Abstract
Depression is a mental disorder that affects more than 300 million people worldwide. The medications available for treatment take a long time to exhibit therapeutic results and present several side effects. Furthermore, there is a decrease in the quality of life of people suffering from this affliction. Essential oils are traditionally used to relieve the symptoms of depression due to the properties of the constituents of these oils to cross the blood–brain barrier acting on depression-related biological receptors associated with reduced toxicity and side effects. In addition, compared to traditional drugs, they have several administration forms. This review provides a comprehensive assessment of studies on plants whose essential oil has exhibit antidepressant activity in the past decade and the mechanism of action of the major components and models tested. An additional in silico study was conducted with the frequent compounds in the composition of these essential oils, providing a molecular approach to the mechanism of action that has been reported in the past decade. This review is valuable for the development of potential antidepressant medications in addition to providing a molecular approach to the antidepressant mechanism of action of the major volatile compounds that have been reported in the past decade.
1. Introduction
Depression is a mental disorder representing a significant and growing public health problem, with an estimated 300 million people afflicted worldwide [1]. The COVID-19 pandemic increased the number of anxiety and depression disorders by 25% during its first year, and the latest data from the World Health Organization estimate that 71% of people with depression do not receive mental health services [2,3]. In addition to the problem of depression itself, this disease brings with it medium- and long-term consequences, such as cognitive disorders, which include deficits in several domains (attention, executive functions, memory and processing speed), dementia and is an initial cause of Parkinson’s disease [4,5].
Although drugs are available, access to pharmacological treatment for depression presents difficulties. The prescribed therapy is expensive, and only a small percentage of patients achieve remission with antidepressant monotherapy alone [6]. Another less discussed factor, a common problem affecting 30% to 50% of people with neurological diseases, is pharmacological refractoriness [7,8].
Refractory patients do not respond adequately to medications, even if administered correctly [9]. This reason is still not fully understood, but it is believed that some neurological diseases, because they are multifactorial, are involved in several biological aspects [10]. Thus, treatment may be effective for depression related to a specific etiological factor but not for depression secondary to another etiology [11].
The main classes of drugs available for the treatment of depression are selective serotonin reuptake inhibitors (SSRIs), serotonin noradrenaline reuptake inhibitors (SNRI), tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) [12]. The class used as the first choice for the treatment of depression is the SSRI, because, according to the pharmacological aspects, they are safe in overdose, have relative tolerability, have a generic form and have a broad spectrum of use compared to other classes [13]. Due to their lipophilic characteristics, SSRIs, especially fluoxetine, have a large volume of distribution between 14 and 100 L/kg, which indicates extensive tissue accumulation, mainly in the lung [14,15]. For this reason, SSRIs can increase systole and diastole time (QT interval), which increases the chances of fatal arrhythmia [16]. Currently, the prescription of other classes of antidepressants is very restricted due to high toxicity and potentially lethal food interactions [17]. For example, the Class of MAOIs interacts with foods containing tyrosine, increasing adrenergic activity at high risk of hypertensive crisis [18]. The class of TCAs also has effects on cardiac function such ashy postural hypo arrest and high-dose cardiotoxicity [19]. Prescription occurs only in patients who do not respond to more tolerable medications or have refractory depression [20]. In recent years, the antidepressant effect of ketamine anesthetic has been reported in cases of treatment-resistant depression [20]. However, ketamine in clinical practice was limited over a period of time due to its side effects on the central nervous system and the characteristics of a drug of abuse [21].
All classes of antidepressants, although with proven efficacy, have their limitations in terms of treatment. In general, the therapeutic effect appears within weeks of drug administration. On the other hand, side effects are felt first, directly interfering with the patient’s quality of life [22]. Among the main side effects, cardiac toxicity in case of overdose, dry mouth, accommodation disorders, constipation, urinary retention, sexual dysfunction and weight gain stand out [23,24,25]. In addition to these side effects, prolonged use of antidepressants can influence cognitive slowness and attention and memory difficulties. Despite being reversible, these effects lead some people to abandon therapy without completing treatment [26]. Given this context, it is important to identify new classes of antidepressant therapeutic agents with rapid onset of action, effective responses and fewer side effects.
The use of essential oils (EOs) is traditionally one of the complementary means or alternatives to drugs used in treating various diseases and symptoms, because they are a rich source of bioactive components [27]. The use of EOs has the advantage of topical or inhaled administration over orally administered drugs, which undergo first-pass metabolism, reducing the oral bioavailability and can provide drug interaction [28]. However, it is not because they are natural products that they can be used without worries. The main adverse events caused by the incorrect and indiscriminate use of EOs are dermatitis by allergic contact, phototoxicity and oral toxicity [29,30]. A review article published in 2022 on the toxicity of selected monoterpenes found that, while most are safe for human food and medical applications, there are monoterpene compounds that, in certain amounts or in particular circumstances (e.g., pregnancy), can cause serious disorders [31]. Therefore, the use of EOs should always be implemented with caution, because all substances can be toxic depending on the conditions of exposure, the dose and the route of administration [32].
In the past decade, many studies have reported the efficiency of EOs in relieving symptoms of diseases related to mental disorders, improving mood and feelings of physical and mental well-being, with the advantage of fewer adverse reactions [33]. The EOs from rose (Rosa damascena Mill.) [34], patchouli (Pogostemon cablin (Blanco) Benth.), lemongrass (Cymbopogon citratus (DC.) Stapf), sandalwood (Santalum album L.), bergamot (Citrus bergamia Risso & Poit.), valerian (Valeriana officinalis L.) and lemon (Citrus limonum Risso) are popularly consumed to relieve symptoms of depression and anxiety [29,35]. Thus, these popular uses stimulate investigations to understand the pharmacological processes of the antidepressant and anxiolytic effects of these EOs [36,37,38].
Lavender EO (Lavandula angustifolia Mill.) has been one of the most studied and has been shown to alleviate symptoms of anxiety and depression in clinical trials [39]. Lavender oil is rich in linalool and linalyl acetate, these two monoterpenoids being the components that determine the antidepressant effect [40]. The importance of natural products in the discovery of bioactive compounds has been extensively documented and has contributed to the development of current drugs [41,42]. For this reason, the identification of molecules in EOs with antidepressant action can contribute to primary or complementary therapy. In this context, in recent years, there has been an increasing number of publications on this topic [43]. However, the most recent review work published was in 2017, which carried out an EO survey of plants with antidepressant activity from 1995 to 2015 [44]. Therefore, this review provides a comprehensive update on EOs with antidepressant activity and aims to present the main results and antidepressant mechanisms obtained for the major constituents of these oils in silico, in vitro and in vivo models. In addition, this article developed an in silico approach to the mechanism of action of key compounds based on in vivo results, illustrating at the molecular level the interaction of these compounds at their corresponding target.
2. Molecular Mechanisms Involved in Depression
Depression is a complex and multifactorial illness triggered by psychological, genetic, social and biological factors [45]. Among the proposals on the biological etiology of depression, the classical theory suggests the hypothesis that the disease is due to a deficiency of monoamine neurotransmitters in the synaptic cleft, as noradrenaline (NA), dopamine (DA) and serotonin (5-HT) [46]. This proposition is reinforced by knowledge of the mechanism of action of antidepressants, including the drug Prozac®, which has the fluoxetine molecule as its active ingredient [47]. Currently, the evidence points to the neurochemical disturbance as a factor for depression disorder, which the 5-HT pathway is involved as one of the neurotransmission systems modified [48]. In addition, antidepressant use was linked to a reduction in 5-HT levels that was not associated to the depression state. Recently, in a systematic review, the authors infer the lack of a robust evidence of association between the reduced 5-HT levels or serotoninergic route hypoactivity per se and depression [48]. Thus, additional components may share the complex pathophysiological mechanisms, in which a cascade initiated in stressor condition on a susceptible genetic profile develops altered responses in an immunologic and endocrine scenario, eliciting functional and biochemical changes in central nervous regions by neurotrophic mediator alterations, neuroinflammation, oxidative stress and also neurochemical modifications [49].
In the past decade, most studies on the antidepressant action of EOs have been associated with more than one mechanism of action. However, the monoaminergic pathway involving the neurotransmitters DA and 5-HT is the most expressively elucidated, probably because of the monoamine theory concerning the most discussed pathway linked to depression. The second most discussed mechanism involves the participation of neurotrophic factors such as the Brain-Derived Neurotrophic Factor (BDNF). BDNF is related to synaptic plasticity and neurogenesis [50]. Thus, recent studies report that its decrease can cause different changes in the nervous system, such as depression, anxiety, schizophrenia and Parkinson’s disease [51]. In addition to the monoaminergic pathway and BDNF, other mechanisms that may be involved in depression have been reported, such as the GABAergic system alteration, increased expression of postsynaptic serotonin receptors (5-HT1A), decreased calcium influx and increased expression of astrocytes. The mechanisms of action of EOs with antidepressant effects reported in the past ten years are shown in Table 1.

Table 1.
Summary of the main information and results found in the research of plant species that showed antidepressant activity in the past ten years.
3. Major Volatile Compounds of Essential Oils with Antidepressant Action
The EOs that showed antidepressant effects have two classes of major compounds in common: terpenoids and phenylpropanoids. The following subtopics describe some studies on the main antidepressant effects and mechanisms of these compounds.
3.1. Monoterpenes with Antidepressant Action
The EO samples rich in monoterpenes (19 samples) showed linalool, d-limonene, α-phellandrene, γ-terpinene and terpinen-4-ol, as the most frequent compounds. EOs of Aniba rosiodora, Aeollanthus suaveolens and Aniba parviflora rich in linalool (88.6%, 49.3% and 45.0%, respectively) were evaluated for the neurobehavioral effect in Wistar rats at doses of 3.5 and 35 mg/kg intraperitoneally (i.p.). Linalool was responsible for the significant improvement in symptoms of depression similar to fluoxetine (10 mg/kg) through its action on the serotonergic pathway [33].
Inhalation of EO from Citrus sinensis and C. reticulata, rich in d-limonene (90.7% and 76.7%, respectively), significantly improved depression-like behavior in mice, suggesting the involvement of the main compound in key mechanisms such as increased expression of BDNF and 5-HT1A receptor, which results in neurogenesis and serotoninergic pathway improvement, respectively [67,80]. Origanum majorana EO rich in terpinen-4-ol (32.6%) showed antidepressant effects in mice at doses of 10 and 80 mg/kg comparable to fluoxetine (20 mg/kg), used as a positive control. The results indicated the involvement of EOs in dopaminergic (D1 and D2), serotonergic (5HT1A, 5-HT2A receptors) and noradrenergic (α1 and α2 adrenoceptors) receptors. On the other hand, terpinen-4-ol is probably responsible for the antidepressant activity involved in the monoaminergic system [80].
3.2. Sesquiterpenes with Antidepressant Action
Despite the wide distribution of sesquiterpenes in essential oils, there are few studies on their antidepressant action [89]. Anthriscus nemorosa EO presented β-caryophyllene (23.6%), caryophyllene oxide (12.3%) and δ-cadinene (12.10%) as major components that were tested in mice by inhalation (1% to 3%). Results demonstrated antidepressant and an anxiolytic response attributed to β-caryophyllene, which positively modulated the GABAA receptor activity similar to the positive control group treated with diazepam (1.5 mg/kg) [61].
EOs from Pinus halepensis having a β-caryophyllene content of 29.4% have been evaluated in vivo by inhalation (1% and 3%) attenuating anxious-depressive behaviors in the model of Alzheimer’s disease induced by Aβ1-42 in rodents [77]. Recent studies published between 2019 and 2022 report the therapeutic potential of β-caryophyllene as a reducer of pro-inflammatory mediators, improving the symptoms of neurological diseases characterized by inflammation and oxidative stress [90,91,92,93]. All these pieces together suggest that β-caryophyllene may exhibits antidepressant activity through more than one mechanism of action in the pathophysiology of depression.
The antidepressant potential of Pogostemon cablin EO was investigated from different fractions with different concentrations of patchoulol. The separation process was carried out by vacuum distillation in the following temperature ranges resulting in patchoulol concentrations of 42.8%, 49.3% and 60.6%. The fraction with the highest patchoulol content (60.66%) showed a better antidepressant effect in animal models, suggesting a monoaminergic mechanism with increased dopamine availability [36].
β-Elemene is the second most widely distributed sesquiterpene in EOs with antidepressant action and was identified in Magnolia sieboldii (22.1%) and Toona ciliata (24.9%) oils. For the EO of M. siebold, at concentrations 625, 1250 and 2500 μL/kg, the antidepressant effect was attributed to the sesquiterpenes β-elemene and germacrene D, which increased the expression of BDNF and 5-HT1A in the brain tissue of mice, in addition to stimulating the secretion of serotonin [85]. T. ciliata EO produced antidepressant effects at 20, 40 and 80 mg/kg concentrations in mice by increasing DA, NE, 5-HT and BDNF levels in the hippocampus in a dose-dependent manner [60].
3.3. Phenylpropanoids with Antidepressant Action
The phenylpropanoid (E)-cinnamaldehyde (87.3%), the main component of the EO of Cinnamomum verum, was responsible for the reduction of the depressive effect in mouse models at doses of 0.5, 1.0 and 2.0 mg/kg; however, its mechanism of action was not elucidated [65]. EOs from Foeniculum vulgare and Pimpinella anisum showed 82.1% and 88.4% (E)-anethole concentrations, respectively. Foeniculum vulgare oil showed an antidepressant effect in mice treated i.p. (100 to 400 mg/kg) through DA and 5-HT pathways in addition to antioxidant activity [82]. Pimpinella anisum oil (0.3 mg/kg) indicated that it could alter the effect of drugs that influence the nervous system. The intake of EO led to a significant increase in the analgesic effect of codeine. Motor impairment caused by midazolam was greater in the group treated with the EO. The diazepam application indicated the drug’s increased effect on motor activity. The pretreatment with EO caused a significant reduction in the pentobarbital-induced sleep time when compared to the control. The pretreatment diminished the decrease in the antidepressant effect of imipramine and fluoxetine with aniseed EO. However, the mechanism of action of Pimpinella anisum EO still needs to be defined. Thus, the evaluation of results gained in our study together with the previously published data indicate that use of aniseed EO can change the effect of drugs that act in central nervous system [53].
4. Molecular Docking
Molecular docking analysis was applied to elucidate the mechanisms of action of the compounds described in this study as antidepressants by in vivo models. The selected molecules (Figure 1) demonstrated a potential effect on the monoaminergic pathway, specifically on the serotonin transporter (SERT) and dopamine transporter (DAT).
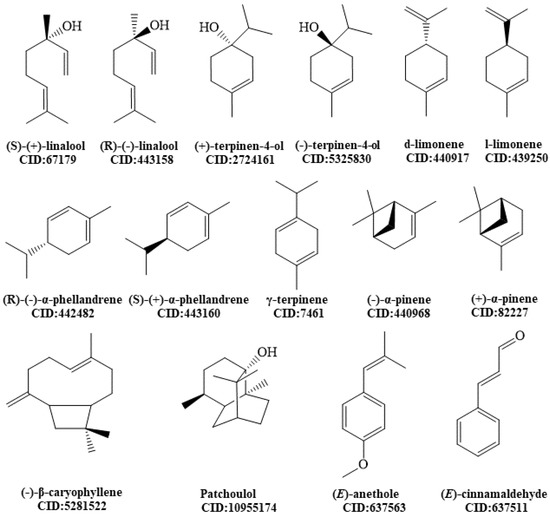
Figure 1.
Chemical structures of molecules analyzed by molecular docking.
Molecules were built and optimized using the Avogadro 1.2.0 software, as well as its main enantiomers, following the MMFF94 method to include all potential conformations in medium-sized rings and bonds, where interconversion between conformations may be impaired [94]. The 3D structures of SERT and DAT were obtained from the protein database (PDB) under code 5I6X and 4M48, respectively. The search sphere for each ligand was 12 Å in radius centered on the sites, and default protonation states of each protein based on neutral pH were used and charges were assigned based on default templates as part of the Molegro Virtual Docker program version 6.0 (MVD). The bonds of the compounds were taken as flexible, and the receptors were considered rigid. Different ligand orientations were generated and classified based on their energy scores. A minimum of 10 runs for each binder were performed. An energy score adjustment was also performed in order to eliminate the bias of the anchoring energies (Edock) with the increase of the second molecular weight, determined as DSnorm, from the equation:
where DSnorm is the normalized docking score, Edock is the Moldock reclassification score, MW is molecular weight and 7.2 is a scale constant to bring the average values of DSnorm comparable to Edock [95]. The best-fitting results are summarized in Table 2.
DSnorm: 7.2 × Edock/MW1/3.

Table 2.
Binding energy (kJ/mol) calculated by molecular docking of the main monoterpenes, sesquiterpenes and phenylpropanoids present in EO with antidepressant effects in the protein SERT and DAT.
Molecular docking was performed for all enantiomers to identify the possible enantioselectivity of these structures against the molecular target. Based on the affinity energy results, a significant difference is observed only from (R)-(−)-linalool at the DAT receptor, with affinity energy higher than (S)-(+)-linalool at the same receptor (−92.76 kJ/mol and −85.86 kJ/mol, respectively). There is evidence that the semantic treatment of linalool enantiomers separately promotes different effects once these compounds are chemically, biosynthetically, electrophysiologically and behaviorally distinct [96]. However, the elucidation of the relative stereochemistry of asymmetric centers of organic molecules is a challenge in the Natural products chemistry, because it requires the simultaneous determination of conformation and configuration, and few studies that discriminate the enantiomers and their mechanisms are related to depression [97].
Continuing the analysis, several studies reported the use of fluoxetine as a positive control in animal models. Interactions between fluoxetine and the SERT receptor are already well established and discussed in the literature [47,98,99]. Molecular interactions that configure the stability and substrate preference for the inhibitor are illustrated in Figure 2.
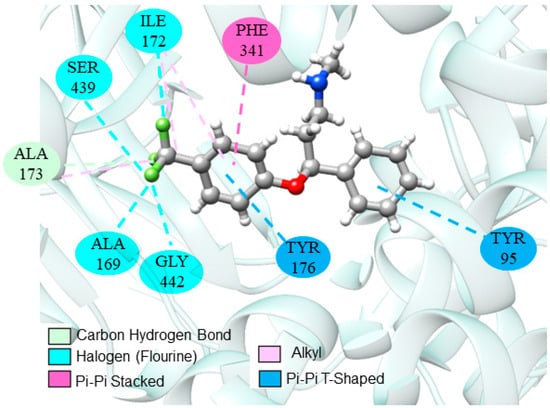
Figure 2.
Interactions of fluoxetine at the SERT catalytic site.
4.1. Monoterpenes
Monoterpenes show more exothermic docking results in the SERT receptor compared to the DAT receptor (Table 2). Specifically, d-limonene, γ-terpinene and α-phellandrene adopted an energy gain above 10 kJ/mol. The results show the compound preference for the SERT receptor, caused by the mechanism of serotonergic inclination, and corroborates the in vivo studies cited in this review.
The values of the affinity energy of monoterpenes are inferior compared to fluoxetine (antidepressant drug reference), which presented a value of −108.28 kJ/mol in the catalytic site. However, it is important to emphasize that it is a synthetic product and structurally differs from monoterpenes.
In addition, the docking energies of compounds are biased by larger molecular mass, since they have a greater number of atoms interacting with the target molecule. There will be a tendency for the selection of larger molecules, even if they are not necessarily as structurally complementary to the target binding site as the smaller compounds; adjustment of the docking score (DSnorm) is required to correct this problem [100]. After that, we can compare the results of the indications of the EOs with the drug fluoxetine. (S)-(+)-linalool displayed an affinity energy value of −93.83 kcal/mol, very close to the value obtained for fluoxetine. The hydroxyl group in both oxygenated monoterpenes allow stronger interactions at the catalytic site and hydrophobic interactions promoting greater stability and affinity for the receptor.
Among the monoterpene hydrocarbons, we can highlight (R)-(−)-α-phellandrene and d-limonene, which showed the best affinity energies (−84.08 kJ/mol and −83.93 kJ/mol, respectively). In the absence of hydrogen bonds, hydrophobic interactions of the π-alkyl or π-pairing influence the stability of molecules at the catalytic site [101]. Despite being a weak interaction, hydrophobic interactions can play a significant role in the conformation and stability of structures and complexes, working cooperatively with energy values of –0.5 and −1.0 kJ/mol per interaction [102]. Figure 3 shows the main monoterpene interactions with the best binding energy results.
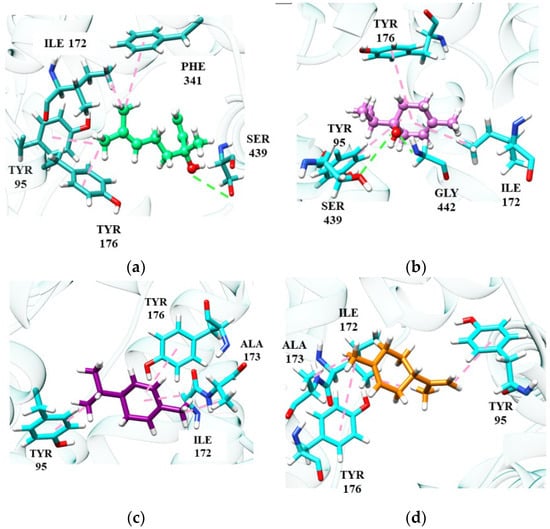
Figure 3.
Monoterpene interactions at the SERT site: linalool (a), terpinen-4-ol (b), α-phellandrene (c), D-limonene (d).
(S)-(+)-Linalool and (+)-terpinen-4-ol perform some important interactions in SERT inhibition, similar to the fluoxetine molecule. (S)-(+)-Linalool makes an π-alkyl interaction with residues Phe341, Tyr95 and Ile172, a significant interaction as it reproduces the docking effect of fluoxetine, as demonstrated in previous studies [103]. (S)-(+)-Linalool also showed relevant interactions with the residues of Ile172, Phe341 and Tyr95. A previous study demonstrated that the mutation in these residues configures a decrease of >10 in the Ki value of fluoxetine, suggesting that these are determinant residues for SERT inhibition [47]. (+)-Terpinen-4-ol replaced the interaction of Phe341 with Tyr176, which may be related to lower energy than that of (S)-(+)-linalool. Specifically, (+)-terpinen-4-ol made an additional energetically favorable hydrogen-bond interaction with the Gly442 residue. The hydrogen-bonding interaction of fluoxetine with Gly442 has previously been demonstrated [98]. The two hydrocarbon monoterpenes, (R)-(−)-α-phellandrene and d-limonene, also do not interact with Phe341 and have lower affinity energy than linalool and (+)-terpinen-4-ol. However, they still manage to interact with essential residues at the catalytic site.
4.2. Sesquiterpenes
Among the sesquiterpenes, we highlight (−)-β-caryophyllene and patchoulol, which are involved in activity against depression, however, acting on different targets (SERT and DAT, respectively). Patchoulol did not show good affinity energy (−56.12 kJ/mol) at the SERT receptor, suggesting a non-compatibility between the target and molecule. Differently, at the DAT receptor, patchoulol presented a better affinity energy (−72.52 kJ/mol), corroborating with the studies that identified the antidepressant activity of patchoulol in the dopaminergic pathway [36,104].
According to Figure 4, it is possible to notice that patchoulol fits better in the catalytic site of the DAT protein, performing interactions with a greater number of amino acid residues, which directly influences the affinity energy. In the patchoulol-DAT complex, hydrogen-bonding interactions occur between the hydroxyl group of the ligand and Ser421 and Tyr124 residues. In contrast, in the SERT protein, patchoulol does not interact strongly due to the 4.99 Å distance to the Tyr176 residue. Consequently, the dipole-induced interaction between Ile172 and Tyr176 is weakened, compromising energy redistribution in the site’s surroundings [69]. On the other hand, (−)-β-caryophyllene has good affinity at the SERT receptor (−96.20 kJ/mol) but has a significant loss in affinity towards the DAT receptor (−58.55 kJ/mol), suggesting greater performance in the serotonergic pathway [36,104].
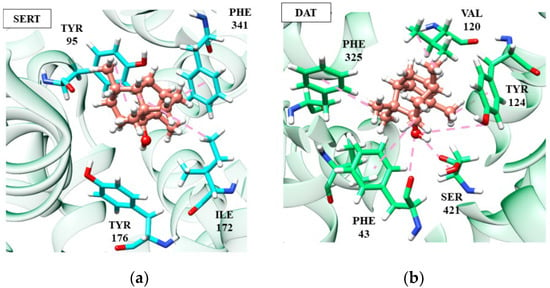
Figure 4.
Patchoulol (salmon) interactions in SERT (a) and DAT receptors (b).
According to Figure 4, it is possible to notice that patchoulol fits better in the catalytic site of the DAT protein, performing interactions with a greater number of amino acid residues, which directly influences the affinity energy. In the patchoulol-DAT complex, hydrogen-bonding interactions occur between the hydroxyl group of the ligand and Ser421 and Tyr124 residues. In contrast, in the SERT protein, patchoulol does not interact strongly due to the 4.99 Å distance to the Tyr176 residue. Consequently, the dipole-induced interaction between Ile172 and Tyr176 is weakened, compromising energy redistribution in the site’s surroundings [103]. On the other hand, (−)-β-caryophyllene has good affinity at the SERT receptor (−96.20 kJ/mol) but has a significant loss in affinity towards the DAT receptor (−58.55 kJ/mol), suggesting greater performance in the serotonergic pathway (Figure 5).
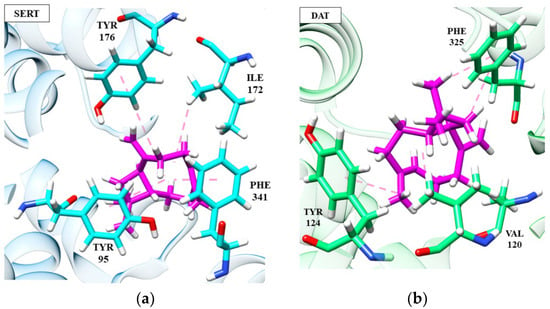
Figure 5.
Interactions of (-)-β-caryophyllene (magenta) at SERT (a) and DAT (b) receptors.
The (−)-β-caryophyllene molecule performs hydrophobic and electrostatic interactions with the SERT residues of Tyr176, Tyr95, Phe341 and Ile172, which are important for blocking serotonin transport, as discussed in previous topics. These interactions, except Tyr95, are absent in patchoulol, compromising its target affinity and indicating its relevance to the stability of the complexes. In the DAT receptor, both (−)-β-caryophyllene and patchoulol interact with the Val120 residue, which is largely conserved and faces the cycloheptene ring of Phe325. This triad of interactions is necessary for DAT blockade [105]. However, we can suggest that the lower affinity of (−)-β-caryophyllene at the DAT receptor is due to the absence of interaction between Ser421 and Phe43, which, on the other hand, is visualized in patchoulol.
Ser421 coordinates the sodium ion (cofactor). It makes a hydrogen interaction with the carbonyl of the Phe43 residue, and then these two residues participate in a network of hydrogen bonds that interconnect patchoulol. Such interactions have already been described as essential for ligand recognition and affinity [103]. The results obtained in this molecular docking analysis correlate with the antidepressant effects observed for the EOs of the species Anthriscus nemorosa and Pogostemon cablin [61].
4.3. Phenylpropanoids
The compound (E)-anethole showed antidepressant activity in vivo by the monoaminergic mechanism, specifically 5-HT and DA. (E)-cinnamaldehyde showed an antidepressant effect without elucidation of the mechanism of action. For this reason, molecular docking of these two phenylpropanoids was carried out against the SERT and DAT receptors. Based on the docking energy result, (E)-anethole showed a higher affinity in the SERT (−87.82 kJ/mol) compared to the DAT (−64.05 kJ/mol), suggesting that the action in the dopaminergic pathway may not only be due to the blockade of the DAT. In contrast, (E)-cinnamaldehyde had similar affinity energies on the two targets with a slight preference for SERT. Figure 6 shows the interactions of the four complexes formed.
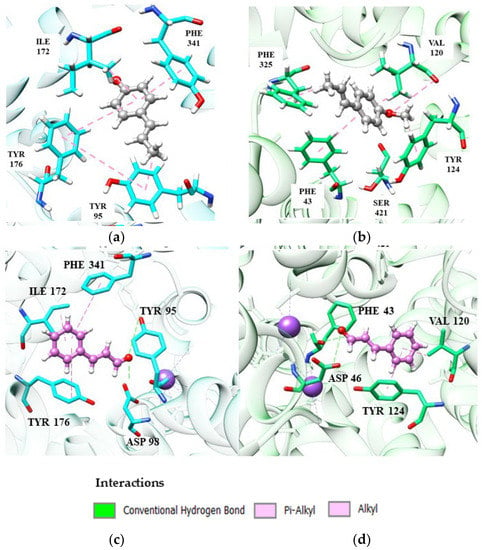
Figure 6.
Interactions of (E)-anethole and (E)-cinnamaldehyde phenylpropanoids at the SERT and DAT site: (a,b) catalytic site of SER and DAT, respectively, complexed with (E)-anethole (dark gray); (c,d) catalytic site of SERT and DAT, respectively, complexed with (E)-cinnamaldehyde (magenta).
(E)-Anethole coordinates π-alkyl-like interactions between Ile172 and Tyr95 and π-pairing between Tyr176 and Phe341 with the receptor SERT (Figure 6). These interactions have been discussed in previous topics and are crucial for the binding and stability of a potential SERT inhibitor. (E)-Cinnamaldehyde (Figure 6C) also repeats the same interactions.
Evaluating the interactions present in DAT, the conformation adopted by the more stable (E)-anethole prevents the interaction between its carbonyl group with Ser421, negatively implying the affinity of the complex. (E)-Cinnamaldehyde also does not interact with Ser421, but its stability is not as compromised due to its conformation, which facilitates an additional hydrogen-bonding interaction between Phe43 and Asp46.
5. Molecular Docking (In Silico) Study: Final Considerations
Among the compounds evaluated in this study by molecular docking analysis, we can highlight those with better affinity energies against the two targets, specifically, (S)-(+)-linalool (SERT: −92.83 and DAT: −85.86), (E)-cinnamaldehyde (SERT: −86.56 kJ/mol and DAT: −72.76 kJ/mol) and γ-terpinene (SERT: −81.86 kJ/mol and DAT: −70.18). Most published studies describe the antidepressant effect of essential oils rich in monoterpenes. However, the results obtained in our in silico evaluation show that this property is not restricted to this class of compounds. For example, the sesquiterpene patchoulol demonstrated the best affinity results on the dopamine target. The phenylpropanoid (E)-anethole showed an affinity energy at the SERT receptor (−87.82 kJ/mol) similar to the monoterpenoid linalool.
The results obtained in the present study reinforce the importance of the synergistic effect between the components of the EOs, which may be involved in different mechanisms related to the pathophysiology of depression. Therefore, the therapeutic effect may not be directly linked to the concentration of a single component since different compounds can express favorable results, such as enhancing the effectiveness of the effect, minimizing or delaying the development of resistance and providing selective synergism against the target [106]. The in silico analysis was limited to evaluating only two targets of the monoaminergic pathway. However, depression, being a multifactorial disease, is far from being entirely explained by monoamine deficiency alone [107]. In this way, for the first time, this review work confirms, at the molecular level, some of the main mechanisms involved in depression described in the past ten years. More robust in silico techniques are needed to fully clarify the mechanism of antidepressant action of the compounds present in EOs. However, our results are in alignment with the in vivo results and demonstrate the binding energy influences of molecules against different targets.
6. Materials and Methods
The present study was based on scientific publications on EOs from plants with antidepressant activity between the years 2012 and 2022. Figure 7 emphasizes the gradual growth that publications on this topic were having from 2016 to 2019. The year 2020 is marked by the COVID-19 pandemic and the whole world joined forces in the search for measures against this disease [108,109]. In 2021 and 2022, there is an increase in publications on the topic of depression. For this reason, the production of this review highlighted aromatic plants with antidepressant activity, as well as the major chemical components of EOs, applied assay models (in silico, in vitro and in vivo) and their corresponding mechanisms of action, which are listed in Table 1.
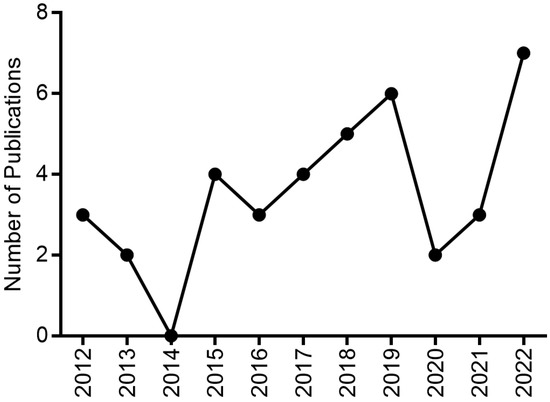
Figure 7.
Graphical representation of the number of publications on EOs with antidepressant activity in the past decade.
6.1. Search Strategy and Inclusion and Exclusion Criteria
The search for information on the chemical composition of EOs and tests performed were implemented considering all articles published in the past ten years (2012–2022) in the literature databases: Scopus (https://www.scopus.com, accessed on 17 November 2022), Science Direct (https://www.sciencedirect.com/, accessed on 18 November 2022) and PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 19 November 2022). The primary keywords “essential oil” or “volatile compounds” and “antidepressant effect” or “antidepressant activity” were searched and combined in the titles and abstract. Inclusion criteria for sections of this study were accessed for chemical composition and assays to support antidepressant activity. Searches performed with commercial samples of EO, review articles, books, book chapters and abstracts were excluded. Figure 8 summarizes the general methodology by highlighting the articles collected in each database, duplicate and deleted files and finally how many were selected for the writing of this review.
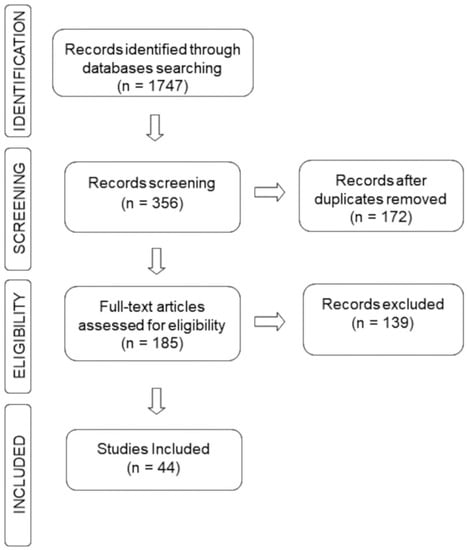
Figure 8.
Search strategy flowchart.
6.2. Study Records: Data Management
The mechanism used for data management is described in Table 1, informing the name of the plant species and main components of the EO, as well as the type of test carried out to evaluate the antidepressant effects of the EO. Two different reviewers completed the reading and revision of the cited articles in each selection phase.
7. Conclusions
EOs have been widely studied for their therapeutic properties and potential health benefits and as an alternative for the treatment of depression [44,108]. In this context, the search for bioactive molecules of natural origin is a promising area in pharmaceutical research, since many plants have compounds with proven antidepressant activity [110,111]. The research of bioactive compounds against depression of natural origin is important not only for the possibility of developing new safer and more effective drugs, but also for the valorization of traditional knowledge and biodiversity [112]. Many plant species that contain EOs with antidepressant activity widely studied, have shown promise for more advanced studies, including Roman chamomile (Chamaemelum nobile L.), rosemary (Rosmarinus officinalis L.) and sweet orange (Citrus sinensis (L.) Osbeck). These plants are good candidates for clinical trials due to their proven effects in reducing symptoms of depression, as well as being of low toxicity and being widely available and used in the cosmetics and food industry [113,114,115]. However, each plant species has a unique chemical composition, and the concentration and activity of bioactive components may vary in different cultivars or geographical origins [116]. For this reason, careful selection of the raw material used in the production of essential oils is fundamental to ensure the quality and effectiveness of the results of clinical studies [117]. Although EOs are often considered safe and natural, it is important to recognize that they are highly concentrated chemical compounds and should be used with caution [118]. Some EOs can cause skin or eye irritation and can be toxic if ingested in large quantities [119,120]. The specific side effects depend on the EO in question, as well as the dose and method of administration [121].
Therefore, this review offers an overview of the evidence found in the past ten years of the use of EOs with antidepressant activity as well as different routes of administration and the main mechanisms. In the general analysis performed, aromatherapy showed its potential to be used as an effective therapeutic option for the relief of depressive symptoms.
Author Contributions
E.C.M.F., L.R.F. and J.K.R.D.S. conducted the bibliographic research, supervision and writing, review and editing. W.N.S., P.L.B.F., C.d.S.F.M. and J.K.R.D.S. revised, edited, and provided critical feedback. All authors approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are grateful the Fundação da Amazonia de Amparo a Estudos e Pesquisas (FAPESPA) and the Coordination for the Improvement of Higher Education Personnel (CAPES) for their financial support, https://aromaticplant.org/).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AM | Action Mechanism |
| BPR | Buspirone |
| COVID-19 | Coronavirus disease 2019 |
| CPM | Clomipramine |
| CUMS | Chronic Unpredictable Mild Stress |
| DPM | Desipramine |
| DZP | Diazepan |
| EPM | Elevated Plus-Maze Test |
| FST | Forced Swimming Test |
| FLU | Fluxetine |
| HBT | Hole Bboard Test |
| ICV | Intracerebroventricular |
| INH | Inhalation |
| IP | Intraperitoneal |
| IPM | Imipramine |
| IT | Immobility Time |
| NSFT | Novelty-Suppressed Feeding Test |
| OFT | Open Field Test |
| PE | Pharmacological Effect |
| PO | Oral Administration |
| RA | Route of Administration |
| RAMT | Radial Arm-Maze Test |
| RD | Reference Drug |
| RSP | Reserpine |
| SER | Sertraline hydrochloride |
| SLM | Scopolamine |
| SPT | Sucrose Preference Teste |
| TST | Tail Suspension Test |
| YMT | Y-Maze Task |
References
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Mental Health Report: Transforming Mental Health for All; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Kola, L.; Kohrt, B.A.; Hanlon, C.; Naslund, J.A.; Sikander, S.; Balaji, M.; Benjet, C.; Cheung, E.Y.L.; Eaton, J.; Gonsalves, P.; et al. COVID-19 Mental Health Impact and Responses in Low-Income and Middle-Income Countries: Reimagining Global Mental Health. Lancet Psychiatry 2021, 8, 535–550. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Xiao, H.X.; Syeda, K.; Vinberg, M.; Carvalho, A.F.; Mansur, R.B.; Maruschak, N.; Cha, D.S. The Prevalence, Measurement, and Treatment of the Cognitive Dimension/Domain in Major Depressive Disorder. CNS Drugs 2015, 29, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Morozova, A.; Zorkina, Y.; Abramova, O.; Pavlova, O.; Pavlov, K.; Soloveva, K.; Volkova, M.; Alekseeva, P.; Andryshchenko, A.; Kostyuk, G.; et al. Neurobiological Highlights of Cognitive Impairment in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 1217. [Google Scholar] [CrossRef]
- Fernandes, M.A.; Ribeiro, H.K.P.; Santos, J.D.M.; de Monteiro, C.F.S.; dos Costa, R.S.; Soares, R.F.S. Prevalence of anxiety disorders as a cause of workers’ absence. Rev. Bras. Enferm. 2018, 71, 2213–2220. [Google Scholar] [CrossRef]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune Mechanisms of Depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The Molecular Neurobiology of Depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- do Rosario-Campos, M.C.; Leckman, J.F.; Mercadante, M.T.; Shavitt, R.G.; da Prado, H.S.; Sada, P.; Zamignani, D.; Miguel, E.C. Adults with Early-Onset Obsessive-Compulsive Disorder. Am. J. Psychiatry 2001, 158, 1899–1903. [Google Scholar] [CrossRef]
- Ferrão, Y.A.; Diniz, J.B.; Lopes, A.C.; Shavitt, R.G.; Greenberg, B.; Miguel, E. Resistance and Refractoriness in Obsessive–Compulsive Disorder [Resistência e Refratariedade No Transtorno Obsessivo–Compulsivo]. Rev. Bras. Psiquiatr. 2007, 29, S66–S76. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant Drugs Act by Directly Binding to TRKB Neurotrophin Receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef] [PubMed]
- Santarsieri, D.; Schwartz, T. Antidepressant Efficacy and Side-Effect Burden: A Quick Guide for Clinicians. Drugs Context 2015, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Catterson, M.L.; Preskorn, S.H. Pharmacokinetics of Selective Serotonin Reuptake Inhibitors: Clinical Relevance. Pharmacol. Toxicol. 1996, 78, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Qazi, R. Religion and Mental Health: A Discussion of How Religion Impacts Mental Health/Bio-Psycho-Social Mode of Treatment. J. Depress. Anxiety 2019, 8, 1. [Google Scholar] [CrossRef]
- Hiemke, C.; Härtter, S. Pharmacokinetics of Selective Serotonin Reuptake Inhibitors. Pharmacol. Ther. 2000, 85, 11–28. [Google Scholar] [CrossRef]
- Funk, K.A.; Bostwick, J.R. A Comparison of the Risk of QT Prolongation among SSRIs. Ann. Pharmacother. 2013, 47, 1330–1341. [Google Scholar] [CrossRef]
- Behlke, L.M.; Lenze, E.J.; Carney, R.M. The Cardiovascular Effects of Newer Antidepressants in Older Adults and Those with or At High Risk for Cardiovascular Diseases. CNS Drugs 2020, 34, 1133–1147. [Google Scholar] [CrossRef]
- Bleakley, S. Antidepressant Drug Interactions: Evidence and Clinical Significance: Antidepressant Drug Interactions. Prog. Neurol. Psychiatry 2016, 20, 21–27. [Google Scholar] [CrossRef]
- Naughton, M.; Clarke, G.; O′Leary, O.F.; Cryan, J.F.; Dinan, T.G. A Review of Ketamine in Affective Disorders: Current Evidence of Clinical Efficacy, Limitations of Use and Pre-Clinical Evidence on Proposed Mechanisms of Action. J. Affect. Disord. 2014, 156, 24–35. [Google Scholar] [CrossRef]
- Niesters, M.; Martini, C.; Dahan, A. Ketamine for Chronic Pain: Risks and Benefits: Ketamine Risks and Benefits. Br. J. Clin. Pharmacol. 2014, 77, 357–367. [Google Scholar] [CrossRef]
- Iyer, R.N.; Favela, D.; Zhang, G.; Olson, D.E. The Iboga Enigma: The Chemistry and Neuropharmacology of Iboga Alkaloids and Related Analogs. Nat. Prod. Rep. 2021, 38, 307–329. [Google Scholar] [CrossRef]
- David, D.J.; Gourion, D. Antidépresseurs et tolérance: Déterminants et prise en charge des principaux effets indésirables. Encéphale 2016, 42, 553–561. [Google Scholar] [CrossRef]
- Trindade, E.; Menon, D.; Topfer, L.; Coloma, C. Adverse Effects Associated with Selective Serotonin Reuptake Inhibitors and Tricyclic Antidepressants: A Meta-Analysis. CMAJ 1998, 10, 1245–1252. [Google Scholar]
- Foong, A.-L.; Grindrod, K.A.; Patel, T.; Kellar, J. Démystifier le Syndrome (ou la Toxicité) Sérotoninergique. Can. Fam. Physician 2018, 64, e422–e430. [Google Scholar]
- Shilyansky, C. Effect of Antidepressant Treatment on Cognitive Impairments Associated with Depression: A Randomised Longitudinal Study. Lancet Psychiatry 2016, 3, 425–435. [Google Scholar] [CrossRef]
- Leherbauer, I.; Stappen, I. Selected Essential Oils and Their Mechanisms for Therapeutic Use against Public Health Disorders. An Overview. Z. Für Nat. C 2020, 75, 205–223. [Google Scholar] [CrossRef]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal Medicine for Depression and Anxiety: A Systematic Review with Assessment of Potential Psycho-Oncologic Relevance. Phytother. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Zárybnický, T.; Boušová, I.; Ambrož, M.; Skálová, L. Hepatotoxicity of Monoterpenes and Sesquiterpenes. Arch. Toxicol. 2018, 92, 1–13. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Heires, A.J.; Bailey, K.L.; Katafiasz, D.M.; Toews, M.L.; Wichman, C.S.; Romberger, D.J. Docosahexaenoic Acid Enhances Amphiregulin-Mediated Bronchial Epithelial Cell Repair Processes Following Organic Dust Exposure. Am. J. Physiol.-Lung Cell Mol. Physiol. 2018, 314, L421–L431. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, É.R.Q.; Maia, J.G.S.; Fontes-Júnior, E.A.; do Socorro Ferraz Maia, C. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef] [PubMed]
- Mahdood, B.; Imani, B.; Khazaei, S. Effects of Inhalation Aromatherapy with Rosa Damascena (Damask Rose) on the State Anxiety and Sleep Quality of Operating Room Personnel during the COVID-19 Pandemic: A Randomized Controlled Trial. J. PeriAnesthesia Nurs. 2022, 37, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Soldatelli, M.V.; Ruschel, K.; Isolan, T.M.P. Valeriana officinalis: Uma alternativa para o controle da ansiedade odontológica? Stomatos 2010, 16, 89–97. [Google Scholar]
- Astuti, P.; Khairan, K.; Marthoenis, M.; Hasballah, K. Antidepressant-like Activity of Patchouli Oil Var. Tapak Tuan (Pogostemon Cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model. Pharmaceuticals 2022, 15, 608. [Google Scholar] [CrossRef]
- Watanabe, E.; Kuchta, K.; Kimura, M.; Rauwald, H.W.; Kamei, T.; Imanishi, J. Effects of Bergamot (Citrus bergamia (Risso) Wright & Arn.) Essential Oil Aromatherapy on Mood States, Parasympathetic Nervous System Activity, and Salivary Cortisol Levels in 41 Healthy Females. Complement. Med. Res. 2015, 22, 43–49. [Google Scholar] [CrossRef]
- Dagli, R.; Avcu, M.; Metin, M.; Kiymaz, S.; Ciftci, H. The Effects of Aromatherapy Using Rose Oil (Rosa damascena Mill.) on Preoperative Anxiety: A Prospective Randomized Clinical Trial. Eur. J. Integr. Med. 2019, 26, 37–42. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, L. Anxiolytic Effect of Essential Oils and Their Constituents: A Review. J. Agric. Food Chem. 2019, 67, 13790–13808. [Google Scholar] [CrossRef]
- Yap, W.S.; Dolzhenko, A.V.; Jalal, Z.; Hadi, M.A.; Khan, T.M. Efficacy and Safety of Lavender Essential Oil (Silexan) Capsules among Patients Suffering from Anxiety Disorders: A Network Meta-Analysis. Sci. Rep. 2019, 9, 18042. [Google Scholar] [CrossRef]
- The International Natural Product Sciences Taskforce; Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Yu, S.; Li, D.; Yang, M.; Guan, Y.; Zhang, D.; Wan, J.; Liu, S.; Shi, A.; et al. Natural Volatile Oils Derived from Herbal Medicines: A Promising Therapy Way for Treating Depressive Disorder. Pharmacol. Res. 2021, 164, 105376. [Google Scholar] [CrossRef]
- de Sousa, D.; Silva, R.; Silva, E.; Gavioli, E. Essential Oils and Their Constituents: An Alternative Source for Novel Antidepressants. Molecules 2017, 22, 1290. [Google Scholar] [CrossRef]
- Villas Boas, G.R.; de Lacerda, R.B.; Paes, M.M.; Gubert, P.; da Almeida, W.L.C.; Rescia, V.C.; de Carvalho, P.M.G.; de Carvalho, A.A.V.; Oesterreich, S.A. Molecular Aspects of Depression: A Review from Neurobiology to Treatment. Eur. J. Pharmacol. 2019, 851, 99–121. [Google Scholar] [CrossRef]
- Delgado, P.L. Depression: The Case for a Monoamine Deficiency. J. Clin. Psychiatry 2000, 61, 7–11. [Google Scholar]
- Andersen, J.; Taboureau, O.; Hansen, K.B.; Egebjerg, J.; Strømgaard, K.; Kristensen, A.S. Location of the Antidepressant Binding Site in the Serotonin Transporter. J. Biol. Chem. 2009, 284, 10276–10284. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry 2022, 1–14. [Google Scholar] [CrossRef]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the Pathophysiology of Depression: From Monoamines to the Neurogenesis Hypothesis Model—Are We There Yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Di Carlo, P.; Punzi, G.; Ursini, G. Brain-Derived Neurotrophic Factor and Schizophrenia. Psychiatr. Genet. 2019, 29, 200–210. [Google Scholar] [CrossRef]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of Lavender Oil Inhalation on Improving Scopolamine-Induced Spatial Memory Impairment in Laboratory Rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Samojlik, I.; Mijatović, V.; Petković, S.; Škrbić, B.; Božin, B. The Influence of Essential Oil of Aniseed (Pimpinella anisum, L.) on Drug Effects on the Central Nervous System. Fitoterapia 2012, 83, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gutiérrez, S.L.; Gómez-Cansino, R.; García-Zebadúa, J.C.; Jiménez-Pérez, N.C.; Reyes-Chilpa, R. Antidepressant Activity of Litsea Glaucescens Essential Oil: Identification of β-Pinene and Linalool as Active Principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, J.F.; Silva, M.I.G.; de Aquino Neto, M.R.; Moura, B.A.; de Carvalho, A.M.R.; Vasconcelos, P.F.; Barbosa Filho, J.M.; Gutierrez, S.J.C.; Vasconcelos, S.M.M.; Macêdo, D.S.; et al. Antidepressant-like Effect of bis-Eugenol in the Mice Forced Swimming Test: Evidence for the Involvement of the Monoaminergic System. Fundam. Clin. Pharmacol. 2013, 27, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.; Bettio, L.E.B.; Oliveira, Á.; Pazini, F.L.; Dalmarco, J.B.; Simionatto, E.L.; et al. Antidepressant-like Effects of Fractions, Essential Oil, Carnosol and Betulinic Acid Isolated from Rosmarinus Officinalis L. Food Chem. 2013, 136, 999–1005. [Google Scholar] [CrossRef]
- Hritcu, L.; Bagci, E.; Aydin, E.; Mihasan, M. Antiamnesic and Antioxidants Effects of Ferulago Angulata Essential Oil against Scopolamine-Induced Memory Impairment in Laboratory Rats. Neurochem. Res. 2015, 40, 1799–1809. [Google Scholar] [CrossRef]
- Gradinariu, V.; Cioanca, O.; Hritcu, L.; Trifan, A.; Gille, E.; Hancianu, M. Comparative Efficacy of Ocimum Sanctum L. and Ocimum Basilicum L. Essential Oils against Amyloid Beta (1–42)-Induced Anxiety and Depression in Laboratory Rats. Phytochem. Rev. 2015, 14, 567–575. [Google Scholar] [CrossRef]
- Liu, B.-B.; Luo, L.; Liu, X.-L.; Geng, D.; Li, C.-F.; Chen, S.-M.; Chen, X.-M.; Yi, L.-T.; Liu, Q. Essential Oil of Syzygium Aromaticum Reverses the Deficits of Stress-Induced Behaviors and Hippocampal p-ERK/p-CREB/Brain-Derived Neurotrophic Factor Expression. Planta Med. 2015, 81, 185–192. [Google Scholar] [CrossRef]
- Duan, D.; Chen, L.; Yang, X.; Tu, Y.; Jiao, S. Antidepressant-like Effect of Essential Oil Isolated from Toona ciliata Roem. var yunnanensis. J. Nat. Med. 2015, 69, 191–197. [Google Scholar] [CrossRef]
- Bagci, E.; Aydin, E.; Ungureanu, E.; Hritcu, L. Anthriscus Nemorosa Essential Oil Inhalation Prevents Memory Impairment, Anxiety and Depression in Scopolamine-Treated Rats. Biomed. Pharmacother. 2016, 84, 1313–1320. [Google Scholar] [CrossRef]
- Cioanca, O.; Hancianu, M.; Mircea, C.; Trifan, A.; Hritcu, L. Essential Oils from Apiaceae as Valuable Resources in Neurological Disorders: Foeniculi vulgare aetheroleum. Ind. Crops Prod. 2016, 88, 51–57. [Google Scholar] [CrossRef]
- Aydin, E.; Hritcu, L.; Dogan, G.; Hayta, S.; Bagci, E. The Effects of Inhaled Pimpinella Peregrina Essential Oil on Scopolamine-Induced Memory Impairment, Anxiety, and Depression in Laboratory Rats. Mol. Neurobiol. 2016, 53, 6557–6567. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, T.; Wang, R.; Ma, Y.; Song, S.; Liu, J.; Hu, W.; Li, S. Inhalation of Roman Chamomile Essential Oil Attenuates Depressive-like Behaviors in Wistar Kyoto Rats. Sci. China Life Sci. 2017, 60, 647–655. [Google Scholar] [CrossRef]
- Sohrabi, R.; Pazgoohan, N.; Rezaei Seresht, H.; Amin, B. Repeated Systemic Administration of the Cinnamon Essential Oil Possesses Anti-Anxiety and Anti-Depressant Activities in Mice. Iran. J. Basic Med. Sci. 2017, 20, 708. [Google Scholar] [CrossRef]
- Ali, S.; Abd El Wahab, M.; Ayuob, N.; Suliaman, M. The Antidepressant-like Effect of Ocimum Basilicum in an Animal Model of Depression. Biotech. Histochem. 2017, 92, 390–401. [Google Scholar] [CrossRef]
- Ayuob, N.N.; Firgany, A.E.-D.L.; El-Mansy, A.A.; Ali, S. Can Ocimum Basilicum Relieve Chronic Unpredictable Mild Stress-Induced Depression in Mice? Exp. Mol. Pathol. 2017, 103, 153–161. [Google Scholar] [CrossRef]
- Akbaba, E.; Hassan, S.; Mohammed Sur, T.; Bagci, E. Memory Enhancing, Anxiolytic and Antidepressant Effects of Achillea biebersteinii (Asteraceae) Essential Oil on Scopolamine-Induced Rats. J. Essent. Oil Bear. Plants 2018, 21, 825–839. [Google Scholar] [CrossRef]
- dos Santos, É.R.Q.; Maia, C.S.F.; Fontes Junior, E.A.; Melo, A.S.; Pinheiro, B.G.; Maia, J.G.S. Linalool-Rich Essential Oils from the Amazon Display Antidepressant-Type Effect in Rodents. J. Ethnopharmacol. 2018, 212, 43–49. [Google Scholar] [CrossRef]
- Parente, M.; Custódio, F.; Cardoso, N.; Lima, M.; Melo, T.; Linhares, M.; Siqueira, R.; Nascimento, A.; Catunda Júnior, F.; Melo, C. Antidepressant-Like Effect of Lippia Sidoides CHAM (Verbenaceae) Essential Oil and Its Major Compound Thymol in Mice. Sci. Pharm. 2018, 86, 27. [Google Scholar] [CrossRef]
- Amiresmaeili, A.; Roohollahi, S.; Mostafavi, A.; Askari, N. Effects of Oregano Essential Oil on Brain TLR4 and TLR2 Gene Expression and Depressive-like Behavior in a Rat Model. Res. Pharm. Sci. 2018, 13, 130. [Google Scholar] [CrossRef]
- Abouhosseini Tabari, M.; Hajizadeh Moghaddam, A.; Maggi, F.; Benelli, G. Anxiolytic and Antidepressant Activities of Pelargonium Roseum Essential Oil on Swiss Albino Mice: Possible Involvement of Serotonergic Transmission. Phytother. Res. 2018, 32, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Diniz, T.C.; de Oliveira Júnior, R.G.; Miranda Bezerra Medeiros, M.A.; Gama E Silva, M.; de Andrade Teles, R.B.; dos Passos Menezes, P.; de Sousa, B.M.H.; Abrahão Frank, L.; de Souza Araújo, A.A.; Russo Serafini, M.; et al. Anticonvulsant, Sedative, Anxiolytic and Antidepressant Activities of the Essential Oil of Annona Vepretorum in Mice: Involvement of GABAergic and Serotonergic Systems. Biomed. Pharmacother. 2019, 111, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Z.; Ren, J.; Fan, G.; Pan, S. Dietary Essential Oil from Navel Orange Alleviates Depression in Reserpine-treated Mice by Monoamine Neurotransmitters. Flavour Fragr. J. 2019, 34, 252–259. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Yang, Z.-Y.; Fan, G.; Ren, J.-N.; Yin, K.-J.; Pan, S.-Y. Antidepressant-like Effect of Citrus Sinensis (L.) Osbeck Essential Oil and Its Main Component Limonene on Mice. J. Agric. Food Chem. 2019, 67, 13817–13828. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Les, F.; Lopez, V.; Galeotti, N. Lavender (Lavandula Angustifolia Mill.) Essential Oil Alleviates Neuropathic Pain in Mice With Spared Nerve Injury. Front. Pharmacol. 2019, 10, 472. [Google Scholar] [CrossRef]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus Halepensis Essential Oil Attenuates the Toxic Alzheimer’s Amyloid Beta (1-42)-Induced Memory Impairment and Oxidative Stress in the Rat Hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef]
- Cahyono, E.; Rimawati, B.C.; Kusuma, E. Antidepressant Activity of Patchouli Alcohol Microcapsule. J. Phys. Conf. Ser. 2019, 1321, 022039. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, A.; Sachan, N.; Aggarwal, I.; Chandra, P. GABAergic and Serotonergic System Mediated Psychoneuropharmacological Activities of Essential Oil from the Leaves of Aegle Marmelos: An in vivo and in silico Approach. J. Essent. Oil Bear. Plants 2020, 23, 1265–1282. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Kadkhoda, Z.; Taghizad-Farid, R. The Antidepressant-like Effects of Origanum Majorana Essential Oil on Mice through Monoaminergic Modulation Using the Forced Swimming Test. J. Tradit. Complement. Med. 2020, 10, 327–335. [Google Scholar] [CrossRef]
- Chang, H.-T.; Chang, M.-L.; Chen, Y.-T.; Chang, S.-T.; Hsu, F.-L.; Wu, C.-C.; Ho, C.-K. Evaluation of Motor Coordination and Antidepressant Activities of Cinnamomum Osmophloeum Ct. Linalool Leaf Oil in Rodent Model. Molecules 2021, 26, 3037. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Maleki, S.G. Antidepressant-like Effects of Foeniculum Vulgare Essential Oil and Potential Involvement of Dopaminergic and Serotonergic Systems on Mice in the Forced Swim Test. PharmaNutrition 2021, 15, 100241. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, A.; Sachan, N.; Chandra, P. Anxiolytic and Antidepressant-like Effects of Essential Oil from the Fruits of Piper Nigrum Linn. (Black Pepper) in Mice: Involvement of Serotonergic but Not GABAergic Transmission System. Heliyon 2021, 7, e06884. [Google Scholar] [CrossRef]
- Tang, D.; Liang, Q.; Zhang, M.; Li, M.; Zhang, Q.; Zhang, S.; Ai, L.; Wu, C. Anti-Depression Effectiveness of Essential Oil from the Fruits of Zanthoxylum bungeanum Maxim. on Chronic Unpredictable Mild Stress-Induced Depression Behavior in Mice. Front. Pharmacol. 2022, 13, 999962. [Google Scholar] [CrossRef]
- Zhang, L.; Ai, Y.; Tang, M.; Ai, Y.; Song, N.; Ren, L.; Zhang, Z.; Rong, B.; Chen, X.; Xu, X.; et al. Antidepressant-Like Effect of Aromatherapy with Magnolia sieboldii Essential Oils on Depression Mice. Rec. Nat. Prod. 2023, 17, 241–255. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Liu, Y.-T.; Jiang, S.-N.; Guo, P.-M.; Wu, X.-Y.; Yu, J. Essential Oil from the Roots of Paeonia lactiflora Pall. Has Protective Effect against Corticosterone-Induced Depression in Mice via Modulation of PI3K/Akt Signaling Pathway. Front. Pharmacol. 2022, 13, 999712. [Google Scholar] [CrossRef]
- Vanegas Andrade, C.; Matera, S.; Bayley, M.; Colareda, G.; Ruiz, M.E.; Prieto, J.; Retta, D.; van Baren, C.; Consolini, A.E.; Ragone, M.I. Antispasmodic, Antidepressant and Anxiolytic Effects of Extracts from Schinus lentiscifolius Marchand Leaves. J. Tradit. Complement. Med. 2022, 12, 141–151. [Google Scholar] [CrossRef]
- Birmann, P.T.; Casaril, A.M.; Zugno, G.P.; Acosta, G.G.; Severo Sabedra Sousa, F.; Collares, T.; Seixas, F.K.; Jacob, R.G.; Brüning, C.A.; Savegnago, L.; et al. Flower Essential Oil of Tagetes minuta Mitigates Oxidative Stress and Restores BDNF-Akt/ERK2 Signaling Attenuating Inflammation- and Stress-Induced Depressive-like Behavior in Mice. Brain Res. 2022, 1784, 147845. [Google Scholar] [CrossRef]
- De Carvalho, R.B.F.; De Almeida, A.A.C.; Campelo, N.B.; Lellis, D.R.O.D.; Nunes, L.C.C. Nerolidol and Its Pharmacological Application in Treating Neurodegenerative Diseases: A Review. Recent Pat. Biotechnol. 2018, 12, 158–168. [Google Scholar] [CrossRef]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-Caryophyllene Enhances Wound Healing through Multiple Routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Hu, Q.; Zuo, T.; Deng, L.; Chen, S.; Yu, W.; Liu, S.; Liu, J.; Wang, X.; Fan, X.; Dong, Z. β-Caryophyllene Suppresses Ferroptosis Induced by Cerebral Ischemia Reperfusion via Activation of the NRF2/HO-1 Signaling Pathway in MCAO/R Rats. Phytomedicine 2022, 102, 154112. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Djafarian, K.; Mofidi Nejad, M.; Yekaninejad, M.S.; Javanbakht, M.H. The Effect of β-Caryophyllene on Food Addiction and Its Related Behaviors: A Randomized, Double-Blind, Placebo-Controlled Trial. Appetite 2022, 178, 106160. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential Oils as Antiviral Agents, Potential of Essential Oils to Treat SARS-CoV-2 Infection: An In Silico Investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef] [PubMed]
- Snow Setzer, M.; Sharifi-Rad, J.; Setzer, W. The Search for Herbal Antibiotics: An In Silico Investigation of Antibacterial Phytochemicals. Antibiotics 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. More Lessons from Linalool: Insights Gained from a Ubiquitous Floral Volatile. Curr. Opin. Plant Biol. 2016, 32, 31–36. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahwan, M.; Shamsi, A.; Alhumaydhi, F.A.; Alsagaby, S.A.; Al Abdulmonem, W.; Abdullaev, B.; Yadav, D.K. Elucidating the Interactions of Fluoxetine with Human Transferrin Employing Spectroscopic, Calorimetric, and In Silico Approaches: Implications of a Potent Alzheimer’s Drug. ACS Omega 2022, 7, 9015–9023. [Google Scholar] [CrossRef]
- Coleman, J.A.; Gouaux, E. Structural Basis for Recognition of Diverse Antidepressants by the Human Serotonin Transporter. Nat. Struct. Mol. Biol. 2018, 25, 170–175. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, N.; Cho, S.; MacKerell, A.D. Consideration of Molecular Weight during Compound Selection in Virtual Target-Based Database Screening. J. Chem. Inf. Comput. Sci. 2003, 43, 267–272. [Google Scholar] [CrossRef]
- Nishio, M.; Umezawa, Y.; Fantini, J.; Weiss, M.S.; Chakrabarti, P. CH–π Hydrogen Bonds in Biological Macromolecules. Phys. Chem. Chem. Phys. 2014, 16, 12648–12683. [Google Scholar] [CrossRef]
- Fokoue, H.; Pinheiro, P.; Fraga, C.; Sant’Anna, C. Há Algo Novo No Reconhecimento Molecular Aplicado à Química Medicinal? Quím. Nova 2020, 43, 78–89. [Google Scholar] [CrossRef]
- Andersen, J.; Stuhr-Hansen, N.; Zachariassen, L.G.; Koldsø, H.; Schiøtt, B.; Strømgaard, K.; Kristensen, A.S. Molecular Basis for Selective Serotonin Reuptake Inhibition by the Antidepressant Agent Fluoxetine (Prozac). Mol. Pharmacol. 2014, 85, 703–714. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, J.; Smolensky, D.; Lee, S.-H. Potential Benefits of Patchouli Alcohol in Prevention of Human Diseases: A Mechanistic Review. Int. Immunopharmacol. 2020, 89, 107056. [Google Scholar] [CrossRef]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-Ray Structure of Dopamine Transporter Elucidates Antidepressant Mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Zięba, A.; Stępnicki, P.; Matosiuk, D.; Kaczor, A.A. Overcoming Depression with 5-HT2A Receptor Ligands. Int. J. Mol. Sci. 2021, 23, 10. [Google Scholar] [CrossRef]
- Harper, L.; Kalfa, N.; Beckers, G.M.A.; Kaefer, M.; Nieuwhof-Leppink, A.J.; Fossum, M.; Herbst, K.W.; Bagli, D. The Impact of COVID-19 on Research. J. Pediatr. Urol. 2020, 16, 715–716. [Google Scholar] [CrossRef]
- Riccaboni, M.; Verginer, L. The Impact of the COVID-19 Pandemic on Scientific Research in the Life Sciences. PLoS ONE 2022, 17, e0263001. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Bai, X.; Wang, M.; Yan, J.; Cao, Y. The Effects of Aromatherapy on Anxiety and Depression in People with Cancer: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 853056. [Google Scholar] [CrossRef]
- Ma, K.; Baloch, Z.; Mao, F. Natural Products as a Source for New Leads in Depression Treatment. Evid. Based Complement. Alternat. Med. 2022, 2022, 9791434. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vidaña, D.I.; Ngai, S.P.-C.; He, W.; Chow, J.K.-W.; Lau, B.W.-M.; Tsang, H.W.-H. The Effectiveness of Aromatherapy for Depressive Symptoms: A Systematic Review. Evid. Based Complement. Alternat. Med. 2017, 2017, 5869315. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ferdousi, F.; Fukumitsu, S.; Kuwata, H.; Isoda, H. Antidepressant- and Anxiolytic-Like Activities of Rosmarinus Officinalis Extract in Rodent Models: Involvement of Oxytocinergic System. Biomed. Pharmacother. 2021, 144, 112291. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Gupta Chamomile: A Herbal Medicine of the Past with a Bright Future (Review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef]
- Muchuweti, M.; Nyamukonda, L.; Chagonda, L.S.; Ndhlala, A.R.; Mupure, C.; Benhura, M. Total Phenolic Content and Antioxidant Activity in Selected Medicinal Plants of Zimbabwe. Int. J. Food Sci. Technol. 2006, 41, 33–38. [Google Scholar] [CrossRef]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia Officinalis Essential Oil: Chemical Analysis and Evaluation of Anti-Enzymatic and Antioxidant Bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Manion, C.R.; Widder, R.M. Essentials of Essential Oils. Am. J. Health. Syst. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef]
- Sergoynne, L.; Mertens, M.; Dendooven, E.; Leysen, J.; Aerts, O. Allergic Contact Dermatitis, Mimicking Atopic Dermatitis, Associated with the Use of Essential Oils in “Home-Made” Cosmetics and Aromatherapy Diffusers. Contact Dermat. 2020, 83, 311–313. [Google Scholar] [CrossRef]
- Posadzki, P.; Alotaibi, A.; Ernst, E. Adverse Effects of Aromatherapy: A Systematic Review of Case Reports and Case Series. Int. J. Risk Saf. Med. 2012, 24, 147–161. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).