselle, M.; Valencia, A.; Zinovyev, A.; Cantini, L. Molecular Inverse Comorbidity between Alzheimer’s Disease and Lung Cancer: New Insights from Matrix Factorization. Int. J. Mol. Sci. 2019, 20, 3114. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.A. Understanding the link between cancer and neurodegeneration. J. Geriatr. Oncol. 2012, 3, 58–67. [Google Scholar] [CrossRef]
- Greene, C.S.; Krishnan, A.; Wong, A.K.; Ricciotti, E.; Zelaya, R.A.; Himmelstein, D.S.; Zhang, R.; Hartmann, B.M.; Zaslavsky, E.; Sealfon, S.C.; et al. Understanding multicellular function and disease with human tissue-specific networks. Nat. Genet. 2015, 47, 569–576. [Google Scholar] [CrossRef]
- Ivanisenko, V.A.; Saik, O.V.; Ivanisenko, N.V.; Tiys, E.S.; Ivanisenko, T.V.; Demenkov, P.S.; Kolchanov, N.A. ANDSystem: An Associative Network Discovery System for automated literature mining in the field of biology. BMC Syst. Biol. 2015, 9 (Suppl. S2). [Google Scholar] [CrossRef]
- Ivanisenko, V.A.; Demenkov, P.S.; Ivanisenko, T.V.; Mishchenko, E.L.; Saik, O.V. A new version of the ANDSystem tool for automatic extraction of knowledge from scientific publications with expanded functionality for reconstruction of associative gene networks by considering tissue-specific gene expression. BMC Bioinform. 2019, 20 (Suppl. S1), 34. [Google Scholar] [CrossRef]
- Advani, D.; Kumar, P. Deciphering the molecular mechanism and crosstalk between Parkinson’s disease and breast cancer through multi-omics and drug repurposing approach. Neuropeptides 2022, 96, 102283. [Google Scholar] [CrossRef]
- Pathak, G.A.; Zhou, Z.; Silzer, T.K.; Barber, R.C.; Phillips, N.R.; Alzheimer’s Disease Neuroimaging Initiative, Breast and Prostate Cancer Cohort Consortium; Alzheimer’s Disease Genetics Consortium. Two-stage Bayesian GWAS of 9576 individuals identifies SNP regions that are targeted by miRNAs inversely expressed in Alzheimer’s and cancer. Alzheimers. Dement. 2020, 16, 162–177. [Google Scholar] [CrossRef]
- Ham, S.; Kim, T.K.; Ryu, J.; Kim, Y.S.; Tang, Y.P.; Im, H.I. Comprehensive MicroRNAome Analysis of the Relationship Between Alzheimer Disease and Cancer in PSEN Double-Knockout Mice. Int. Neurourol. J. 2018, 22, 237–245. [Google Scholar] [CrossRef]
- Lakra, P.; Aditi, K.; Agrawal, N. Peripheral Expression of Mutant Huntingtin is a Critical Determinant of Weight Loss and Metabolic Disturbances in Huntington’s Disease. Sci. Rep. 2019, 9, 10127. [Google Scholar] [CrossRef] [PubMed]
- Aditi, K.; Shakarad, M.N.; Agrawal, N. Altered lipid metabolism in Drosophila model of Huntington’s disease. Sci. Rep. 2016, 6, 31411. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Agrawal, N. Deciphering the key mechanisms leading to alteration of lipid metabolism in Drosophila model of Huntington’s disease. Biochim. Biophys. Acta Mol Basis Dis. 2021, 1867, 166127. [Google Scholar] [CrossRef]
- Russo, M.; Russo, G.L. Autophagy inducers in cancer. Biochem. Pharmacol. 2018, 153, 51–61. [Google Scholar] [CrossRef]
- Cai, H.; Cong, W.N.; Ji, S.; Rothman, S.; Maudsley, S.; Martin, B. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr. Alzheimer. Res. 2012, 9, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Dickson, E.; Dwijesha, A.S.; Andersson, N.; Lundh, S.; Björkqvist, M.; Petersén, Å.; Soylu-Kucharz, R. Microarray profiling of hypothalamic gene expression changes in Huntington’s disease mouse models. Front. Neurosci. 2022, 16, 1027269. [Google Scholar] [CrossRef]
- Podvin, S.; Rosenthal, S.B.; Poon, W.; Wei, E.; Fisch, K.M.; Hook, V. Mutant Huntingtin Protein Interaction Map Implicates Dysregulation of Multiple Cellular Pathways in Neurodegeneration of Huntington’s Disease. J. Huntingtons. Dis. 2022, 11, 243–267. [Google Scholar] [CrossRef]
- Andréasson, C.; Fiaux, J.; Rampelt, H.; Druffel-Augustin, S.; Bukau, B. Insights into the structural dynamics of the Hsp110-Hsp70 interaction reveal the mechanism for nucleotide exchange activity. Proc. Natl. Acad. Sci. USA 2008, 105, 16519–16524. [Google Scholar] [CrossRef] [PubMed]
- Tsapara, A.; Matter, K.; Balda, M.S. The heat-shock protein Apg-2 binds to the tight junction protein ZO-1 and regulates transcriptional activity of ZONAB. Mol. Biol. Cell. 2006, 17, 1322–1330. [Google Scholar] [CrossRef]
- Yang, Z.; Zhuang, L.; Szatmary, P.; Wen, L.; Sun, H.; Lu, Y.; Xu, Q.; Chen, X. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int. J. Med. Sci. 2015, 12, 256–263. [Google Scholar] [CrossRef]
- Scior, A.; Buntru, A.; Arnsburg, K.; Ast, A.; Iburg, M.; Juenemann, K.; Pigazzini, M.L.; Mlody, B.; Puchkov, D.; Priller, J.; et al. Complete suppression of Htt fibrilization and disaggregation of Htt fibrils by a trimeric chaperone complex. EMBO J. 2018, 37, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, T.; Palm, K.; Metsis, M.; Reintam, T.; Paalme, V.; Saarma, M.; Persson, H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron 1993, 10, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Puehringer, D.; Orel, N.; Lüningschrör, P.; Subramanian, N.; Herrmann, T.; Chao, M.V.; Sendtner, M. EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat. Neurosci. 2013, 16, 407–415. [Google Scholar] [CrossRef]
- Zuccato, C.; Tartari, M.; Crotti, A.; Goffredo, D.; Valenza, M.; Conti, L.; Cataudella, T.; Leavitt, B.R.; Hayden, M.R.; Timmusk, T.; et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat. Genet. 2003, 35, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.R.; Charrin, B.C.; Borrell-Pagès, M.; Dompierre, J.P.; Rangone, H.; Cordelières, F.P.; De Mey, J.; MacDonald, M.E.; Lessmann, V.; Humbert, S.; et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 2004, 118, 127–138. [Google Scholar] [CrossRef]
- Wu, L.L.; Fan, Y.; Li, S.; Li, X.J.; Zhou, X.F. Huntingtin-associated protein-1 interacts with pro-brain-derived neurotrophic factor and mediates its transport and release. J. Biol. Chem. 2010, 285, 5614–5623. [Google Scholar] [CrossRef] [PubMed]
- Park, H. Cortical Axonal Secretion of BDNF in the Striatum Is Disrupted in the Mutant-huntingtin Knock-in Mouse Model of Huntington’s Disease. Exp. Neurobiol. 2018, 27, 217–225. [Google Scholar] [CrossRef]
- Radin, D.P.; Patel, P. BDNF: An Oncogene or Tumor Suppressor? Anticancer Res. 2017, 37, 3983–3990. [Google Scholar] [CrossRef]
- Vanhecke, E.; Adriaenssens, E.; Verbeke, S.; Meignan, S.; Germain, E.; Berteaux, N.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin. Cancer Res. 2011, 17, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, Y.; Song, Y.; Li, X.; Lan, D.; Zhang, P.; Xiao, Y.; Xing, Y. Activation of BDNF/TrkB pathway promotes prostate cancer progression via induction of epithelial-mesenchymal transition and anoikis resistance. FASEB J. 2020, 34, 9087–9101. [Google Scholar] [CrossRef]
- Lee, J.M.; Gillis, T.; Mysore, J.S.; Ramos, E.M.; Myers, R.H.; Hayden, M.R.; Morrison, P.J.; Nance, M.; Ross, C.A.; Margolis, R.L.; et al. Common SNP-based haplotype analysis of the 4p16.3 Huntington disease gene region. Am. J. Hum. Genet. 2012, 90, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wen, Y.; Al-Kuwari, N.; Chen, X. Association Between Parkinson’s Disease and Melanoma: Putting the Pieces Together. Front. Aging Neurosci. 2020, 12, 60. [Google Scholar] [CrossRef]
- Bragina, E.Y.; Tiys, E.S.; Rudko, A.A.; Ivanisenko, V.A.; Freidin, M.B. Novel tuberculosis susceptibility candidate genes revealed by the reconstruction and analysis of associative networks. Infect. Genet. Evol. 2016, 46, 118–123. [Google Scholar] [CrossRef]
- Yankina, M.A.; Saik, O.V.; Demenkov, P.S.; Khusnutdinova, E.K.; Rogaev, E.I.; Lavrik, I.N.; Ivanisenko, V.A. Analysis of the interactions of neuronal apoptosis genes in the associative gene network of Parkinson’s disease. Vavilovskii. Zhurnal. Genet. Sel. 2018, 22, 153–160. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef]
- Saik, O.V.; Demenkov, P.S.; Ivanisenko, T.V.; Bragina, E.Y.; Freidin, M.B.; Goncharova, I.A.; Dosenko, V.E.; Zolotareva, O.I.; Hofestaedt, R.; Lavrik, I.N.; et al. Novel candidate genes important for asthma and hypertension comorbidity revealed from associative gene networks. BMC Med. Genom. 2018, 11 (Suppl. S1). [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Mahi, N.A.; Najafabadi, M.F.; Pilarczyk, M.; Kouril, M.; Medvedovic, M. GREIN: An Interactive Web Platform for Re-analyzing GEO RNA-seq Data. Sci. Rep. 2019, 9, 7580. [Google Scholar] [CrossRef]
- Polonikov, A.V.; Klyosova, E.Y.; Azarova, I.E. Bioinformatic tools and internet resources for functional annotation of polymorphic loci detected by genome wide association studies of multifactorial diseases (review). Res. Results Biomed. 2021, 7, 15–31. [Google Scholar] [CrossRef]
- Siddiqui, S.; Deshmukh, A.J.; Mudaliar, P.; Nalawade, A.J.; Iyer, D.; Aich, J. Drug repurposing: Re-inventing therapies for cancer without re-entering the development pipeline-a review. J. Egypt. Natl. Canc. Inst. 2022, 34, 33. [Google Scholar] [CrossRef]
- Forés-Martos, J.; Boullosa, C.; Rodrigo-Domínguez, D.; Sánchez-Valle, J.; Suay-García, B.; Climent, J.; Falcó, A.; Valencia, A.; Puig-Butillé, J.A.; Puig, S.; et al. Transcriptomic and Genetic Associations between Alzheimer’s Disease, Parkinson’s Disease, and Cancer. Cancers 2021, 13, 2990. [Google Scholar] [CrossRef] [PubMed]
- Dreher, R.D.; Theisen, E.R. Lysine specific demethylase 1 is a molecular driver and therapeutic target in sarcoma. Front. Oncol. 2023, 12, 1076581. [Google Scholar] [CrossRef] [PubMed]

| Gene * | Chromosome | Entrez Gene ID | Protein Name |
|---|---|---|---|
| APOE | 19 | 348 | apolipoprotein E |
| PSEN1 | 14 | 5663 | presenilin 1 |
| INS | 11 | 3630 | insulin |
| IL6 | 7 | 3569 | interleukin 6 |
| SQSTM1 | 5 | 8878 | sequestrum 1 |
| SP1 | 12 | 6667 | Sp1 transcription factor |
| HTT | 4 | 3064 | huntingtin |
| LEP | 7 | 3952 | leptin |
| HSPA4 | 5 | 3308 | heat shock protein family A (Hsp70) member 4 |
| BDNF | 11 | 627 | brain derived neurotrophic factor |
| Study ID | Overall design | N (Case vs. Control) | Platforms |
|---|---|---|---|
| GSE61405 | RNA-seq profiles of blood taken from normal controls and Huntington’s disease patients | 11 vs. 8 | GPL9115 Illumina Genome Analyzer II |
| GSE174431 | RNA-Seq and single cell RNA sequencing of PBMCs from metastatic breast cancer patients | 6 vs. 2 | GPL24014 Ion Torrent S5 XL |
| GSE97901 | Whole blood miRNA samples from both controls and patients where sequences and a differential expressional analysis was conducted to identify possible biomarkers to distinguish patients from controls | 28 vs. 12 | GPL11154 Illumina HiSeq 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragina, E.Y.; Gomboeva, D.E.; Saik, O.V.; Ivanisenko, V.A.; Freidin, M.B.; Nazarenko, M.S.; Puzyrev, V.P. Apoptosis Genes as a Key to Identification of Inverse Comorbidity of Huntington’s Disease and Cancer. Int. J. Mol. Sci. 2023, 24, 9385. https://doi.org/10.3390/ijms24119385
Bragina EY, Gomboeva DE, Saik OV, Ivanisenko VA, Freidin MB, Nazarenko MS, Puzyrev VP. Apoptosis Genes as a Key to Identification of Inverse Comorbidity of Huntington’s Disease and Cancer. International Journal of Molecular Sciences. 2023; 24(11):9385. https://doi.org/10.3390/ijms24119385
Chicago/Turabian StyleBragina, Elena Yu., Densema E. Gomboeva, Olga V. Saik, Vladimir A. Ivanisenko, Maxim B. Freidin, Maria S. Nazarenko, and Valery P. Puzyrev. 2023. "Apoptosis Genes as a Key to Identification of Inverse Comorbidity of Huntington’s Disease and Cancer" International Journal of Molecular Sciences 24, no. 11: 9385. https://doi.org/10.3390/ijms24119385
APA StyleBragina, E. Y., Gomboeva, D. E., Saik, O. V., Ivanisenko, V. A., Freidin, M. B., Nazarenko, M. S., & Puzyrev, V. P. (2023). Apoptosis Genes as a Key to Identification of Inverse Comorbidity of Huntington’s Disease and Cancer. International Journal of Molecular Sciences, 24(11), 9385. https://doi.org/10.3390/ijms24119385






