Abstract
Low temperatures restrict the growth of the grapevine industry. The DREB transcription factors are involved in the abiotic stress response. Here, we isolated the VvDREB2A gene from Vitis vinifera cultivar ‘Zuoyouhong’ tissue culture seedlings. The full-length VvDREB2A cDNA was 1068 bp, encoding 355 amino acids, which contained an AP2 conserved domain belonging to the AP2 family. Using transient expression in leaves of tobacco, VvDREB2A was localized to the nucleus, and it potentiated transcriptional activity in yeasts. Expression analysis revealed that VvDREB2A was expressed in various grapevine tissues, with the highest expression in leaves. VvDREB2A was induced by cold and the stress-signaling molecules H2S, nitric oxide, and abscisic acid. Furthermore, VvDREB2A-overexpressing Arabidopsis was generated to analyze its function. Under cold stress, the Arabidopsis overexpressing lines exhibited better growth and higher survival rates than the wild type. The content of oxygen free radicals, hydrogen peroxide, and malondialdehyde decreased, and antioxidant enzyme activities were enhanced. The content of raffinose family oligosaccharides (RFO) also increased in the VvDREB2A-overexpressing lines. Moreover, the expression of cold stress-related genes (COR15A, COR27, COR6.6, and RD29A) was also enhanced. Taken together, as a transcription factor, VvDREB2A improves plants resistance to cold stress by scavenging reactive oxygen species, increasing the RFO amount, and inducing cold stress-related gene expression levels.
1. Introduction
Grapevine (Vitis vinifera L.) is a commercially grown fruit tree planted worldwide. Most of the commonly planted grapevine varieties in China are Eurasian, which are sensitive to low-temperature stress and have poor cold tolerance [1]. Moreover, frost during the winter and early spring seriously affects the yield and quality of grapevines in northern China. Extremely cold weather has occurred frequently in recent years, and frost damage, as a “bottleneck”, has greatly restricted the development of the grapevine industry.
Some progress has been made in understanding the action mechanism of the plant’s response to cold stress. The low-temperature signaling pathway in plant cells is transmitted in a particular sequence: membrane receptors—Ca2+—protein kinases—transcription factors—cold-related genes—physiological and biochemical reactions—cold resistance [2]. Under cold stress conditions, plants regulate gene expression through a complex signal transduction network, ultimately increasing the content of osmotic substances, regulating osmotic pressure, scavenging reactive oxygen species (ROS), and stabilizing cell membranes to alleviate cell damage at low temperatures [3,4]. Transcription factors are the pivots in the response to low temperatures. Among these transcription factors, CBF/DREB binds to the DRE/CRT motif, which activates the transcriptional expression of the downstream COR gene and participates in the resistance to cold stress [5,6].
The DREB transcription factor plays a crucial roles in the abiotic stress response. It consists of a highly conserved ERF or AP2 domain, about 60 amino acids, which is in charge of the specific binding with DNA [7]. According to the conserved domain, the DREB transcription factor family members can be divided into six subfamilies (A1–A6), and many studies have been conducted on the A1 and A2 subfamilies [8,9]. DREB/CBF research in the A1 subfamily has concentrated on low temperatures [10,11,12]. Many DREB1/CBF genes have been identified in Arabidopsis, Malus domestica, Medicago truncatula, Hordeum vulgare, Triticum aestivum, and Zea mays, and they play a critical role under cold stress. Research on the A2 subfamily of DREB2 transcription factors has mainly focused on the resistance to salt, drought, and heat stress [10,13]. However, this classification is not absolute and the classification in different literatures is not completely consistent. Some DREB2 are not only involved in drought stress, but also in cold tolerance [14,15,16].
Thirty-eight VvDREB members have been identified from the entire grapevine genome. These members were divided into six groups (A1–A6), as in the Arabidopsis DREBs [17]. Many studies have focused on the A1 subfamily, revealing that overexpression of VvCBF4/VvDREB1D in wine grapes improves cold resistance [18]. The grapevine VrCBF1/DREB1B and VrCBF4/DREB1D overexpression in Arabidopsis improves cold tolerance through increasing the activities of protective enzymes and activating the expression of the cold response genes COR15A, COR6.6, COR47, and RD29A [19]. Nevertheless, the function of the DREB A2 subfamily members in the grapevine has rarely been reported, and the mechanism has not been revealed.
In this study, we reported a DREB A2 subfamily member, VvDREB2A, from the cold-tolerant grapevine ‘Zuoyouhong.’ Its characteristics were identified, and the expression pattern was detected. Furthermore, we also generated VvDREB2A ectopic overexpressing Arabidopsis lines, observed the phenotypes after cold treatment, and measured some cold-related physiological indicators and the expression level of cold-response genes. The data reveal the function and mechanism of VvDREB2A under cold stress and contributes to providing candidate genes for grapevine breeding.
2. Results
2.1. Isolation and Sequence Analysis of VvDREB2A
First, we used the AtDREB (AT2G40340) amino acid sequence from TAIR to obtain the homolog in grapevine using WU-BLAST. The homologous sequence from the grapevine cultivar ‘Pinot Noir’ was XP_002273838.1 (VIT_13s0067g01960). We designed primers according to the sequence to clone the gene from the cold-tolerant grapevine cultivar ‘Zuoyouhong’, and the cloned gene was named VvDREB2A (GenBank Accession number: MH089405.1). Sequence analyses showed that the VvDREB2A cDNA was 1068 bp in full length, and encoded 355 amino acids. The molecular weight of VvDREB2A is approximately 38.74 kDa, and its isoelectric point is 4.93. The 125, 162, 224, and 266 amino acids in ‘Zuoyouhong’ were T, L, H, and S, respectively (GenBank Accession number: AZI71501.1), while the corresponding positions in ‘Pinot Noir’ were M, V, R, and G. The sequence contained an AP2 conserved domain (66–119 aa) and belonged to the AP2 family (Figure 1A). The phylogenetic tree showed that VvDREB2A is closely related to M. domestica DREB2A (Figure 1B).
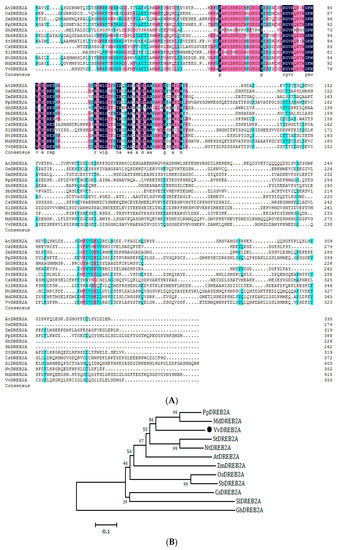
Figure 1.
VvDREB2A sequence analysis. (A) Alignment of amino acid sequences of VvDREB2A and DREB2A from other species (GenBank Accession numbers: AtDREB2A: NP_196160; OsDREB2A: XP_025878770; ZmDREB2A: NP_001105876; PpDREB2A: XP_020413813; GhDREB2A: NP_001314301; SbDREB2A: XP_002457289; StDREB2A: NP_001305480; CsDREB2A: XP_004148625; SlDREB2A: XP_010327719; NtDREB2A: XP_009788693; MdDREB2A: NP_001280947; VvDREB2A: AZI71501). (B) Phylogenetic tree of VvDREB2A and DREB2A from other species.
2.2. VvDREB2A Acts as a Transcription Factor
To identify the subcellular localization of VvDREB2A, the pSuper1300-VvDREB2A-GFP was constructed and transiently expressed in tobacco leaves. The results indicated that green fluorescence was only observed in the nucleus of the tobacco leaves (Figure 2A), implying that VvDREB2A was located in the nucleus.
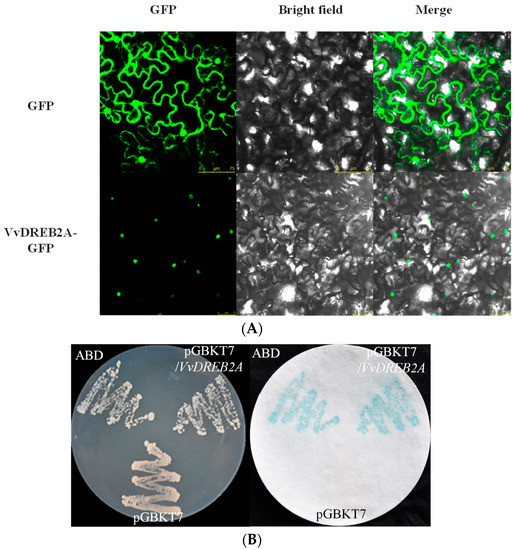
Figure 2.
Subcellular localization and transcription activation of VvDREB2A. (A) Tobacco leaves were agroinfiltrated with 35S-GFP (control) or 35S-VvDREB2A-GFP (fusion plasmid) and visualized under a confocal microscope after 2 days. From left to right, panels display dark-field images of GFP, bright-field images of cell morphology, and the merged images. (B) Yeast cells containing pGBKT7-VvDREB2A, pGBKT7(negative control), and ABD (positive control) were cultured on SD/Trp- medium. X-gal staining was conducted for β-galactosidase activity.
The positive control (ABD) plasmid, the negative control (pGBKT7), and the recombinant plasmid pGBKT7-VvDREB2A were transformed into yeast strain Y187 for the transcriptional activation assay. The positive control and the yeast of the recombinant plasmid pGBKT7-VvDREB2A both turned blue for β-galactosidase activity (Figure 2B), demonstrating that VvDREB2A has transcriptional activity. Therefore, VvDREB2A could probably act as a transcription activator in the nucleus.
2.3. VvDREB2A Expression Pattern
The expression of VvDREB2A in the grapevine cultivar ‘Zuoyouhong’ was detected in different tissues, including the roots, stems, leaves, flowers, the shoot tip, tendrils, and fruit, implying that VvDREB2A was expressed in various tissues and had a relatively high expression level in leaves (Figure 3A). Thus, we speculated that VvDREB2A is mainly involved in leaf-related functions in grapevines.
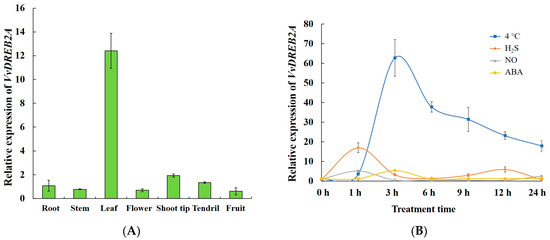
Figure 3.
VvDREB2A expression pattern in grapevine. (A) VvDREB2A expression pattern in different grapevine tissues. (B) VvDREB2A expression level in response to cold stress and signal molecules. Three repetitious experiments were conducted. Bars are the means ± SD of three repeats.
Further, the tissue culture seedlings of ‘Zuoyouhong’ were subjected to cold stress (4 °C) and cold-related signaling molecules, such as H2S, NO, and ABA, were determined. The results showed that 4 °C, H2S, NO, and ABA-induced VvDREB2A expression. The 4 °C and H2S treatments strongly induced VvDREB2A expression, which peaked at 3 h and 1 h, respectively (Figure 3B). These data indicate that VvDREB2A is induced by cold stress as well as signaling molecules related to cold stress.
2.4. VvDREB2A Enhances Cold Tolerance of Transgenic Arabidopsis
VvDREB2A was overexpressed in Arabidopsis, and the overexpressing lines were identified by PCR (Supplementary Figure S1A). The relative expression of VvDREB2A in transgenic lines was detected by quantitative real-time PCR, and three independent lines (OE1, OE3, and OE4) were screened for subsequent experiments (Supplementary Figure S1B). The 2-week-old WT and VvDREB2A-overexpressing Arabidopsis seedlings were treated at −10 °C for 1 h, and then cultured at 22 °C for 2–3 days to observe the phenotype and calculate the survival rate. The results showed that the WT and VvDREB2A-overexpressing lines had similar growth and development status under normal conditions, while the VvDREB2A-overexpressing lines grew better than the WT plants after the −10 °C treatment. We calculated the survival rates of OE1, OE3, and OE4, which were approximately 26%, 43%, and 60%, whereas the survival rate of the WT was approximately 8% (Figure 4A).
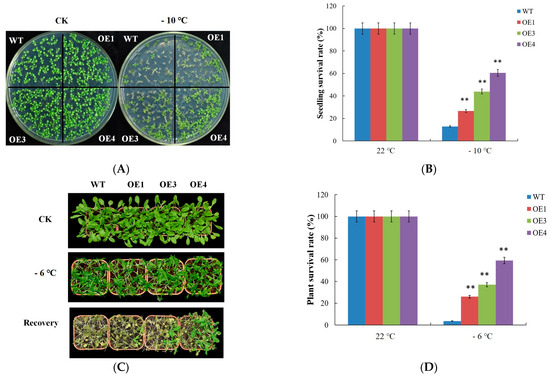
Figure 4.
Phenotypes of the VvDREB2A transgenic Arabidopsis responses to cold stress. (A) Phenotypes of 2-week-old Arabidopsis seedlings after −10 °C treatment. Each experiment was repeated three times, and there were over 30 seedlings in each line. (B) Survival rates of VvDREB2A-overexpressing Arabidopsis seedlings after the −10 °C treatment. Two asterisks show a significant difference between WT and transgenic lines (** p < 0.01). (C) Phenotypes of 4-week-old Arabidopsis plants under −6 °C treatment. Each experiment was repeated three times, and there were over t 30 seedlings in each line. (D) Survival rates of VvDREB2A transgenic plants after the −6 °C treatment. Two asterisks show a significant difference between WT and transgenic lines (** p < 0.01).
The 4-week-old WT and transgenic Arabidopsis were subjected to 4 °C for 36 h, followed by −6 °C for 12 h in the dark, then at 4 °C for 12 h, and lastly cultured at 22 °C for 3–4 days to observe the phenotypes. The results showed that the VvDREB2A-overexpressing lines grew better than the WT plants after the cold treatment. The survival rates of OE1, OE3, and OE4 were 25%, 37%, and 59% compared to 3% in the WT plants (Figure 4B). These data indicate that VvDREB2A improves cold tolerance in plants.
2.5. Overexpression of VvDREB2A Reduces Reactive Oxygen Species Accumulation in Transgenic Arabidopsis under Cold Stress
Under normal conditions, cell membrane permeability and MDA, H2O2, and O2 contents were not significantly different between the 4-week-old WT and VvDREB2A-overexpressing Arabidopsis. However, all of these parameters increased in the WT and VvDREB2A-overexpressing Arabidopsis after the 4 °C and −6 °C treatments, yet the degree of the increase was lower in the VvDREB2A-overexpressing lines (Figure 5A–D). These data indicate that VvDREB2A improved cold resistance by reducing cell membrane permeability, membrane lipid peroxidation, and the amount of ROS.
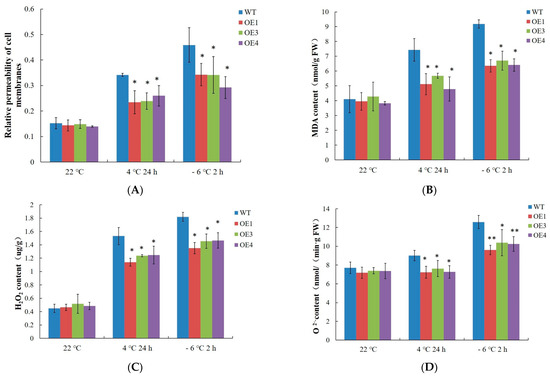
Figure 5.
ROS accumulation in VvDREB2A-overexpressing Arabidopsis under cold stress Relative permeability of the cell membranes (A), MDA content (B), H2O2 content (C), and O2− content (D) in the leaves of transgenic Arabidopsis after being treated at 4 °C for 24 h or at −6 °C for 2 h. Asterisks show a significant difference between WT and transgenic lines (* p < 0.05; ** p < 0.01).
As shown in Figure 6, the activity of the antioxidant enzymes was stable under normal conditions, and the genes related to the antioxidant enzymes were accordingly at a low expression level. In contrast, the antioxidant enzyme activity and the related genes (Cu/Zn SOD, POD2, and CAT1) expression levels all increased after the 4 °C and −6 °C treatments, and their levels were higher in the VvDREB2A-overexpressing lines compared with WT. These data demonstrated that VvDREB2A enhanced cold resistance by promoting antioxidant enzyme activity.
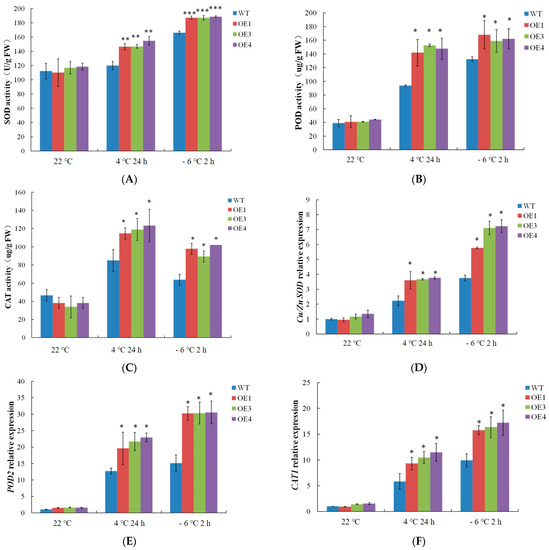
Figure 6.
Effects of cold stress on antioxidant enzymes in VvDREB2A-overexpressing Arabidopsis. The activities of SOD (A), POD (B), and CAT (C), and the expression levels of Cu/Zn SOD (D), POD2 (E), and CAT1 (F) in transgenic Arabidopsis leaves treated at 4 °C for 24 h or at −6 °C for 2 h. Asterisks show significant differences between WT and transgenic lines (* p < 0.05; ** p < 0.01; *** p < 0.001).
2.6. Overexpression of VvDREB2A Accumulates More Raffinose Family Oligosaccharides under Cold Stress
The RFOs are the second most abundant class of soluble sugars in higher plants after sucrose. As an important osmotic regulator in plants, RFOs play an important role in responses to abiotic stressors [4]. The synthesis of RFOs begins with raffinose, and the galactose groups on galactinol are transferred sequentially to produce raffinose and stachyose. The RFO contents were detected in this experiment. As shown in Figure 7, the WT and VvDREB2A-overexpressing Arabidopsis showed little difference in the levels of galactinol, raffinose, and stachyose at 22 °C. Nevertheless, the contents of galactinol, raffinose, and stachyose increased after the 4 °C and −6 °C treatments, and were significantly higher than the WT. As a result, VvDREB2A increases the cold tolerance of plants by increasing RFO contents.
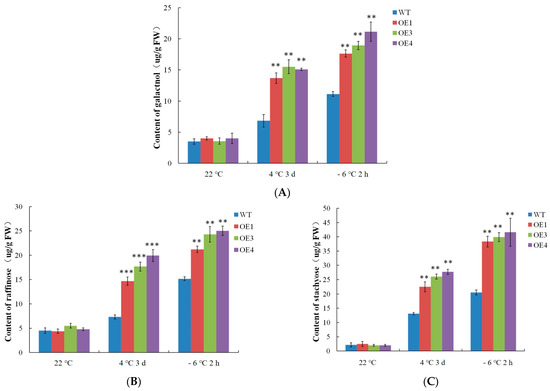
Figure 7.
Detection o of raffinose family oligosaccharide contents in VvDREB2A-overexpressing lines under cold stress. The amounts of galactinol (A), raffinose (B), and stachyose (C) in transgenic Arabidopsis leaves after being treated at 4 °C for 3 days or at −6 °C for 2 h. Asterisks show significant differences between WT and transgenic lines (** p < 0.01; *** p < 0.001).
2.7. Overexpression of VvDREB2A Promotes Cold-Related Gene Expression
The expression profiles of the cold-related gene were measured. The expression levels of COR15A, COR27, COR6.6, and RD29A in WT and VvDREB2A-overexpressing Arabidopsis were not significantly different at 22 °C. Meanwhile, the cold-related gene expression levels increased after the 4 °C and −6 °C treatments, and the gene expression levels in the transgenic Arabidopsis were markedly higher than those in the WT plants (Figure 8), indicating that VvDREB2A enhances cold resistance by increasing cold-related gene expression.
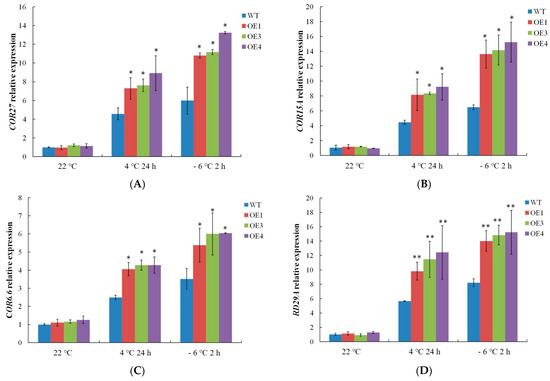
Figure 8.
Measurement of cold-related gene expression in VvDREB2A-overexpressing lines after cold treatment COR27 expression (A), COR15A expression (B), COR6.6 expression (C), and RD29A expression (D) in transgenic Arabidopsis leaves after being treated at 4 °C for 24 h or at −6 °C for 2 h. Asterisks show significant differences between WT and transgenic lines (* p < 0.05; ** p < 0.01).
3. Discussion
DREB2A is a member of the AP2 transcription factor family. It binds to the CRT/DRE response element of downstream genes to regulate stress-related gene expression and participate in the transduction of abiotic stress signals. The alignment analysis of the VvDREB2A gene (MH089405) from the grapevine cultivar “Zuoyouhong” and VIT_13s0067g01960 from the grapevine cultivar “Pinot Noir” revealed 99% similarity. The amino acid sequence alignment indicated that VvDREB2A belonged to the A2 subfamily, which was consistent with the sequence-based protein classification predicted by Zhao et al. The conservative region of VvDREB2A contained seven key amino acid residues, including one valine (V) residue (68th), two tryptophan (W) residues (76th and 90th), and four arginine (R) residues (66th, 69th, 81st, and 88th) (Figure 1A). These amino acids may directly interact with the DREB protein and the CRT/DRE cis element, which agrees with some previous reports [9,10]. Our subcellular localization and transcriptional activity analyses (Figure 2) proposed that VvDREB2A may function as a transcription factor.
Research on the function of the DREB2 transcription factor family has mainly focused on drought, salinity, and high-temperature resistance. Some transcription factors from this family are widely involved in drought stress, such as AtDREB2A from Arabidopsis [8], GmDREB2A from soybean [20], VuDREB2A from cowpea beans [21], and FpDREB2A from Fraxinus pennsylvanica [1]. Other members, such as VrDREB21 from green beans [22] and OsDREB2A from Oryza sativa [23,24], may be multi-functional and play roles in the salt and drought stress processes. AtDREB2A in Arabidopsis binds to the AtHsfA3 promoter and regulates the response to heat stress [25]. A few DREB2 family members participate in cold stress. The CpDreb2-type gene from Carica papaya L is expressed under cold stress, and CpDreb2 ectopic overexpression in tobacco shows an increased amount of proline and greater tolerance under cold stress [16]. PeDREB2L from Populus euphratica Oliva was transformed into Arabidopsis and the transgenic lines showed improved tolerance against cold stress [15]. In this research, VvDREB2A was regulated by cold stress and cold-related signaling molecules (Figure 3B). We used VvDREB2A ectopically overexpressing Arabidopsis as the material and observed that transgenic Arabidopsis had a greater tolerance to cold stress and a higher survival rate (Figure 4). These data demonstrated that VvDREB2A enhanced the cold tolerance of plants.
Furthermore, we explored the underlying mechanism by which VvDREB2A is involved in cold resistance. According to literature reports, potato (Solanum tuberosum L.) StDREB2-overexpressing cotton plants decrease ROS content by enhancing the activities of the antioxidant enzymes SOD, CAT, POD, and GST as well as the expression of related genes. As a result, transgenic cotton has improved drought resistance [26]. PgDREB2A was cloned from Pennisetum glaucum and the PgDREB2A-overexpressing Arabidopsis showed tolerance to osmotic, salt, cold, and heat stress by alleviating the degree of membrane damage, and increasing proline content [27]. We found that cell membrane permeability, and MDA, H2O2, and O2 contents of the VvDREB2A-overexpressing lines decreased significantly (Figure 5), and antioxidant enzyme activities and related gene expression levels increased significantly (Figure 6). These data suggest that VvDREB2A alleviated cell damage and improved cold resistance by enhancing antioxidant enzyme activities and reducing ROS content.
Notably, the RFO content increased during cold stress in the Arabidopsis lines with heterologous overexpression of VvDREB2A (Figure 7). As important osmotic regulators in plants, RFOs respond to abiotic stress [4]. In contrast, RFOs also protect cells from oxidative damage. They could scavenge ROS in the vicinity of organellar membranes so as to maintain cellular ROS homeostasis [28]. Chai et al. measured the metabolic changes in V. amurensis and V. vinifera cv. grapevine plants after a 4°C treatment and found that galactinol and raffinose contents increased [29]. Sun et al. reported that the grape transcription factor AQUILO increases low-temperature tolerance by increasing raffinose content in transgenic Arabidopsis and the grape callus [30]. In our study, the RFO contents increased in the VvDREB2A-overexpressing lines under cold stress, demonstrating that VvDREB2A improves cold tolerance by enhancing RFO contents. Further exploration is needed to determine whether VvDREB2A can regulate the genes related to raffinose synthesis and improve the cold tolerance of grapevine.
It is well known that DREB binds to the DRE/CRT motif, which activates the transcriptional expression of downstream genes and participates in the resistance to abiotic stress process [9,31,32]. However, whether VvDREB2A regulates cold-related gene expression is unknown. Therefore, we measured the expression level of the downstream genes. Higher expression of these cold response-related genes was detected compared with wild type, suggesting that VvDREB2A improves cold tolerance through enhancing these marker genes expressions in the transgenic Arabidopsis.
4. Materials and Methods
4.1. Plant Growth and Treatment Conditions
Grapevine tissue culture seedling, Arabidopsis, and Nicotiana tabacum cultivation: under light conditions of 200 μmol·m−2·s−1 (12 h/12 h light cycle, 25 ± 1 °C), the grapevine variety ‘Zuoyouhong’ tissue culture seedlings were inoculated on root media (1/2 MS + 0.1 mg·L−1 IAA) and continuously cultured for 45–55 d. Arabidopsis seeds were sterilized and placed at 4 °C for 2 days to break dormancy, after which they were sown on MS solid medium and incubated in light conditions (16 h/8 h light cycle, 22 ± 2 °C) for 14 days. Then, the seedlings were planted in nutrient-rich soil. N. tabacum was grown at 25 °C and with a 12 h/12 h light cycle for the subcellular localization assay.
Grapevine tissue culture seedling treatment: Grapevine tissue culture seedlings growing for 45–55 days were subjected to 4 °C for the cold stress, an H2S donor sodium hydrosulfide (NaHS, 0.1 mmol/L), a NO donor sodium nitroprusside (SNP, 0.1 mmol/L), and abscisic acid (ABA, 10 μmol/L) for 0, 1, 3, 6, 9, 12 and 24 h. The seedlings’ leaves were collected to measure the expression level of VvDREB2A.
Arabidopsis treatment: 2-week-old Arabidopsis were treated at −10 °C for 1 h, and then cultured at 22 °C for 2–3 days to observe the phenotypes and collect the survival rate data; 4-week-old Arabidopsis were treated at 4 °C for 36 h, followed by the −6 °C dark treatment for 12 h, 4 °C treatment for 12 h, and then cultured at 22 °C for 3–4 days for phenotypic observations and collecting the survival rate data; 4-week-old Arabidopsis were treated at 4 °C for 24 h (chilling treatment), or at 4 °C for 36 h followed by −6 °C for 2 h (freezing treatment). Cell membrane permeability; the H2O2, O2−, and malondialdehyde (MDA) amounts; antioxidant enzyme activities;and cold response genes expression levels were detected. 4-week-old Arabidopsis were treated at 4 °C for 3 days (chilling treatment), or at 4 °C for 36 h followed by −6 °C for 2 h (freezing treatment). The galactinol, raffinose, and stachyose contents were detected.
4.2. Cloning and Sequence Analysis of VvDREB2A
The AtDREB2A (AT2G40340) sequence of Arabidopsis was subjected to WU-BLAST alignment to search for homologous sequences (XP_002273838.1) from the grapevine cultivar ‘Pinot Noir’. According to the sequences, we designed primers to clone the gene from the cold-tolerant grapevine cultivar ‘Zuoyouhong’. The forward primer was 5′-GCGGATCCATGTCGTCCGGAGTCAT-3′, BamH I site underlined. The reverse primer was 5′-CGAGCTCTTAGAACCCCATATCTGATA-3′, Sac I site underlined. The PCR program was 94 °C for 5 min; 30 s at 94 °C, 15 s at 56 °C, and 70 s at 72 °C, 35 cycles; 72 °C for 10 min, and at 4 °C for ∞. The physical and chemical properties of AtDREB2A were analyzed by ProtParam online software. The evolutionary tree was constructed via MEGA 5.0 software with the neighbor-joining method.
4.3. Subcellular Localization of VvDREB2A
The full-length cDNA of VvDREB2A was amplified with 5′-TCTAGAATGTCGTCCGGAGTCAT-3′ (FP) and 5′-ACTAGAGAACCCCATATCTGATA-3′ (RP), and the amplified sequence was inserted into the pSuper1300-GFP vector driven by a CaMV 35S promoter. The recombinant plasmid pSuper1300-VvDREB2A-GFP was transformed into Agrobacterium tumefaciens strain EHA105. Transient expression of the fluorescent protein in tobacco leaf cells was performed as described previously [33]. GFP fluorescence was detected at 488 nm by a laser confocal scanning microscope (SP5, Leica, Wetzlar, Germany).
4.4. Transcriptional Activation of VvDREB2A
The primers for transcriptional activation assays were as follows: pGBKT7-VvDREB2A-F, 5′-CCCATGGATGTCGTCCGGAGTCAT-3′, Nco I site underlined, pGBKT7-VvDREB2A-R, 5′-GCGGATCCTTAGAACCCCATATCTGATA-3′, BamH I site underlined. The construct, as well as pGBKT7 (the negative control) and ABD (the positive control), were transformed into yeast strain Y187 on SD/Trp- medium plates. The growth of the transformants on SD/Trp- medium plates was observed after 3 days. A β-galactosidase activity was detected based on the manufacturer’s instructions (Clontech, Palo Alto, CA, USA).
4.5. RNA Extraction and qRT-PCR Assay
Total RNA was extracted using the CTAB method according to our previous work [34]. Quantitative real-time PCR was performed at 95 °C for 60 s; 40 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s. The genes relative expression was analyzed by the 2−ΔΔCT method. The primers are shown in Table S1 (Supplementary data).
4.6. Transformation of Arabidopsis
The VvDREB2A fragment was obtained after the pMD18-T-VvDREB2A plasmid was digested with BamH I and Sac I. The pSuper1300 vector was digested with the same restriction endonuclease, and the vector was recovered. Then, the VvDREB2A fragment and the pSuper1300 vector were ligated to construct the pSuper1300-VvDREB2A. Agrobacterium tumefaciens GV3101 containing a recombinant plasmid was transformed into Arabidopsis using the floral-dip method. The homozygous T3 progeny were named OE1, OE3, and OE4.
4.7. Determination of Physiological Indicator
O2 and H2O2 contents were detected using the methods in our previous work [35]. SOD, POD, and CAT activities were measured using the method of Wang et al. [36]. MDA content and electrolyte leakage were detected according to Fu et al. [37]. Each experiment was repeated three times, and there were at least 30 seedlings in each line.
4.8. Determination of Raffinose Family Oligosaccharide Contents
Galactinol, stachyose, and raffinose were extracted from Arabidopsis as described by Sui et al. [38]. Plant leaves were pulverized in liquid nitrogen, then 1 mL of 80% ethanol (v/v) was added and placed at 85 °C for 30 min. After centrifugation at 14,000 rpm for 20 min, the supernatant was collected, then extracted twice with 80% ethanol, dried at 42 °C, and the solid precipitate was dissolved in 1 mL of ddH2O. The RFOs were quantified by high-performance liquid chromatography (HPLC), according to Sui et al. [38]. Each experiment was repeated three times, and there were at least 30 seedlings in each line.
4.9. Statistical Analysis
All physiological data were calculated by the t-test via SPSS software (SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was regarded as significant differences.
5. Conclusions
In summary, we cloned VvDREB2A from grapevine cultivar ‘Zuoyouhong,’ and its GenBank accession number is MH089405.1. The amino acids sequence contained an AP2 conserved domain and belonged to the AP2 family. Subcellular localization and transcriptional activation assays indicated that VvDREB2A acted as a transcription factor. VvDREB2A was significantly induced by cold and the stress-signaling molecules. Moreover, VvDREB2A plays a positive regulatory function under cold stress by alleviating the levels of ROS, increasing RFO content, and enhancing the expression of cold stress response genes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24119381/s1.
Author Contributions
L.H. performed experiments, interpreted data, and wrote the article. Q.W. and X.Z. cloned the VvDREB2A gene and performed subcellular localization assays, transcriptional activation assays and Arabidopsis transformation. X.L. (Xin Liu) conceived and designed experiments and edited the article. X.L. (Xiangyu Li), X.F. and M.H. performed stress experiments. Q.Y. and G.L. performed all the qRT-PCR and statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Improved Variety Program of Shandong Province” (2020LZGC008 and 2022LZGCQY019), and the “National Natural Science Foundation of China” (Grant No. 31872082 and 32102352).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, D.; Zhang, L.; Zhao, K.; Niu, R.; Zhai, H.; Zhang, J. VaERD15, a Transcription factor gene associated with cold-tolerance in Chinese wild Vitis amurensis. Front. Plant Sci. 2017, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Orvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. 2000, 23, 785–794. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.K.; Sunkar, R. Gene regulation during cold stress acclimation in plants. Methods Mol. Biol. 2010, 639, 39–55. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef]
- Zhao, T.; Liang, D.; Wang, P.; Liu, J.; Ma, F. Genome-wide analysis and expression profiling of the DREB transcription factor gene family in Malus under abiotic stress. Mol. Genet. Genom. 2012, 287, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Zhu, J.J.; Wei, C.J.; Guo, Q.; Bian, C.K.; Xiang, Z.H.; Zhao, A.C. Genome-wide identification and characterization of the DREB transcription factor gene family in mulberry. Biol. Plantarum. 2015, 59, 253–265. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozakim, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Egawa, C.; Kobayashi, F.; Ishibashi, M.; Nakamura, T.; Nakamura, C.; Takumi, S. Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes Genet. Syst. 2006, 81, 77–91. [Google Scholar] [CrossRef]
- Chen, J.; Xia, X.; Yin, W. A poplar DRE-binding protein gene, PeDREB2L, is involved in regulation of defense response against abiotic stress. Gene 2011, 483, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ana, A.H.; Luis, F.Y.; Carlos, R.Z.; Alejandro, P.S.; Jorge, E.A. A novel DREB2-type gene from Carica papaya confers tolerance under abiotic stress. Plant Cell Tiss. Org. 2016, 125, 119–133. [Google Scholar] [CrossRef]
- Zhao, T.; Xia, H.; Liu, J.; Ma, F. The gene family of dehydration responsive element-binding transcription factors in grape (Vitis vinifera): Genome-wide identification and analysis, expression profiles, and involvement in abiotic stress resistance. Mol. Biol. Rep. 2014, 41, 1577–1590. [Google Scholar] [CrossRef]
- Tillett, R.L.; Wheatley, M.D.; Tattersall, E.A.; Schlauch, K.A.; Cramer, G.R.; Cushman, J.C. The Vitis vinifera C-repeat binding protein 4 (VvCBF4) transcriptional factor enhances freezing tolerance in wine grape. Plant Biotechnol. J. 2012, 10, 105–124. [Google Scholar] [CrossRef]
- Siddiqua, M.; Nassuth, A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ. 2011, 34, 1345–1359. [Google Scholar] [CrossRef]
- Mizoi, J.; Ohori, T.; Moriwaki, T.; Kidokoro, S.; Todaka, D.; Maruyama, K.; Kusakabe, K.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. GmDREB2A;2, a canonical DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN2-type transcription factor in soybean, is posttranslationally regulated and mediates dehydration-responsive element-dependent gene expression. Plant Physiol. 2013, 161, 346–361. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Kobayashi, Y.; Kobayashi, Y.; Tokizawa, M.; Yamamoto, Y.Y.; Iuchi, S.; Koyama, H.; Panda, S.K.; Sahoo, L. VuDREB2A, a novel DREB2-type transcription factor in the drought-tolerant legume cowpea, mediates DRE-dependent expression of stress-responsive genes and confers enhanced drought resistance in transgenic Arabidopsis. Planta 2014, 240, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, L.; Wang, L.; Wang, S.; Cheng, X. VrDREB2A, a DREB-binding transcription factor from Vigna radiata, increased drought and high-salt tolerance in transgenic Arabidopsis thaliana. J. Plant Res. 2016, 129, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, W.; Zhang, Q.; Xu, Z.; Zhu, Z.; Duan, F.; Wu, R. Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiol. Biochem. 2011, 49, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, G.; Mallikarjuna, K.; Reddy, M.K.; Kaul, T. Expression of OsDREB2A transcription factor confers enhanced dehydration and salt stress tolerance in rice (oryza sativa L.). Biotechnol. Lett. 2011, 33, 1689–1697. [Google Scholar] [CrossRef]
- Schramm, F.; Larkindale, J.; Kiehlmann, E.; Ganguli, A.; Englich, G.; Vierling, E.; von Koskull-Döring, P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008, 53, 264–274. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of StDREB2 Transcription Factor Enhances Drought Stress Tolerance in Cotton (Gossypium barbadense L.). Genes 2019, 10, 142. [Google Scholar] [CrossRef]
- Meena, R.P.; Ghosh, G.; Vishwakarma, H.; Padaria, J.C. Expression of a Pennisetum glaucum gene DREB2A confers enhanced heat, drought and salinity tolerance in transgenic Arabidopsis. Mol. Biol. Rep. 2022, 49, 7347–7358. [Google Scholar] [CrossRef]
- Van den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef]
- Chai, F.; Liu, W.; Xiang, Y.; Meng, X.; Sun, X.; Cheng, C.; Liu, G.; Duan, L.; Xin, H.; Li, S. Comparative metabolic profiling of Vitis amurensis and Vitis vinifera during cold acclimation. Hortic. Res. 2019, 1, 6–8. [Google Scholar] [CrossRef]
- Sun, X.; Matus, J.T.; Wong, D.C.J.; Wang, Z.; Chai, F.; Zhang, L.; Fang, T.; Zhao, L.; Wang, Y.; Han, Y.; et al. The GARP/MYB-related grape transcription factor AQUILO improves cold tolerance and promotes the accumulation of raffinose family oligosaccharides. J. Exp. Bot. 2018, 69, 1749–1764. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.; Zhang, S.; Zhang, B.; Zhao, Q.; Li, G.; Yang, X.; Wang, C.; He, J.; Yi, M. A canonical DREB2-Type transcription factor in lily is post-translationally regulated and mediates heat stress response. Front. Plant Sci. 2018, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, G.; Hou, L.; Wang, W.; Hao, J.; Liu, X. Vitis vinifera VvWRKY13 is an ethylene biosynthesis-related transcription factor. Plant Cell Rep. 2015, 34, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Fan, X.; Hao, J.; Liu, G.; Zhang, Z.; Liu, X. Negative regulation by transcription factor VvWRKY13 in drought stress of Vitis vinifera L. Plant Physiol. Bioch. 2020, 148, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Hou, L.; Xiao, P.; Guo, Y.; Deyholos, M.K.; Liu, X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2019, 280, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, C.; Wang, C.; Wang, Y. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance. BMC Plant Biol. 2012, 12, 118. [Google Scholar] [CrossRef]
- Fu, P.; Wang, W.; Hou, L.; Liu, X. Hydrogen sulfide is involved in the chilling stress response in vitis vinifera L. Acta Soc. Bot. Pol. 2013, 82, 295–302. [Google Scholar] [CrossRef]
- Sui, X.L.; Meng, F.Z.; Wang, H.Y.; Wei, Y.X.; Li, R.F.; Wang, Z.Y.; Hu, L.P.; Wang, S.H.; Zhang, Z.X. Molecular cloning, characteristics and low temperature response of raffinose synthase gene in Cucumis sativus L. J. Plant Physiol. 2012, 169, 1883–1891. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).