Comparative Physiological and Transcriptome Analysis Reveals Potential Pathways and Specific Genes Involved in Waterlogging Tolerance in Apple Rootstocks
Abstract
1. Introduction
2. Results
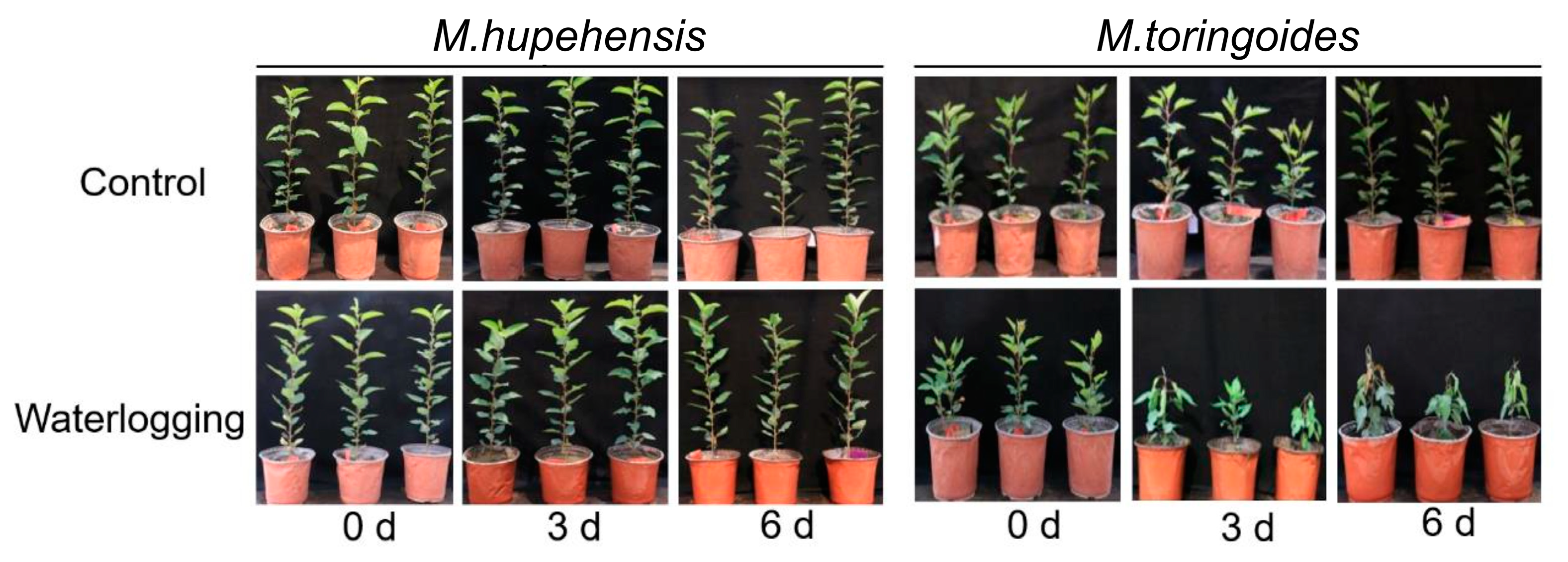
2.1. Differences in Growth and Morphology between M. toringoides and M. hupehensis under Waterlogging Stress
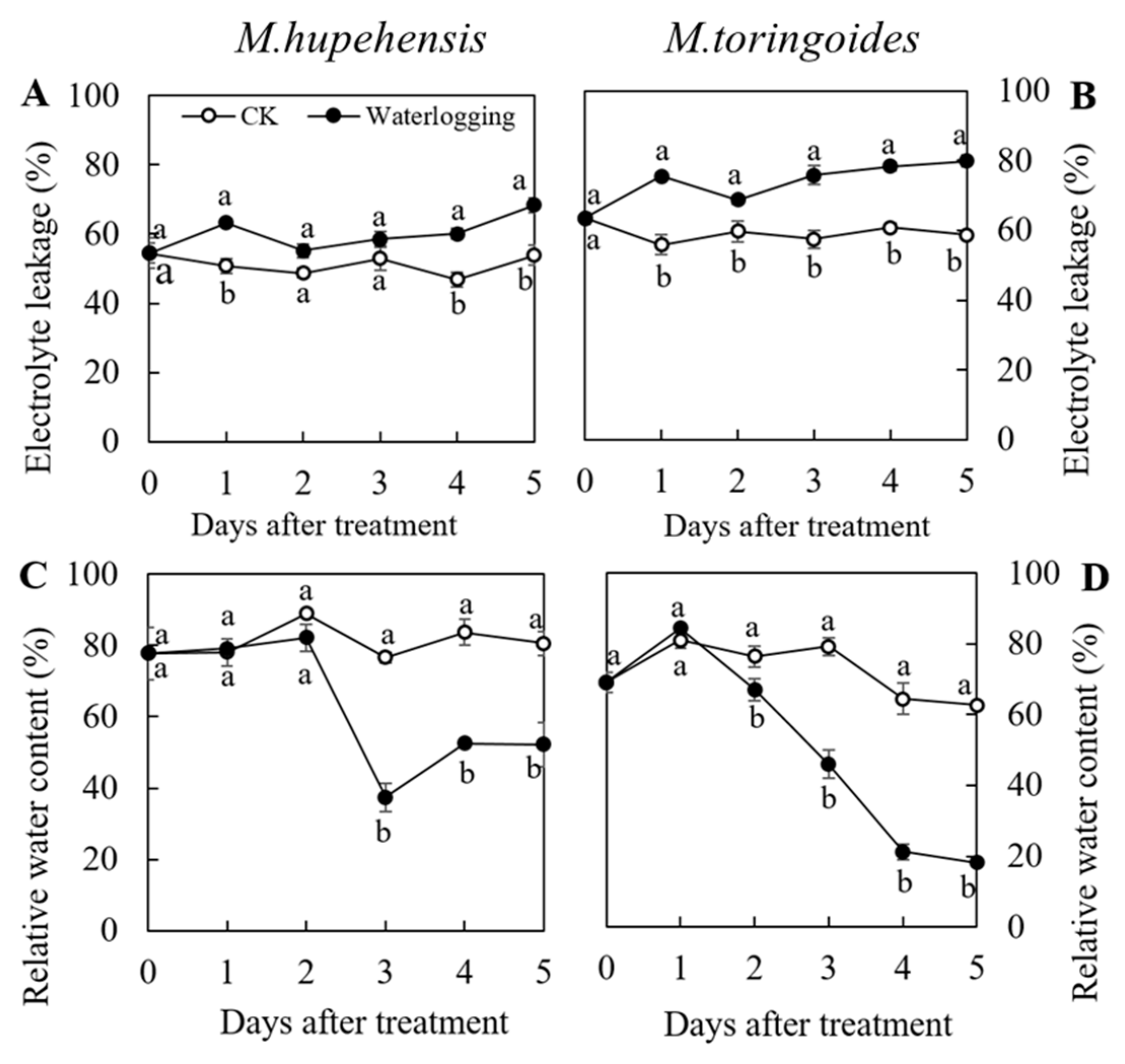
2.2. Differences in Electrolyte Leakage and Relative Water Content Changes between M. toringoides and M. hupehensis under Waterlogging Stress
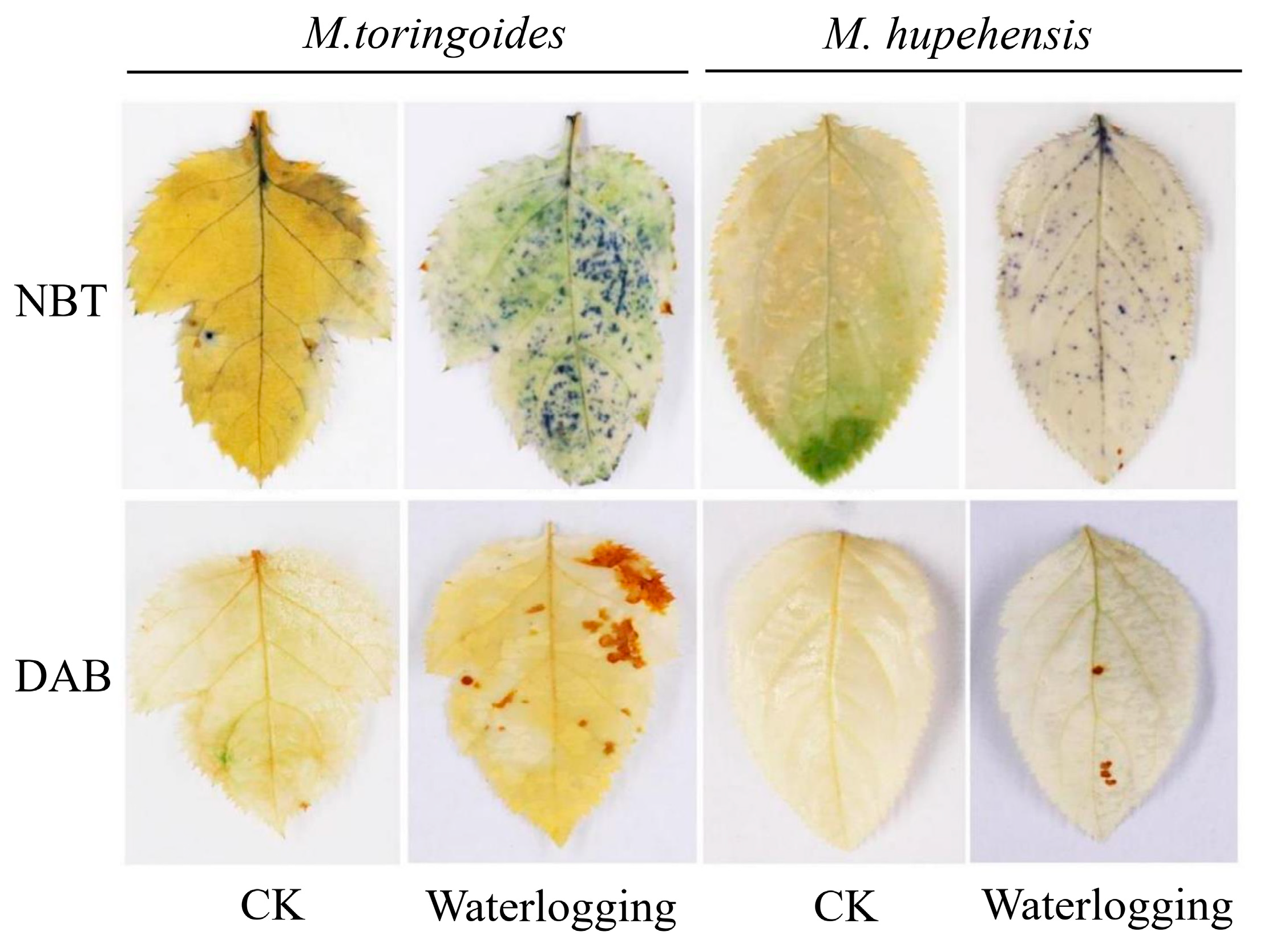
2.3. Differences in O2•− and H2O2 Accumulations between M. toringoides and M. hupehensis under Waterlogging Stress
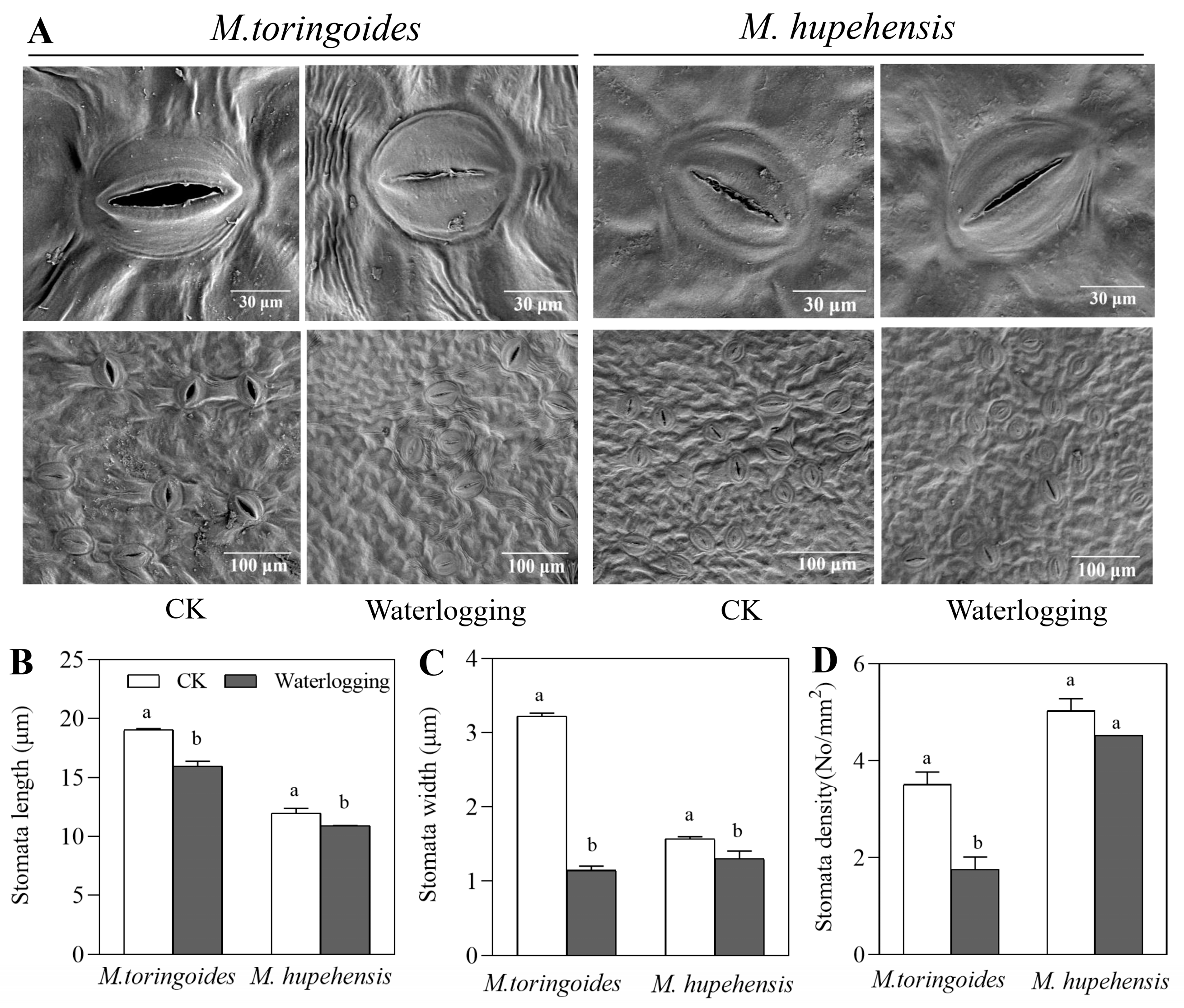
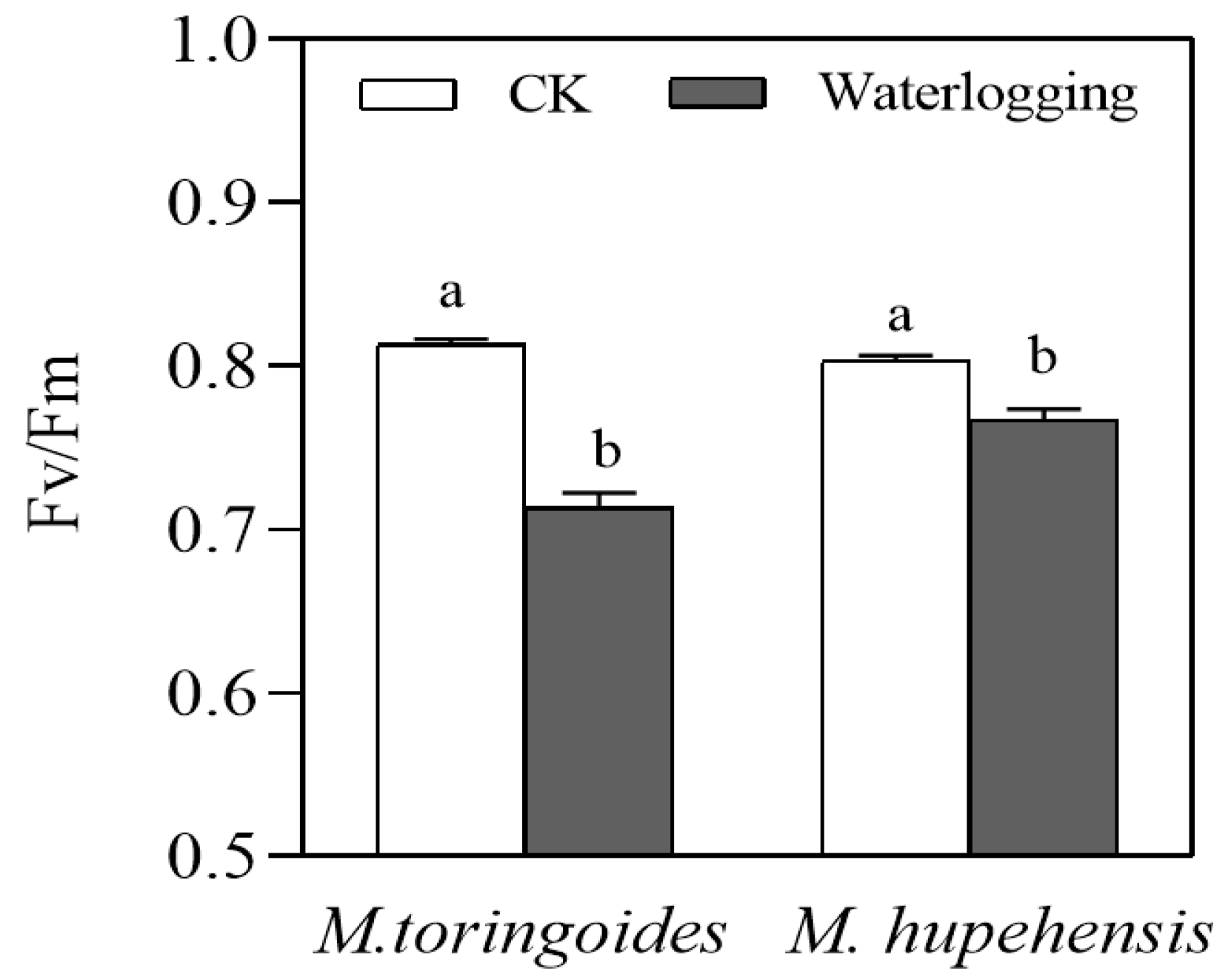
2.4. Differences in Stomatal Behavior and the Leaf Maximal Photochemical Efficiency (Fv/Fm) between M. toringoides and M. hupehensis under Waterlogging Stress
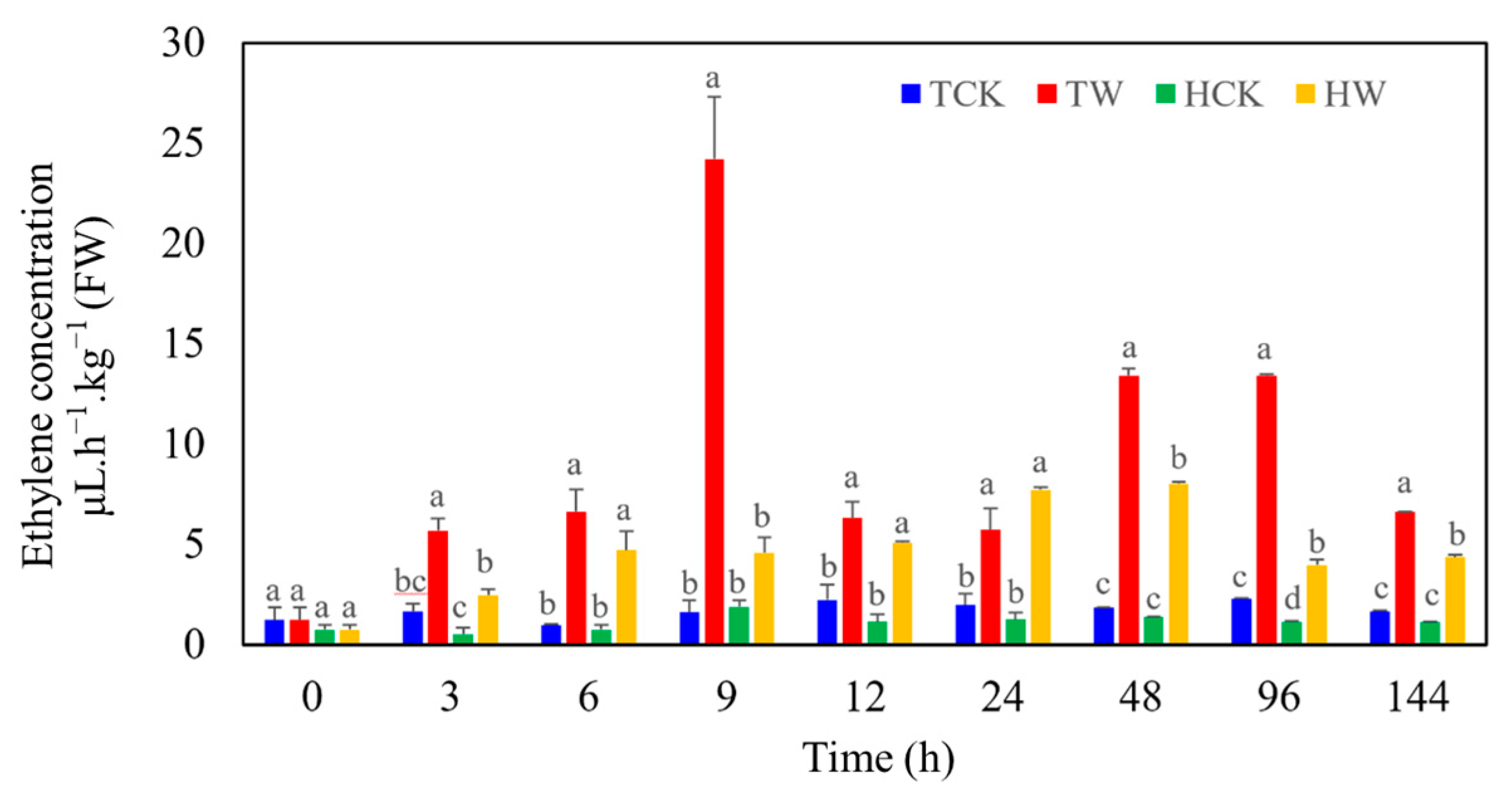
2.5. Differences in Ethylene Production between M. toringoides and M. hupehensis under Waterlogging Stress
2.6. Transcriptome Sequencing and Mapping to the Reference Genome
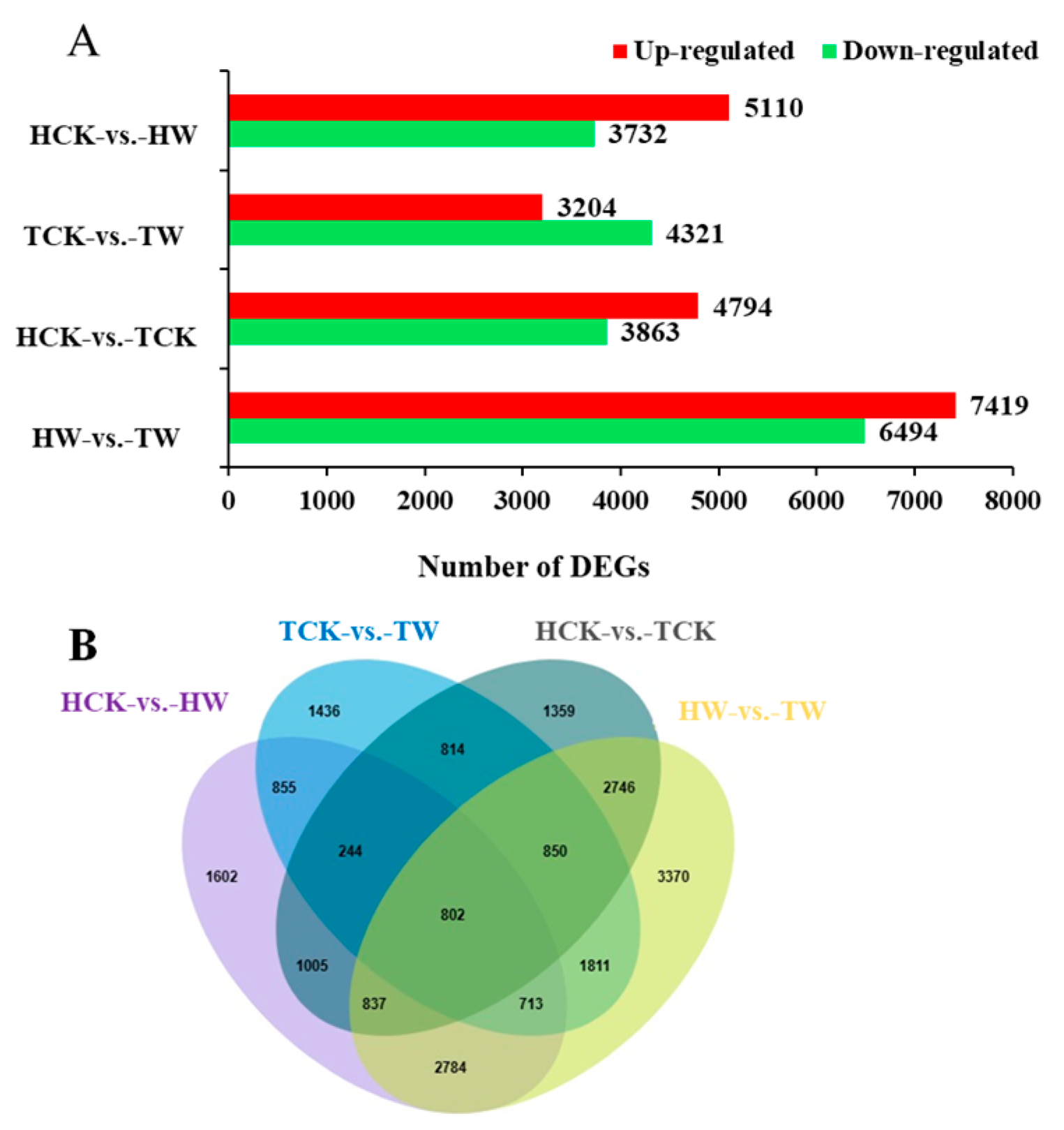
2.7. Identifying the Differentially Expressed Genes (DEGs)
2.8. GO and KEGG Pathway Enrichment Analysis
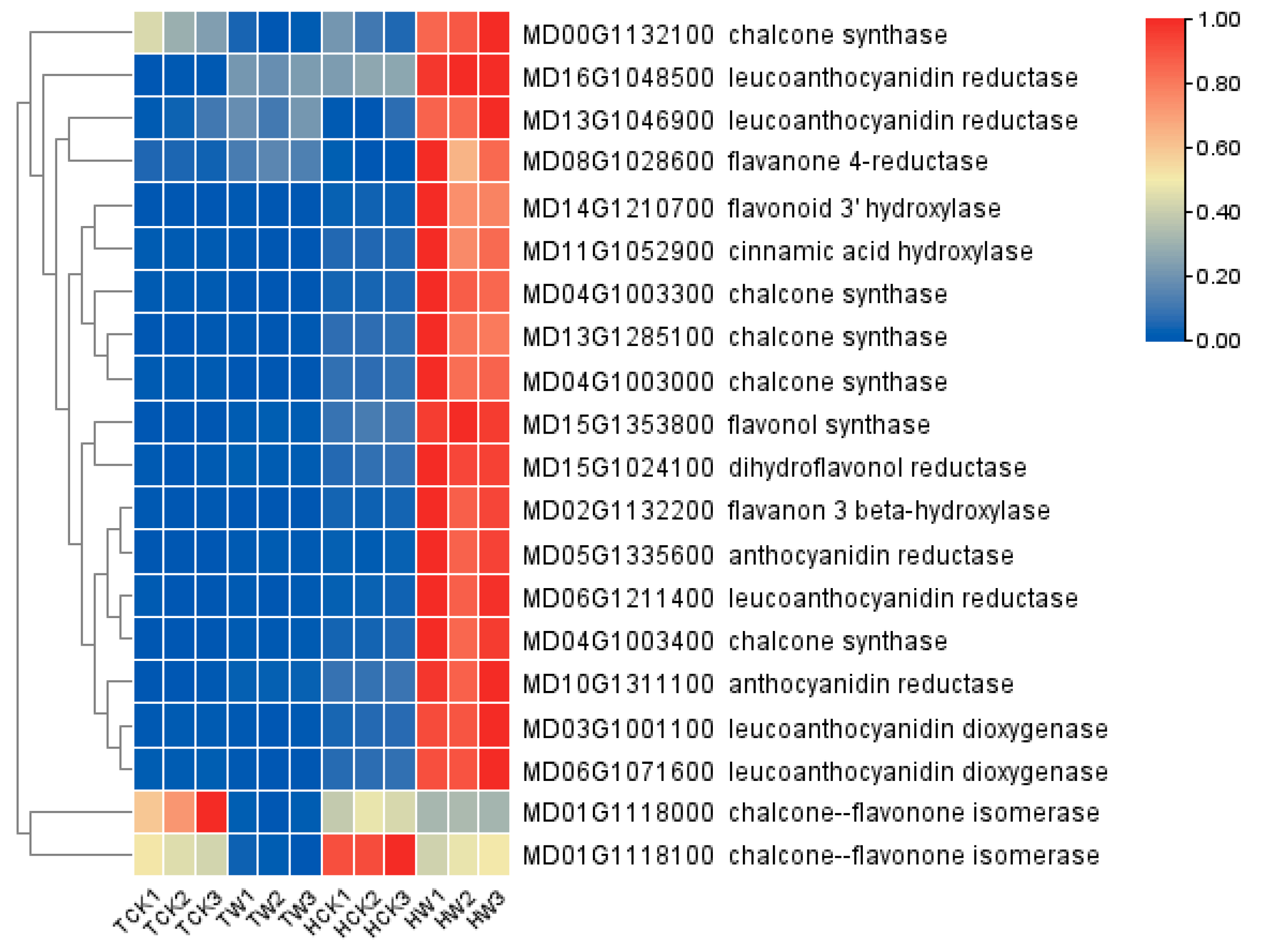
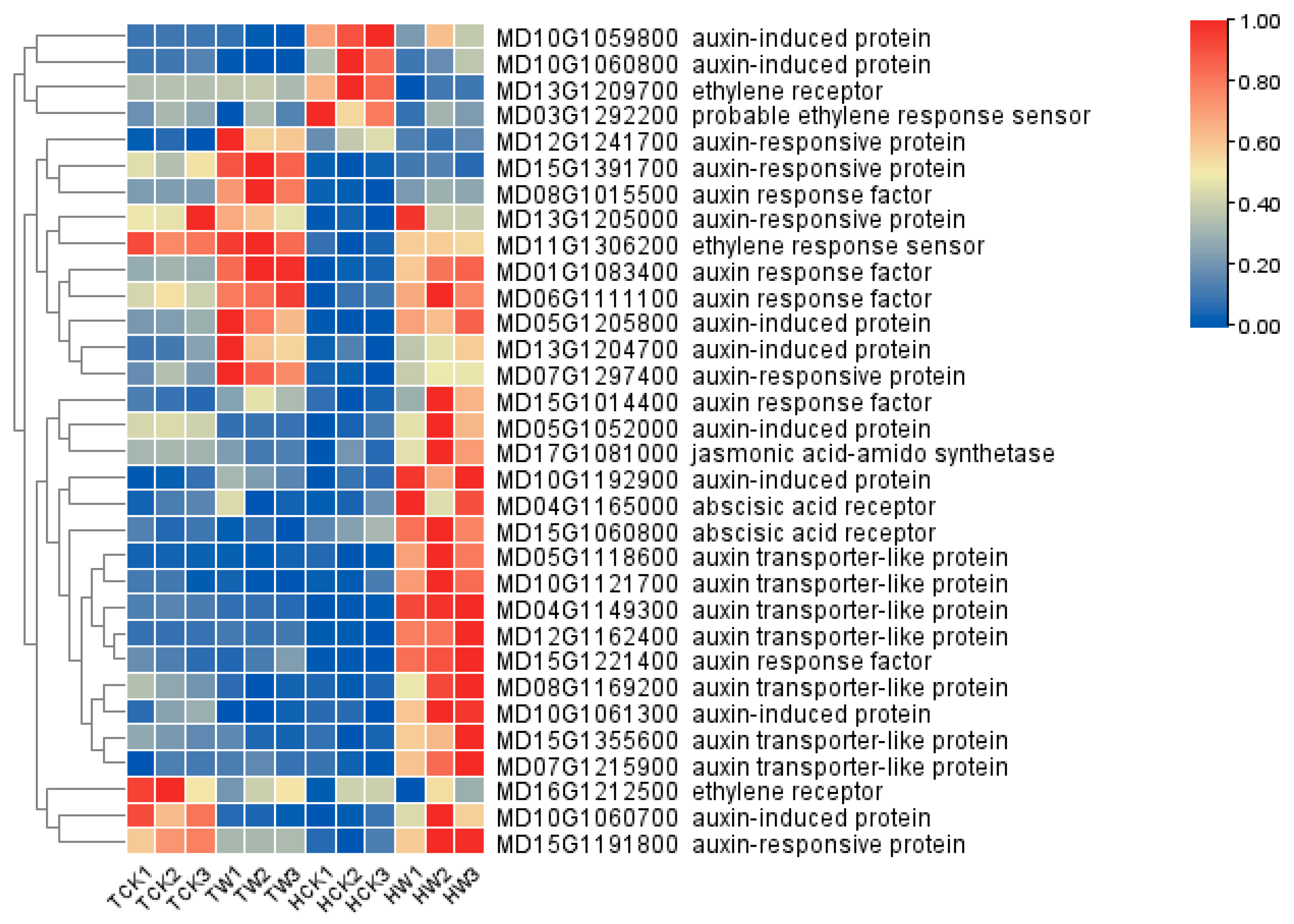
2.9. Expression Analysis of Flavonoid-Related DEGs and Hormone-Related DEGs
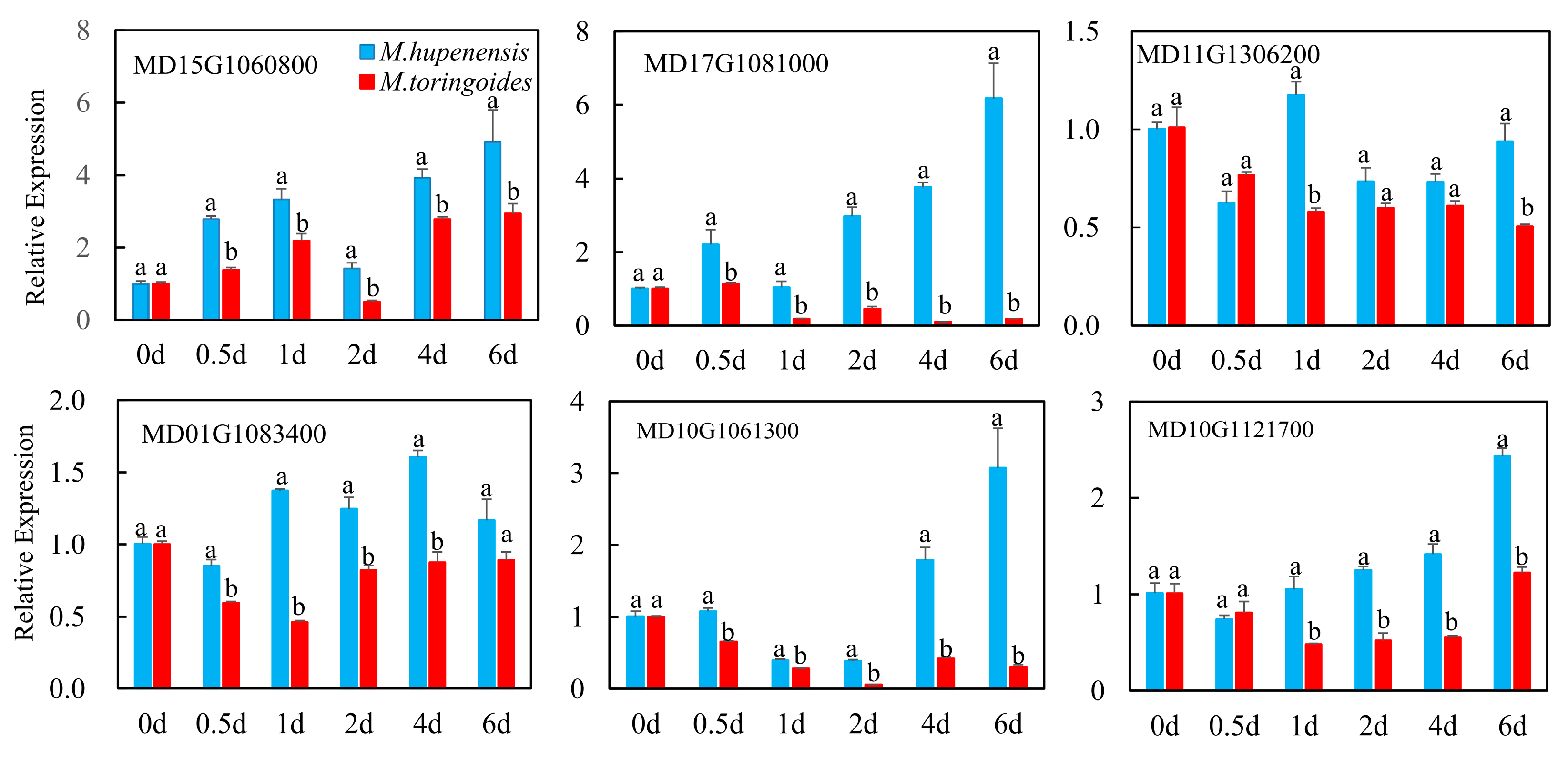
2.10. qRT-PCR Validation and Analysis of Hormone-Related Genes
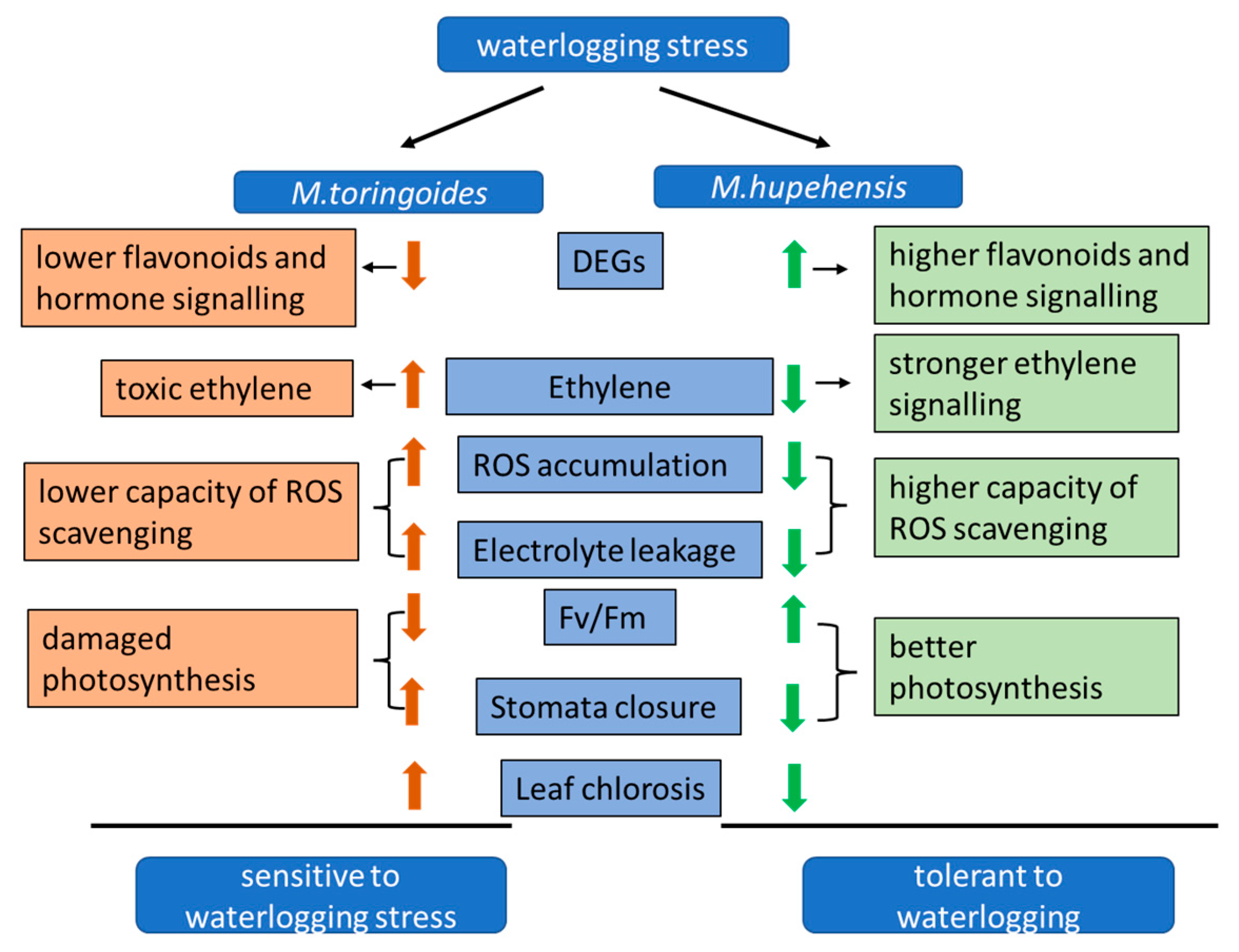
3. Discussion
3.1. Physiological and Morphological Changes under Waterlogging Stress
3.2. Differentially Expressed Genes Involved in Flavonoid Biosynthesis under Waterlogging Stress
3.3. Differentially Expressed Genes Involved in Hormone Biosynthesis and Signaling under Waterlogging Stress
4. Materials and Methods
4.1. Plant Materials and Cultivation
4.2. Waterlogging Treatment
4.3. Measurement of Electrolyte Leakage (EL) and Leaf Relative Water Content
4.4. Detection of O2•− and H2O2 Accumulation
4.5. Stomata Observation and Analysis
4.6. Measurement of Ethylene Production
4.7. Measurement of the Leaf Maximal Photochemical Efficiency (Fv/Fm)
4.8. RNA Extraction, cDNA Library Construction, and Sequencing
4.9. Identification of the DEGs
4.10. qRT-PCR Validation and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kim, H.; Kanae, S. Global flood risk under climate change. Nat. Clim. Change 2013, 3, 816–821. [Google Scholar] [CrossRef]
- Ding, J.; Liang, P.; Wu, P.; Zhu, M.; Li, C.; Zhu, X.; Gao, D.; Chen, Y.; Guo, W. Effects of waterlogging on grain yield and associated traits of historic wheat cultivars in the middle and lower reaches of the Yangtze River, China. Field Crops Res. 2020, 246, 107695. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Drew, M.C. Root growth and metabolism under oxygen deficiency. In Plant Roots; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 1139–1187. [Google Scholar]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Colmer, T. Plant tolerance of flooding stress–recent advances. Plant Cell Environ. 2014, 37, 2211–2215. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef]
- Sairam, R.; Kumutha, D.; Ezhilmathi, K.; Deshmukh, P.; Srivastava, G. Physiology and biochemistry of waterlogging tolerance in plants. Biol. Plantarum. 2008, 52, 401–412. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef]
- Bashar, K.K. Hormone dependent survival mechanisms of plants during post-waterlogging stress. Plant Signal. Behav. 2018, 13, e1529522. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; van Veen, H.; Yeung, E.; Voesenek, L.A. Signal dynamics and interactions during flooding stress. Plant Physiol. 2018, 176, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liang, K.; Fang, T.; Zhao, H.; Han, X.; Cai, M.; Qiu, F. A group VII ethylene response factor gene, ZmEREB180, coordinates waterlogging tolerance in maize seedlings. Plant Biotechnol. J. 2019, 17, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Y.; Yue, Z.; Chen, X.; Cao, X.; Xu, X.; Xing, Y.; Jiang, B.; Ai, X.; Huang, R. Changes in photosynthesis, chloroplast ultrastructure, and antioxidant metabolism in leaves of sorghum under waterlogging stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef]
- Thomson, C.J.; Greenway, H. Metabolic evidence for stelar anoxia in maize roots exposed to low O2 concentrations. Plant Physiol. 1991, 96, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Liu, T.; Shahid, M.A.; Schaffer, B.; Sarkhosh, A. Physiological, biochemical, and molecular responses of fruit trees to root zone hypoxia. Environ. Exp. Bot. 2023, 206, 105179. [Google Scholar] [CrossRef]
- Wegner, L.H. Oxygen transport in waterlogged plants. In Waterlogging Signalling and Tolerance in Plants; Mancuso, S., Shabala, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–22. [Google Scholar]
- Xu, X.; Wang, H.; Qi, X.; Xu, Q.; Chen, X. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar]
- Zhang, K.; Wen, T.; Dong, J.; Bai, T.; Wang, K.; Li, C. Comprehensive evaluation of tolerance to alkali stress by 17 genotypes of apple rootstocks. J. Integr. Agric. 2017, 15, 1499–1509. [Google Scholar] [CrossRef]
- Smethurst, C.F.; Shabala, S. Screening methods for waterlogging tolerance in lucerne: Comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Funct. Plant Biol. 2003, 30, 335–343. [Google Scholar] [CrossRef]
- Wang, A.; Roitto, M.; Lehto, T.; Sutinen, S.; Heinonen, J.; Zhang, G.; Repo, T. Photosynthesis, nutrient accumulation and growth of two Betula species exposed to waterlogging in late dormancy and in the early growing season. Tree Physiol. 2017, 37, 767–778. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, J.; Tan, D.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS Migration. Front. Plant Sci 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Chang, R. Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann. Bot. 2005, 96, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C.; Parlanti, S.; Banti, V.; Novi, G.; Perata, P. Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol. 2012, 159, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Jia, L.; Qin, X.; Lyu, D.; Qin, S.; Zhang, P. ROS production and scavenging in three cherry rootstocks under short-term waterlogging conditions. Sci. Hortic. 2019, 257, 108647. [Google Scholar] [CrossRef]
- Justin, S.; Armstrong, W. Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytol. 1991, 118, 49–62. [Google Scholar] [CrossRef]
- Gunawardena, A.; Pearce, D.; Jackson, M.; Hawes, C.; Evans, D. Rapid changes in cell wall pectic polysaccharides are closely associated with early stages of aerenchyma formation, a spatially localized form of programmed cell death in roots of maize (Zea mays L.) promoted by ethylene. Plant Cell Environ. 2001, 24, 1369–1375. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Purcell, L.C. Soybean root morphological and anatomical traits associated with acclimation to flooding. Crop Sci. 1999, 39, 143–149. [Google Scholar] [CrossRef]
- Zúñiga-Feest, A.; Bustos-Salazar, A.; Alves, F.; Martinez, V.; Smith-Ramírez, C. Physiological and morphological responses to permanent and intermittent waterlogging in seedlings of four evergreen trees of temperate swamp forests. Tree Physiol. 2017, 37, 779–789. [Google Scholar] [CrossRef]
- Núnez-Elisea, R.; Schaffer, B.; Fisher, J.B.; Colls, A.; Crane, J.H. Influence of flooding on net CO2 assimilation, growth and stem anatomy of Annona species. Ann. Bot. 1999, 84, 771–780. [Google Scholar] [CrossRef]
- Guillermo, T.; Manuel, P.; Paula, P. Root respiratory components of Prunus spp. rootstocks under low oxygen: Regulation of growth, maintenance, and ion uptake respiration. Sci. Hortic. 2018, 239, 259–268. [Google Scholar]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat–a review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Glick, B.R. Ethylene and abiotic stress tolerance in plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 395–412. [Google Scholar]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Bradford, K.J.; Yang, S. Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol. 1980, 65, 322–326. [Google Scholar] [CrossRef]
- Stünzi, J.T.; Kende, H. Gas composition in the internal air spaces of deepwater rice in relation to growth induced by submergence. Plant Cell Physiol. 1989, 30, 49–56. [Google Scholar] [CrossRef]
- Choi, B.H.; Bhusal, N.; Jeong, W.T.; Park, I.H.; Han, S.G.; Yoon, T.M. Waterlogging tolerance in apple trees grafted on rootstocks from G, CG, and M series. Hortic. Environ. Biotechnol. 2020, 61, 685–692. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Ma, F.; Shu, H.; Han, M.; Wang, K. Physiological responses and analysis of tolerance of apple rootstocks to root-zone hypoxia stress. Sci. Agric. Sin. 2008, 41, 4140–4148. [Google Scholar]
- Wang, H.; Song, C.; Fang, S.; Wang, Z.; Song, S.; Jiao, J.; Wang, M.; Zheng, X.; Bai, T. Genome-wide identification and expression analysis of the ASMT gene family reveals their role in abiotic stress tolerance in apple. Sci. Hortic. 2022, 293, 110683. [Google Scholar] [CrossRef]
- Rodriguez-Gamir, J.; Ancillo, G.; González-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner-Giner, M.A. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef]
- Drew, M.; Sisworo, E. Early effects of flooding on nitrogen deficiency and leaf chlorosis in barley. New Phytol. 1977, 79, 567–571. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Jia, X.; Huo, L.; Che, R.; Ma, F. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018, 16, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Zhang, X.; Ma, F.; Guo, T.; Li, C. Activation of the ABA signal pathway mediated by GABA improves the drought resistance of apple seedlings. Int. J. Mol. Sci. 2021, 22, 12676. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, H.; Geng, Y.; Schneider, R. Effects of submergence on photosynthesis and growth of Pterocarya stenoptera (Chinese wingnut) seedlings in the recently-created Three Gorges Reservoir region of China. Wetl. Ecol. Manag. 2010, 18, 485–494. [Google Scholar] [CrossRef]
- Kuai, J.; Liu, Z.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Zhou, Z.; Oosterhuis, D.M. Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton boll and its relationship with boll weight. Plant Sci. 2014, 223, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zhao, S.; Cui, M.; Han, G.; Wen, P. Vulnerability of photosynthesis and photosystem I in Jerusalem artichoke (Helianthus tuberosus L.) exposed to waterlogging. Plant Physiol. Biochem. 2018, 125, 239–246. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- van Loon, L.C.; Glick, B.R. Increased plant fitness by rhizobacteria. In Molecular Ecotoxicology of Plants; Sandermann, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 177–205. [Google Scholar]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Bakker, P.A.H.M., Raaijmakers, J.M., Bloemberg, G., Höfte, M., Lemanceau, P., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 329–339. [Google Scholar]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids-biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophy. 2000, 373, 102–109. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; van Breusegem, F.; Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of auxin in growth and stress response of cucumber. Veg. Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, C.; Yan, Y.; Cao, W.; Song, A.; Wang, H.; Chen, S.; Jiang, J.; Chen, F. Comparative transcriptome analysis of waterlogging-sensitive and waterlogging-tolerant Chrysanthemum morifolium cultivars under waterlogging stress and reoxygenation conditions. Int. J. Mol. Sci. 2018, 19, 1455. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Ma, X.; Xu, Q.; Qi, X.; Chen, X. Comparative proteomic analysis provides insight into the key proteins involved in cucumber (Cucumis sativus L.) adventitious root emergence under waterlogging stress. Front. Plant Sci. 2016, 7, 1515. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.H.M.; Komatsu, S. Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J. Proteom. 2016, 133, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hwang, S.J.; Waqas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef]
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Bio. 2012, 79, 609–622. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Chen, X.; Yuan, P.; Song, C.; Song, S.; Jiao, J.; Wang, M.; Hao, P.; Zheng, X.; Bai, T. Comparative Physiological and Transcriptome Analysis Reveals Potential Pathways and Specific Genes Involved in Waterlogging Tolerance in Apple Rootstocks. Int. J. Mol. Sci. 2023, 24, 9298. https://doi.org/10.3390/ijms24119298
Zhang K, Chen X, Yuan P, Song C, Song S, Jiao J, Wang M, Hao P, Zheng X, Bai T. Comparative Physiological and Transcriptome Analysis Reveals Potential Pathways and Specific Genes Involved in Waterlogging Tolerance in Apple Rootstocks. International Journal of Molecular Sciences. 2023; 24(11):9298. https://doi.org/10.3390/ijms24119298
Chicago/Turabian StyleZhang, Kunxi, Xiaofei Chen, Penghao Yuan, Chunhui Song, Shangwei Song, Jian Jiao, Miaomiao Wang, Pengbo Hao, Xianbo Zheng, and Tuanhui Bai. 2023. "Comparative Physiological and Transcriptome Analysis Reveals Potential Pathways and Specific Genes Involved in Waterlogging Tolerance in Apple Rootstocks" International Journal of Molecular Sciences 24, no. 11: 9298. https://doi.org/10.3390/ijms24119298
APA StyleZhang, K., Chen, X., Yuan, P., Song, C., Song, S., Jiao, J., Wang, M., Hao, P., Zheng, X., & Bai, T. (2023). Comparative Physiological and Transcriptome Analysis Reveals Potential Pathways and Specific Genes Involved in Waterlogging Tolerance in Apple Rootstocks. International Journal of Molecular Sciences, 24(11), 9298. https://doi.org/10.3390/ijms24119298





